Ascophyllum nodosum and Fucus vesiculosus ameliorate restenosis via improving inf lammation and regulating the PTEN/PI3K/AKT signaling pathway
2024-01-24CrystlNgofiZumbiChunHsuPnHuiYuHungChiehHsiWu
Crystl Ngofi Zumbi, Chun-Hsu Pn, Hui-Yu Hung, Chieh-Hsi Wu,
a Program in Clinical Drug Development from Herbal Medicine, College of Pharmacy, Taipei Medical University, Taipei 11031, China
b Program in Drug Discovery and Development Industry, College of Pharmacy, Taipei Medical University, Taipei 11031, China
c School of Pharmacy, Taipei Medical University, Taipei 11031, China
d Graduate Institute of Metabolism and Obesity Sciences, Taipei Medical University, Taipei 11031, China
Keywords: Ascophyllum nodosum Fucus vesiculosus PTEN/PI3K/AKT Restenosis Macrophage Vascular smooth muscle cells Gut microbiota
ABSTRACT Restenosis is a common complication following coronary angioplasty. The traditional use of seaweeds for health benef its has increasingly been explored, however few studies exist reporting its protective effects on the development of restenosis and gut dysbiosis. The aim of this study was to investigate the potential of seaweed extracts (SE) of Ascophyllum nodosum and Fucus vesiculosus in inhibiting intimal hyperplasia in a rat model of restenosis and its underlying mechanisms in macrophages and vascular smooth muscle cells(vSMCs). 16S rRNA sequencing was done to investigate the regulatory effect of SE on the gut microbiome of injured rats. As indicated by the results, SE signif icantly inhibited the progression of intimal hyperplasia in vivo, attenuated inflammation in macrophages and could inhibit the proliferation, dedifferentiation and migration of vSMCs. It was observed through immunoblotting assays that treatment with SE significantly upregulated PTEN expression in macrophages and inhibited the upregulation of PI3K and AKT expression in vSMCs. Meanwhile, according to the 16S rRNA gene sequencing analysis, supplementation with SE modulated gut microbiota composition in injured rats. In conclusion, SE could ameliorate intimal hyperplasia by inhibiting inf lammation and vSMCs proliferation through the regulation of the PTEN/PI3K/AKT pathway and modulating the gut microbiome.
1. Introduction
Coronary heart diseases (CHDs) in conjunction with other diseases have been reported as part of the predominant causes of disability globally[1]. The best treatment option for CHD patients is percutaneous coronary intervention (PCI), which, despite it being the best, has for many years now, remained the focus of most research and development. However, with the advances made in improving the outcome of this procedure (mainly by employing stents coated with drugs), delayed healing, restenosis and the onset of late thrombosis remains a major disadvantage, hence there is need for effective alternative treatments[2-4]. The pathogenesis of restenosis is a quite complex one and is yet to be fully understood. However, existing evidence show that, in the development of restenosis, persistent inf lammation, migration and increased proliferation of vSMCs are the notable hallmarks present. At the onset phases, in response to vascular injury, immune cells and platelets produce inf lammatory mediators,cytokines and growth factors (e.g. platelet-derived growth factor BB; PDGF-BB) which promotes the dedifferentiation of vSMCs from a contractile to a synthetic phenotype, which intend accelerates migration and proliferation of these cells[5]. In addition to other signalling pathways, the PI3K/AKT signalling pathway has been reported to being activated upon vascular injury and is involved in regulating proliferation, survival and inflammatory status of cells[6-8].Another important protein related to this pathway is the phosphatase and tensin homolog (PTEN). It’s a protein known to suppress many tumour cells and achieves that by antagonizing the function of PI3K hence, negatively regulating cell proliferation and survival[9]. With respect to intimal hyperplasia, PDGF-BB induced migration and proliferation in vSMCs was attenuated by PTEN’s over-expression[10].Moulton et al., also reported a significant downregulation of PTEN in human atherosclerotic hyperplasia and vascular fibrosis[11].
Currently, the use of drug eluting stents (mainly anti-proliferative drugs) are the main choices in preventing restenosis after PCI[12].However, the development of stent thrombosis and bleeding complications challenges its long term clinical use[13]. Therefore, new treatments are needed urgently. Seaweeds are edible marine algae known to contain high amounts of phlorotannins and polysaccharides,hence possess both anti-inflammation and antioxidant properties. Crude extracts of the seaweedsAscophyllum nodosumandFucus vesiculosusextracts are commercially available for lowering blood glucose and obesity[14-15]. According to a study conducted by Gabbia et al.[14],using1H-NMR, GC-MS and HPLC, they found this extract to be composed mainly of polysaccharides, phloroglucinols and fatty acids. Very few studies exist showcasing the effects of these constituents on restenosis.However, Jean-François Deux and his colleagues reported the ability of low molecular weight fucoidan (a polysaccharide obtained from brown seaweeds) to inhibit VSMCs proliferationin vitroand also ameliorate restenosis by decreasing intimal hyperplasia by 56%. This study also demonstrated the uptake of fucoidan by VSMC vesicles[16].Another study also demonstrated the beneficial effects of pyrogallolphloroglucinol-6,6-bieckol (a phloroglucinol derivative) on restenosis by inhibiting VSMCs dedifferentiation and proliferation[17]. Despite limited evidence of these bioactive components on restenosis, many studies have reported their activities on the individual hallmarks in restenosis’ pathogenesis. They have also been reported to elicit anti-inflammatory[18-20], anti-proliferative[21-22]and gut microbiota modulation[23-24]activities. In a study conducted by Ahmad and colleagues, fucoidans were isolated from extracts of several seaweeds includingA. nodosumandF. vesiculosus, and they reported the ability of these compounds to significantly reduce/modulate the production of IL-6, IL-1β and TNF-α (pro-inflammatory cytokines) in macrophages and PBMCs cells treated with LPS[25]. Also, using anex-vivomodel of porcine colonic tissues, Bahar et al., report the inflammatory inhibitory properties of a water extract ofF. vesiculosus. They show this extract could inhibit the MAPK, NF-κB, TLRs (TLR4,TLR7), cell adhesion molecules, chemokines, cytokines and other inflammatory mediators[26].
In addition to the above, patients with restenosis also present with changes in their gut microbiota composition and it has been implicated to contribute to its severity[27-28]. A pool of evidence exist reporting the association between gut dysbiosis and the severity of neointimal formation in both animals and humans[29-31]. Compared to healthy human controls, alterations in the abundance of Firmicutes and Bacteroidetes were found to be higher and lower respectively in coronary heart disease patients[32]. Gut microbiota affects health via either a direct or indirect way. The direct effects of the gut microbiome on health is via the production of metabolites such as but not limited to; short chain fatty acids (SCFAs), coprostanols or trimethylamine-N-oxides (TMAO). SCFAs such as propionate,butyrate and acetate are the most abundant SCFAs present in humans[33]. Bacteria from the phylum Bacteriodetes can produce butyrate and acetate while a few in the phylum Firmicuteshave the potential of producing butyrate. SCFAs have also been found to produce by the bacteriaRoseburia,Bacteroidesspp.,Faecal prausnitziiandEubacterium rectale. SCFAs also affects the immune system by regulating cell signalling in macrophages during innate immune responses and is known to also affect both T and B cells function[34]. On the other hand, TMAO, which is produced as a result of the breakdown of dietary choline,L-carnitine and lecithin by gut microbes, positively correlates with CADs[35]. TMAO positively correlates with high serum levels of C-reactive protein (CRP) and is also associated with endothelial dysfunction[36]. TMAO microbe producers include bacteria from the phylum Actinobacteria,Proteobacteria and Firmicutes but have not been found to be produced by those in the phylum Bacteriodetes[37].Actinobacter,Pseudomonas,Proteus,KlebsiellaandEnterobacter(from the phylum Proteobacteria),Streptococcus,EnterococcusandClostridium(from the phylum Firmicutes) have all been found to have a positive correlation with TMAO production[36]. Therefore, for this reason,modulation of the gut microbiome have come up as one of the strategies for treating or preventing most coronary artery diseases including restenosis. In this regard, seaweeds have been considered as prebiotics with numerous effects on the gut microbiome[38-39]. In anin-vitrostudy conducted by Chen et al.[40], they reported an increase in the phylum Bacteroidetes in addition to increased production of SCFAs byA. nodosum. They also recorded an increase in bacteria of the genusFaecalibacterium,OscillospiraandBacteroideswhile decreasing those ofDorea,ClostridiumandFusobacterium.
Since few studies exist reporting the protective effects of seaweeds on the development of restenosis, for this purpose, we investigated the effects of the seaweedsA. nodusumandF. vesiculosusextracts(herein, referred to as seaweed extracts; SE) on the development of intimal hyperplasiain vivoand evaluated its anti-inflammatory and anti-proliferation activity in macrophages and vSMCs. Additionally,the underlying mechanisms involved and alterations of the gut flora were assessed.
2. Materials and methods
2.1 Materials
Antibodies including AKT (#GTX121937), phospho-AKT(#ab28821), COX-2 (#160126), iNOS (#610432) and GAPDH(#NB300-221), were obtained from GeneTex (Ir-vine, USA), Novus Biologicals (Centennial, USA), BD Transduction Laboratories(Lexington, USA) and Cayman Chemical (Ann-Arbor, USA).PTEN (#9552) and TLR4 antibodies were purchased from Cell signalling and Proteintech respectively. Anti-mouse HRP-conjugated(#GTX213112-01) and Biotin anti-rabbit (ab6720) secondary antibodies were bought from Genetex and Abcam respectively.
2.2 Seaweed extract (SE)
InSea2® (innoVactiv Inc.; Rimouski, Canada), a commercially available nutraceutical was used for intervention purposes in this study. This extract is prepared from the seaweedsF. vesiculosusandA. nodosum. The preparation method and its phytoconstituents have previously been reported[14]. For its use in ourin vitrostudy, 25 mg/mL(m/V) stock concentration of extract was prepared using double distilled water, sonicated and centrifuged. Supernatants were collected and used for all experiments.
2.3 Cell culture
Rat thoracic aorta derived smooth muscle cells (A10 cells,#60127) and mouse BALB/c macrophages (RAW264.7 cells, #60001)used in this study are purchased from the Bioresource Collection and Research Centre (BCRC; Hsinchu city, Taiwan, China). For culturing,Dulbecco’s modified Eagle’s medium (DMEM) containing 100 mg/L streptomycin, 4 mmol/LL-glutamine, 100 U/L penicillin, 4.5 g/LD-glucose, 1.5 g/L sodium bicarbonate, and 10% fetal bovine serum(FBS) was used as culture media. For culturing A10 cells, 1 mmol/L sodium pyruvate was included in the culture medium preparation. A humidified 5% CO2incubator maintained at 37 °C was used.
2.4 Cell viability assay
For cell viability studies, we conducted the sulforhodamine B(SRB) assay following the protocol of Orellana et al.[41]. Briefly, cells(RAW264.7 and A10) were seeded on 24-well plates (2 × 104cells/well)and grown overnight. They were later treated with or without SE for 24 h, following stimulation with LPS or PDGF-BB respectively.After that, fixation was done by incubating cells in Trichloroacetic acid (50%,m/V) at 4 °C overnight. Next day, cells were rinsed with tap water and stained with SRB solution (0.04%,m/V) at room temperature for 30 min. Acetic acid (1%,V/V) was used in rinsing cells, followed by air drying. 10 mmol/L Tris base was later added to dissolve the protein bound dye. Measuring the absorbance at 510 nm was used to determine the viability status of the cells.
For subsequent experiments, cells were pre-treated with SE for 2 h prior to stimulation and co-treatment with either PDGF-BB,conditioned medium (CM) or lipopolysaccharide (LPS).
2.5 Nitric oxide (NO) level measurements
NO levels in cell culture supernatants were measured using Greiss assay as previously described[42]. RAW264.7 cells(1 × 105cells/well) were grown in 6 well plates overnight. Following pre-treatment, cells were stimulated with LPS (0.2 μg/mL) with or without SE for 24 h. Supernatants were collected and NO levels measured by adding Greiss’ reagents (50 μL of 0.1% naphthyl ethylenediamine dihydrochloride in 5% phosphoric acid and 50 μL of 1% sulphanilamide) to 50 μL of cell supernatant and incubating in the dark for 15 min at room temperature. Measuring the absorbance at 540 nm was used to determine NO levels.
2.6 Macrophage conditioned medium (CM) preparation
RAW264.7 macrophages cultured on 10 cm culture plates were stimulated with LPS (0.2 μg/mL) for 24 h. Subsequently, the supernatants of these LPS-stimulated RAW264.7 cells were obtained and centrifuged at 1 250 r/min for 3 min. These were kept at -80 °C until use. For conditioned medium experiments, the supernatants were diluted 1:1 with normal cell medium before use[43].
2.7 Cell migration assays
For the wound healing and transwell methods, procedures were done as reported previously[44]. Briefly, A10 cells (5 × 104cells/well)were seeded on 6-well plates, and allowed to grow to about 80%confluent. Following that, the culture media was replaced with serum free medium and grown overnight. With the use of a 200 μL pipette tip, a ‘wound’ was made by drawing cross-like lines over the confluent cells and then cultured with or without SE, following stimulation with PDGF-BB or CM for 24 h. Images of the wound was taken using an inverted microscope (magnification, 100×, Leica,Germany) and Image J software was used to measure the percentage of cell free area.
For Transwell assay, cell culture inserts were used. Using 24-well plates containing PDGF-BB infused culture media, A10 cells were seeded into inserts (104cells/insert) and placed unto the 24-well plates and allowed to incubate for 24 h. Using PBS, the inserts were washed twice, followed by fixing in methanol. To remove un-migrated cells, cotton buds were used to wipe the inner surface of the inserts and those on the lower surface of the inserts were stained with 0.9% crystal violet. The inserts were photographed using an inverted microscope. Cells in four random areas were counted for each image and the mean taken.
2.8 Cell cycle analysis
Progression of the cell cycle was analysed using an already described protocol[45]. After PDGF-BB or CM stimulation for 18‒24 h, cells were suspended in PBS after harvesting. Using 70%ethanol, the cells were fixed at -20 °C for 20 min. Following fixation,PBS was used to rinse the cells and staining was done using 50 μg/mL of propidium iodide (PI) in PBS (with 100 μg/mL RNase A). Cells were incubated in the dark, at room temperature for 30 min and then measured using the Attune Nxt Flow Cytometer (Thermofisher scientific. Inc; USA). The percentage number of cells at the various cell cycle stages were ascertained by using the Modfit LT software(BD, Topsham, ME, USA).
2.9 Immunoblot analysis
Immunoblotting was carried out as previously illustrated with little adjustments[44]. Briefly, protein extraction from cells was carried out using the iNtRON PROPREP protein extraction solution(Korea). Cells were lysed and centrifuged for 15 min (4 °C) at 12 000 ×g. Protein concentration of supernatants were measured using the BioRad protein assay kit (BioRad, USA). The same amounts of proteins were electrophoresed using either 10% or 15% SDS-PAGE and transferred unto polyvinylidene difluoride(PVDF) membranes (Immuneblot, BioRad). Following that, PVDF membranes were incubated at room temperature for an hour in 1%BSA solution prepared in Tris-base saline containing 0.1% Tween 20(TBST) (blocking). Primary antibodies, were incubated (4 °C) with blots overnight. The next day, after rinsing with TBST, the blots were reacted for 2 h with the respective secondary antibodies at 4 °C.WesternBright ECL kit (#K12045D50, USA) was used to develop the chemiluminescent signals. Photos were taken using the Azure C300 imaging system. Quantification analysis of band intensities was done using AzureSpot (Azure Biosystems) and standardized to that of the internal control (GAPDH).
2.10 RNA extraction and qualitative real-time PCR (qRT-PCR)
The NucleoSpin RNA extraction kit (11922402, Fischer Scientific,England) was used to extract total RNA as per the manufacturers guide, followed by reverse transcription into cDNAs using ReverTra Ace Set (#PUTRT200; Japan), as illustrated by its manufacturers.Then using SYBR Green qPCR mix (Invitrogen) and StepOne RT-PCR machine (Invitrogen), qRT-PCR was performed. The primer sequences shown in Table 1 were used for PCR analyses.
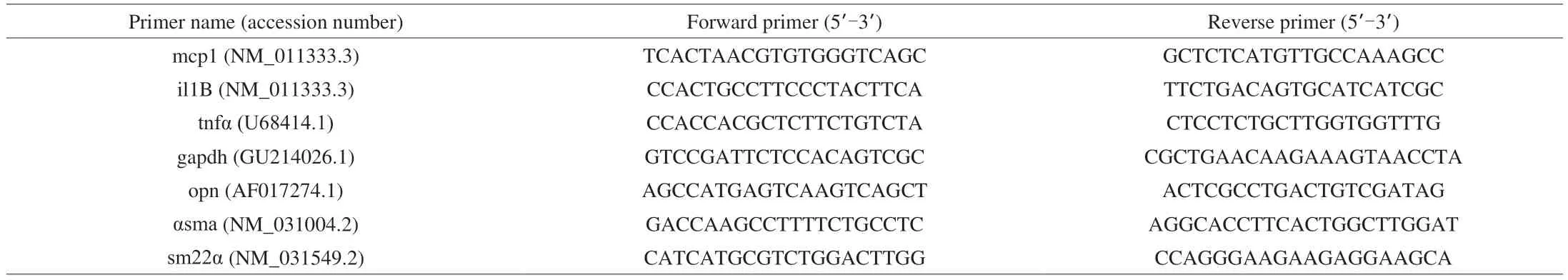
Table 1 Primer sequences
2.11 Balloon angioplasty rat model
Male Sprague-Dawley rats (6 weeks old; (230 ± 20) g)were obtained from BioLASCO (Taipei, Taiwan). They were accommodated at the Taipei Medical University (TMU) Centre for Laboratory animals, and they were placed as single rats in each cage. Their accommodation animal centre included a timed exposure to a light/dark cycle of 12 h each. They ate and drank at liberty.The animal protocol (LAC 2019-0417) was accepted by the TMU Animal Care and Use Committee and executed following its animal ethical guidelines. The animals were separated into three groups(n= 5‒6/group) which included: sham group, balloon angioplasty (BA)and BA + 1X SE (51.66 mg/kg/day). SE was dissolved in distilled water and the animal’s treatment dose was calculated from the human dose according to the method outlined by Nair et al.[46]. The BA group was given only double distilled water.
The left common carotid artery was ligated using a 2F × 80 cm arterial embolectomy catheter (Biosensors International Technology,Singapore) and used to induce neointimal hyperplasia. Briefly,5 mg/kg Rompun (Canada) and 20 mg/kg Zoletil 50 (France) were administered intraperitoneally to anesthetize the rats. When sedated,an opening was made at the external carotid artery and the catheter inserted through it into the left common carotid artery. Once inside,the catheter’s balloon was inflated under pressure (1.3 kg/cm2),pushed and pulled thrice, to ensure damage of the arterial wall. SE was administered by oral gavage three weeks prior to balloon injury to 2 weeks’ post injury[44]. At the end of the study, blood and fecal samples were collected prior to sacrifice.
2.12 Hematoxylin and eosin staining and immunohistochemistry
The animals were sacrificed and the left carotid common arteries collected. Animals were perfused using PBS, and fixed by per fusing with 4% formaldehyde. The arteries were further fixed in formaldehyde overnight, rehydrated in 30% sucrose and using a cryostat, were cut into 20 μm frozen sections. To observe the neointimal formation, hematoxylin and eosin were used to stain the sections. The intima and media areas were measured using ImageJ(USA), and the intima-to-media area ratio (I/M ratio) was used as a determinant for the severity of intimal hyperplasia.
Immunohistochemistry was done according to previous procedures[47]. Briefly, antigen retrieval was done using Tris-EDTA (pH 9). The sections were later incubated with 1%BSA at room temperature for 1 h to avert inaccurate binding of primary antibodies. Desired antibodies were then incubated on sections overnight at 4 °C. Following that a biotinylated secondary antibody, Vectastain ABC reagent (PK-4000, Vector Labs; USA) and diaminobenzidine (DAB, Diagnostic BioSystems; USA) were used sequentially, and then counter-stained with haematoxylin. Images were taken and signal intensities measured following the protocol by Crowe AR et al.[48]. 1% BSA was used to replace primary antibodies for negative controls.
2.13 Measurement of pro-inflammatory cytokines and PDGF-BB
The serum levels of the IL-1β, TNF-α and PDGF-BB (conditioned medium) were measured using the following ELISA kits; Rat IL6,Legend MaxTM, USA, Cat. No. 437107; Rat TNF-α, ABCAM, USA,ab100785; PDGF-BB, Proteintech, Cat. No. KE10034. Procedures were done according to the manufacturer’s instructions.
2.14 Microbial genomic DNA (gDNA) extraction
Two weeks post balloon injury and prior to sacrifice, fecal samples (about 300 mg) were collected from individual rats. The defecation reflex was triggered by massaging the abdomen of the rats.The QIAamp DNA Stool Minikit (Germany) was used for extracting total gDNA, and its concentration measured using the NanoDrop2000(Thermo Scientific, USA).
2.15 16S rRNA amplicon sequencing and data analysis
Here, the 341 forward primer (5’-CCTAYGGGRBGCASCAG-3’)and 806 reverse primer (5’-GGACTACNNGGGTATCTAAT-3’) was used in PCR analysis to produce libraries from the V3-V4 regions of the 16S rRNA gene. This was generated following the Illumina 16S Metagenomic Sequencing Library Preparation protocol as illustrated by Klindworth et al.[49].
Employing the Illumina High-Sequence 2000 platform, pairedend sequencing was performed on the amplicons (PE 2 × 250), as illustrated by the manufacturer. Analysis of the data was done using Quantitative Insights into Microbial Ecology (QIIME). Following these and quality processes, merging of the paired forward and reverse reads was done. In order to find the representative operational taxonomic unit (OTU), alignment of identical sequences was performed with the use of the UPARSE algorithm. OTU clusters contained sequences that had ≥97% similarities. Chi-meraSlayer was used to remove chimeric sequences. OTU annotations were given using the SILVA v132 database. Alpha diversity (Shannon index) was analyzed by QIIME. The linear discriminant analysis (LDA) effect size (LefSe), was evaluated by using the online Galaxy workflow framework.
2.16 SCFA analysis
Serum samples were collected from ratsduring sacrifice, two weeks post-PCI. The SCFAs were quantified in serum using gas chromatography-mass spectrophotometry (GC-MS), as previously described[50]. Concentrations of acetic, propionic and butyric acids were analysed. Briefly, both SCFAs standards and serum SCFAs were extracted using methyl tert-butyl ether (MTBE). Followed by centrifugation and collection of the supernatant. 100 μL MTBE extracts were filled into cuvettes and analysed using the Agilent 5977 MS coupled with 7820 GC machine. The rationale for this procedure is according to Cummings et al.[51], who found out that a percentage of SCFA could seep into the blood circulation and as such have beneficial effects on other tissues other than those present in the gut.
2.17 Statistical analysis
Values for all results were displayed as mean ± standard deviations (SD). Graphpad Prism 9 was used for all statistical analyses. One-way analysis of variance (ANOVA) was used to ascertain significant differences between groups, followed by Turkey’s test. Statistical significance was considered atPvalues greater the 0.05 versus either the control or diseased group.
3. Results
3.1 SE inhibits neo-intimal hyperplasia formation and local inflammation following balloon injury
To investigate SE ability in mitigating the development and progression of intimal hyperplasia, balloon injury was done on rats to induce intimal hyperplasia. Two weeks after injury, compared to rats in the sham group, there was a visible increase in the thickness of the intima in the arteries of the balloon injured rats(BA), which subsided upon supplementation with SE (BA-1X)(Fig. 1A). Measuring the intima/media (I/M) area ratio of all groups confirmed this (Fig. 1B).
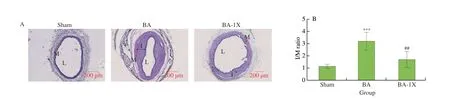
Fig. 1 Effect of SE on the development of restenosis. (A) Representative images of the arteries in sham and balloon-injured rats, 2 weeks after injury with or without SE treatment. (B) Quantitative measurements of the I/M area ratio for the left carotid artery in SD rats. (C) Local expressions of TLR4, PTEN and PI3K.Values are shown as mean ± SD (n=3‒5). *P < 0.05, ***P < 0.001 vs sham group, ##P < 0.01 vs BA group. I: intima layer; M: media layer; L: lumen.
With regards to inflammation, no changes in cytokines and PDGFBB were found in the serum of rats with or without injury(data not shown). This could partly be because balloon angioplasty performed in rats is a local injury and doesn’t sustain the ability to make profound changes in the blood. However, after performing IHC,we found that supplementing with SE could reduce the expression of the inflammation marker, toll-like receptor 4 (TLR4) in the arteries and increase PTEN (anti-inflammation and anti-proliferation marker) expression compared to the BA group (Fig. 1C). We also see an increase in PI3K’s expression in balloon injured rats, while supplementation with SE significantly decreased its expression.
3.2 Effects of SE on gut microbiota composition
The relationship between gut microbiome and restenosis has recently been acknowledged. With regards to the persistent inflammation created following balloon injury, we hypothesized that this could alter the gut microbiota composition, since numerous studies have highlighted the contributions of the gut microbiota in the pathogenesis and severity of inflammatory diseases[52]. Likewise,pharmacological interventions modulating the gut microbiome have been reported to ameliorate many diseases, especially those related to inflammation. Herein, we explored the effects SE has on the gut microbial community and diversity.
3.2.1 α- and β-Diversity
In this study, faecal samples from 5 rats of each group were used in performing 16s RNA sequencing.α-Diversity which measures the microbial diversity within an ecosystem is usually indicated by the Shannon indices[53]. An ecosystem with a high diversity is represented with a larger Shannon index. According to Fig. 2A, compared to the sham group, balloon injury significantly reduced the Shannon index(P< 0.001), suggesting a significant difference in the microbial diversity (and richness of species). However, following supplementation with SE, there was a drastic increase and an enrichment of species in BA-1X rats. In order to determine the changes occurring in the gut microbial communities within different treatment groups, the relative abundances at the phylum and genus levels were measured as shown in Figs. 2B and C.

Fig. 2 Relative abundance and microbial diversity of different rats’ groups. (A) Balloon injury and SE supplementation significantly changed the microbial α-diversity, the relative abundance of bacteria at both the (B) phylum and (C) genus levels. The β-diversity indicated by (D) Bray-curtis and (E) Jaccard indices.
On the other hand,β-diversity measures the similarity between species in a given sample or habitat. It usually indicates the comprehensive structure and composition of the gut community[53].Based on the Bray-Curtis and Jaccard distance matrix index (used for measuringβ-diversity), the results show that biological replicates which had undergone the same experimental treatment, clustered together (Figs. 2D and E). This suggest that the microbial community in these different groups are distinct.
3.2.2 Effect of SE on gut microbial taxa.
According to Fig. 3, the histograms show the differences in the abundance of several bacteria taxa in the experimental groups. As shown in Fig. 3A, rats with balloon injury (BA) showed a significant increase in the abundance of bacteria belonging to the phylum Firmicutes and a decreased abundance in those belonging to Bacteroidetes. However, following supplementation, only bacteria in the Firmicutes phyla were significantly reduced. Although there were subtle changes in the number of Bacteroidetes in the supplemented group compared to the BA group, results show it wasn’t significant.
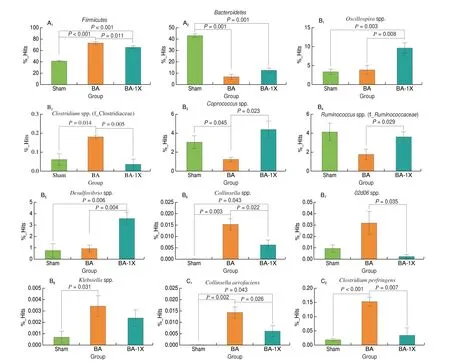
Fig. 3 Effect of SE on gut microbial taxa. Supplementation with SE significantly modulated some gut microbes at the (A) phylum, (B) genus, (C) species levels in balloon injured rats. (D) Numerical points of the histograms. Values are reported as mean ± SD (n = 5). * is P < 0.05 compared to sham group, # is P < 0.05 compared to BA group.
Furthermore, exploring at the genus level, supplementation with SE could significantly increase the relative abundance ofOscillospira(P= 0.006) (which have potentials as probiotics)[54],Coprococcus(P= 0.023),Rumminococcus(P= 0.029) andDesulfovibriospp.(P= 0.004) which were impaired in the BA group. Interestingly,Clostridium,CollinsellaandKlebsiellaspp. which are pathogenic bacteria were enriched in the BA group. However, supplementation with SE subdued them. Overall, these results indicate that supplementation with SE was able to promote the enrichment of commensal bacteria and on the other hand decrease those in the pathogenic group, hence reshaping the gut flora.
3.2.3 Effect of SE on SCFAs production
Seeing that supplementation with SE significantly increased the relative abundances of some SCFA producing bacteria, we further investigated the effects of SE on the serum concentration of SCFA.As shown in Fig 4, serum concentrations of acetic acid, propionic acid and butyric acids were significantly reduced in the BA group compared to the sham group. However, supplementation with SE slightly increased these SCFAs in the serum of rats, though these changes were not statistically significant.
3.3 Effect of SE on LPS stimulated inflammatory pathways in macrophages
After finding out that SE could inhibit inflammationin-vivo, we went on to investigate the underlying mechanisms involved in SE’s anti-inflammatory activity. Here, lipopolysaccharide (LPS) was used as an inducer to stimulate inflammatory responses in RAW264.7 macrophage cells.
3.3.1 Cytotoxic study of SE and its effect on inflammatory cytokines in LPS-stimulated macrophage cells.
The sulforhodamine B (SRB) assay was used to investigate the cytotoxicity effects of SE on macrophage cells. Little or no cytotoxic effects were detected after treating cells with SE doses up to 100 μg/mL (Fig. 5A). Hence for further experiments, SE was used at concentrations 12.5 and 50 μg/mL.
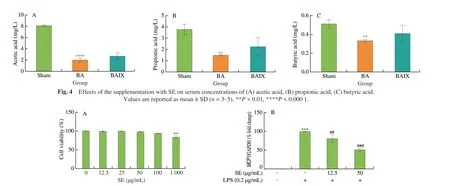
Fig. 5 Effect of SE on cell viability and inflammatory cytokines in LPS-stimulated RAW264.7 cells. (A) Effects of SE on cell viability. (B-D) LPS-induced mRNA expression of MCP1, IL-1β and TNF-α in RAW264.7 macrophages. Pre-treatment of cells with SE for 2 h was performed prior to stimulation with LPS(0.2 μg/mL) for 6 h and mRNA levels were detected with RT-PCR. For cell viability, cells were treated with SE only, for 24 h. Values are shown as mean ± SD(n = 3). **P < 0.01, ***P < 0.001 vs control group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs LPS group.
Also, to examine the effects of SE on cytokine release, mRNA expressions of monocyte chemoattractant protein-1 (MCP1),tumour necrosis factor α (TNF-α), and interleukin-1β (IL-1β) were detected using RT-PCR. As demonstrated on Figs. 5B-D, there was a remarkable upregulation in mRNA levels of these cytokines upon treatment with LPS. However, in contrast to the LPS group,co-treating with SE significantly downregulated these cytokines.
3.3.2 Effect of SE on inflammatory responses in LPSstimulated macrophage cells
To study the effect SE has on the production of inflammatory mediators in macrophages, nitric oxide (NO) levels, cyclooxygenase 2(COX2) and inducible nitric oxide synthase (iNOS) protein expressions were measured after stimulation with LPS. NO production (Fig. 6A), iNOS and COX2 (Fig. 6B) protein levels in RAW264.7 cells were remarkably increased upon stimulation with LPS. Treatment with SE significantly decreased these adverse effects.
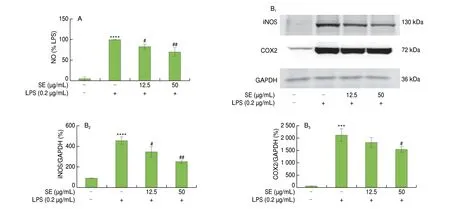
Fig. 6 Effect of SE on inflammatory responses in LPS-stimulated RAW264.7 cells. (A) NO and (B) protein expression of iNOS and COX-2. Pre-treatment of cells with SE for 2 h was performed prior to stimulation with LPS (0.2 μg/mL) for 24 h. Values are shown as mean ± SD (n = 3). ***P < 0.05, ****P < 0.000 1 vs control group, #P < 0.05, ##P < 0.01 vs LPS group.
3.3.3 Regulation of SE on NF-κB-p65 nuclear translocation,TLR4 and PTEN protein expression
NF-κB-p65 is a crucial transcription factor involved in inducing inflammation and it is a downstream molecule of TLR4. To further elucidate the effect of SE on inflammation signalling, TLR4 and nuclear translocation of NF-κB-p65 protein expressions were detected by immunoblotting. As depicted in Figs. 7A and B, TLR4 and nuclear expression of NF-κB-p65 were significantly increased upon stimulation with LPS and there was a resulting decrease in this effect after co-treatment with SE.
PTEN, a phosphatase molecule which was originally associated with tumour suppression, has also been found to regulate inflammation[55]. As shown in Fig. 7C, in comparison with the control group, LPS-stimulation decreases the expression of PTEN in the RAW264.7 cells. However, after co-treating with SE, there was a significant upregulation in PTEN’s protein expression.

Fig. 7 Regulation of SE on LPS-induced inflammatory related proteins. (A) Western blot images of NF-κB-p65, LAMINB1, TLR4, PTEN, GAPDH.Densitometric analyses of (B) NF-κB-p65 nuclear translocation, (C) TLR4, (D) PTEN protein expressions in RAW264.7 cells. Pre-treatment of cells with SE for 2 h was done prior to stimulation with LPS (0.2 μg/mL) for 30 min and 24 h for NF-κB-p65 nuclear expression levels and TLR4/PTEN respectively. Values are shown as mean ± SD (n = 3). *P < 0.05 vs control group, #P < 0.05, ##P < 0.01 vs LPS group.
3.4 SE prevents proliferation and migration of vSMCs induced by CM
To understand whether SE could inhibit vSMC proliferation and migration, induced by the inflammatory micro-environment induced during balloon injury, vSMCs were incubated with conditioned medium (CM) obtained from LPS-stimulated RAW264.7 cells.Proliferation and migration indices were studied using SRB assay,Flow cytometry and wound healing analysis. First, cytotoxicity analysis of CM on vSMCs was done using different concentrations of SE (Fig. 8A).
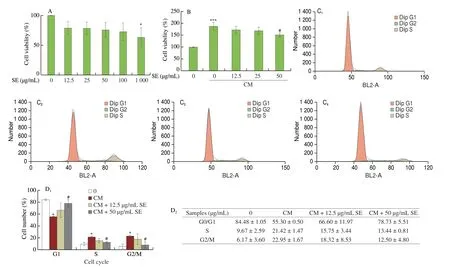
Fig. 8 Effect of SE on activated RAW264.7 conditioned medium induced vSMCs proliferation. (A) Pre-treatment of A10 cells with SE for 2 h was done prior to stimulation with CM with or without SE for cell viability with SE alone. (B) Cell viability. (C) Cell cycle progression. (C1-C4) 0, CM, CM + 12.5 μg/mL SE,CM + 50 μg/mL SE, respectively. (D) Quantitative data for cell cycle. (E) Cell migration. (F) PDGF-BB concentration in conditioned medium. Values are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 vs control group, #P < 0.05 vs CM group. (CM; CML; conditioned medium from RAW264.7 cells stimulated with LPS, CM0; conditioned medium from unstimulated RAW264.7 cells).
Figs. 8B-E shows that CM increased the cell viability, cell cycle progression and migratory capacity of A10 cells compared to the control; and co-treatment with SE (50 μg/mL), significantly inhibited this effect.
In addition to cytokines, RAW264.7 cells also produce PDGFBB when stimulated with LPS as compared to its unstimulated counterpart (Fig. 8F). This proves the LPS-activated RAW264.7 cells’conditioned medium as a good representative of the environment created after PCI.
3.5 Effect of SE on PDGF-BB induced vSMC dysfunction
Since PDGF-BB is a potent mitogen and is released after balloon injury, it was used to induce dedifferentiation, proliferation and migration in vSMCs. And also used as a means to confirm results observed in A10 cells when using CM.
3.5.1 SE effects on PDGF-BB induced vSMC proliferation.
As shown in Fig. 9A, the increased proliferation conferred upon treatment with PDGF-BB was significantly decreased when co-treated with SE.

Fig. 9 SE inhibits PDGF-BB induced proliferation in A10 cells. Pre-treatment of A10 cells with SE for 2 h was done prior to 24 h stimulation with PDGF-BB(20 ng/mL) with or without SE for (A) cell viability, (B) cell cycle analysis. (B1-B4) 0, PDGF-BB, PDGF-BB + 12.5 μg/mL SE, PDGF-BB + 50 μg/mL SE,respectively. (C) Quantitative data for cell cycle. Data represented as mean ± SD (n = 3). ***P < 0.001, ****P < 0.000 1 vs control group, #P < 0.05, ##P < 0.01,###P < 0.001 vs PDGF-BB group.
Progression of the cell cycle strictly controls cell proliferation.As shown in Fig. 9B, upon PDGF-BB stimulation, A10 cells were significantly reduced in the G0/G1 phase leading to a subsequent increase in the S and G2/M phases. However, after co-treating with SE at the different concentrations, the fraction of A10 cells arrested in the G0/G1 phase was significantly increased whilst decreasing those in the S and G2/M-phases.
SE concentration at 50 μg/mL showed the most potent effect on the cells. Hence subsequent analyses were performed using solely this dose.
3.5.2 Effects of SE on PDGF-BB induced vSMC migration and dedifferentiation
As shown, PDGF-BB promoted the wound healing capacity(Fig. 10A) and the migration of cells to beneath the insert upon contact with PDGF-BB supplemented medium (Fig. 10B). Both indices were significantly inhibited after co-treatment with different concentrations of SE. For the effect of SE on vSMC dedifferentiation,the mRNA expressions of both contractile (alpha smooth muscle actin; αSMA, smooth muscle protein 22 alpha; SM22α) and synthetic markers osteopontin (OPN) were determined. Also, treatment with PDGF-BB caused morphological changes on the cells from an elongated and spindled-shape to a rhomboidal and cobblestoned-shape morphology (Fig. 10C)[56]. Fig. 10D confirms the ability of PDGF-BB to significantly reduce the mRNA levels of the contractile markersαSMAandSM22αwhile increasing that of the synthetic markerOPN,and co-treatment with SE counteracted these effects. Thus, returning the cells to a contractile phenotype.

Fig. 10 SE inhibits PDGF-BB induced migration and dedifferentiation in A10 cells. Pre-treatment of A10 cells with SE for 2 h was done prior to a 24 h stimulation with PDGF-BB (20 ng/mL) with or without SE for (A) wound healing assay, (B) transwell assay. (C) Microscope images. (D) mRNA expression analysis for phenotypic switching markers. Data represented as mean ± SD (n = 3). **P < 0.01, ***P < 0.001, ****P < 0.000 1 vs control group, #P < 0.05,##P < 0.01, ###P < 0.001, ####P < 0.000 1 vs PDGF-BB group.
3.5.3 Regulation of the PTEN/PI3K/AKT signalling pathway by SE
To investigate the involvement of the PTEN/PI3K/AKT pathway in SE anti-proliferative effects, the protein levels of their active forms were analysed by immunoblotting. As expected, stimulating A10 cells with PDGF-BB markedly upregulated the protein expression of PI3K,hence inducing the activated forms of AKT (pAKT) compared to that of the control group. Co-treating with SE resulted in a significant reduction in the expression levels of these activated proteins(Figs. 11A and B).
Unexpectedly, PDGF-BB had little or no effect on PTEN (a negative regulator of this pathway), however, there were observable increases in its expression after co-treatment with SE though it wasn’t statistically significant. (Fig. 11C).

Fig. 11 SE effects on PDGF-BB induced PTEN/PI3K/AKT signalling pathway. (A) Western blot images of pAKT, AKT, PI3K, PTEN and GAPDH.Pre-treatment of A10 cells with SE for 2 h was done prior to stimulation with PDGF-BB (20 ng/mL) with or without SE for (B) pAKT/AKT (30 min) (C) PI3K (24 h)(D) PTEN (24 h) (data presented as mean ± SD). ***P < 0.001, ****P < 0.000 1 vs control group, #P < 0.05, ##P < 0.01 vs PDGF-BB group.
4. Discussion
The development of restenosis following percutaneous coronary intervention (PCI) is a complicated process involving several interactions between vascular cells. After vascular injury, the recruitment of macrophages is the key feature at the early stages of restenosis development, which corresponds to the persistent inflammation reported at this stage. These macrophages secrete inflammatory factors such as growth factors, chemokines, cytokines and increase production of nitric oxide, which exert paracrine effects on other neighbouring cells[57]. In addition to the inflammatory mediators, PDGF-BB, a known potent mitogen, is also released from injured vessels[58]. One of the adverse effects these molecules have on vSMCs is a change in its phenotype, switching it from a quiescent contractile to a proliferative and migratory synthetic phenotype. This causes the cells to migrate from the media layer into the intima layer,where they proliferate excessively, leading to the development of intimal hyperplasia. This is the major pathophysiological basis for restenosis and other coronary heart diseases (CHDs)[59]. Hence, the pharmacological inhibition of vascular inflammation, phenotypic switching and proliferation/migration in vSMCs, have proven to be fundamental in the management of most CHDs. A growing body of pharmacological studies have implicated the use of marine products for CVDs. Herein, ourin vivomodel for restenosis included rats whose blood vessels were damaged with a balloon catheter and forin vitromodels, conditioned medium (CM) from activated macrophages was used as an indirect co-culture system to study the cross-talk between macrophage secreted proteins and vSMCs, hence mimicking the microenvironment created following vascular injury.For confirmatory studies, PDGF-BB was also used as anin vitrostimulant.
In this study, firstly we demonstrate that supplementation with SE attenuated intimal hyperplasia in SD rats which underwent balloon angioplasty (Figs. 1A and B) and also decreased the expression of some inflammatory markers on the vessel walls (Fig. 1C). However,ELISA assay for serum concentrations of IL-1β and TNF-α were performed but results showed no significant differences amongst groups (data not shown). This could partly be due to the fact that PCI in itself, induces an acute inflammatory response with reports indicating an increase in C-reactive protein within 24 h post PCI.Meanwhile for those with extended inflammation post-PCI, it is usually as a result of atherosclerosis and other underlying coronary pathologies[60]. Secondly, SE supplementation could enhance gut microbial diversity and vice versa (Figs. 2 and 4). Finally, SE inhibited LPS-induced pro-inflammatory cytokines production in macrophages and consequently inhibited the proliferation and migration of vSMCs stimulated with either CM or PDGF-BB (Figs. 5–11).
Gut microbiota has been reported to have a potential role in the pathogenesis of CHDs, restenosis, heart failure and other inflammatory driven diseases, where inflammation is known to drive the changes in the gut microbiome composition[61]. A significant shift or difference in the gut microbiome of coronary artery disease (CAD)patients with hyperplasia or stenosis have been reported as compared to CAD patients without[29]. The gut microbiome elicits its effects on health and disease through the production of metabolites that are carried in the blood stream to other parts of the body. Gut metabolites such as TMAO and phenylacetylglutamine have been reported to be positively correlated with the onset and severity of most cardiovascular related diseases and are associated with high Firmicutes bacteria compared to Bacteroidetes[29,62]. Similar to our study, NGS results show an increase in the relative abundance of Firmicutesin the BA group compared to those in the sham control and the opposite is true for the phylum Bacteroidetes(Fig. 3A). Interestingly, despite the abundant evidence illustrating a link between gut dysbiosis and restenosis development, there are no previous studies as to the best of our knowledge demonstrating a causal relationship between PCI and gut microbiota changes. However, it is reported that PCI induces acute systemic inflammation[63]and it’s been proven clearly that the inflammatory status of the host can alter the gut microbiome composition, favouring inflammatory related bacteria which intend aggravates inflammation[64-65]. In our study, balloon injured rats had a positive relationship with some known pathogenic bacteria such asCollinsellaspp.,02d06spp. (Clostridiaceae),Clostridiumspp. andKlepsiellaspp, which are potential pro-inflammatory inducers[66],while supplementation with SE could reduce their abundance(Figs. 3B and C). On the other hand, the relative abundances ofOscillospira,Coprococcus,RuminococcusandDesulfovibriospp.were significantly elevated upon supplementation with SE (Fig. 3B).Though limited reports exists on gut microbiota and restenosis, most studies report on the role played by the gut flora on cardiovascular diseases as a whole. Li et al.[67]reported on the increase inPrevotella,KlebsiellaandCollinsellaspp., in patients with hypertension, which was linked to the inflammatory status of the patients. Likewise, a randomised controlled trial conducted by Altemani et al., reported a decrease in the diversity of gut microbes and the abundance SCFA producing bacteria likeCoprococcus[68]. On the other hand, seaweed components have been shown to modulate the gut flora, as well as increase the abundance of SCFA-producing bacteria[38]. In a study conducted by Chen et al.[40],A. nodosumincreased SCFA production,as well as the genusOscillospira.
AlthoughOscillospira,CoprococcusandRuminococcusare gut microbes that haven’t been thoroughly studied, next generation sequencing data including metabolomics and other literature, have suggested the likelihood of them being short chain fatty acid (SCFA)producing bacteria (especially butyrate), and SCFA are known to regulate the immune system, inflammation and play a key role in preserving the gut barrier functions[54,69-72]. Liu et al.[73]found a positive correlation betweenOscillospiraenrichment and a reduction in atherosclerotic plaques, as well as a decrease in inflammation in ApoE-/-mice supplemented with polyphenols. Additionally,Lim et al.[74]reported a decrease in the abundance ofCoprococcusin older Korean adults with frailty (involves dysregulation of the immune system) and was negatively correlated with inflammatory status and depression. While,Ruminococcuswhich is an enterotype,has been reported to be negatively correlated with arterial stiffness and inflammatory adipokine levels[75]. Also, Yang et al.[76]reported changes made to the phylum upon supplementation with fucoidan.Lastly,Desulfovibriois one of the major sulfate-reducing bacteria in the human gut microbiome and the major metabolite (hydrogen sulfite) produced by this bacterium has been shown to have antiinflammatory, anti-proliferative effects and its potential in inhibiting restenosis[77-78].
With respect to how the gut flora could affect health, we are aware that these changes affect the host’s health primarily through the secretion of its metabolites into circulation[33]. Therefore, we investigated the serum concentration of SCFAs. However, our results(Fig. 4) show that there were no significant changes in the serum concentrations of SCFA, though slight increases were observed. This could probably be explained as a result of more SCFAs confined within the gut to ward off or elicit growth inhibiting activities on pathogenic bacteria[79]. Which on one part, could explain the shift differences we see in theβ-diversity cluster pattern of the rats supplemented with SE compared to the injured only group(Figs. 2D, E). Gosalbes et al.[80]reported the inability of antibiotic resistant enterobacteria to colonise guts that were rich in the abundance ofOscillospira,CoprococcusandDesulfovibrio. Seeing that these bacteria were enriched in the rats in our study upon supplementation with SE, this could probably be the reason for the decrease in pathogenic bacteria (Fig. 3). Catarino et al., had also found out that extracts fromF. vesiculosuscould enhance the levels of propionic and butyric acids in a simulated large intestine[23].Taking these into perspective, the above suggest the likelihood of SE ameliorating restenosis primarily by inhibiting local inflammation and partially by modulating the gut microbiome.
To investigate the molecular and underlying mechanisms,in vitrostudies were performed. As mentioned earlier, macrophages(which are responsible for inflammation) are actively involved in the pathogenesis of restenosis. Under healthy conditions, tissue macrophages remain quiescent and can be polarized into an inflammatory phenotype in disease conditions, thereby releasing increasing amounts of pro-inflammatory cytokines such as MCP-1,IL1B, TNF-α, nitric oxide, and other chemoattractant to stimulate and recruit other immune cells[81]. NF-κB is a downstream signalling molecule of toll-like receptors (TLRs) and is a crucial transcription factor responsible for regulating phenotype changes and cytokine production in macrophages, when exposed to infection, injury or stress. We showed that, upon stimulation with LPS, the mRNA expressions of cytokines such asMCP1,IL-1βandTNF-αwere upregulated and treatment with SE could inhibit it (Fig. 5). Likewise,SE treatment reversed the upregulation of pro-inflammatory markers such as NO, iNOS and COX2 caused by the stimulation with LPS (Fig. 6). Furthermore, the TLR4/NF-κB signalling pathway was activated upon stimulation with LPS, but co-treatment with SE significantly inhibited it by decreasing the protein expression of TLR4, hence preventing the nuclear translocation of NF-κB(Figs. 7A and B). Studies demonstrating the anti-inflammatory effect of this phytocomplex are lacking, however, Marlene et al. reported a decrease in inflammatory cytokines in obese and diabetic patients after supplementation with this nutraceutical, SE[82]. In addition,numerous studies have reported the ability of the individual seaweeds or their bioactivities to be anti-inflammatory and immunosuppressive.These studies demonstrated an inhibition of NF-κB signalling, NO and cytokine production in macrophage cells[18,83-84]. The secretions of these cytokines have paracrine effects on VSMCs. For example,interleukin 1β has been shown to increase VSMCs proliferation and could cause phenotypic switching in these cells[85-86]. Prostaglandin E2 (PGE2) have also been shown to modulate the dedifferentiation,proliferation of VSMCs and promote vascular intimal hyperplasia.PGE2 which is an inflammatory mediator is regulated by cyclooxygenase-2 (COX2)[85,87]. Seeing that SE could inhibit the production of these proteins (IL-1β and COX2), it could insinuate that the decrease of these proteins could affect its paracrine effects,thereby decreasing the dedifferentiation of VSMCs, and hence slowing down the proliferation of these cells. In addition to these,the clinical use of anti-inflammatory products coated on stents have proven effective in ameliorating neointimal hyperplasia formation after PCI[88-89].
One of the pathophysiology in the development of restenosis is intimal hyperplasia which is characterized by the excessive proliferation and migration of vSMCs from the media into the intima layer. Inhibiting this process is another effective strategy for preventing restenosis progression[90]. In this study, PDGF-BB and CM were used to stimulate this processin vitro. Our results show that SE could significantly inhibit PDGF-BB and CM-induced vSMC proliferation and migration (Figs. 8 and 9). To further confirm its anti-proliferative activity, using flow cytometry, we show that SE significantly inhibited the cell cycle progression into the S phase(Figs. 8 and 9). Furthermore, SE also inhibited PDGF-BB induced vSMC migration (Fig. 10). Consistent with our study, fucoidan (a bioactive component of seaweeds) was reported to inhibit PDGF-BB induced vSMC proliferation and cell cycle progression[91]. The inhibition of proliferation, migration and cell cycle progression in many cancer studies have also been reported after treatment with fractions of seaweed extracts or its constituents[22,92]. As mentioned earlier, the basis for vSMC’s excessive proliferation and migration observed after vascular injury is as a result of the phenotypic switching, where contractile quiescent vSMCs in the media layer transform to a synthetic proliferative phenotype and migrate into the intimal, where they uncontrollably proliferate[5,56].αSMA,SM22αand OPN are well known markers for the contractile and synthetic phenotype respectively[93-94]. We reported that SE could remedy the adverse effects caused by PDGF-BB on vSMC dedifferentiation.Consistent with the results on proliferation and migration, PDGF-BB increased the synthetic marker,OPNmRNA expression whilst downregulating that ofαSMAandSM22α(contractile markers) (Fig. 10D).Co-treating with SE remedied these adverse effects, thereby allowing the cells to remain in the contractile phenotype (Fig. 10C).
The PTEN/PI3K/AKT pathway plays an essential role in regulating several biological activities such as cell proliferation and survival, dedifferentiation, apoptosis, neuron signalling, and of recent,has also been implicated in regulating inflammation and the immune system[95-96]. This signalling pathway can be activated by several factors including growth factors, mechanical stress and inflammatory mediators[97]. PTEN is a negative regulator of the PI3K/AKT signalling pathway. PTEN activators can attenuate intimal hyperplasia formation in injured vessels[98], prevent phenotypic switching in vSMCs as well as inhibiting proliferation and migration of SMCs and cancer cells[99]. Though no studies have reported the activity of seaweed on PTEN in vSMCs, a handful of studies have reported its activity in upregulating PTEN expression in cancer cells[100]. Because PDGF-BB is known to activate the PI3K/AKT pathway[101-103], it was used as anin vitrostimulant. Herein, we found that PDGF-BB induced the protein expression of PI3K and phosphorylated levels of AKT in vSMCs, which was inhibited by treating the cells with SE(Figs. 11A and B). Even though PDGF-BB could not downregulate PTEN expression in vSMCs, co-treating with SE slightly increased the protein expression of PTEN, though it was not statistically significant(Fig. 11C). Inflammation in macrophage cells has however been reported to downregulate PTEN expression[104-106], which is consistent with our findings and we show that, co-treating with SE upregulated its expression (Fig. 7C), which by nature of its biological function,would further deactivate PI3K and AKT, leading to amelioration of inflammation. Consistent with ourin vitrostudies, IHC staining of the injured rats’ aortic tissues showed less PTEN protein expression compared to TLR4 and PI3K but was increased after supplementation with SE (Fig. 1C). These results indicate that SE could activate PTEN,leading to the downregulation of the PI3K/AKT signalling pathway which diminishes inflammation and proliferation, hence ameliorating intimal hyperplasia.
In light of the above, a limitation in this study is that we couldn’t provide evidence as to whether SE elicited its anti-restenosis effectsin-vivo, solely by directly activating PTEN or indirectly, using the gut microbiota to regulate PTEN/PI3K/AKT signalling. Also, limited(5 rats’ fecal) samples were used for analysing gut microbiota.
Putting together these findings, our results suggest that supplementation with seaweed extracts may produce beneficial effects in patients with restenosis by exerting anti-proliferation and anti-inflammatory activities, partly by modulating the gut microbiome,thereby reducing the degree of intimal hyperplasia development.
5. Conclusion
In conclusion, this study reveals that extracts from the seaweedsA. nodosumandF. vesiculosuscould inhibit intimal hyperplasia by suppressing inflammation (partly by modulating gut microbiota composition) and vSMCs proliferation and migration. Moreover,these extracts exhibited its anti-inflammatory and anti-proliferative effects by regulating the PTEN/PI3K/AKT signalling pathway.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
This work has been supported by the research grants from the Ministry of Science and Technology of Taiwan (MOST108-2320-B-038-040-MY3 and MOST 111-2320-B-038-049) and the National Science and Technology Council (NSTC111-2320-B-038-049).
Institutional review board statement
The animal protocol was approved by the Institutional Animal Care and Use Committee and performed according to TMU animal ethical guidelines (approval no. LAC 2019-0417)
杂志排行
食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Call for Papers from Food Science of Animal Products
- Alleviatory effect of isoquercetin on benign prostatic hyperplasia via IGF-1/PI3K/Akt/mTOR pathway
- Adsorption, in vitro digestion and human gut microbiota regulation characteristics of three Poria cocos polysaccharides
- Voluntary wheel running ameliorated the deleterious effects of high-fat diet on glucose metabolism, gut microbiota and microbial-associated metabolites
- Effect of simulated gastrointestinal digestion on antioxidant, and anti-inflammatory activities of bioactive peptides generated in sausages fermented with Staphylococcus simulans QB7
