Adsorption, in vitro digestion and human gut microbiota regulation characteristics of three Poria cocos polysaccharides
2024-01-24FngmingZhngHuiZhengToZhengPnXuYoXuYuxinCoFnJiYiqiongZengYubingFnKiHeXinwenDiFengfeiHouYongYng
Fngming Zhng, Hui Zheng, To Zheng, Pn Xu, Yo Xu, Yuxin Co, Fn Ji,Yiqiong Zeng, Yubing Fn, Ki He, Xinwen Di, Fengfei Hou, Yong Yng,
a College of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, China
b School of Pharmaceutical Science, Hunan University of Medicine, Huaihua 418000, China
c Hunan Fuling Engineering Technology Research Center, Huaihua 418100, China
Keywords: Poria cocos polysaccharides Alkali-soluble poria polysaccharide Carboxymethyl poria polysaccharide Adsorption characteristics In vitro simulated digestion In vitro simulated gut microbiota fermentation
ABSTRACT Poria cocos (PC) is a famous traditional Chinese medicine (TCM) and a widely used healthcare ingredient,which has antiobesity, enhancing immunity and improving sleep effects. Traditionally, only water-soluble poria polysaccharide (WSP) is extracted and applied for clinical application, while insoluble polysaccharide(alkali-soluble poria polysaccharide, ASP) is discarded as herb residue. However, the whole PC has also been historically utilized as functional herbal food. Considering the benef icial role of dietary f iber and the traditional use of PC, ASP may also contribute substantially to the therapy function of PC. Compared to WSP, little attention has been paid to ASP and ASP modif ied product carboxymethyl poria polysaccharide(CMP) which has been used as an antitumor adjuvant drug. In this study, the oil, cholesterol, metal ions and polyphenols adsorption ability, in vitro simulated digestive and the gut microbiota fermentation characteristics of WSP, ASP and CMP were studied to evaluate the functional values of three P. cocos polysaccharides(PCPs). The results showed that all three PCPs had good adsorption capacity on cholesterol, polyphenols and metal ions (Cd2+/Zn2+/Mg2+), among which ASP showed the highest capacity than WSP and CMP. The adsorption capacity of all three PCPs on heavy metal ions (Cd2+/Zn2+) was stronger than that of non-heavy metal ions (Mg2+); The in vitro digestibility of all three PCPs was very low, but WSP was slightly higher than ASP and CMP; Moreover, the indigestible residue of all three PCPs could improve the richness and diversity of gut microbiota, among which ASP had the greatest inf luence. In general, ASP and CMP could signif icantly promote the proliferation of some probiotics and inhibit the growth of some harmful bacteria. The gut microbiota diversity of CMP was reduced, but the richness of probiotics, especially Parabacteroides distasonis was significantly enhanced compared with the ASP group, and the growth of harmful bacteria Klebsiella pneumoniae was inhibited after CMP treatment. The short-chain fatty acids (SCFAs) analysis results showed that all three PCPs could signif icantly promote the production of acetic acid, propionic acid and the total acid content compared with blank control group, and SCFAs producing activity was positively correlated with the proliferative capacity of probiotics. Taken together, the good adsorption characteristics and gut microbiota regulatory activity of ASP may lay foundation for its lipid-lowering and immune-improving function.Additionally, the probiotic effect of CMP and ASP indicated that except for only use the water extract of PC in clinic, CMP and ASP also can be used in healthcare to take full advantage of this valuable medicine.
1. Introduction
Poriacocos(Schw.) Wolf (PC) is the dried sclerotium of Fu Ling Indian Bread Poria, which is believed by traditional Chinese medicine (TCM) practitioner to have the functions of invigorating spleen, dispelling dampness and calming the mind[1]. It is an important medicine in TCM clinical and is also commonly used in traditional diet therapy. Modern pharmaceutical studies have confirmed that PC has many health functions similar to dietary fiber, such as enhancing immunity[2], improving sleep[3], reducing weight and improving gastrointestinal function[4]. Polysaccharides and triterpenoids are considered to be their main effective components. The content of water-soluble poria polysaccharide (WSP) in PC is only 0.29%–3.36%, but the water-insoluble polysaccharide, namely alkali-soluble poria polysaccharide (ASP), accounts for 70%–90%[5]. Unlike WSP,ASP normally discarded as herb residue in the preparation of TCM decoction or formula granules because its insolubility in water.Previous study showed that WSP and ASP could play protective roles on cisplatin-induced intestinal injury by regulating the gut microbiota, and their protective roles were significantly stronger than that of the total triterpenes[6]; Xu et al.[7]found that WSP could improve the antibiotic-associated diarrhea in mice by regulating gut microbiota and intestinal mucosal barrier homeostasis; WSP could play an immunomodulatory role by regulating gut microbiota in rats with spleen deficiency[8]; Sun et al.[9]found that ASP could significantly improve the glycolipld metabolism disorder and reduce hepatic steatosis inob/obmice by increasing the relative abundance of Lachnospiracea andClostridium; As a water-soluble product modified by ASP carboxymethylation, carboxymethyl poria polysaccharide (CMP) has been shown to enhance the immune function of immunosuppressed mice by regulating gut microbiota[10].Currently, a great number of animal studies have revealed thatP.cocospolysaccharides (PCPs) may exert their bioactivities via regulating the gut microbiota.
Since the non-digestive polysaccharide is the main source of dietary fiber, and the whole PC is traditionally used as functional foods, a large amount of ASP naturally becomes the main component of various medicinal diets of PC. It is not difficult to speculate that ASP is also responsible for the beneficial role of the whole PC and may play the healthcare function similar to dietary fiber. Thein vitroadsorption experiment is beneficial to study the interaction between PCPs and various food components (such as cholesterol,polyphenol and metal ions, etc.), and to explore the health function and mechanism of PC from the perspective of dietary fiber function.However, few studies have been conducted to compare the adsorption of oil, cholesterol, metal ions and polyphenols by three PCPs (WSP,ASP and CMP), and the regulation role of three PCPs on human gut microbiota. To give a more comprehensive interpretation of the functional value of PC, this study investigated the adsorption characteristics, gastrointestinal digestion characteristics and the regulation function of the human gut microbiota of three PCPs from the perspective of dietary fiber. Our findings may lay a foundation to take full advantage of this valuable herbal medicine.
2. Materials and methods
2.1 Materials and chemicals
PC were purchased from Hunan Butian Pharmaceutical Co.,LTD. Sodium tauroglycocholate (≥ 99%) was purchased from Shanghai Aladdin, China.α-Amylase (50 U/mg), pepsin (3 000 U/mg),lipase (100 000 U/mg), hemin (≥ 95%),L-cysteine (≥ 99%), vitamin K1(≥ 98%), resazurin (≥ 90%) and SCFAs standard (GC ≥ 99.5%)were purchased from Shanghai Macklin, China. Trypsin (1:250) was purchased from Fuzhou Phygene, China. Anhydrous glucose standard(HPLC ≥ 98%) was purchased from Chengdu Desite, China.3,5-Dinitrosalicylic acid (DNS) reagent and 10× phosphate buffered solution (PBS) (pH 7.4) were purchased from Beijing Solarbio, China.All other reagents were analytical grade.
2.2 Preparation of PCPs
WSP was prepared by water extraction and alcohol precipitation according to the method of Jiang et al.[11]. The degreasing poria powder was obtained after heating reflux by petroleum ether for 2 h, and was extracted with water at 100 °C for 2 h according to the liquid-material ratio 1:20 and the supernatant was obtained by centrifugation. The extraction was repeated twice. The centrifuged precipitation, namely poria residue, was retained for extracting ASP.The two supernatants were combined and concentrated by rotary evaporation at 75 °C, and the concentrated solution was added with 80% ethanol to precipitate for 24 h, and the precipitated crude WSP was obtained by centrifugation. WSP was obtained by freeze-drying after protein removal with Sevage reagent.
ASP was prepared by alkali extraction and acid precipitation according to the method of Sun et al.[8]. The poria residue was soaked with 0.5 mol/L NaOH overnight according to the liquid-material ratio 1:40. After centrifugation, the supernatant was added with dilute HCl to reach pH 11. After centrifugation, precipitation was repeatedly washed to neutral, and ASP was obtained by freeze-drying.
CMP was prepared by the water-medium method according to the method of Chen et al.[12]. ASP was dissolved in 400 mL of 2.25 mol/L NaOH to obtain solution A; 300 mL of 6.25 mol/L NaOH was slowly added into the same volume of 5.3 mol/L chloroacetic acid solution,the reaction mixture was stirred to ensure complete reaction and then cooling to room temperature to obtain solution B. Solution A and B were mixed, and the mixture was slowly heated to 75 °C for 2.5 h.The reaction solution was neutralized with 6 mol/L HCl, and 3 times the volume of 95% ethanol was added to precipitate the product, and crude CMP was obtained after repeated ethanol washing and drying.Crude CMP was dissolved in water, decolorized by H2O2and filtered by DH-UF hollow fiber ultrafilter. The filtrate was settled with 3 times the volume of 95% ethanol, and CMP was obtained by freeze-drying ethanol precipitation.
2.3 Identification and analysis of PCPs
The structures of WSP, ASP and CMP were identified by ultraviolet spectroscopy (UV), infrared spectroscopy (IR),13C nuclear magnetic resonance spectroscopy (NMR) and1H NMR respectively.The WSP, CMP and ASP samples were prepared into 1 mg/mL solution with water and 0.5 mol/L NaOH, respectively, and scanned by UV spectrophotometer (UV-1780) in the range of 200–500 nm to determine purity. The samples were scanned by KBr method and infrared spectrometer (Thermo Nicolet iS5) in the range of 4 000–400 cm–1. The samples13C NMR and1H NMR were analyzed with an NMR spectrometer (AVANCE NEO 600 MHz). The degree of substitution (DS) of CMP was determined according to the method of Liu et al.[13]. CMP was dissolved in water and neutralized with excessive standard NaOH. After the reaction was complete, the excess base was neutralized with standard HCl. The DS of CMP can be calculated as follows:
Wheremrepresent CMP mass (g) ;CNaOHrepresent NaOH concentration (0.01 mmol/mL);VNaOHrepresent NaOH volume(25 mL);CHClrepresent HCl concentration (0.01 mmol/mL) andVHClrepresent HCl volume (mL).
2.4 Determination of the adsorption capacity of PCPs
2.4.1 Oil holding capacity (OHC)
According to the method of Si et al.[14]and modified it. WSP,ASP and CMP were respectively weighed about 0.5 g (dry base),and their mass was denoted asm1. Oil was added to the sample at the ratio of 1:30 (m/V), mixed well, placed at room temperature for 1 h, centrifuged at 3 000 r/min for 15 min, discarded the oil layer, and collected the sediment, whose mass was denoted asm2. OHC can be calculated as follows:
2.4.2 Cholesterol adsorption capacity (CAC)
According to the method of Xin et al.[15]and modified it. Mix fresh egg yolk with water at a ratio of 1:9 (m/V) to make the yolk liquid. Take 0.5 g (dry base) each for WSP, ASP and CMP added with 25 mL yolk liquid respectively, mix well, and shake for 2 h at 37 °C at 240 r/min in a shaker. After centrifugation at 4 000 r/min for 10 min, 0.1 mL supernatant was taken and added with acetic acid. [FeNH4(SO4)2·12H2O] was used as a chromogenic reagent. The absorbance was determined at 570 nm. And according to the standard curve (y= 16.049 0x–0.014 1,R2= 0.999 1), the cholesterol concentration of the yolk liquid (c0) and the centrifugal supernatant after adsorption (ci)were calculated. CAC can be calculated as follows:
2.4.3 Polyphenol adsorption capacity (PAC)
According to the method of Wu et al.[16]and modified it. The solutions of gallic acid, chlorogenic acid, epigallocatechin gallate(EGCG) and tea polyphenol with standard concentration (c0) were prepared, respectively. WSP, ASP and CMP (0.5 g, dry base) were added with 50 mL of different polyphenol solutions, and shaken for 2 h at 37 °C at 240 r/min in a shaker. After the adsorption reaction,the polyphenol adsorption reaction liquid of ASP was centrifuged at 4 000 r/min for 10 min, and the supernatant was taken to obtain the liquid to be tested. The polyphenol adsorption reaction liquid of WSP and CMP was ethanol-precipitated, the centrifuged supernatant was dealcoholized by rotary evaporation, and constant volume to obtain the liquid to be tested. All absorbance of the liquid to be tested was determined by the Foline-phenol method and the polyphenol concentration (ci) was calculated according to the standard curve (y=19.361 0x+ 0.014,R2= 0.999 1). PAC can be calculated as follows:
2.4.4 Metal ion adsorption capacity (MIAC)
According to the method of Huang et al.[17]and modified it. The polysaccharide solution was prepared with 0.5 g (dry base) WSP,ASP and CMP and 10 mL deionized water, put into a dialysis bag(interception aperture 3 500 Da) and then placed in 0.5 L Cd2+,Zn2+and Mg2+solutions with standard concentration (c0). After the adsorption reaction at room temperature for 3 h, the dialysis bag was taken out, and the solution outside the dialysis bag was diluted and the absorbance was measured by atomic absorption spectrometer(PinAAcle 900T). The concentrations of Cd2+, Zn2+and Mg2+in the solution outside the dialysis bag were calculated by the standard curve equation (Cd2+:y= 0.047 3x+ 0.000 7,R2= 0.999 2; Zn2+:y= 0.034 4x–0.001 6,R2= 0.999 2; Mg2+:y= 0.061 3x+ 0.001 7,R2= 0.999 0),denoted asc1.
After the adsorption, the dialysis bag was placed in 0.1 L deionized water for stirring dialysis to remove Cd2+, Zn2+and Mg2+that were not bound by PCP. The deionized water was replaced once every 1 h, 3 times in the same method. The replaced deionized water was combined, and the absorbance was determined by an atomic absorption spectrometer and the ion concentration was calculated,denoted asc2. The sum concentration of the residual ions in the solution after adsorption and those that were not adsorbed is denoted asci. MIAC can be calculated as follows:
2.5 In vitro simulated digestion
According to INFOGEST standard method[18], 400 mL electrolyte solution for simulated saliva fluid (SSF), simulated gastric fluid (SGF)and simulated small intestine fluid (SIF) were prepared respectively.Four milliliters of SSF electrolyte solution, 0.025 mL of 0.3 mol/L CaCl2solution and 0.75 mL of 750 U/mLα-amylase solution were mixed to obtain SSF for later use. Eight milliliters of SGF electrolyte solution, 0.005 mL of 0.3 mol/L CaCl2solution, 0.667 mL of 300 U/mL pepsin solution and 0.48 mL of 60 U/mL lipase solution were mixed, adjust pH to 3.0 with 6 mol/L HCl, and mix well to obtain SGF for later use. Eight milliliters of SIF electrolyte solution,0.04 mL of 0.3 mol/L CaCl2solution, 5 mL of 204 U/mL trypsin solution and 3 mL of 10 mmol/L sodium tauroglycocholate solution were mixed, adjust pH to 7.0 with 5 mol/L NaOH, mix well to obtain SIF for later use.
2.5.1 In vitro SSF digestion
Accurately weigh about 0.5 g of WSP, ASP and CMP, denoted asm0, add SSF preheated to 37 °C, add ultrapure water to 10 mL volume, and shake at 37 °C at 200 r/min for 5 min to SSF digestion.Immediately after digestion, put the mixture in a boiling water bath to inactivate the enzyme.
2.5.2 In vitro SGF digestion
Invitro, SGF digestion was performed on the basis ofinvitroSSF digestion. The method was as follows: add SGF preheated to 37 °C into the digestive fluid obtained during the SSF digestion phase,add ultrapure water to 20 mL, and shake at 37 °C at 200 r/min for 1,2 and 3 h to prepare SGF digestive fluid with different digestion time.Immediately after digestion, put the mixture in a boiling water bath to inactivate the enzyme, and centrifuge at 4 °C at 12 000 r/min for 15 min. The supernatant was denoted as GF-0 (digestion time 0 h),GF-1, GF-2, GF-3, and frozen at –80 °C for storage to be tested. The reduced sugar content (mg) of each supernatant was determined by the DNS method.
2.5.3 In vitro SIF digestion
Invitro, SIF digestion was performed on the basis ofinvitroSGF digestion. The method was as follows: add SIF preheated to 37 °C into the digestive fluid obtained after 3 h SGF digestion, add ultrapure water to 40 mL, and shake at 37 °C at 200 r/min for 1, 2 and 3 h to prepare SIF digestive fluid with different digestion time. Immediately after digestion, put the mixture in a boiling water bath to inactivate the enzyme, and centrifuge at 4 °C at 12 000 r/min for 15 min. The supernatant was denoted as IF-0 (digestion time 0 h), IF-1, IF-2, IF-3,and frozen at –80 °C for storage to be tested. The reduced sugar content (mi) of each supernatant was determined by the DNS method and the rate of residual polysaccharide (R) was calculated as follows:
2.6 In vitro simulated gut microbiota fermentation
Invitro, fermentation of digestive residue of PCPs was according to the method of Tian et al.[19]and modified it. WSP, ASP and CMP were simulatedly digested to the SIF digestion phase according to the above section 2.5. After 3 h of SIF digestion, the digestive fluid was dialyzed for 24 h (interception aperture 3 500 Da), and the remaining contents of the dialysis bag were freeze-dried and frozen at –20 °C for storage forinvitrosimulated intestinal fermentation experiment.
2.6.1 Intestinal microbiota collection
Three healthy volunteers who have not used antibiotics within three months were selected and their feces were collected, both ends and surfaces of the feces were discarded, and equal amounts of feces were taken and stirred evenly. A total of 10 g fresh feces were weighed and added to 100 mL sterile PBS (pH 7.4), and mixed to obtain 10% (m/V) fecal suspension. The fecal inoculum was obtained by filtration with 4 layers of sterilized gauze, and the whole operation should be completed within 15 min.
2.6.2 Mixed culture
According to the method of Amorim et al.[20], the culture medium was prepared and autoclaved for use. There were 4 groups ofinvitrofermentation experiment, which were the WSP, ASP, CMP and BC(distilled water, blank control) groups, with 4 parallel experiments in each group. Each polysaccharide sample (200 mg) was mixed with 9 mL of culture medium and autoclaved at 121 °C for 20 min. After cooling, 1 mL of fecal inoculum was added and then placed in an anaerobic air pouch for 24 h of oscillating fermentation at 37 °C. The fermentation broth was centrifuged at 4 °C at 12 000 r/min for 10 min,the supernatant was collected and frozen at –80 °C for subsequent short-chain fatty acids (SCFAs) detection, and the precipitate was frozen at –80 °C for microbial flora sequencing analysis.
2.6.3 Full-length 16S rRNA gene sequencing and SCFAs analysis
Microbial full-length 16S rRNA gene sequencing was completed by the third-generation microbial sequencing technology commissioned by Shanghai Personal Biotechnology Co., Ltd. Total DNA was extracted from the samples after fermentation and frozen at –20 °C for further analysis and detection. The DNA concentration was detected by UV quantitative equipment, and the purity was determined by agarose gel electrophoresis. Then, the extracted DNA was diluted to 20 ng/μL with sterile water. The full-length 16S rRNA gene in each sample was amplified using pre-primer 27F (AGAGTTTGATCMTGGCTCAG) and post-primer 1492R(ACCTTGTTACGACTT). The PCR reaction system consisted of 5 μL 5 × reaction buffer, 5 μL 5 × GC buffer, 2 μL dNTP (2.5 mmol/L),1 μL Forwardprimer (10 μmol/L), 1 μL Reverseprimer (10 μmol/L),2 μL DNA Template, 8.75 μL ddH2O, 0.25 μL Q5 DNA Polymerase.The reaction procedure of thermal PCR was as follows: initial denaturation 98 °C 2 min, denaturation 98 °C 15 s, annealing 55 °C 30 s, extension 72 °C 30 s, final extension 72 °C 5 min, 10 °C hold;25–30 cycles. After purification of PCR amplification products,TruSeq®DNA PCR-Free Sample Preparation Kit (Illumina, USA)was used for library construction, and Illumina NovaSeq platform was used to sequence the prepared library.
The contents of acetic acid, propionic acid, isobutyric acid,butyric acid, isovaleric acid and valeric acid in the fermentation were determined by gas chromatograph (Agilent-7890B). Chromatographic conditions: Stabilwax gas chromatographic column (30 m ×0.32 mm × 0.25 μm), carrier gas was nitrogen, flow rate of 1 mL/min,sample injection volume of 1 μL, shunt ratio of 10:1. The heating procedure is to maintain the initial column temperature at 100 °C for 0.5 min, then maintain 1 min after rising to 160 °C at 6 °C/min, and then maintain 0.5 min after rising to 200 °C at 20 °C/min.
2.7 Statistic analysis
The NMR spectral data were processed and analyzed using MestReNova software; TheP-value of theα-diversity index was tested by Kruskal-Wallis; The association between SCFAs and the gut microbiota was characterized by the Pearson correlation analysis;SPSS 23.0 was used for ANOVA of other data, and the data were presented as mean ± standard deviation (SD),P< 0.05 indicates significant difference and has statistical significance. The gene sequencing data were analyzed and drawn through the genes cloud platform (https://www.genescloud.cn); other parts were plotted by Origin 2018.
3. Results and analysis
3.1 Structure identification and analysis of PCPs
3.1.1 UV scan results
As shown in Fig. 1, no obvious absorption peaks could be found at 260 and 280 nm in three PCPs samples. This indicated that after purification, the protein and nucleic acid of three PCPs were removed,and the purity reached the experimental requirement.
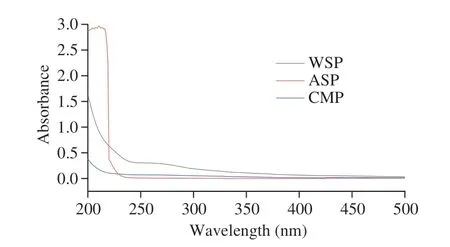
Fig. 1 UV scanning of three PCPs.
3.1.2 IR scan results
As shown in Fig. 2, the infrared spectrums of three PCPs are extremely similar, with characteristic absorption peaks of polysaccharides, including a strong absorption peak of 3 300–3 500 cm–1and a weak absorption peak near 2 900 cm–1.The absorption peak of CMP at 3 300–3 500 cm–1was weaker than that of ASP, which resulted from the substitution of partial–OH. The stretching vibration absorption peak of –COO appeared at 1 601 cm–1and the shear vibration peak of methine linked to–COO appeared at 1 420 cm–1, which constituted the characteristic signal of carboxymethylation products. The obvious absorption peaks of ASP and CMP near 890 cm–1indicate that the end-group carbon configuration of ASP and CMP isβ-type, but the absorption peak of WSP at 890 cm–1is not obvious, which requires further analysis of the glycosidic bond configuration.
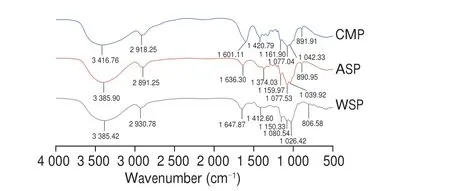
Fig. 2 IR spectrums of three PCPs.
3.1.3 NMR scan results
In the13C NMR spectrum (Figs. 3A–C), WSP, ASP and CMP showed signal values atδ102.34, 103.10 and 102.38 respectively,indicating that three PCPs containedβ-anomeric carbon. WSP had multiple signal values atδ95–110, indicating that WSP was a heteropolysaccharide. ASP and CMP had only one signal value,indicating that they were homopolysaccharides and inferred to beβ-glucan. Studies have shown that WSP was heteropolysaccharide containingD-glucose,D-galactose,D-mannose,D-fucose and a trace ofD-xylose[21]. ASP was aβ-glucan[22]. CMP was homogeneous containing onlyD-glucose[23].
ASP had 6 signal peaks atδ60–105, which wereδ103.10, 86.20,76.40, 72.92, 68.45 and 60.91, respectively, while CMP had signal peaks atδ102.38, 83.82, 75.50, 74.33, 73.21, 70.98, 67.97 and 60.57,respectively. It was speculated that the chemical shift of CMP moved to the high field than ASP, and two new signal peaks were appeared atδ60–105. Compared with the ASP, the13C NMR spectrum of CMP had a new carbon signal nearδ178, which should be the carbonyl characteristic peak of carboxymethyl, and a new carbon signal atδ57.34, which should be the –CH2of carboxymethyl signal peak.
In the1H NMR spectrum, WSP, ASP and CMP showed typical polysaccharide characteristics. Generally, theα-end-group proton signal is in theδ5–6 region, and theβ-end-group proton signal is inδ4–5. According to Figs. 3D and E, the chemical shifts of WSP and ASP occurred inδ4.2–5.3, indicating that they hadαandβpyranyl glycosidic bonds. The end-group proton signal of CMP wasδ4.35,and the signal peak larger thanδ5 was masked by the solvent peak(Fig. 3F).
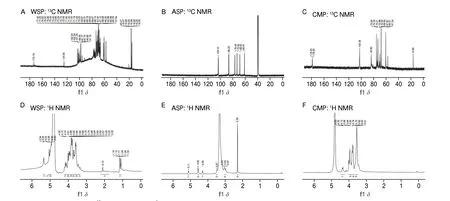
Fig. 3 13C NMR (A–C) and 1H NMR spectra (D–F) of three PCPs. (A, D) WSP; (B, E) ASP; (D, F) CMP.
The DS of CMP measured by titration was 1.3, indicating that on average 1.3 –OH was substituted by carboxymethyl on each glucose unit. Based on the data and analysis results, we could confirm that WSP, ASP and CMP have been successfully prepared.
3.2 The adsorption capacity of PCPs
3.2.1 OHC
As shown in Fig. 4A, the OHC of three PCPs was: ASP > WSP>CMP in decreasing order. The OHC of ASP was significantly higher than WSP and CMP (P< 0.05), but the difference between WSP and CMP was not significant (P> 0.05). It is assumed that ASP surface structure is loose and porous, with high oil adsorption space; while WSP and CMP have better hydrophilicity and low affinity for oil.
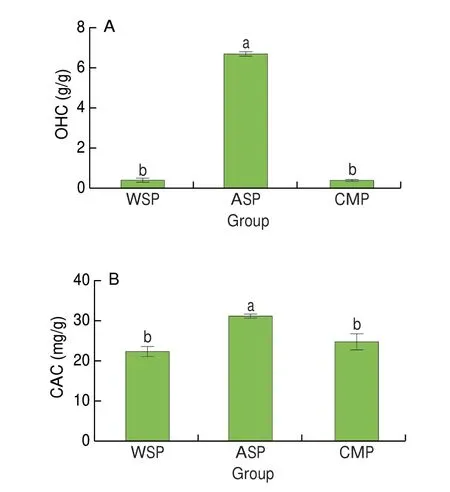
Fig. 4 OHC (A) and CAC (B) of WSP, ASP and CMP. Different letters indicate significant differences (P < 0.05).
3.2.2 CAC
The CAC was also used to evaluate the adsorption capacity of lipophilic substances of polysaccharides. As shown in Fig. 4B, the CAC of three PCPs was: ASP > CMP > WSP in decreasing order. ASP showed the highest CAC among the three samples (P< 0.05). Compared with the OHC, the CAC difference of the three PCPs was small.
3.2.3 PAC
As shown in Fig. 5A, the adsorption capacity of the same polysaccharide for different polyphenols is different. The adsorption capacity of WSP for four kinds of polyphenols was tea polyphenols > EGCG > chlorogenic acid > gallic acid in decreasing order; As for ASP, the order was EGCG > tea polyphenols >chlorogenic acid > gallic acid; As for CMP, the order was chlorogenic acid > tea polyphenols > EGCG > gallic acid. The three PCPs did not show the same optimal polyphenol adsorption, but the gallic acid adsorption capacity of three PCPs was lowest among four polyphenols.This result was consistent with that reported by Wu et al.[15].The adsorption capacity of different polysaccharides for the same polyphenol was also different. In general, the polyphenols adsorption capacity of ASP was significantly higher than that of WSP and CMP(P< 0.05). It can be inferred that ASP could be acted as a polyphenol carrier or polyphenol embedding agent to increase the polyphenol content in large intestine and exhibit antioxidant activities.
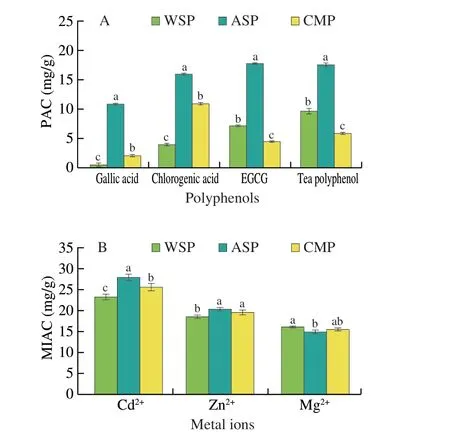
Fig. 5 PAC (A) and MIAC (B) of WSP, ASP and CMP. Different letters in the same index indicate significant differences (P < 0.05).
3.2.4 MIAC
The experimental results showed that all three PCPs had strong MIAC (Fig. 5B). The adsorption capacity of three PCPs for different ions was Cd2+> Zn2+> Mg2+, consistent with the principle that the adsorption rate of mental ion is positively correlated with the electronegativity and radius[24-25]. Comparison between different polysaccharides, the adsorption capacity of ASP for Cd2+and Zn2+was significantly stronger than that of WSP and CMP, and was slightly weaker than WSP and CMP for Mg2+, suggesting that the intake of PC or PCPs may participate in the digestion and absorption of minerals in the human body. The strong adsorption capacity of ASP for heavy metals deserves attention and research.
3.3 Reduced sugar release during in vitro digestion
Non-starch polysaccharides are important components of dietary fiber, and many non-starch polysaccharides are not easy to be decomposed by human digestive enzymes[26]. Some non-starch polysaccharides can be digested and degraded into reducing sugars by human enzymes[27]. The reducing sugars released quantity duringinvitrosimulated digestion can be used to assess the difficulty of polysaccharide digestion and degradation. As shown in Fig. 6, more reducing sugar was produced in WSP during SGF digestion, and increased with the digestion time, while the reducing sugar release in ASP and CMP was less, and the release quantity was not obvious with the digestion time. The reducing sugar release of three PCPs during SIF digestion was increased and significantly higher than that during SGF digestion, but compared with WSP, the reducing sugar release of ASP and CMP was still at a very low level. Afterinvitrodigestion,the polysaccharide residual rate of WSP, ASP and CMP was 91.41%,98.97% and 98.83%, respectively, indicating that ASP and CMP could reach the large intestine completely in the human digestive tract.
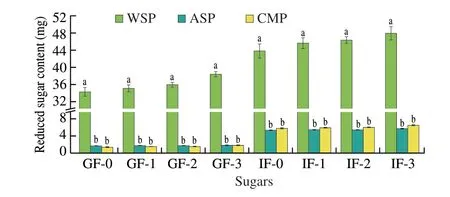
Fig. 6 The changes of reduced sugar content of WSP, ASP and CMP during digestion in vitro. Different letters in the same index indicate significant differences (P < 0.05).
3.4 Effects of PCPs on human gut microbiota
16S rRNA sequencing was performed on 16 samples from WSP,ASP, CMP and BC groups by Illumina platform, and a total of 317 050 raw sequences were obtained, 261 364 optimized sequences were obtained by DADA2 analysis, including sequence filtering, denoising, and dechimerism[28]. The average number of sequences in each sample was 16 335, and the sequence length was distributed between 1 407 and 1 549 bp, with an average sequence length of 1 455 bp, which met the requirements. Amplicon Sequence Variants (ASV) was obtained after clustering based on optimized sequence data.
The rarefaction curve (Fig. 7) indicated that the current sequencing depth was sufficient to reflect the diversity of the gut microbiota of samples in each group, and further increasing the sequencing depth could not find a new ASV, so data analysis could be performed.
Fig. 7 Rarefaction curve of each group
3.4.1 Venn diagrams analysis
Fig. 8 showed that a total of 363 ASVs were obtained in the 4 groups, among which the number of ASVs in WSP, ASP, CMP and BC groups was 39, 321, 29 and 28, respectively. The number of ASVs shared by 4 groups was 18. The ASVs unique to ASP group showed the highest value (303), indicating ASP may exert the strongest gut microbiota regulation role among the three PSPs samples.

Fig. 8 ASV Venn diagrams of each group.
3.4.2 α-Diversity analysis
α-Diversity refers to the richness, diversity, and evenness of the species within the group. QIIME software was used to calculate theα-diversity index. As can be seen from Fig. 9, the Good’s coverage index of all groups was greater than 0.99, indicating that the sequencing results could truly reflect the actual situation of the samples. Compared with BC, the Chao1 and Observed species index of the ASP group was increased, indicating that ASP could improve the richness of gut microbiota afterinvitrofermentation. Compared with BC, ASP incubation substantially increased the Shannon index of the human gut microbiota, while this index in WSP and CMP only slightly increased. Compared with BC, the remarkable increase of Faith’s PD index of ASP group indicating that ASP could improve the genetic diversity of gut microbiota which is important to the adaptability of gut microbiota to environmental changes. Compared with BC, the Pielou’s evenness index of WSP and ASP were decreased, but that value of CMP group was increased, indicating that species distribution of CMP group was more even. In conclusion,the comprehensive comparison of allα-diversity indexes showed that ASP could strongly improve the richness and diversity of gut microbiota compared with WSP and CMP.
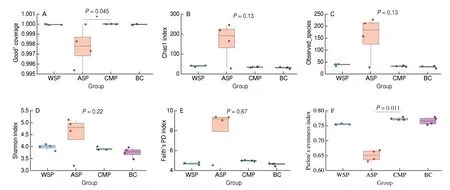
Fig. 9 Effects of WSP, ASP, CMP and BC on α-diversity of human gut microbiota. (A) Good’ coverage; (B) Chao1 index; (C) Observed_species; (D) Shannon index; (E) Faith’s PD index; (F) Pielou’s evenness index.
3.4.3 β-Diversity analysis
β-Diversity refers to differences in species composition between groups. According to principal coordinate analysis (PCoA), the proportions of PCo1 and PCo2 to explain the difference data of each group of samples were 57.2% and 10.4%, respectively (Fig. 10A).The stress value of the nonmetric multidimensional scale (NMDS)analysis was 0.022 7 ( < 0.05) (Fig. 10B). These data demonstrated that the microbiota species of three experimental groups were distant from BC group. The boxplot for difference analysis between groups was shown in Fig. 10B. The median line of the 3 experimental groups, especially ASP group, was higher than that of the BC group(Fig. 10C), indicating that there were significant microbiota differences between three PCPs and BC (P< 0.05). TheRvalue was 0.784 722, indicating that the difference between groups was greater than the difference within groups. These results showed that among the three PCPs, ASP could exert strongest effect on diversity of human gut microbiota.

Fig. 10 Effects of WSP, ASP, CMP and BC on β-diversity of human gut microbiota. (A) PCoA diagram; (B) NMDS graph; (C) Boxplot for difference analysis between groups.
3.4.4 Species composition analysis
Fig. 11A showed that Proteobacteria, Firmicutes and Bacteroidetes were the common dominant bacteria at the phylum level of the four experimental groups. Fusobacteria existed in ASP,CMP and BC groups, and was not detected in the WSP group.Actinobacteria existed only in the ASP group and was not detected in the other groups. Compared with the BC group, WSP, ASP and CMP all increased the relative abundance of Firmicutes, and only CMP increased the relative abundance of Bacteroidetes. Both ASP and CMP decreased the relative abundance of Proteobacteria, and ASP exhibited a stronger effects than CMP.

Fig. 11 Relative abundance of dominant bacteria at the phylum (A) and species (B) level in WSP, ASP, CMP and BC group.
Fig. 11B showed the relative abundances of the top 10 dominant bacteria at the species level.Escherichia coliis an opportunistic pathogen. Compared with the BC group, ASP and CMP could inhibit the growth ofE. coli, and ASP had a more obvious inhibition effect.Parabacteroidesdistasonisis a probiotic. Compared with the BC group, only CMP increased the relative abundance ofP. distasonis.Klebsiellapneumoniaeis an opportunistic pathogen. Compared with the BC group, the three PCPs could inhibit the growth ofK. pneumoniae, and the inhibitory effect of WSP and CMP was better than ASP.Enterococcus faeciumis a probiotic, and belongs toEnterococcus. Compared with the BC group, both ASP and CMP could promote the growth of theE. faecium.Weissella confusahas been recently proposed for their probiotic potential which can inhibit the growth of many harmful bacteria[29]. WSP and ASP incubation promoted the growth ofW. confusa.
In conclusion, the three PCPs can promote the growth of some beneficial bacteria and inhibit the growth of some harmful bacteria or opportunistic pathogens, but there are some differences among different polysaccharides. For instance, ASP and CMP can inhibit the growth ofE. coliand promote the growth of beneficial bacteriaE. faecium. CMP can promote the growth of beneficial bacteriaP. distasonis; WSP can promote the growth ofW. confusa. All three PCPs can inhibit the growth of the opportunistic pathogenK. pneumoniae.
3.4.5 Mark species analysis
Fig. 12 showed the heatmap of the species composition. There were some differences in microbial composition among the groups.WSP group mainly containedMegasphaera elsdeniiandWeissella confusa. ASP group mainly containedAnaerostipes hadrus,Bacteroides ovatus,Phascolarctobacterium faecium,Enterobacter hormaechei,Citrobacter freundiiandLactococcus lactis. The CMP group mainly containedP. distasonisandBacteroides thetaiotaomicron. While the BC group mainly containedKlebsiella pneumoniae. Studies suggested thatA. hadrus,P. faecium,L. lactis,P. distasonisandB. thetaiotaomicronare beneficial bacteria[30].Therefore, ASP and CMP can promote the growth of intestinal beneficial bacteria and regulate intestinal microecology.
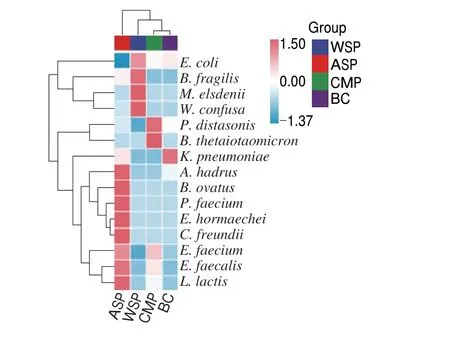
Fig. 12 Heatmap of species composition (species level) in WSP, ASP, CMP and BC group.
LDA effect size (LEfSe) analysis and random forest analysis can be used to find differential species of gut microbiota in each group,and the results are shown in Fig. 13. LEfSe analysis results showed that with the LDA threshold greater than 4 as the evaluation criteria,there were 7 characteristic microorganisms at the species level among all groups.E. coli,M. elsdeniiandW. confusawere differential species in the WSP group.B. doreiwas the differential specie in the ASP group.P. distasonisandB. thetaiotaomicronwere differential species in the CMP group. The random forest analysis results showed thatE. coli,M. elsdenii,W. confusa,P. distasonis,B. thetaiotaomicronandK. pneumoniaeobtained by LEfSe analysis had influence values.The area under the curve (AUC) of these six differential species were greater than 0.8 by receiver operating characteristic (ROC)analysis (E. coli,M. elsdeniiandW. confusa, AUC = 1;P. distasonis,AUC = 0.958 3;B. thetaiotaomicron, AUC = 0.875;K. pneumoniaeAUC = 0.958 3) verifying that the data were not overfitted.
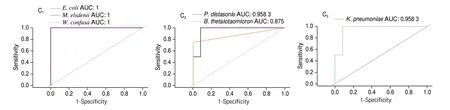
Fig. 13 (Continued)
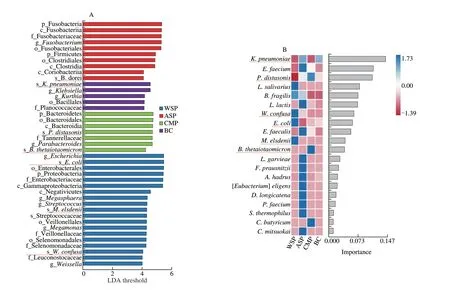
Fig. 13 Marker species of gut microbiota in WSP, ASP, CMP and BC group. (A) Histogram of LDA value distribution of differential species; (B) Random forest map; (C) ROC curve, AUC value represents the size of the area below the ROC curve, the closer to 1, the more reliable.
BothP. distasonisandB. thetaiotaomicron[31]are probiotics,indicating that CMP can improve the intestinal environment by increasing the relative abundance of beneficial bacteria.
3.5 SCFAs content
According to Table 1, the total acid content of the three experimental groups was significantly higher than that of the BC group (P< 0.05), indicating that the three PCPs could promote gut microbiota to produce SCFAs by using themselves as a metabolic substrate. The total acid content was CMP (30.207 mmol/L) >WSP (29.827 mmol/L) > ASP (23.228 mmol/L). Compared with the BC group, CMP, WSP and ASP incubation increased the total acid content by 2.30, 2.26 and 1.54 times, respectively. In general,acetic acid production of the three PCPs was the highest, followed by propionic acid and butyric acid. There were differences in SCFAs production among all groups. Water-soluble WSP and CMP were superior to alkali-soluble ASP in promoting acetic acid and propionic acid production. The content of butyric acid in the WSP group was significantly lower than that in CMP and ASP groups(P< 0.05). Three PCPs incubation showed little effect on isovaleric acid production. Compared with BC group, only WSP significantly increased the valeric acid and isobutyric acid.
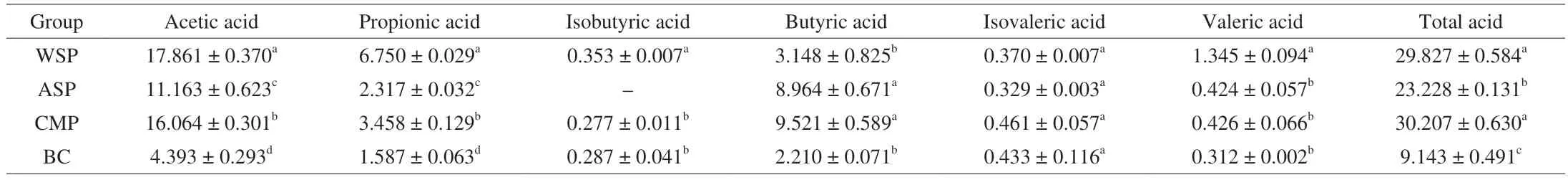
Table 1 SCFAs concentration in fermentation broth of WSP, ASP, CMP and BC group of samples in vitro (mmol/L).
In conclusion, all three PCPs could significantly promote the production of acetic acid, propionic acid and the total acid content compared with BC group. Hence, it can be speculated that all three PCPs can maintain a healthy intestinal micro ecological environment by promoting the growth of some beneficial bacteria and producing health beneficial short chain fatty acids.
3.6 Correlation analysis between SCFAs and gut microbiota
Pearson correlation analysis was used to explore the correlation between differential species and SCFAs, as shown in Fig. 14.M. elsdeniiandW. confusawere positively correlated with acetic acid and propionic acid content, indicating thatM. elsdeniiwas the main species causing the higher acetic acid and propionic acid content in WSP group than in other groups.P. distasonisandB. thetaiotaomicronwere positively correlated with butyric acid.P. distasonisandB. thetaiotaomicronwere differential species in the CMP group, so it was inferred that CMP can promote the growth of beneficial bacteria and produce butyric acid.K. pneumoniaewas negatively correlated with acetic acid (P< 0.01) and propionic acid.K. pneumoniaewas the differential species in the BC group. The relative abundance ofK. pneumoniaein the BC group was the highest, and the contents of acetic acid and propionic acid were the lowest. Therefore, it was concluded that the three PCPs could inhibit the growth ofK.pneumoniaeby promoting the growth of probiotics and producing acetic acid and propionic acid.
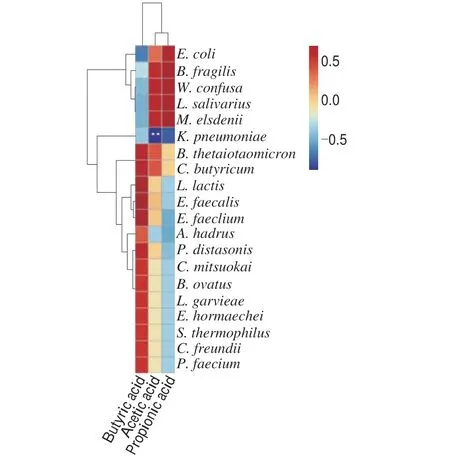
Fig. 14 Correlation analysis between SCFAs and differential species. ** P < 0.01.
4. Discussion
PC decoction has been widely used in TCM clinics for thousands of years. The insoluble polysaccharide (ASP) is often discarded as herb residue. However, the whole PC has also been historically utilized as functional herbal food to improve cognitive and immune functions, as well as to reduce levels of serum lipids. Considering the health benefits of ASP similar to dietary fiber and the traditional application of whole PC, ASP may also contribute substantially to the therapy function of PC. In this study, ASP showed the highest OHC, cholesterol and various polyphenols adsorption capacity.Additionally, ASP and CMP exhibited probiotic effect via stimulating beneficial bacteria proliferation and inhibiting the growth of some harmful bacteria or opportunistic pathogens. Additionally, the content of short-chain fatty acids, one of the main metabolic products of gut bacteria, was increased after three PCPs treatment. Therefore, except for only use the water extract of PC in clinic, ASP also contribute to the efficacy of dietary PC therapy, and can be used in healthcare to take full advantage of this valuable herbal medicine.
Good adsorption capacity of PCPs on cholesterol and oil may guarantee the lipids lowering activity of PC diet. Consistent with the results of Wu et al.[32], we found that the adsorption capacity of ASP on oil and cholesterol was significantly stronger than water-soluble WSP and CMP. Since ASP is one of the main components in PC, we concluded that ASP is the material basis of PC to exert lipids lowering activity and weight reducing effects in human body. These effects also conform to the TCM application characteristics of PC, such as invigorating the spleen and removing dampness, and hyperlipidemia and obesity therapy.
Free polyphenols in food are almost completely absorbed in the small intestine during human digestion, while when combined with protective carriers such as dietary fiber, polyphenols can reach the large intestine and play a protective role in the intestinal tract.In this study, the adsorption capacity of ASP on four polyphenols was significantly stronger than that of water-soluble WSP and CMP, which indicated that combined with polyphenols, ASP can maintaining a healthy environment in intestinal tract by increasing the antioxidant activity.
This study also found that the three PCPs had strong MIAC,suggesting that the intake of PC or PCPs may participate in the digestion and absorption of minerals in the human body. The adsorption capacity of three PCPs on heavy metal ions was stronger than that on non-heavy metal ions. From the perspective of food safety and nutrition, PCPs in food could be conducive to reducing the absorption of Cd2+in food.
The regulation of gut microbiota is another major mechanism underlying the pharmaceutical activities of PC and PCPs. ASP and its modified product CMP have better probiotic effects on gut microbiota than WSP. Insoluble ASP was superior to water-soluble WSP and CMP in increasing the richness and diversity of intestinal microorganisms. It has also been found that insoluble dietary fiber(IDF) is better than soluble dietary fiber (SDF) in alleviating colitis in mice by regulating gut microbiota[33], and the addition of dietary insoluble polysaccharides can establish a health microbial community,which is conducive to intestinal health[34]. In this study, ASP and its modified product CMP showed stronger effects of promoting the proliferation of probiotics and inhibiting the growth of harmful bacteria than that of WSP. At the phylum level, only CMP increased the relative abundance of Bacteroidetes which was negatively correlated with obesity[35-36]. Studies have demonstrated that supplementation of diets rich in dietary fiber or polysaccharides could reduce weight gain by increasing the abundance of Bacteroidetes[37-38].Bacteroidetes could also activate T cell-mediated responses by interacting with the immune system to limit the colonization of potentially pathogenic bacteria in the gastrointestinal tract, thus exerting a healthy effect on the host[39]. These studies suggested that CMP has benefical functions on human body, such as weight loss and immune regulation. Both ASP and CMP decreased the relative abundance of Proteobacteria. Proteobacteria contains a large number of potential pathogenic bacteria, which can cause intestinal disorders or directly contribute to inflammatory bowel disease. The increased abundance of Proteobacteria may lead to intestinal microecological instability or energy imbalance in the host[40-42], suggesting that ASP and CMP had the function of improving intestinal disorders.At the species level, only CMP increased the relative abundance of probioticP. distasoniswhich can improve the symptoms of diarrhea, constipation or aging by regulating human gut microbiota.P. distasonisis negatively correlated with obesity, diabetes, nonalcoholic fatty liver and other diseases[43]. Both ASP and CMP could promote the growth of the probioticE. faeciumwhich can inhibit the proliferation of harmful intestinal bacteria, promote the digestion and absorption of nutrients[44-45]. The three PCPs could inhibit the growth of the opportunistic pathogenK. pneumoniae. ASP and CMP could inhibit the growth of the opportunistic pathogenE. coli. Therefore,more attention should be paid to the function of ASP and its modified products on regulating gut microbiota. In this study, it is particularly noteworthy that CMP reduced the diversity of flora, but it remarkably increased the relative abundance ofP. distasonis, indicating CMP may serve as a good prebiotics candidate. One limitation of the present study is that we only investigated the effects of PCPs on human gut microbiotainvitro, further human study is needed to uncover the underlying mechenisms.
SCFAs are the main metabolites of intestinal microbial fermentation of undigested polysaccharides in the colon, with the number of carbon atoms generally ranging from 1 to 6[46-47]. The common SCFAs are including acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid and isovaleric acid, et al. Acetic acid can cross the blood-brain barrier and be metabolized in the muscle, kidneys, heart and brain to provide energy[48]. Propionic acid can provide energy to cells, participate in gluconeogenesis and inhibit cholesterol synthesis[49]. Butyric acid has anti-inflammatory,immunomodulation, antitumor and other effects[50]. The yield of SCFAs can reflect the changes in intestinal microorganismsin vivo[51].The probiotic activity of PCPs can be evaluated by the analysis of SCFAs after the fermentation of gut microbiota[52]. In this study, all three PCPs promoted the production of SCFAs in gut microbiota fermentation. The proliferation of the main beneficial bacteria was positively correlated with butyric acid, while the proliferation of the main harmful bacteria was negatively correlated with acetic acid and propionic acid. Therefore, it can be inferred that PCPs can change the intestinal micro ecological environment by promoting the growth of probiotics and the production of their metabolite SCFAs.
5. Conclusion
In conclusion, ASP showed the highest OHC, cholesterol and various polyphenols adsorption capacity than WSP and CMP. ASP incubation could strongly improve the richness and diversity of gut microbiota. ASP and CMP can inhibit the growth ofE. coliand promote the growth of beneficial bacteriaE. faecium. CMP can promote the growth of beneficial bacteriaP. distasonis. WSP can promote the growth ofW. confusa. All three PCPs can inhibit the growth of the opportunistic pathogenK. pneumoniae. Additionally,the three PCPs significantly promoted the production of acetic acid,propionic acid and the total acid content compared with BC group.Therefore, among three PCPs, ASP has great potential to be developed as an excellent candidate for the treatment of obesity, hyperlipidemia and immune regulation. Moreover, in the healthcare field, it is a waste of resources to discard the ASP of PC as TCM extract residues, and the processing and utilization of only water-soluble extract of PC need to be re-examined. The healthcare effect of PCPs based oninvitroobservation needs to be further verified byin vivoand even human experiments.
Conflict of interest
The authors declare there is no conflict of interest.
Acknowledgements
This work was supported by the Province Natural Science Foundation of Hunan, China (2022JJ5410) and Special Project on Modern Agricultural Industrial Technology System Construction of Hunan, China (2022-67).
杂志排行
食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Call for Papers from Food Science of Animal Products
- Ascophyllum nodosum and Fucus vesiculosus ameliorate restenosis via improving inf lammation and regulating the PTEN/PI3K/AKT signaling pathway
- Alleviatory effect of isoquercetin on benign prostatic hyperplasia via IGF-1/PI3K/Akt/mTOR pathway
- Voluntary wheel running ameliorated the deleterious effects of high-fat diet on glucose metabolism, gut microbiota and microbial-associated metabolites
- Effect of simulated gastrointestinal digestion on antioxidant, and anti-inflammatory activities of bioactive peptides generated in sausages fermented with Staphylococcus simulans QB7