Alleviatory effect of isoquercetin on benign prostatic hyperplasia via IGF-1/PI3K/Akt/mTOR pathway
2024-01-24YoungJinChoiMeiqiFanNishalaEraniWeamullaYujiaoTangEunKyungKim
Young-Jin Choi, Meiqi Fan, Nishala Erani Weamulla,e, Yujiao Tang, Eun-Kyung Kim
a Department of Food Science and Nutrition, Dong-A University, Busan 49315, Republic of Korea
b Center for Silver-targeted Biomaterials, Brain Busan 21 Plus program, Dong-A University, Busan 49315, Republic of Korea
c Department of Health Sciences, the Graduate School of Dong-A University, Busan 49315, Republic of Korea
d Division of Food Bioscience, College of Biomedical and Health Sciences, Konkuk University, Chungju 27478, Republic of Korea
e Department of Export Agriculture, Faculty of Animal Science and Export Agriculture, Uva Wellassa University, Badulla 90000, Sri Lanka
f School of Bio-Science and Food Engineering, Changchun University of Science and Technology, Changchun 130600, China
g Nutritional Education Major, Graduate School of Education, Dong-A University, Busan 49315, Republic of Korea
h Nutrinomics Lab. Co., Ltd., Busan 49315, Republic of Korea
Keywords: Isoquercetin Benign prostatic hyperplasia Androgen receptor signaling PI3K/Akt/mtor pathway
ABSTRACT We evaluated the effect of isoquercetin (quercetin-O-3-glucoside-quercetin, IQ) as a functional component of Abeliophyllum disistichum Nakai ethanol extract (ADLE) on prostate cell proliferation and apoptosis and its effects on the IGF-1/PI3K/Akt/mTOR pathway in benign prostatic hyperplasia (BPH). Metabolites in ADLE were analyzed using UHPLC-qTOF-MS and HPLC. IQ was orally administered (1 or 10 mg/kg) to a testosterone propionate-induced BPH rat model, and its effects on the prostate weight were evaluated. The effect of IQ on androgen receptor (AR) signaling was analyzed in LNCaP cells. Whether IGF-1 and IQ affect the IGF-1/PI3K/Akt/mTOR pathway in BPH-1 cells was also examined. The metabolites in ADLE were identif ied and quantif ied, which conf irmed that ADLE contained abundant IQ (20.88 mg/g). IQ signif icantly reduced the prostate size in a concentration-dependent manner in a BPH rat model, and signif icantly decreased the expression of AR signaling factors in the rat prostate tissue and LNCaP cells in a concentration-dependent manner. IQ also inhibited the PI3K/AKT/mTOR pathway activated by IGF-1 treatment in BPH-1 cells. In BPH-1 cells, IQ led to G0/G1 arrest and suppressed the expression of proliferation factors while inducing apoptosis. Thus, IQ shows potential for use as a pharmaceutical and nutraceutical for BPH.
1. Introduction
Benign prostatic hyperplasia (BPH) is a chronic disease associated with aging in men but its exact cause remains unknown[1]. BPH often causes bladder outlet obstruction, lower urinary tract symptoms,and, in severe cases, renal failure and bladder decompensation[2].The number of patients with BPH will continue to increase as society ages[3]. Approved medical therapies for BPH are limited to alpha-blockers, 5-alpha reductase inhibitors, and phosphodiesterase type 5 inhibitors[4].
BPH is thought to be caused mainly by decreased secretion of male hormones due to the deterioration of endocrine function with aging and is associated with metabolic syndrome, hyperinsulinemia,race, obesity, high blood pressure, diabetes, low-density cholesterol,and smoking[5-10]. The combination of androgen and AR, ligandinduced transcription factor are essential for the growth and development of the prostate. However, excessive AR sign aling is considered as a major factor promoting BPH and prostate cancer[11]. On the other hand, men tend to decrease circulating androgen levels with age, but it has been reported that testosterone levels in the prostate of patients with benign prostatic hyperplasia are similar to those of normal people[12]. Several times higher dihydrotestosterone (DHT) level in the prostate have been reported in hyperplastic tissue compared to normal prostate[13]. This suggests that the androgen-binding androgen receptor (AR), which converts testosterone to DHT, and the activity of 5-alpha reductase type 2(5AR2) are involved in the development of BPH. Additionally,the phosphoinositide 3-kinase/protein kinase B/mechanistic target of rapamycin (PI3K/Akt/mTOR) signaling pathway is involved in abnormal prostate development[14]. Excessive activation of this pathway is associated with abnormal proliferation of prostate cells.AR has been suggested to induce insulin-like growth factor (IGF)-1 production in prostate stromal cells and promote proliferation through IGF-1 receptor (IGF-1R) in the surrounding prostate epithelium[15].Previous studies showed that in patients with BPH treated with rapamycin, prostate proliferation was inhibited[16-17]. Metformin,a widely used treatment for diabetes, activates AMP-activated protein kinase and inhibits mTOR signaling. According to recent studies, metformin also inhibits the overgrowth of prostate cells,demonstrating its potential for use as a treatment for benign prostatic hyperplasia[18-19]. Therefore, BPH treatment may not only inhibit AR signaling but also target IGF-1/PI3K/Akt/mTOR signaling.
Quercetin also inhibits PI3K/Akt/mTOR signaling and has anticancer[20], anti-inflammatory[21], anti-allergy[22], and anti-diabetic effects[23]. Quercetin is largely distributed in natural plants as isoquercetin (quercetin-O-3-glucoside-quercetin, IQ) and rutin(quercetin 3-rutinoside) rather than as aglycone[24]. Previous studies showed that the ethanolic leaf extract ofAbeliophyllum distichumNakai (ADLE), a plant native to Korea, affects BPH[25]. In a follow-up study, the metabolites of ADLE were analyzed using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-qTOF MS), confirming the high abundance of IQ (Figs. 1A-B). However the effects of IQ on BPH are not known.Therefore, we examined whether IQ inhibits BPH by inhibiting AR signaling and the PI3K/AKT/mTOR pathway.
2. Materials and methods
2.1 Materials
DriedA. distichumleaves were obtained from Woorinamoo Agricultural Union Corporation (Goesan, Korea). RPMI 1640 medium and fetal bovine serum (FBS) were purchased from Welgene(Daegu, Korea). Penicillin/streptomycin (P/S) was obtained from Gibco (Grand Island, NY, USA). Testosterone propionate was provided by the Tokyo Chemical Industry Co. (Tokyo, Japan). Rutin,IQ, verbascoside, DHT, finasteride (Fi) enzalutamide, LY294002,and rapamycin were purchased from Sigma-Aldrich (St. Louis,MO, USA). Human IGF-1 was purchased from R&D Systems(Minneapolis, MN, USA).β-Actin, steroid receptor coactivator-1,prostate-specific antigen (PSA), AR, proliferating cell nuclear antigen (PCNA), and glyceraldehyde-3-phosphate dehydrogenase(GAPDH) antibodies were purchased from Santa Cruz Biotechnology(Dallas, TX, USA). Antibodies against p21, cyclin D1, cyclin D2,cyclin D3, cyclin-dependent kinase 4 (CDK4), CDK6, cyclin A2,IGF-1R, p-IGF-1R, p-Akt, PI3K, and p-mTOR were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against 5AR2 and IGF-1 were purchased from Abcam (Cambridge,UK). Horseradish peroxidase-conjugated goat anti-rabbit IgG,horseradish peroxidase-conjugated goat anti-mouse IgG, and FSD™594 conjugated goat anti-rabbit IgG were acquired from BioActs(Incheon, Korea).
2.2 UPLC-qTOF MS analysis
A Vion UPLC™ system (Vion, Waters, Milford, MA, USA) was used in this study. The LC conditions were optimized as 9 min on an Acquity UPLC BEH C18column (2.1 mm × 100 mm, 1.7 μm; Waters).The column temperature was set to 55 °C and the flow rate was set to 0.35 mL/min. The mobile phases were water containing 0.1%formic acid (solvent A) and acetonitrile containing 0.1% formic acid (solvent B). Spectrometry using electrospray ionization was performed in negative mode. The MS operating conditions were as follows: capillary voltage 2.5 kV, sample cone, 20 V; ion source temperature, 200 °C; desolvation temperature, 400 °C; cone gas,30 L/h; desolvation gas, 900 L/h; scan time 0.2 s, scan range,m/z50–1 500; collision energy ramp from 10–30 eV (m/z50–1 000).
2.3 HPLC
A UltiMate3000 HPLC System (Thermo Fisher Scientific,Waltham, MA, USA) was used in this study. IQ, rutin, and verbascoside were used as standard samples. An Acclaim™ C18reversed-phase HPLC column (4.6 mm × 250 mm, 5 μm) was used.The column temperature was 30 °C and flow rate was 1.0 mL/min.The gradient elution was changed linearly from 0% to 20% B over 5 min, from 20% to 40% B for 20 min, and from 40% to 80% B for 35 min. The mobile phase composition was returned to the initial condition (solvent B:solvent A = 0:100) and allowed to run for another 5 min before injecting another sample. The total analysis time per sample was 40 min. The detector wavelength was measured using ultraviolet light at a wavelength of 272 nm.
2.4 Cell culture
LNCaP cells (KCLB number: 21740) were obtained from the Korean Cell Line Bank (Seoul, Republic of Korea). BPH-1 cells(SCC256) were purchased from Merck (Kenilworth, NJ, USA). The cells were cultured in RPMI 1640 supplemented with 10% FBS and 100 mg/mL P/S in 5% CO2at 37 °C. LNCaP cells were seeded into 6-well plates (1 × 106cells/well) in 2 mL of RPMI 1640 supplemented with 10% FBS and 100 mg/mL P/S. After 24 h, the LNCaP cells were co-treated with testosterone propionate (TP; 100 nmol/L), ADLE (25,50, or 100 μg/mL), or IQ (5, 10, or 20 μmol/L) for 24 h. LNCaP cells treated with enzalutamide (En, 10 μmol/L) or Fi (1 μg/mL) were used as positive controls. The cells were then collected for western blot analysis.
BPH-1 cells were seeded in 6-well plates (2 × 106cells/well) in 2 mL of RPMI 1640 supplemented with 10% FBS and 100 mg/mL P/S. After 24 h of culture, the BPH-1 cells were starved in serum-free medium for 24 h. The BPH-1 cells were then incubated for 24 h in FBS-free RPMI 1640 medium containing IGF-1 (100 ng/mL), ADLE(25, 50, or 100 μg/mL), or IQ (10, 25, or 50 μmol/L). Cells treated with LY294002 (10 μmol/L) or rapamycin (10 μmol/L) were used as positive controls. The cells were collected for western blot analysis,immunofluorescence, and flow cytometry.
2.5 MTT assay
LNCaP and BPH-1 cells were seeded into 96-well plates at a density of 5 × 103cells/well and incubated in 5% CO2at 37 °C for 24 h.After 24 h of incubation, the cells were replaced with fresh medium and treated with various concentrations of ADLE or IQ at 37 °C for 24 h. Subsequently, each well was treated with MTT working solution(5 mg/mL in phosphate buffer) for 4 h. The medium was removed,and 100 μL of dimethyl sulfoxide was added to each well. Finally,measurements were performed at a wavelength of 540 nm using an absorbance meter. The data are expressed as percentages relative to the absorbance values of the untreated group.
2.6 Reverse transcription quantitative PCR
LNCaP cells were seeded into 6-well plates at a density of 5 × 105cells/well and incubated in 5% CO2at 37 °C for 24 h. LNCaP cells were harvested after treatment with TP (100 nmol/L), ADLE (25, 50, or 100 μg/mL), or IQ (5, 10, or 20 μmol/L) for 48 h. En (10 μmol/L) or Fi (1 μg/mL) served as a positive control. RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA synthesis was performed using AccuPower® RT PreMix (Bio-Rad, Hercules,CA, USA). Quantitative PCR was performed for PSA, UGT2B28,FGF-2, EGF, IGF-1R, and GAPDH, using an MIC qPCR cycler(Bio Molecular Systems, Upper Coomera, Australia). The ΔCt =Target gene Ct - GAPDH Ct value was calculated using the Ct value provided by the micPCR software and expressed as 2-ΔΔCt.
2.7 Immunofluorescence
BPH-1 cells were treated with IGF-1 (100 ng/mL) and ADLE(25, 50, or 100 μg/mL), IQ (10, 25, or 50 μmol/L), LY294002(10 μmol/L), or rapamycin (10 μmol/L). The expression of p-AKT was visualized using immunofluorescence. Briefly, BPH-1 cells(5 × 103cells/well) were seeded into an 8-well slide chamber (SPL Life Science Co., Seoul, Republic of Korea). The cells were fixed in ice-cold methanol, followed by permeabilization with 0.1%Triton X-100 for 15 min. After blocking in 5% normal goat serum,the slides were incubated overnight at 4 °C with p-AKT antibody(1:300 dilution), followed by incubation with the corresponding goat anti-rabbit IgG, FSD 594 (1:1 000 dilution) for 1 h. Mounting medium containing DAPI was added to the slides, and coverslips were mounted. Images were captured using a Zeiss 700 confocal microscope (Oberkochen, Germany).
2.8 Flow cytometry for cell cycle analysis
The cell cycle was analyzed using a Guava Muse Cell Analyzer(Luminex Corp., Austin, TX, USA). BPH-1 cells were seeded into a 12-well plate at density of 1 × 105cells/well and incubated for 24 h. The cells were washed three times with phosphate-buffered saline (PBS), and then harvested by treatment with 0.25% trypsin-EDTA in PBS, fixed in ice-cold 70% ethanol, and stored at -20 °C overnight. The fixed cells were centrifuged to remove the supernatant,and the cell pellet was washed with PBS. Following treatment of the cells with 200 μL Muse Cell Cycle Reagent (MCH100106, Merck Millipore, Billerica, MA, USA) in the dark for 30 min at room temperature (20–25 °C), the cell cycle was analyzed using a Guava®Muse® cell analyzer.
2.9 Animal experiments for TP-induced BPH
Male Sprague-Dawley rats (n= 40, 8 weeks old) were obtained from Nara Biotech Co., Ltd. (Pyeongtaek, Korea). The setup of the rat cage was carefully controlled under the following conditions: a temperature of 23–25 °C, relative humidity of 40%–60%, and lightdark cycle of 12 h, with a 2-h cycle of 40%–60% alternating day and night. Water and feed were provided ad libitum. All experimental and animal care procedures were approved by the Dong-A University Animal Care and Use Committee (DIACUC-21-05). To eliminate endogenous testosterone secretion after a one-week adaptation period,the BPH group was anesthetized with phenobarbital (50 mg/kg),scrotal skin of the rats was incised, vas deferens was tied with sutures,and testes and epididymis were removed. In the control group, the scrotal skin was incised and sutured. After 3 days, BPH was induced by subcutaneous injection of TP (3 mg/kg·day) dissolved in corn oil daily for four weeks. When TP was administered, IQ (1 mg/kg)and IQ (10 mg/kg) were orally administered daily to the BPH+IQ 1 and BPH+IQ 10 groups for 4 weeks. In the BPH+Fi group, Fi(1 mg/kg·day) was orally administered for 4 weeks. After the final injection, the rats were anesthetized with pentobarbital (50 mg/kg,anesthetized by intraperitoneal injection), the prostate was harvested,and its weight (g) was measured. Sections of the ventral prostate lobes were fixed with 10% formaldehyde for hematoxylin and eosin staining, and the remaining ventral prostate lobes were stored at -80 °C for western blot analysis.
2.10 Hematoxylin and eosin staining
The ventral prostate lobe was fixed with 10% formaldehyde and embedded in paraffin. The sections were cut to a thickness of 4 μm using a Leica microtome (RM 2135, Wetzlar, Germany). The sections were deparaffinized and hydrated prior to staining with hematoxylin for 5 min, and then washed with water for 5 min. The sections were stained with eosin for 30 s. Dehydrated sections were cleared with xylene and mounted. Histological examination was performed using a Leica DMi1 Research Inverted Phase microscope. In each sample, we analyzed three areas under magnification (×100).
2.11 Masson’s trichrome staining
Prostate tissue sections (4 μm) were deparaffinized and hydrated using a common xylene-alcohol series. Nuclei were counterstained with Weigert’s hematoxylin solution for 5 min. After thoroughly washing with distilled water, it was stained with biebrich scarletacid fuchsin solution (Sigma-Aldrich, St. Louis, MO, USA) for 10 min. Following the washing with distilled water, the sections were reacted with 1% phosphomolybdic acid (Sigma-Aldrich, St. Louis,MO, USA) solution for 10 min. It was rinsed with water for 1 min and stained with aniline blue solution (Sigma-Aldrich, St. Louis,MO, USA) for 5 min. After being dehydrated quickly through 95%alcohol, absolute alcohol, the sections were cemented using mounting medium for observation. In each sample, we analyzed three areas under magnification (×50).
2.12 Immunohistochemistry
Sections (4 μm) were deparaffinized and hydrated using a common xylene-alcohol series. For antigen retrieval, the sections were treated in a microwave oven for 10 min with 0.01 mol/L citrate buffer (pH 6.0), incubated at room temperature for 10 min, and washed with distilled water. Endogenous peroxidase activity was quenched by treatment with 3% H2O2. The cells were treated with normal goat serum to block non-specific binding and the sections were then incubated overnight at 4 °C with anti-AR. After treatment with the secondary antibody for 1 h, all sections were counted and stained with Mayer›s hematoxylin. In each sample, we analyzed three areas under magnification (×100).
2.13 Western blotting
Harvested LNCaP and BPH-1 cells and homogenized prostate tissues were lysed using RIPA lysis buffer. The supernatant was separated by centrifugation at 13 000 r/min (15 928 ×g) and 4 °C for 20 min. Protein concentrations were estimated using by performing a bicinchoninic acid assay, and proteins were separated using 12%dodecyl sulfate-polyacrylamide gel electrophoresis (90 min at 120 V).The gel was transferred onto a nitrocellulose membrane using a Mini Trans-Blot cell (Bio-Rad). The blocking procedure was performed using 5% skimmed milk for 1 h at room temperature. The membrane was incubated with the primary antibody overnight at 4 °C. After washing the membrane three times (5 min each) with Tris-buffered saline containing 0.05% Tween 20 (TBST), AffiniPure IgG bound to horseradish peroxidase was added and incubated for 1 h, followed by washing three times for 5 min each with TBST.The chemiluminescent membrane was imaged by processing with an horseradish peroxidase substrate (Advansta, Inc., San Jose, CA, USA)using an Azure c300 imaging system (Azure Biosystems, Dublin,CA, USA). The chemiluminescent intensity of the protein signal was quantified using the ImageJ 1.47v software.
2.14 Statistical analysis
The data are shown as the mean ± standard error of the mean. The data were analyzed using SPSS version 11.5 software for Windows(SPSS, Inc., Chicago, IL, USA). The means of two continuous normally distributed variables were compared using independent samples Student’st-test. Multiple group evaluations of the means were performed using one-way analysis of variance and Scheffe’s multiple comparisons post-hoc analysis.P< 0.05 was considered to be statistically significant.
3. Results
3.1 UPLC–QTOF/MS for compounds from leaves of A. distichum
The metabolites of ADLE that affected BPH in previous experiments were investigated using UPLC-tandem MS(Figs. 1A-B and Table 1). ADLE was rich in verbascoside, rutin, and IQ(Fig. 1C). In a previous study, LNCaP cells were treated with ADLE harvested in spring and autumn[24]. ADLE harvested in autumn had a greater inhibitory effect on AR signaling. The HPLC results revealed that ADLE harvested in autumn contained IQ (20.88 mg/g), rutin(29.03 mg/g), and verbascoside (72.16 mg/g). In contrast, ADLE harvested in spring contained IQ (12.49 mg/g), rutin (27.77 mg/g),and verbascoside (112.46 mg/g).

Table 1 Ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry identification of metabolites in Abeliophyllum disistichum Nakai ethanol extract.

Fig 1 Ultra-high performance liquid chromatography-quadrupole time-offlight mass spectrometry profiles of ADLE. (A) ADLE analyzed in electrospray ionization-positive mode. (B) ADLE extract analyzed in electrospray ionization-negative mode. (C) High-performance liquid chromatography profiling of ADLE (D) Chemical structures of IQ.
3.2 Effect of IQ administration on prostate tissue in the BPH rat model
A TP-induced BPH rat model was used to confirm whether IQ alleviated BPH. The prostate size in the BPH group was significantly larger than that in the control group (Figs. 2A-C). However, the BPH group administered IQ showed a concentration-dependent reduction in the prostate size compared with that in the BPH group. According to the prostate index, IQ was less effective than Fi but the difference was not significant. The BPH group showed excessive proliferation of prostate epithelial tissue and a decreased lumen area, as revealed by histological examination using hematoxylin and eosin staining. In contrast, IQ administration to the BPH group suppressed epithelial tissue hyperproliferation and a larger luminal area, similar to the results in the control group (Fig. 2D). In the prostate, the stroma is a fibromuscular matrix that supports the epithelium. The prostate stroma consists of two different types of fibroblasts and smooth muscle cells,and the interconnected collagen, proteoglycans, and extracellular matrix (ECM)[26]. Masson’s trichrome staining revealed smooth muscle cells to be red and collagen fibers to be blue (Fig. 2E). Fibrosis in the prostate was observed in the BPH group compared to the Con group, and the amount of fibrous connective tissue was increased compared to the Con group (Fig. 2E). On the other hand, when IQ was treated, it was confirmed that fibrosis in the prostate was improved to a level similar to that of the Con group, which was similar to that of the Fi administration group. Therefore, IQ had an effect similar to that of Fi in BPH-induced rats and showed concentration-dependent improvement, suggesting that IQ can alleviate BPH.
3.3 Effects of IQ on AR signaling in BPH

Fig 2 Effect of IQ administration on prostate tissue in TP-induced benign prostatic hyperplasia (BPH) rats. (A) Photographs of the prostate tissues (VP, ventral prostate; DLP, dorsolateral prostate; and anterior prostate; AP). (B) Total prostate tissue weight and (C) prostate indices of the rats. (D) Hematoxylin and eosin-stained prostate tissues (magnification 100×). (E) Masson’s trichrome stained prostate tissues (magnification 50×). Prostatic smooth muscle cells were stained red, collagen fibers were stained blue. Con, corn oil, subcutaneous injection (s.c.) and distilled water-treated rats; BPH, TP (3 mg/kg, s.c.) and distilled water-treated castrated rats; BPH+IQ1, TP (3 mg/kg, s.c.), and IQ 1 mg/kg-treated castrated rats; BPH+IQ10, TP (3 mg/kg, s.c.) and IQ 10 mg/kg-treated castrated rats; BPH+finasteride (Fi), TP (3 mg/kg, s.c.), and Fi 1 mg/kg-treated castrated rats. The data are expressed as the means ± SEMs (n = 8 per group). Groups with different letters in the same row are significantly different by one-way ANOVA test with Scheffe’s multiple comparisons post-hoc analysis (P < 0.05).
The inhibitory effect of IQ on AR signaling was confirmed in rat tissues and LNCaP cells, an androgen-dependent prostate cancer cell line. The expression of 5AR2, AR, SRC-1, and PSA increased after BPH induction in rats and was decreased by treatment with IQ(1 or 10 mg/kg) (Fig. 3A). The immunohistochemistry results showed that AR expression in the prostate tissue of rats was higher in the BPH group than in the control group, which was reduced by IQ (1 or 10 mg/kg) and Fi administration (Fig. 3B). We also compared the effects of ADLE (25, 50, and 100 µg/mL) and IQ (10, 25, and 50 μmol/L) on LNCaP cells, confirming that IQ is a functional component of ADLE. The concentration-dependent effects of ADLE and IQ on LNCaP cell viability were evaluated in an MTT assay(Fig. 3C). Cell viability was significantly reduced at IQ concentrations of 10 μmol/L and ADLE concentrations of 50 μg/mL. Based on the results of the MTT assay, the high ADLE concentration was set to 100 μg/mL and IQ was set to 20 μmol/L. Subsequently, we examined whether IQ inhibits AR signaling when TP was applied to LNCaP cells. When LNCaP cells were treated with TP, the expression of 5AR2, AR, and PSA was significantly increased compared to that in the untreated group. When LNCaP cells were treated with ADLE and IQ, the expression of 5AR2, AR, and PSA was inhibited in a concentration-dependent manner (Fig. 3D). Analysis of the expression of representative AR target genes through quantitative PCR showed that ADLE and IQ significantly inhibited AR target gene expression (Fig. 3E). Interestingly, IQ and ALDE suppressed AR expression, similar to the AR inhibitors and enzalutamide.Therefore, IQ interferes with AR signaling, and the results suggest that IQ, abundantly contained in ADLE, is a functional component that inhibits AR signaling.
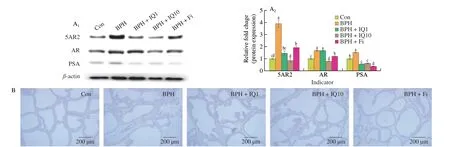
Fig. 3 Effect of IQ on protein expressions of AR signaling in TP-induced BPH rats. (A) Western blot showing expression of AR signaling in prostate tissues of rat. (B) Immunohistochemical staining strength for AR. Con, corn oil, s.c. and distilled water (D.W.)-treated rats; BPH, TP (3 mg/kg, s.c.) and D.W.-treated castrated rats; BPH+IQ1, TP (3 mg/kg, s.c.) and IQ 1 mg/kg-treated castrated rats; BPH+IQ10, TP (3 mg/kg, s.c.) and IQ 10 mg/kg-treated castrated rats;BPH+finasteride (Fi), TP (3 mg/kg, s.c.) and Fi 1 mg/kg-treated castrated rats. (C) Cell viability was measured in an MTT assay after ADLE or IQ treatments in LNCaP cells. LNCaP cells were treated with various concentrations of ADLE (1–1 000 μg/mL) or IQ (0.5–1 000 μmol/L) for 24 h. (D) Western blot showing expression of AR signaling in LNCaP cells. (E) Quantitative PCR showing expression of AR signaling target gene in LNCaP cells. LNCaP cells were incubated for 24 h in culture medium containing TP (100 nmol/L), ADLE (25, 50, or 100 μg/mL), IQ (5, 10, or 20 μmol/L), Fi (1 μg/mL) or enzalutamide (En, 10 μmol/L).Data are expressed as the mean ± SEM. Groups with different letters in the same row are significantly different by one-way ANOVA test with Scheffe’s multiple comparisons post-hoc analysis (P < 0.05).
3.4 Effects of IQ on PI3K/AKT pathway in BPH
IGF-1 is a major factor that regulates stromal-epithelial interactions and plays a key role in BPH development via the PI3K/AKT/mTOR pathway by activating IGF-1R. We investigated whether IQ and ADLE inhibit the PI3K/Akt/mTOR pathway activity induced by IGF-1 treatment in BPH-1 cells. To determine the appropriate experimental concentration, BPH cell viability after treatment with different ADLE and IQ concentrations was confirmed in an MTT assay (Fig. 4A). Cell viability was significantly reduced by 50 μg/mL for ADLE and 10 μmol/L for IQ. Based on the results of the MTT assay, ADLE (100 μg/mL) and IQ (50 μmol/L) were used at high concentrations. As shown in Fig. 5B, the PI3K/Akt/mTOR pathway was activated in BPH-1 cells treated with IGF-1. BPH-1 cells treated with IQ or ADLE showed a concentration-dependent inhibitory effect on activation of the PI3K/Akt/mTOR pathway mediated by IGF-1.IQ downregulated the phosphorylation of AKT similar to the effect of LY294002, a representative PI3K inhibitor. Analysis of the expression of p-AKT in BPH-1 cells using immunofluorescence showed that ADLE decreased the expression of p-AKT in a concentrationdependent manner (Fig. 4C). IQ also decreased p-AKT expression in a concentration-dependent manner; this result was similar to that of LY294002. Moreover, IQ inhibited the phosphorylation of mTOR,similar to the mTOR inhibitor rapamycin. According to these results,IQ and ADLE inhibited the PI3K/Akt/mTOR pathway in prostate epithelial cells.
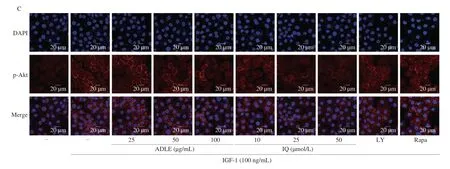
Fig. 4 (Continued)

Fig 4 Effect of IQ administration on IGF-1/PI3K/Akt/mTOR pathway in BPH-1 cells. (A) Cell viability was measured by MTT assay after ADLE or IQ treatments. BPH-1 cells were treated with various concentrations of ADLE (1–1 000 μg/mL) or IQ (1–1 000 μmol/L) for 24 h. (B) Western blot showing expression of IGF-1/PI3K/Akt/mTOR pathway in BPH-1 cells. (C) Immunofluorescence showing of p-Akt in BPH-1 cells. BPH-1 cells were incubated for 6 h in culture medium containing IGF-1 (100 ng/mL), ADLE (25, 50, or 100 μg/mL), IQ (10, 25, or 50 μmol/L), LY (LY294002, 10 μmol/L), or Rapa (rapamycin, 10 μmol/L).Groups with different letters in the same row are significantly different by one-way ANOVA test with Scheffe’s multiple comparisons post-hoc analysis (P < 0.05).
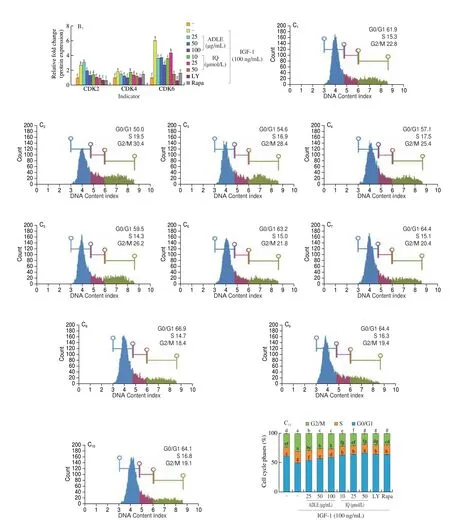
Fig. 5 (Continued)
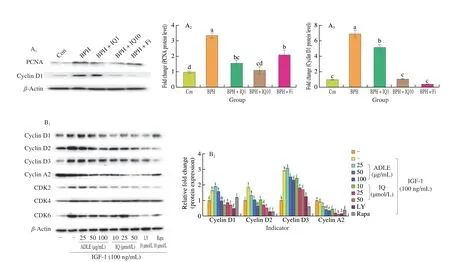
Fig 5 Anti-proliferative effect of IQ administration in BPH. (A) Western blot showing expression of proliferating cell nuclear antigen (PCNA) and cyclin D1 proteins in prostate tissues of BPH rats. Con, corn oil, s.c. and distilled water (D.W.)-treated rats; BPH, TP (3 mg/kg, s.c.), and D.W.-treated castrated rats;BPH+IQ1, testosterone propionate (TP) (3 mg/kg, s.c.) and IQ 1 mg/kg-treated castrated rats; BPH+IQ10, TP (3 mg/kg, s.c.), and IQ 10 mg/kg-treated castrated rats; BPH+finasteride (Fi), TP (3 mg/kg, s.c.) and Fi 1 mg/kg-treated castrated rats. (B) Western blot showing expression of p21, cyclin D1, CDK4, cyclin A2,and cyclin E1 proteins in BPH-1 cell. BPH-1 cells were incubated for 6 h in culture medium containing IGF-1 (100 ng/mL), ADLE (25, 50, or 100 μg/mL), IQ(10, 25, or 50 μmol/L), LY (LY294002, 10 μmol/L), or Rapa (rapamycin, 10 μmol/L). (C) Cell cycle analysis in BPH-1 cells incubated for 24 h in culture medium containing IGF-1 (100 ng/mL), ADLE (25, 50, or 100 μg/mL), IQ (10, 25, or 50 μmol/L), LY (LY294002, 10 μmol/L), or Rapa (rapamycin, 10 μmol/L).C1-C10 were treated the same as columns in C11 respectively. Groups with different letters in the same row are significantly different by one-way ANOVA test with Scheffe’s multiple comparisons post-hoc analysis (P < 0.05).

Fig 6 Apoptosis effect of IQ administration in BPH. (A) Western blot showing expression of Bcl-2 and Bax proteins in prostate tissues of BPH rats Con, corn oil, s.c. and distilled water (D.W.)-treated rats; BPH, TP(3 mg/kg, s.c.), and D.W.-treated castrated rats; BPH+IQ1, TP (3 mg/kg, s.c.)and IQ 1 mg/kg-treated castrated rats; BPH+IQ10, TP (3 mg/kg, s.c.) and IQ 10 mg/kg-treated castrated rats; BPH+finasteride (Fi), TP (3 mg/kg, s.c.)and Fi 1 mg/kg-treated castrated rats. (B) Western blot showing expression of apoptosis related proteron in BPH-1 cells. BPH-1 cells were incubated for 6 h in culture medium containing IGF-1 (100 ng/mL), ADLE (25, 50,or 100 μg/mL), IQ (10, 25, or 50 μmol/L), LY (LY294002, 10 μmol/L), or Rapa (rapamycin, 10 μmol/L). Groups with different letters in the same row are significantly different by one-way ANOVA test with Scheffe’s multiple comparisons post-hoc analysis (P < 0.05).
3.5 Effects of IQ on proliferation in BPH
AR signaling and the expression of cell proliferation-related factors via the IGF-1/PI3K/Akt/mTOR pathway contribute to BPH development. IQ suppresses the expression of proliferation factors by inhibiting AR signaling and the IGF-1/PI3K/Akt/mTOR pathway.In the BPH rat model, PCNA and cyclin D1 were overexpressed compared to their expression in the control group (Fig. 6A). The expression of PCNA and cyclin D1 was significantly lower in the BPH+IQ10 group than in the BPH group. We also examined the expression of cell growth factors induced by IGF-1 in BPH-1 cells.IGF-1 treatment of BPH-1 cells significantly increased the expression of cyclin D1, cyclin D2, cyclin D3, CDK2, CDK4, and CDK6 compared to that in the IGF-1 untreated group (Fig. 5B). ADLE and IQ suppressed the expression of cyclins and CDKs compared to in the IGF-1 treatment group. Cell cycle analysis using fluorescenceactivated cell sorting showed that IGF-1 treatment of BPH-1 cells decreased the G0/G1 phase ratio and increased the G2/M phase ratio(Fig. 5C). In contrast, in cells treated with ADLE or IQ, the ratio of G0/G1 phase cells increased in a concentration-dependent manner and the ratio of G2/M phase cells decreased.
3.6 Effects of IQ on apoptosis in BPH
An imbalance between cell proliferation and apoptosis causes BPH. Previous experiments showed that IQ suppresses BPH cell proliferation. We confirmed the expression of apoptosis-related factors in IQ-treated BPH and BPH-1 cell lines. In BPH-induced rats,IQ downregulated the expression of Bcl-2, which inhibits apoptosis,and upregulated the expression of BAX, which induces apoptosis.(Fig. 6A). In BPH-1 cells treated with IGF-1, IQ downregulated the expression of Bcl-2 and upregulated the expression of Bax in a concentration-dependent manner (Fig. 6B). The apoptosis-inducing effect of ADLE treatment in BPH-1 cells is thought to be influenced by the IQ contained in ADLE. Therefore, IQ inhibited BPH development by inhibiting excessive proliferation and inducing apoptosis.
4. Discussion
This follow-up study was conducted to identify the functional components in ADLE responsible for the prostate size reduction in rats with BPH. Our results confirmed that ADLE contains abundant verbascoside, rutin, and IQ (Figs. 1A-C). In addition, ADLE harvested in autumn contained approximately two-fold more IQ compared to that harvested in spring. During BPH development, androgens appear to activate AR signaling and the PI3K/Akt/mTOR pathway to stimulate cell growth and proliferation and reduce the apoptosis rate in prostate tissue[27]. Numerous studies reported that IQ inhibits the PI3K/Akt/mTOR pathway[28-32]. Therefore, we hypothesized that IQ can alleviate BPH by inhibiting the androgen-activated PI3K/Akt/mTOR pathway. Our results showed that IQ reduced the prostate size of BPH rats in a concentration-dependent manner (Figs. 2A-C). We then examined whether IQ inhibits androgen-activated AR signaling.IQ significantly decreased the expression of AR signaling factors in rat prostate tissue and LNCaP cells, similar to the currently used treatment, Fi (Fig. 3C). In a previous study, quercetin was shown to reduce the expression of AR and AR signaling target genes in a concentration-dependent manner, which is similar to our results[33]. In a previous study, ADLE also reduced the prostate size by effectively inhibiting AR signaling[25]. We showed that IQ, which is abundant in ADLE, inhibits AR signaling, suggesting that IQ is a functional ingredient in ADLE and affects BPH.
Moreover, high blood IGF-1 levels, along with sex hormone imbalances due to relatively low blood testosterone levels associated with aging, appear to accelerate BPH onset in individuals with diabetes and obesity. BPH is also correlated with metabolic syndrome[34-35]. As a representative example, BPH patients with metabolic syndrome show a significantly higher annual prostate growth rate than those without metabolic syndrome[36]. The mechanism appears to be related to hyperinsulinemia, a cause of metabolic syndrome and major endocrine abnormalities, and is thought to be due to the action of insulin on cell proliferation and anti-apoptotic effect of insulin, mediated by IGF-1[37-38]. The expression of IGF-1R is increased by AR signaling, which triggers cell growth and proliferation by activating IGF-1-mediated PI3K/AKT/mTOR signaling[39]. Therefore, drugs that inhibit activation of the PI3K/AKT/mTOR pathway by IGF-1 may be effective against BPH. This effect may expand the choice of BPH treatment options,which are currently limited. Therefore, we investigated activation of the PI3K/AKT/mTOR pathway and whether ADLE and IQ effectively inhibited this mechanism after IGF-1 treatment in BPH-1 cells. ADLE and IQ inhibited PI3K/AKT/mTOR pathway activation in a concentration-dependent manner, similar to the PI3K inhibitor LY294002 and mTOR inhibitor rapamycin. A previous study showed that administration of rapamycin was clinically effective in reducing prostate size in patients with BPH[17]. As IQ inhibited the PI3K/AKT/mTOR pathway, the expression of cyclin proteins was significantly reduced, and the cells were arrested in G0/G1 phase (Fig. 5C). In contrast, IQ induced apoptosis by decreasing the expression of Bcl-2 and increasing the expression of Bax in a TP-induced BPH rat model and BPH-1 cells. Therefore, our results show that IQ inhibits androgen-induced AR signaling activation, and IGF-1 induced PI3K/Akt/mTOR pathway activation, thus inhibiting cell proliferation and increasing the apoptosis rate.
Rapamycin induces autophagy[40], slows aging[41], and shows efficacy in cancer[42]as well as metabolic diseases, such as type 2 diabetes mellitus and cardiovascular diseases[43-44]. Thus, drugs that activate autophagy may alleviate BPH. Furthermore, IQ induces autophagy in several cancer cells and induces autophagic apoptosis.BPH also activates autophagy to inhibit cell proliferation and increase the cell death rate[45-47]. However, it remains unclear whether IQ induced autophagy and inhibited the PI3K/Akt/mTOR pathwayin vivoandin vitro. Further studies are needed to investigate the autophagy-inducing activity of IQ in BPH.
In contrast, several studies reported that IQ, which uses quercetin as an aglycon, exhibits a higher absorption rate than quercetin[48-49].Quercetin is absorbed only by passive transport, whereas IQ is absorbed not only in the glycoside form but also in the quercetin form by lactase-phlorizin hydrolase secreted by intestinal epithelial cells[50]. Therefore, although IQ has similar efficacy as quercetin, it is more bioavailable than quercetin in many tissues, indicating its greater effect at lower concentrations than that of quercetin. However,we did not compare the differences in the bioavailability and efficacy of quercetin and IQ in the prostate; therefore, whether mechanistic differences exist between the effects of quercetin and IQ in BPH remains unclear, and further studies are needed. In addition, it is unknown whether mechanistic differences exist between the effects of quercetin and its glucoside, IQ, and rutin on BPH. Therefore, the activities of quercetin, IQ, and rutin in BPH should also be compared.In conclusion, IQ effectively reduced the prostate size in a BPH rat model and effectively inhibited AR signaling and the PI3K/AKT/mTOR pathwayin vitro, suggesting its potential as a treatment for BPH.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2020R1A2C1014798 to E-K Kim).
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango