低频超声在抗感染领域的应用现状
2024-01-01闫开成陈丽刘晓丽梁文馨蔡芸
闫开成 陈丽 刘晓丽 梁文馨 蔡芸
摘要:抗菌药物的不合理使用,导致耐药菌感染成为影响人类健康的一大危机,虽然近些年研发了一些新型抗菌药物,但耐药趋势并未逆转,目前急需替代疗法来应对这一危机。低频超声作为一个安全且有应用前景的物理方法,在抗感染领域的应用越来越被受到重视,与抗菌药物联合应用能产生协同杀菌作用,杀菌机制包括热效应、机械效应和空化效应。本文综述了体内外低频超声联合抗菌药物在抗浮游菌和生物被膜、促进植入物药物释放和临床应用的特点,旨在对低频超声进一步研究和未来在抗感染领域的应用提供指导。
关键词:低频超声;抗感染;空化效应;浮游菌;生物被膜;植入物
中图分类号:R978.1 文献标志码:A
Application status of low-frequency ultrasound in the field of anti-infection
Yan Kaicheng1,2, Chen Li3, Liu Xiaoli4, Liang Wenxin2, and Cai Yun2
(1 Medical School of Chinese PLA, Beijing 100853; 2 Center of Medicine Clinical Research, Department of Pharmacy, Medical Supplies Center, Chinese PLA General Hospital, Beijing 100853; 3 Department of Information, Medical Supplies Center, Chinese PLA General Hospital, Beijing 100853; 4 Department of Dermatology, the First Medical Center, Chinese PLA General Hospital, Beijing 100853)
Abstract The irrational use of antibiotics has led to the infection of drug-resistant bacteria, which has become a major crisis affecting human health. Although some new antibiotics have been developed in recent years, the trend of drug resistance has not reversed. At present, there is an urgent need for alternative therapy to deal with this crisis. As a safe and promising physical method, low-frequency ultrasound has attracted more and more attention in the field of anti-infection. Combined with antibiotics, it can produce synergistic bactericidal effects. The bactericidal mechanism includes thermal effects, mechanical effects and cavitation effects. This review summarizes the characteristics of low-frequency ultrasound combined with antibiotics in anti-planktonic bacteria and biofilm, promoting implant drug release and clinical application in vivo and in vitro, in order to provide guidance for the further research of low-frequency ultrasound and its application in the field of anti-infection in the future.
Key words Low-frequency ultrasound; Anti-infection; Cavitation effect; Planktonic bacteria; Biofilm; Implants
1 背景
細菌感染仍然是人类社会面临的主要挑战之一,而抗菌药物依旧是目前治疗相关感染的最有效方法。但是在过去的几十年中,由于不谨慎地使用抗菌药物导致细菌耐药性的流行急剧增加,耐药菌感染仍然是人类健康的严重威胁[1]。在这种情况下,抗菌药物联合应用已成为治疗耐药菌感染的一种选择,因为它具有广泛的覆盖范围和协同效应,但也提高了药物不良反应风险,最终导致治疗失败、抗菌药物使用量增加以及可能加速多重耐药菌产生[2]。生物被膜是指细菌黏附于接触表面或形成聚集体,并显示出对抗菌药物和宿主防御的极端耐受性,抗菌药物消除细菌生物被膜所需的药量是根除浮游菌所需用量的500~5000倍,这远远超出了人体的承受能力[3]。因此,抗菌药物治疗通常无法完全根除生物膜,导致复发性生物被膜相关感染[4]。为了应对这些挑战,有必要寻求一种辅助方法来协助去除细菌生物被膜和缓解细菌耐药趋势。
物理方法是疾病治疗的一个重要辅助手段,其中低频超声(low frequency ultrasound,LFU)作为一个安全且有应用前景的物理方法,已经在临床研究和诊断中应用了很多年。LFU指频率范围在20 kHz~1 MHz,具有长波长的声波,这种声波在各种组织中具有很强的穿透力,可以引起热效应、机械效应和空化效应[5]。关于超声治疗的最早报道可以追溯到19世纪50年代,氢化可的松软膏联合超声“按摩”治疗手指关节炎和手滑囊炎,与单纯注射氢化可的松相比疗效更佳,在后来的研究中也得到了类似的结果,证实了超声可以增强药物的皮肤渗透性[6-7]。随着对这些作用机制的深入研究,LFU在治疗中的潜力逐渐显现。针对多种临床疾病进行了充分研究,包括促进组织再生、疼痛管理、神经调节、抗感染和抗癌症治疗[8]。
LFU在抗感染领域的应用也越来越受到重视,多项研究表明,LFU与抗菌药物联合应用能产生协同杀菌作用,可以提高抗菌药物对浮游细菌、细菌生物被膜、真菌和其他生物体的杀菌作用[9]。因此,根据现有的体内和体外的研究数据,综述LFU的作用机制及应用效果,旨在评估LFU协同抗菌药物在抗感染领域的应用现状,为未来的临床实践提供指导。
2 LFU的生物学机制
2.1 热效应
一般认为,LFU的抗菌效果取决于其生物学影响,包括热效应和非热效应。LFU的热效应也称为热疗效应,当超声波穿过组织时,组织颗粒介质界面会产生摩擦,介质吸收这些能量并将其转化为热能,引起生物体的某些变化[10]。LFU的热效应与超声参数和组织密度有关,超声的受阻和衰减度决定了组织中产生的热量水平,这些热量随后对机体的血管舒张、氧合功能和营养交换等生理现象发挥作用。当LFU强度<0.1 W/cm2主要表现为多普勒效应,而0.1和1 W/cm2之间的强度用于诊断成像[11]。当超声强度>10 W/cm2会产生大量热量,高强度聚焦超声是一种新的无创治疗技术,可以瞬间将靶组织加热到60℃,导致蛋白质变性或凝固性坏死,因此,高强度聚焦超声主要用于抗癌和消融[12]。相比之下,低频低强度超声(0.02至1 W/cm2)随着时间的推移产生的热量相对较少[13],具体取决于频率、波长和治疗持续时间,许多研究已经验证了低强度脉冲超声和低强度连续超声在组织再生、疼痛缓解、血栓形成、抗微生物、骨折愈合和骨关节炎等疾病治疗中的潜在有效性,可见LFU在低强度下对抗炎、抗菌、修复和再生等有积极疗效。
2.2 机械效应
机械效应是超声波的最基本的效应,当超声波在体液介质中产生驻波时,悬浮在介质中的微粒在机械力的作用下凝聚,发生位移,这些作用力包括空化效应、微束流和辐射力。而LFU最重要的生理效应是产生空化效应,当超声波在机体传播时,使组织中的液体形成微小气泡核(空化核),这些空化核闭合时会发生一系列动态过程,包括振荡、膨胀、收缩和崩溃。当声压达到一定数值时,气泡迅速膨胀,然后突然关闭,随后的冲击波可以诱导周围空化核的形成以及细胞膜和质膜的破裂。LFU的这种空化和微流特性归因于惯性流体中微泡的连续压缩和折射循环,引发细胞膜和质膜的局部扩展松动,从而增加营养物质的交换或促进药物输送[14]。
空化效应通常分为稳定型和惯性型,在低强度下,空化核围绕平衡半径周期性振荡,产生辐射压力和微束,微束可以在气泡表面附近产生高剪切力,然后气泡变形和破裂,影响相邻的组织结构并使周围的细胞或血管壁破裂。随着频率的降低,共振气泡的大小和因振荡而被迫移动的液体体积也会增加,这表明微束在低频下可能具有更重要的作用[15]。当强度增加时,空化气泡在负压下迅速膨胀,在正压下急剧收缩内爆,称为惯性空化。在此过程中,气泡振荡比较剧烈,从剧烈膨胀到急剧塌陷再到瞬间破裂,惯性空化对超声强度呈总体依赖性,超声强度必须高于阈值强度,并且超声频率越高,惯性空化的阈值强度越大。Tezel等[16]发现惯性空化增强通常与空化的能量密度相关,而与强度和频率无关。这些数据表明惯性空化在低频超声中起着重要作用。
LFU的生理效应受多因素影响,其效应机制也是多样复杂的,生物学效应可能主要归因于机械振动,因为一般在低强度下对组织和细胞水平上的热效应很小[17]。在过去的几十年中,LFU应用取得了重大进展,随着对超声生物效应的了解不断增加,已经确定机械效应可以增强超声化学效应,在促进药物渗透中,LFU介导的微束流和内吞作用可能具有协同作用;在抗肿瘤和抗感染的治疗中,LFU的热效应和空化效应也是相辅相成的;多数研究发现LFU的空化作用在多种治疗作用中具有主导作用[18]。
3 LFU的协同抗菌效应
3.1 对浮游菌的作用
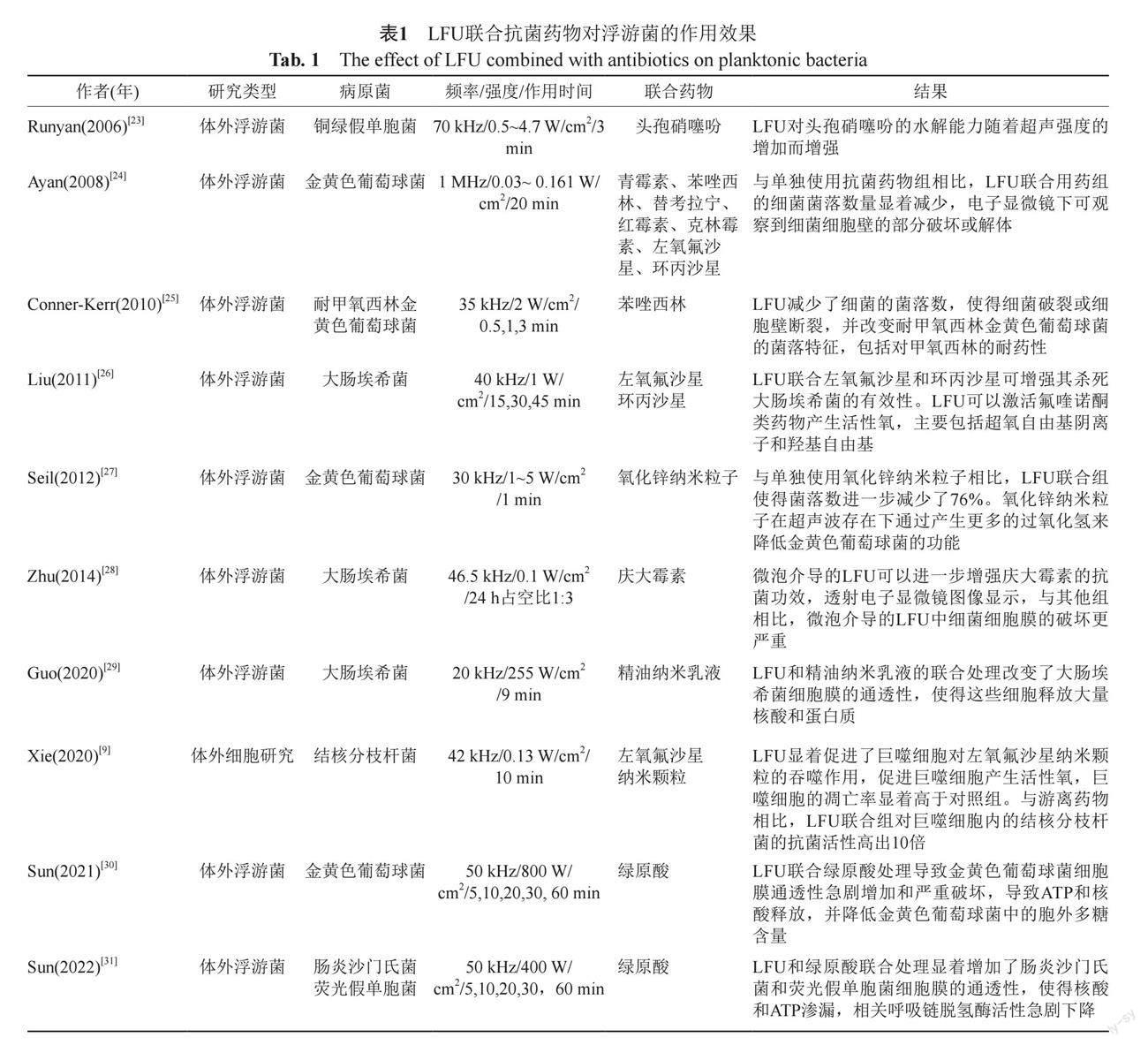
LFU对浮游菌作用效果的相关文献大多是体外研究,尽管有些研究表明单独使用LFU可以降低细菌的数量[19-20],但这样的杀菌效果并不明显,设定的强度也较高,容易产生热损伤。本课题组胡杏等[21]通过体内小鼠肺炎模型,考察了LFU對小鼠肺炎克雷伯菌肺炎的作用效果,设定频率为29.36 kHz,强度为0.25~0.3 W/cm2,发现对小鼠活体肺组织中发光肺炎克雷伯菌荧光强度无影响。因此,LFU在抗感染领域的应用主要表现为和抗菌药物的协同作用,Pitt等[22]首次证实了LFU联合庆大霉素对铜绿假单胞菌、大肠埃希菌和葡萄球菌的协同抗菌作用,吸引了很多学者对LFU在抗感染方面的探索。LFU对浮游菌的联合作用主要是在体外孔板中进行,容易操作和孵育,作用时间短且不易生成生物被膜(表1)。
Runyan等[23]使用70 kHz的LFU在不同强度下联合头孢硝噻吩作用于铜绿假单胞菌,在0.5~4.7 W/cm2范围内,杀菌效果随着超声强度增强而增高,进一步研究发现LFU作用于铜绿假单胞菌时,会增大细菌的孔膜,可以使大分子的β-内酰胺酶从细胞中排出,而小分子头孢硝噻吩更容易进入细菌内,从而杀死细菌。其他研究也证实了LFU增强了一些抗菌药物对某些特定细菌的活性,特别是氨基糖苷类对革兰阴性菌的活性,Zhu等[28]运用LFU介导的微泡法进一步增强了庆大霉素对大肠埃希菌的抗菌活性,透射电镜下显示细菌细胞膜比单用药物组破坏更严重。此外,Liu等[26]发现LFU联合左氧氟沙星或环丙沙星可增强其杀死大肠埃希菌的有效性,LFU可以激活氟喹诺酮类药物产生活性氧,主要包括超氧自由基阴离子和羟基自由基。研究发现一些革兰阳性菌也容易受到LFU的作用,使得病原菌对抗菌药物更敏感,甚至能降低耐药性,Ayan等[24]发现LFU可以增强金黄色葡萄球菌对青霉素、替考拉宁、红霉素等多种抗生素的敏感性,对细菌的形态学和遗传学都产生了改变。LFU联合苯唑西林不仅减少了耐甲氧西林金黄色葡萄球菌(methicillin-resistant Staphylococcus aureus,MRSA)的菌落数,使得细胞壁破裂,并且改变MRSA的菌落特征,包括对甲氧西林的耐药性[25]。
除了常规抗菌药物,LFU还可以增强新型抗菌物质对病原菌杀伤能力,比如金属氧化物纳米颗粒、生物聚合物和绿原酸[27,29-30]。
3.2 对生物被膜的作用
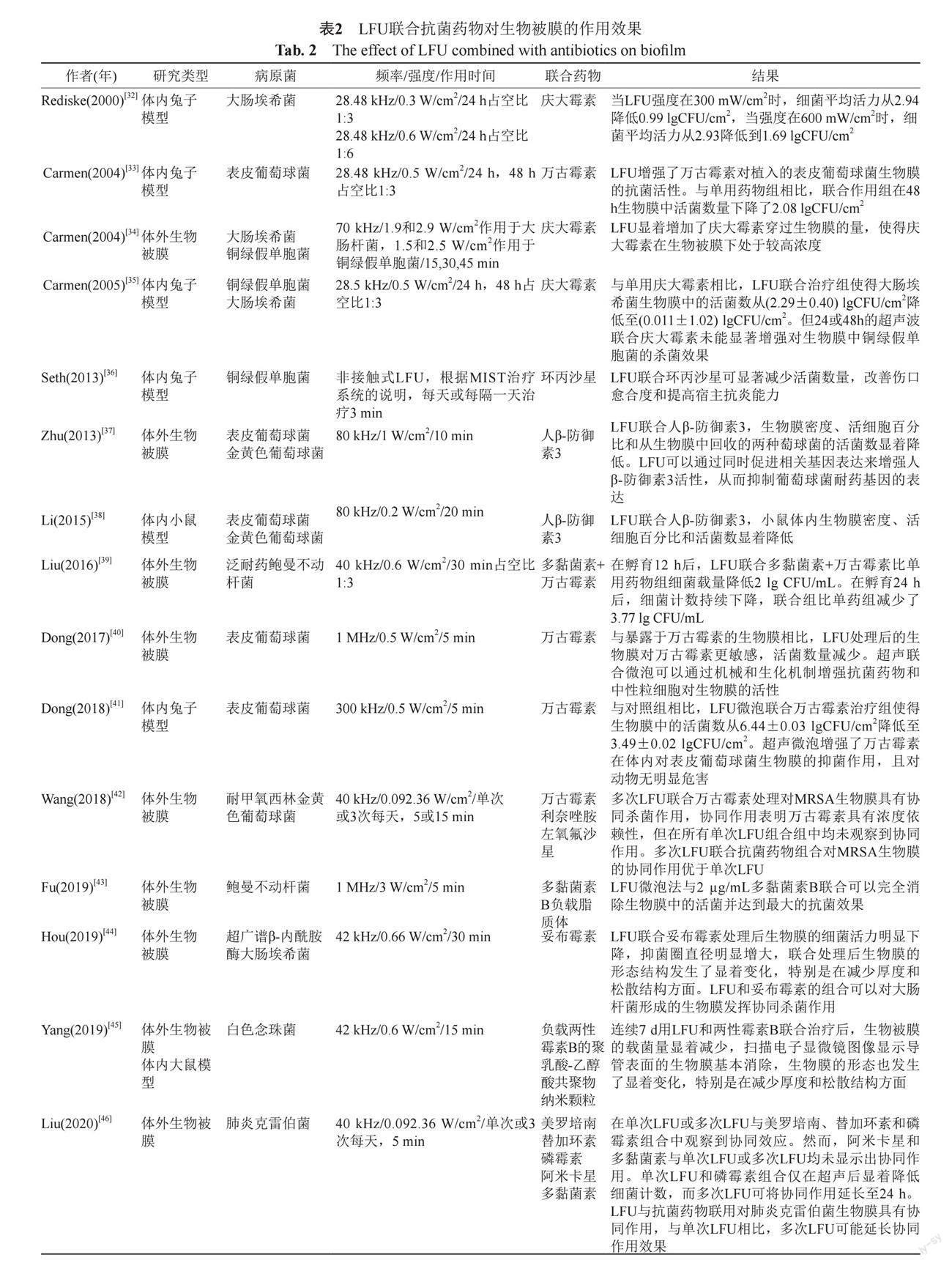
与浮游菌相比,生物被膜内的细菌对多种抗菌药物表现为耐药,耐药性的增加是由于细菌代谢特征和基因表达的变化。除了表型变化外,生物被膜本身可能会结合或减缓抗菌药物的运输,从而保护内部的细菌免于接触致死水平的抗菌药物。大多细菌在繁殖过程中都会产生生物被膜,金黄色葡萄球菌、铜绿假单胞菌、大肠埃希菌产生的生物被膜更常见、耐药性更强。很多研究报道了LFU在体外和体内可有效增强某些抗菌药物杀灭细菌生物膜的作用,体内模型大多为兔子和大小鼠皮下植入物模型(表2)。
Rediske等[32]报道LFU作用于家兔皮下的载有大肠埃希菌的聚乙烯圆盘,皮下在超声作用前注射庆大霉素,治疗24 h后分别测量圆盘载菌量,研究分为8个组别,分别设置了阴性对照组、阳性对照组、单用药物组、单用LFU组以及不同强度的LFU组。在24.48 kHz频率下,单用强度0.1 W/cm2
的LFU未见杀菌效果,而与单用药物组相比,LFU联合庆大霉素在0.1 W/cm2时,细菌活力未见明显降低;在0.3 W/cm2时,细菌平均活力从2.94降低到
0.99 lgCFU/cm2;在0.6 W/cm2时,细菌平均活力从2.93降低到1.69 lgCFU/cm2,结果显示在强度为0.3 W/cm2时联合庆大霉素有更好的杀菌效果,这也提示并不是超声强度越高越好,在实际应用中需要探索合适的作用强度,才可能产生更好地杀菌效果。同样,Carmen等[34]研究了LFU联合庆大霉素在不同强度下作用于体外大肠埃希菌和铜绿假单胞菌被膜,随着作用强度的增加LFU显著增加了庆大霉素穿过生物膜的量,使得庆大霉素在生物被膜下处于较高浓度。对于金黄色葡萄球菌和表皮葡萄球菌生物被膜的治疗主要是协同万古霉素,LFU增强了万古霉素在体内外对葡萄球菌生物膜的抑菌作用,特别是对于MRSA[42],这种联合作用更具殺菌效果,且对动物无明显危害,其他研究也显示多次LFU联合抗菌药物组合对细菌生物膜的协同作用优于单次LFU[46]。另外,人β-防御素3抗菌肽对于葡萄球菌具有杀菌效果,LFU可以通过同时促进细菌相关基因表达来增强人β-防御素3活性,从而抑制葡萄球菌耐药基因的表达,在80 kHz时体内外都有明显的协同杀菌作用[37-38]。LFU协同杀死有生物被膜的细菌,一是可能增加了细胞的通透性,提高了抗菌物质通过生物膜的效率;二是LFU作用后的局部温度升高和细胞内活性氧的产生增多。
3.3 促进植入物抗菌药物释放
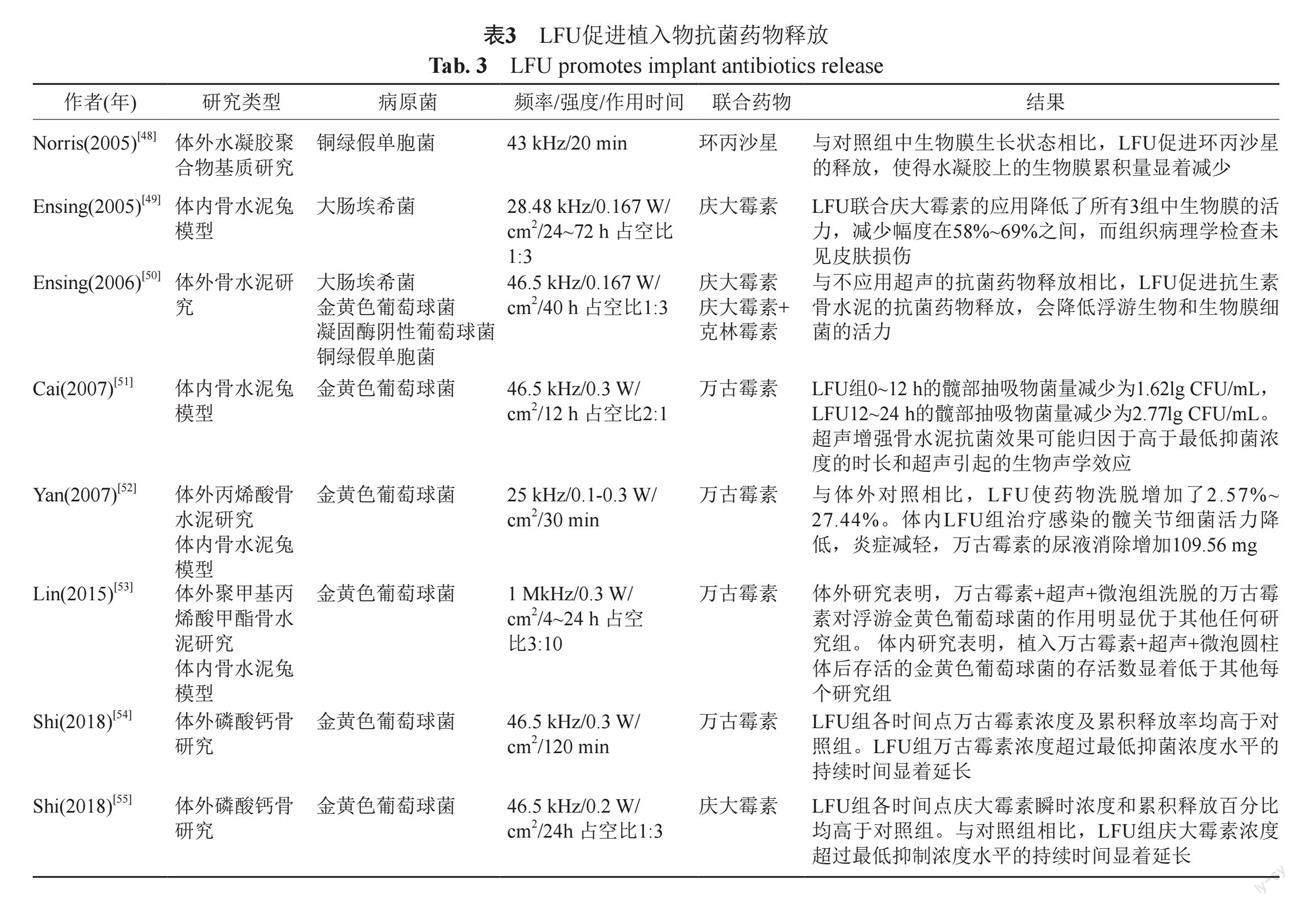
临床植入物的的使用越来越广范,心脑血管和骨科每年有大量的手术都涉及医疗器械植入人体,这就导致这些“外来品”容易造成局部或全身感染,并且细菌在植入物表面更易形成生物被膜。近年来,人们致力于通过制备生物相容性材料来抑制细菌及生物被膜的形成,以防止或减少生物被膜的感染。其中一种策略是在器械或材料中加入抗菌药物,通过药物缓慢释放来预防植入部位的细菌感染以及生物被膜的形成,当感染发生时,LFU可促进这些预制抗菌药物的加速释放而起到积极的效果[47]。目前相关研究主要集中在体内外模拟预制骨水泥抗菌药物的释放,而主要的病原菌和临床感染相似,以常见的金黄色葡萄球菌为主(表3)。
Cai等[51]模拟髋关节置换术预制万古霉素丙烯酸骨水泥,置入家兔髋关节部位,然后人为造成金黄色葡萄球菌急性感染,加用LFU后测量髋部抽取物的细菌载量,LFU组0~12 h的髋部抽吸物菌量减少了1.62 lg CFU/mL,LFU组12~24 h的髋部抽吸物菌量减少了2.77 lg CFU/mL。LFU增强骨水泥抗菌效果可能归因于促进释放的药物浓度始终高于最低抑菌浓度,以及超声引起的相关生物声学效应。在预防植入物葡萄球菌引发的感染主要以预制万古霉素和庆大霉素骨水泥为主[50,55],而对于革兰阴性菌大肠埃希菌和铜绿假单胞菌的植入物感染,LFU主要联合环丙沙星或庆大霉素[48-49],LFU可促进相应抗菌药物的释放,使得水凝胶上的生物膜累积量显著减少。LFU的这种可靶向或控制药物释放能力,使得在感染发生时能够使预制骨水泥大量释放抗菌药物,局部药物浓度高于最低抑菌浓度,有效杀灭感染菌,此外,LFU可以更有效地让药物透过深层组织,发挥更大疗效。
3.4 临床应用
LFU作为一种新型的物理辅助抗感染手段,目前研究大多处于体外和动物实验阶段,临床研究主要集中在表皮清创术后的康复和慢性伤口愈合,具有减少抗菌药物的使用时间、降低感染复发率和促进组织再生等功效。相关研究主要以临床病例回顾性为主,主要针对葡萄球菌,抗菌药物全身使用,部分是局部协同用药,LFU局部治疗时间一般较长,3个月左右(表4)。
Tewarie等[59]回顾性对比了胸骨切开术后心脏手术患者胸骨皮肤瘘使用超声辅助治疗的效果,设定超声频率为25 kHz,强度35~40 W/cm2 ,LFU辅助伤口组18人,常规治疗组19人,61%为革兰阳性菌,16.5%为革兰阴性菌,10.5%白色念珠菌,结果显示LFU辅助治疗组伤口愈合时间和患者住院时间明显缩短,抗菌药物使用时间减少,感染复发率降低。LFU辅助伤口清创系统是一种能够破坏细菌生物被膜、优先清除坏死组织、减少细菌数量、减少出血量和相对无痛的清创方式。在胸骨深部感染及下肢血管移植术后感染,LFU辅助清除促进了表面和深部坏死物质的分离和脱落,而不损害周围正常的组织[56-57]。除了外科手术的优势外,LFU辅助技术在伤口清创和促进愈合等方面,将通过缩短住院时间和降低抗微生物治疗的时间来节约成本,提高疗效。
4 总结
根据近20年的体外和体内研究数据,可以得出LFU在对浮游菌和细菌生物被膜的联合治疗中起到了很好的辅助作用。对于含药植入物,LFU可以促进抗菌药物的释放,达到最佳疗效,但也有报道显示,经LFU处理会降低负载万古霉素的丙烯酸骨水泥的界面剪切强度和稳定性[60]。另外,LFU与抗菌药物联合治疗的临床应用可能还有很长的路要走,因为临床应用除了考察其有效性外,安全性是放在首位的,目前虽然对LFU的作用机制有一定了解,但作用频率、强度及应用时间在体外研究中尚存在很大差异,能够使用于临床的参数还需要进一步评估。人们对一种新生事物的研究总会经历一个漫长的过程,从理论到实践都是必经之路,从目前来看,LFU这一物理抗感染手段在未来应对细菌耐药及抗生物被膜是很有前景的。
參 考 文 献
Kong Q, Yang Y. Recent advances in antibacterial agents[J]. Bioorg Med Chem Lett, 2021, 35(6): 127-134.
Wang Y, Li H, Xie X, et al. In vitro and in vivo assessment of the antibacterial activity of colistin alone and in combination with other antibiotics against Acinetobacter baumannii and Escherichia coli[J]. J Glob Antimicrob Resist, 2020, 20(3): 351-359.
Maszewska A, Moryl M, Wu J, et al. Amikacin and bacteriophage treatment modulates outer membrane proteins composition in Proteus mirabilis biofilm[J]. Sci Rep, 2021, 11(1): 1522-1529.
Tzeng A, Tzeng T H, Vasdev S, et al. Treating periprosthetic joint infections as biofilms: Key diagnosis and management strategies[J]. Diagn Microbiol Infect Dis, 2015, 81(3): 192-200.
Schoellhammer C M, Srinivasan S, Barman R, et al. Applicability and safety of dual-frequency ultrasonic treatment for the transdermal delivery of drugs[J]. J Control Release, 2015, 202(37): 93-100.
Fellinger K S J. Klinik and therapies des chromi-schen gelenkreumatismus[J]. Vienna: Maudrich Verlag, 1954, 6(5): 549-552.
Santoianni P, Nino M, Calabro G. Intradermal drug delivery by low-frequency sonophoresis(25 kHz)[J]. Dermatol Online J, 2004, 10(2): 24-29.
Uddin S M Z, Komatsu D E, Motyka T, et al. Low-intensity continuous ultrasound therapies-a systematic review of current state-of-the-art and future perspectives[J]. J Clin Med, 2021, 10(12): 325-334.
Xie S, Li G, Hou Y, et al. A synergistic bactericidal effect of low-frequency and low-intensity ultrasound combined with levofloxacin-loaded PLGA nanoparticles on M. smegmatis in macrophages[J]. J Nanobiotechnology, 2020, 18(1): 107-112.
Jiang B L, Gao X, Xiong J, et al. Experimental study on synergistic effect of HIFU treatment of tumors using Bifidobacterium bound with cationic phase-change nanoparticles[J]. Eur Rev Med Pharmacol Sci, 2020, 24(10): 5714-5725.
Kingwill A, Barker G, Wong A. Point-of-care ultrasound: its growing application in hospital medicine[J]. Br J Hosp Med(Lond), 2017, 78(9): 492-496.
Quadri S A, Waqas M, Khan I, et al. High-intensity focused ultrasound: Past, present, and future in neurosurgery[J]. Neurosurg Focus, 2018, 44(2): 16-25.
Jiang X, Savchenko O, Li Y, et al. A review of low-intensity pulsed ultrasound for therapeutic applications[J]. IEEE Trans Biomed Eng, 2019, 66(10): 2704-2718.
Uddin S M Z, Komatsu D E. Therapeutic potential low-intensity pulsed ultrasound for osteoarthritis: Pre-clinical and clinical perspectives[J]. Ultrasound Med Biol, 2020, 46(4): 909-920.
Bader K B, Gruber M J, Holland C K. Shaken and stirred: Mechanisms of ultrasound-enhanced thrombolysis[J]. Ultrasound Med Biol, 2015, 41(1): 187-196.
Tezel A, Sens A, Mitragotri S. Investigations of the role of cavitation in low-frequency sonophoresis using acoustic spectroscopy[J]. J Pharm Sci, 2002, 91(2): 444-453.
De Lucas B, Perez L M, Bernal A, et al. Ultrasound therapy: Experiences and perspectives for regenerative medicine[J]. Genes(Basel), 2020, 11(9): 356-367.
Petit B, Bohren Y, Gaud E, et al. Sonothrombolysis: The contribution of stable and inertial cavitation to clot lysis[J]. Ultrasound Med Biol, 2015, 41(5): 1402-1410.
Gao S, Lewis G D, Ashokkumar M, et al. Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria[J]. Ultrason Sonochem, 2014, 21(1): 446-453.
Al Bsoul A, Magnin J P, Commenges-Bernole N, et al. Effectiveness of ultrasound for the destruction of Mycobacterium sp. strain(6PY1)[J]. Ultrason Sonochem, 2010, 17(1): 106-110.
Hu X, Cai Y, Wang Y, et al. Imaging of bioluminescent Klebsiella pneumoniae induced pulmonary infection in an immunosuppressed mouse model[J]. J Int Med Res, 2020, 48(10): 1-13.
Pitt W G, Mcbride M O, Lunceford J K, et al. Ultrasonic enhancement of antibiotic action on Gram-negative bacteria[J]. Antimicrob Agents Chemother, 1994, 38(11): 2577-2582.
Runyan C M, Carmen J C, Beckstead B L, et al. Low-frequency ultrasound increases outer membrane permeability of Pseudomonas aeruginosa[J]. J Gen Appl Microbiol, 2006, 52(5): 295-301.
Ayan I, Aslan G, Comelekoglu U, et al. The effect of low-intensity pulsed sound waves delivered by the Exogen device on Staphylococcus aureus morphology and genetics[J]. Acta Orthop Traumatol Turc, 2008, 42(4): 272-277.
Conner-Kerr T, Alston G, Stovall A, et al. The effects of low-frequency ultrasound(35 kHz) on methicillin-resistant Staphylococcus aureus(MRSA) in vitro[J]. Ostomy Wound Manage, 2010, 56(5): 32-43.
Liu B, Wang D J, Liu B M, et al. The influence of ultrasound on the fluoroquinolones antibacterial activity[J]. Ultrason Sonochem, 2011, 18(5): 1052-1056.
Seil J T, Webster T J. Antibacterial effect of zinc oxide nanoparticles combined with ultrasound[J]. Nanotechnology, 2012, 23(49): 495-502.
Zhu H X, Cai X Z, Shi Z L, et al. Microbubble-mediated ultrasound enhances the lethal effect of gentamicin on planktonic Escherichia coli[J]. Biomed Res Int, 2014, 14(6): 142-151.
Guo M, Zhang L, He Q, et al. Synergistic antibacterial effects of ultrasound and thyme essential oils nanoemulsion against Escherichia coli O157:H7[J]. Ultrason Sonochem, 2020, 66(5): 104-113.
Sun J, Wang D, Sun Z, et al. The combination of ultrasound and chlorogenic acid to inactivate Staphylococcus aureus under planktonic, biofilm, and food systems[J]. Ultrason Sonochem, 2021, 80(6): 105-111.
Sun J, Huang L, Sun Z, et al. Combination of ultrasound and chlorogenic acid for inactivation of planktonic and biofilm cells of Pseudomonas fluorescens[J]. Food Res Int, 2022, 155(9): 111-118.
Rediske A M, Roeder B L, Nelson J L, et al. Pulsed ultrasound enhances the killing of Escherichia coli biofilms by aminoglycoside antibiotics in vivo[J]. Antimicrob Agents Chemother, 2000, 44(3): 771-772.
Carmen J C, Roeder B L, Nelson J L, et al. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo[J]. J Biomater Appl, 2004, 18(4): 237-245.
Carmen J C, Nelson J L, Beckstead B L, et al. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli[J]. J Infect Chemother, 2004, 10(4): 193-199.
Carmen J C, Roeder B L, Nelson J L, et al. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics[J]. Am J Infect Control, 2005, 33(2): 78-82.
Seth A K, Nguyen K T, Geringer M R, et al. Noncontact, low-frequency ultrasound as an effective therapy against Pseudomonas aeruginosa-infected biofilm wounds[J]. Wound Repair Regen, 2013, 21(2): 266-274.
Zhu C, He N, Cheng T, et al. Ultrasound-targeted microbubble destruction enhances human beta-defensin 3 activity against antibiotic-resistant Staphylococcus biofilms[J]. Inflammation, 2013, 36(5): 983-996.
Li S, Zhu C, Fang S, et al. Ultrasound microbubbles enhance human beta-defensin 3 against biofilms[J]. J Surg Res, 2015, 199(2): 458-469.
Liu X, Yin H, Weng C X, et al. Low-frequency ultrasound enhances antimicrobial activity of colistin-vancomycin combination against pan-resistant biofilm of Acinetobacter baumannii[J]. Ultrasound Med Biol, 2016, 42(8): 1968-1975.
Dong Y, Xu Y, Li P, et al. Antibiofilm effect of ultrasound combined with microbubbles against Staphylococcus epidermidis biofilm[J]. Int J Med Microbiol, 2017, 307(6): 321-328.
Dong Y, Li J, Li P, et al. Ultrasound microbubbles enhance the activity of vancomycin against Staphylococcus epidermidis biofilms in vivo[J]. J Ultrasound Med, 2018, 37(6): 1379-1387.
Wang J, Wen K, Liu X, et al. Multiple low frequency ultrasound enhances bactericidal activity of vancomycin against methicillin-resistant Staphylococcus aureus biofilms[J]. Biomed Res Int, 2018, 18(6): 602-609.
Fu Y Y, Zhang L, Yang Y, et al. Synergistic antibacterial effect of ultrasound microbubbles combined with chitosan-modified polymyxin B-loaded liposomes on biofilm-producing Acinetobacter baumannii[J]. Int J Nanomedicine, 2019, 14(8): 1805-1815.
Hou Y, Yang M, Jiang H, et al. Effects of low-intensity and low-frequency ultrasound combined with tobramycin on biofilms of extended-spectrum beta-lactamases(ESBLs) Escherichia coli[J]. FEMS Microbiol Lett, 2019, 366(3): 326-331.
Yang M, Du K, Hou Y, et al. Synergistic antifungal effect of amphotericin b-loaded poly(lactic-co-glycolic acid) nanoparticles and ultrasound against Candida albicans biofilms[J]. Antimicrob Agents Chemother, 2019, 63(4): 765-763.
Liu X, Wang J, Weng C X, et al. Low-frequency ultrasound enhances bactericidal activity of antimicrobial agents against Klebsiella pneumoniae biofilm[J]. Biomed Res Int, 2020, 2020(8): 591-626.
Delaney L J, Macdonald D, Leung J, et al. Ultrasound-triggered antibiotic release from PEEK clips to prevent spinal fusion infection: Initial evaluations[J]. Acta Biomater, 2019, 93(2): 12-24.
Norris P, Noble M, Francolini I, et al. Ultrasonically controlled release of ciprofloxacin from self-assembled coatings on poly(2-hydroxyethyl methacrylate) hydrogels for Pseudomonas aeruginosa biofilm prevention[J]. Antimicrob Agents Chemother, 2005, 49(10): 4272-4279.
Ensing G T, Roeder B L, Nelson J L, et al. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo[J]. J Appl Microbiol, 2005, 99(3): 443-448.
Ensing G T, Neut D, Van Horn J R, et al. The combination of ultrasound with antibiotics released from bone cement decreases the viability of planktonic and biofilm bacteria: An in vitro study with clinical strains[J]. J Antimicrob Chemother, 2006, 58(6): 1287-1290.
Cai X Z, Yan S G, Wu H B, et al. Effect of delayed pulsed-wave ultrasound on local pharmacokinetics and pharmacodynamics of vancomycin-loaded acrylic bone cement in vivo[J]. Antimicrob Agents Chemother, 2007, 51(9): 3199-3204.
Yan S, Cai X, Yan W, et al. Continuous wave ultrasound enhances vancomycin release and antimicrobial efficacy of antibiotic-loaded acrylic bone cement in vitro and in vivo[J]. J Biomed Mater Res B Appl Biomater, 2007, 82(1): 57-64.
Lin T, Cai X Z, Shi M M, et al. In vitro and in vivo evaluation of vancomycin-loaded PMMA cement in combination with ultrasound and microbubbles-mediated ultrasound[J]. Biomed Res Int, 2015, 15(8): 309-315.
Shi M, Chen L, Wang Y, et al. Effect of low-frequency pulsed ultrasound on drug delivery, antibacterial efficacy, and bone cement degradation in vancomycin-loaded calcium phosphate cement[J]. Med Sci Monit, 2018, 24(8): 797-802.
Shi M, Chen L, Wang Y, et al. Low-intensity pulsed ultrasound enhances antibiotic release of gentamicin-loaded, self-setting calcium phosphate cement[J]. J Int Med Res, 2018, 46(7): 2803-2809.
Breuing K H, Bayer L, Neuwalder J, et al. Early experience using low-frequency ultrasound in chronic wounds[J]. Ann Plast Surg, 2005, 55(2): 183-187.
Carmo M, Mazzaccaro D, Barbetta I, et al. Use of ultrasound debridement as an adjunctive tool for treating infected prosthetic vascular grafts in the lower extremities[J]. Ann Vasc Surg, 2015, 29(3): 607-615.
Tewarie L, Moza A K, Zayat R, et al. Ultrasound-assisted treatment of sternocutaneous fistula in post-sternotomy cardiac surgery patients[J]. Eur J Cardiothorac Surg, 2015, 47(5): 180-187.
Tewarie L, Chernigov N, Goetzenich A, et al. The effect of ultrasound-assisted debridement combined with vacuum pump therapy in deep sternal wound infections[J]. Ann Thorac Cardiovasc Surg, 2018, 24(3): 139-146.
Zhao Q H, Zhu F B, Cai X Z, et al. Effects of low-frequency pulsed wave ultrasound on the shear properties of the interface of vancomycin-loaded acrylic bone cement-stem[J]. Zhonghua Yi Xue Za Zhi, 2017, 97(7): 545-550.
