Facet-dependent catalytic activity of two-dimensional Ti3C2Tx MXene on hydrogen storage performance of MgH2
2023-12-27HaiguangGaoRuiShiYanaLiuYunfengZhuJiguangZhangLiquanLiXiaohuiHu
Haiguang Gao ,Rui Shi ,Yana Liu,∗ ,Yunfeng Zhu,∗ ,Jiguang Zhang ,Liquan Li ,Xiaohui Hu
aCollege of Materials Science and Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, PR China
bJiangsu Collaborative Innovation Centre for Advanced Inorganic Function Composites, Nanjing Tech University, Nanjing 211816, PR China
Abstract Two-dimensional Ti3C2Tx MXenes exposing different active facets are introduced into MgH2,and their catalytic effects are systematically investigated in depth through experimental and theoretical approaches.Excluding factors such as interlayer space,surface functional groups and experimental contingency,the exposed facets is considered to be the dominant factor for catalytic activity of Ti3C2Tx towards MgH2.More exposed edge facets of Ti3C2Tx displays higher catalytic activity than that with more exposed basal facets,which also leads to different rate-controlling steps of MgH2 in the de/hydrogenation process.The low work function,strong hydrogen affinity and high content of in situ metallic Ti for the edge facet contribute the high catalytic activity.This work will give insights into the structural design of two-dimensional Ti3C2Tx MXene used for enhancing the catalytic activity in various fields.
Keywords: Hydrogen storage materials;Magnesium hydride;MXene;Catalyst;Facet design.
1.Introduction
Hydrogen is widely regarded as one of the most promising energy carriers in studies on clean energy.However,the low density at standard atmospheric and temperature conditions for hydrogen gas brings a great challenge for storage [1].The high energy consumption and insecurity of traditional storage technology have stimulated the appearance of materialsbased hydrogen storage featuring high hydrogen capacity and safety.As a typical representative of hydrogen storage materials,magnesium hydride (MgH2) has owned great attention due to its high hydrogen content (7.6 wt.%),good reversibility and low cost.However,the high thermodynamic stability and kinetic barrier lead to the high operating temperature and sluggish reaction kinetics,limiting its practical application [2,3].Various methods such as catalysts doping [4,5],nanosizing [6,7],alloying [8,9] have been widely used to alleviate these problems,of which catalysts doping is deemed as the most effective method [10].
As an important branch of catalysts,transition metal-based catalysts (transition metals,their chloride,oxide and composites) such as Ni@rGO [11],TiNb2O7[12],Co@C [13],VB2[14] and so on [15–17],have been proved experimentally to play a positive role for enhancing hydrogen storage performance of MgH2.Wang et al.reported that the peak dehydrogenation temperature for MgH2doped with Ni90@PHCNSs is 242 °C,which is 75 °C lower than that for pure MgH2[18].The MgH2–Ni3S2@C-4 composite prepared by Zeng et al.shows faster dehydrogenation kinetics than pure MgH2,releasing 6.15 wt.% hydrogen in 8 min at 300 °C [19].Theoretical researches confirm that the transitional metals with special 3d orbital states tend to form covalent bonds with hydrogen atoms,affecting the stability of ionic bond Mg-H and destabilizing the MgH2[20,21].The dissociation and recombination of hydrogen molecules on the transitional metals surface can be effectively accelerated,enhancing the hydrogen ab/desorption kinetics of MgH2[22–24].As another important branch of catalysts,catalysts with special morphology have also shown superb catalytic activity for enhancing hydrogen storage performance of MgH2[25–27].Chen et al.reported that 5.32 wt.% hydrogen can be rapidly absorbed at 50 °C by MgH2doped with two-dimensional graphene-like TiO2due to the in-situ formed Ti and wrinkled Ti2O3[25].ZrO2wrapped in carbon can reduce the initial dehydrogenation temperature of MgH2from 309 °C to 208 °C due to the synergistic effect of ZrO2and C [26].The morphology of catalysts is closely related to its catalytic activity [28,29].The TiO2nanosheets exposed with high surface energy {001}facets show better catalytic activity than other TiO2nanoparticles for improving hydrogen storage performance of MgH2[30,31].Among four carbon materials,the layered structural carbon materials with interconnected wrinkles (coconut shell charcoal)show the largest promotion for the de/hydrogenation kinetics of MgH2[32].The large surface area,the exposure of active surface brought by the special morphology can explain its excellent catalytic activity [25,29].
Recently,two-dimensional transitional metal carbide,nitride or carbonitrides (MXene) have attracted a lot of attentions due to its unique structure,rich element composition,functional surface in super capacitors,optoelectronics,purifiers,etc [33,34].Luckily,development in the field of hydrogen storage have also been attained by MXene,showing excellent catalytic activity in many hydrogen storage systems such as LiAlH4[35],NaAlH4[36],2LiH+MgB2[37] and MgH2[38].Especially in MgH2system,various MXene and its derivatives,composites have been introduced to greatly improve the hydrogen storage performance,such as Ti2CTx[39],Nb4C3Tx[40],NbTiCTx[41],TiVO3.5[42],(Ti0.5V0.5)3C2Tx[43],K2Ti6O13[44] and Ni/Ti3C2Tx[45,46].However,the catalytic mechanism is mainly attributed to the in situ formed transitional metal or transitional metal hydride,and the unique layered structure which has not been thoroughly explored.Although an indelible effect on the catalytic activity for the layered structure has been further proved via our previous research [47],the specific relationship between structure and catalytic activity is unclear.
Herein,Ti3C2TxMXenes exposing with different amounts of basal and edge facets were designed and then were introduced into MgH2via ball milling.The different structures of Ti3C2TxMXenes make a great difference for enhancing hydrogen storage performance of MgH2.The different exposed facets of Ti3C2TxMXenes are verified to be the dominant factor through excluding the other structural parameters (interlayer space and functional groups) and experimental contingency.To our knowledge,this is the first in-depth study of the mechanism behind the effect of Ti3C2TxMXenes on MgH2from the perspective of Ti3C2Txstructure,both experimentally and theoretically.The proposed mechanism here provides far-reaching guidance for further designing MXene materials and improving its effects on hydrogen storage materials.
2.Experimental
2.1.Sample preparation
The F-Ti3C2Txstands for the Ti3C2Txobtained by etching Ti3AlC2MAX (MAX: ternary transitional metal carbide,nitride or carbonitrides) with hydrofluoric acid.1 g Ti3AlC2powder (400 mesh,Jilin 11 technology Co.,Ltd.) was added into 10 ml hydrofluoric acid(HF,≥40%,Sinopharm Chemical Reagent Co.,Ltd.),fully stirred and reacted at room temperature for 72 h Then,the reactant was cleaned several times with deionized water (DI) to ensure that the supernatant pH was above 6.The F-Ti3C2Txcould be obtained after filtration and freeze drying [47].
The E-F-Ti3C2Txstands for the F-Ti3C2Txafter exfoliating,filtering and drying.0.5 g F-Ti3C2Txpowder was dispersed in 25 ml dimethyl sulfoxide (DMSO,>99.8%,Aladdin) and stirred at room temperature overnight for further treatments.The intercalated F-Ti3C2Txwas collected by centrifugation and washed multiple times with ethanol and DI water to remove the residual DMSO.Then the intercalated F-Ti3C2Txwas dispersed in DI water and sonicated under ice bath to express oxidation.After centrifugation (3500 rpm for 1 h),the E-F-Ti3C2Txcould be obtained via filtration and freeze drying of supernatant [48].
The L-F-Ti3C2Txstands for the F-Ti3C2Txwith expanded layers.The intercalated F-Ti3C2Txwas dispersed in DI water and sonicated under ice bath to express oxidation.After centrifugation (3500 rpm for 1 h),the L-F-Ti3C2Txcould be obtained via filtration and freeze drying of sediment.
The S-Ti3C2Txstands for the Ti3C2Txobtained by etching Ti3AlC2MAX with in situ hydrochloric acid method.Firstly,2 g of lithium fluoride (LiF,99%,Aladdin) powers were dispersed in 40 ml 9 M hydrochloric acid(HCl,36–38%,Shanghai lingfeng chemical reagent Co.,Ltd.) and stirred for 30 min.Then 2 g Ti3AlC2powders were slowly added into the above mixed solution and stirred for 48 h at 50 °C.Following on,the reactant was washed with DI water for several times to ensure that the supernatant pH was above 6.After filtration and freeze drying,the S-Ti3C2Txcould be obtained.
The E-S-Ti3C2Txstands for the S-Ti3C2Txafter exfoliating,filtering and drying.The prepared S-Ti3C2Txpowders were added into the ethanol and were sonicated under ice bath for 1 h.After centrifugation (10,000 rpm for 10 min),DI water was used instead of ethanol and the sonication was once again under the same conditions.Finally,the E-S-Ti3C2Txcould be obtained via centrifugation (3500 rpm for 1 h),filtration and freeze drying of supernatant [45].
MgH2powders were prepared through hydriding combustion synthesis,with a purity of about 98 wt.% [49];.Under Ar atmosphere,5 wt.% as-synthesized catalysts were added into MgH2to prepare catalyzed-MgH2composites through a planetary ball mill.The detailed ball milling parameters can be found in our previous reports [47].The procedure was carried out in a glovebox with the oxygen/water concentrations below 1 ppm.
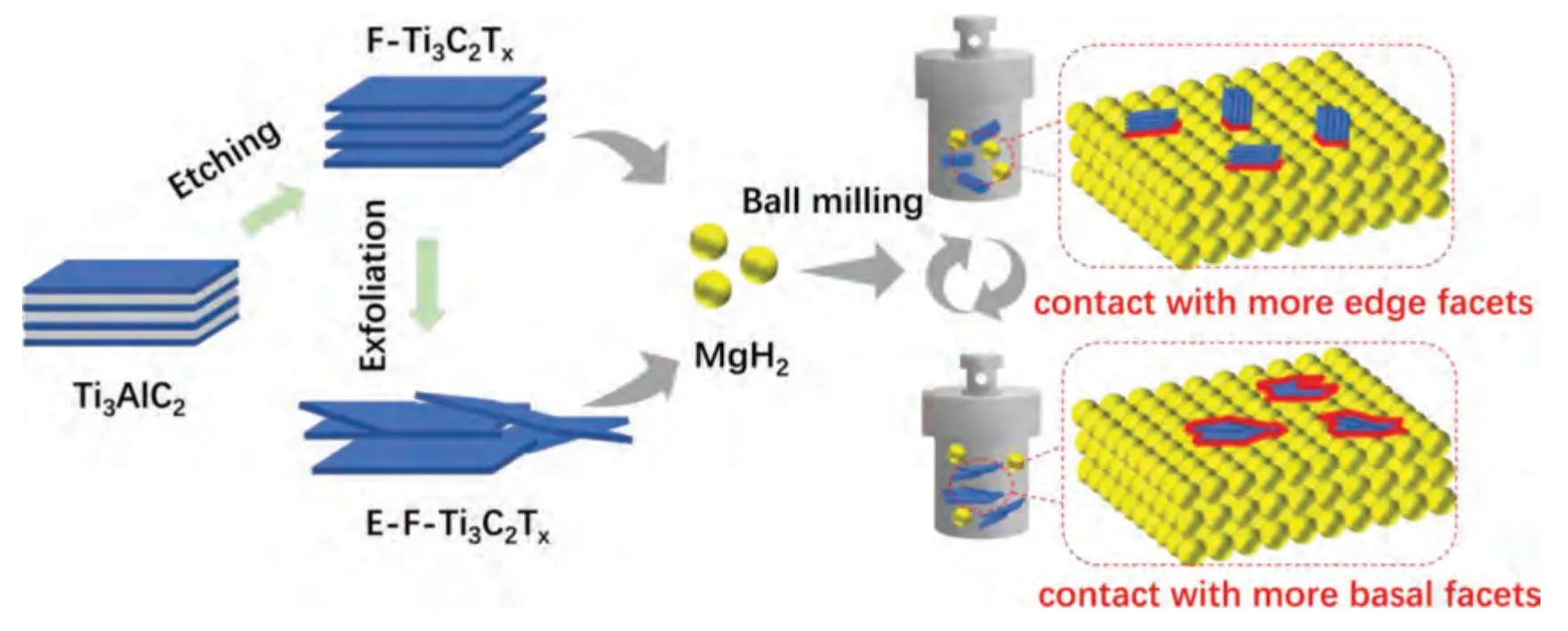
Fig.1.Schematic digram for the preparation of F-Ti3C2Tx,E-F-Ti3C2Tx and their combination with MgH2.
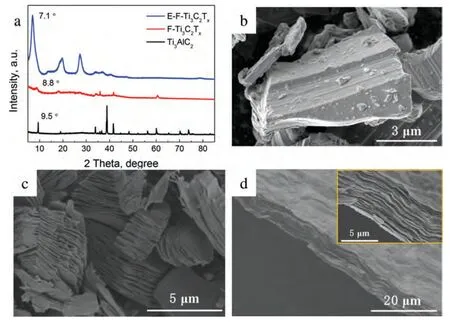
Fig.2.(a) XRD curves of Ti3AlC2,F-Ti3C2Tx and E-F-Ti3C2Tx.SEM images of (b) Ti3AlC2,(c) F-Ti3C2Tx and (d) E-F-Ti3C2Tx (inset is the corresponding image under high magnification).
2.2.Characterization
The crystal structure of the samples was analyzed via X-ray diffraction (XRD,ARL X’TRA diffractometer,Cu-Kαradiation,40 kV and 35 mA).Scanning electron microscope (SEM,JEOL JSM-7600F) and transmission electron microscopy (TEM,FEI Titan80–300Cs) were used to analyze the chemical composition and microstructure characteristics of the samples.X-ray photoelectron spectroscopy (XPS) was carried out in a Kratos AXIS ULTRA DLD system.
Hydrogen storage performances of samples including isothermal hydrogen ab/desorption kinetics (an initial hydrogen pressure of 3.0 MPa and 0.005 MPa for isothermal hydrogen ab/desorption,respectively) and thermal desorption profiles (heat up from 30 °C to 360 °C at a rate of 10 °C/min)were carried out in a Sieverts type volumetric apparatus(GRC,Advanced Materials Co.).The dehydrogenation behavior was investigated by differential scanning calorimetry(DSC,TA Q2000) at different heating rates (5,8,10 and 12 °C/min) from 30 °C to 500 °C at a flow rate of 50 ml/min Ar.The detailed test information can be found in our previous works [40].

Fig.3.(a) XRD patterns of MgH2-5 wt.% F-Ti3C2Tx and MgH2-5 wt.% E-F-Ti3C2Tx.(b) TPD curves of MgH2-5 wt.% F-Ti3C2Tx and MgH2-5 wt.%E-F-Ti3C2Tx.Comparison of hydrogen storage properties between MgH2-5 wt.% F-Ti3C2Tx and the MgH2-5 wt.% E-F-Ti3C2Tx: (c) desorption curves at different temperatures with an initial hydrogen pressure of 0.005 MPa.(d) absorption curves at different temperatures with an initial hydrogen pressure of 3.0 MPa.
2.3.Calculation methods
The density functional theory(DFT)calculations were performed via VASP 5.4.4 software package [50].The projectoraugmented wave (PAW) method [51] and the generalized gradient approximation (GGA) within Perdew–Burke–Ernzerhof(PBE) were selected to perform the electronic structure calculations [52].The Ti3C2Tx(001) slab and (010) slab were constructed and further optimized.The position of hydrogen atoms on Ti3C2Txslabs were the most stable position determined by optimization.In order to avoid the interactions between repeating images,a∼15 ˚A vacuum space was set.The convergence criteria for energy and force are set to 10-5eV and 0.02 eV/˚A,respectively.
3.Results and discussion
The schematic diagram for the preparation of two kinds of Ti3C2Txwith different morphologies and their combination with MgH2is shown in Fig.1.Firstly,accordion-like Ti3C2Txdenoted as F-Ti3C2Txwas obtained by etching Ti3AlC2,selectively removing the Al layer in Ti3AlC2with HF [38].In order to facilitate the exfoliation of the F-Ti3C2Tx,DMSO was used to interlaminate F-Ti3C2Txto extend the distance between layers [48].After ultrasonic exfoliation and filtration,paper-like Ti3C2Txdenoted as E-F-Ti3C2Txcould be obtained.Then two different morphologies of Ti3C2Txwere introduced into MgH2through ball milling separately.The F-Ti3C2Txmay have more edge facets contact with MgH2while the E-F-Ti3C2Txmay have more basal facets contact with MgH2.
The XRD curves and SEM images of Ti3AlC2,F-Ti3C2Txand E-F-Ti3C2Txare displayed in Fig.2.In Fig.2a,the strongest diffraction peak of Ti3AlC2(2θ=39°) disappearing and the peak of (002) shifting to a lower angle (2θ=8.8°),prove the formation of F-Ti3C2Txderived from Ti3AlC2[38].In Fig.2b and c,the distinct accordion-like morphology of F-Ti3C2Txis completely different from that of the precursor Ti3AlC2,further indicating the successful preparation of FTi3C2Txby the selective etching of Al layers in Ti3AlC2via HF.Through ultrasonic exfoliation and filtration,the peak of(002) shifts further to the left from 2θ=8.8° (F-Ti3C2Tx) to 2θ=7.1° (E-F-Ti3C2Tx),indicating that the interlayer space is expanded.The increase in interlayer space is consistent with the results reported in reference [48] regarding Ti3C2Txexfoliation.As shown in Fig.2d,E-F-Ti3C2Txpresents a paperlike morphology,with a large basal/edge ratio.The exposed basal facets size of E-F-Ti3C2Txis significantly different from that of F-Ti3C2Tx,while the exposed edge facets size of E-FTi3C2Txis similar to that of F-Ti3C2Tx.Although F-Ti3C2Txand E-F-Ti3C2Txshare the same chemical composition,exposed active facets are distinctly different.

Fig.4.HRTEM images of (a) MgH2-5 wt.% F-Ti3C2Tx (b) MgH2-5 wt.% E-F-Ti3C2Tx.
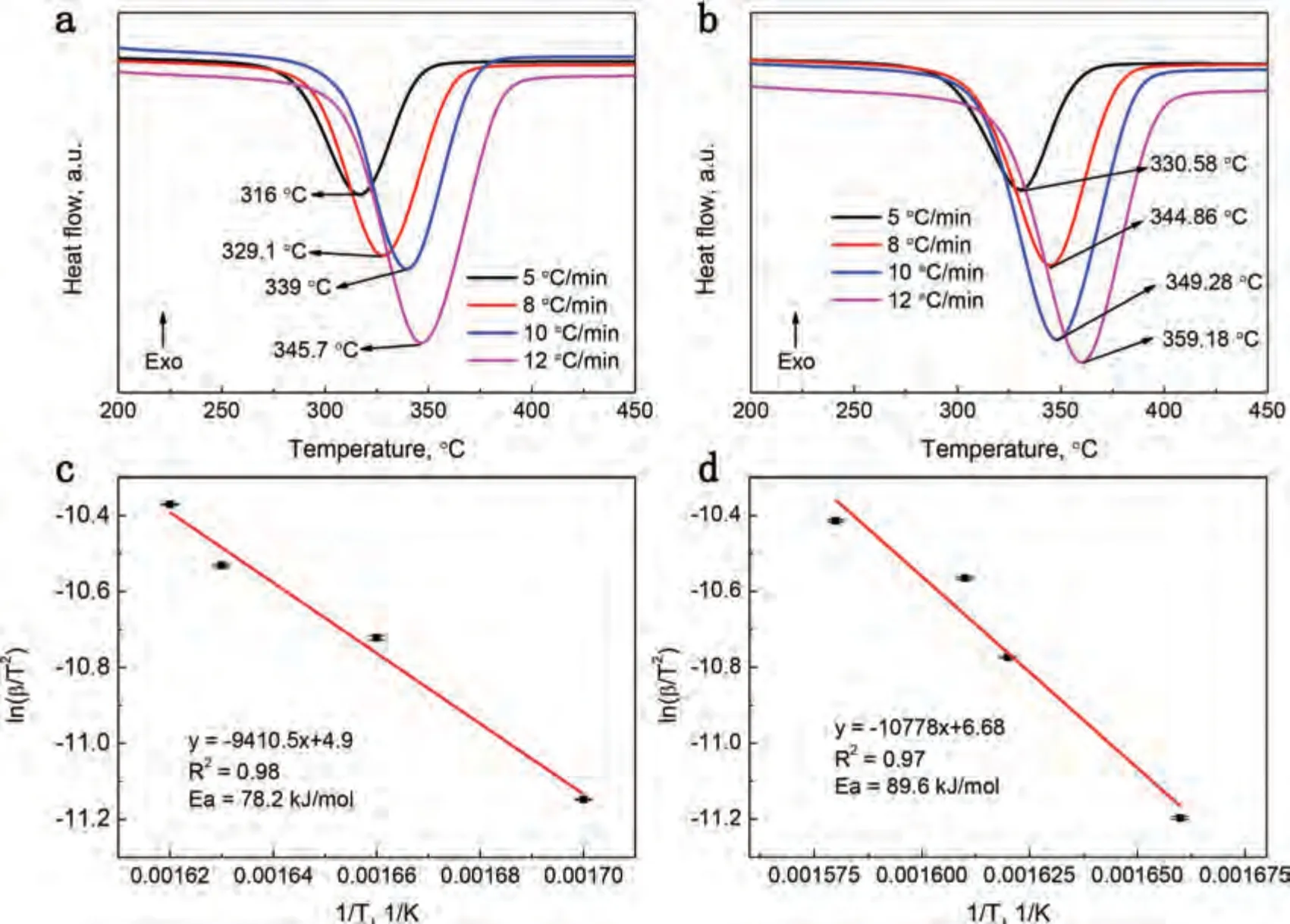
Fig.5.(a) and (b) DSC curves of MgH2-5 wt.% F-Ti3C2Tx and MgH2-5 wt.% E-F-Ti3C2Tx at different heating rates;(c) and (d) the corresponding Kissinger plots of activation energy.
To explore the effects of different exposed active facets on the catalytic activity,5 wt.% F-Ti3C2Txand E-F-Ti3C2Txwere introduced into MgH2via ball milling,respectively.The XRD curves of MgH2-5 wt.% F-Ti3C2Txand MgH2-5 wt.%E-F-Ti3C2Txare shown in Fig.3(a).For the two composites,the main diffraction peaks are the same,belonging to the MgH2.The existence of small quantity of MgO is ascribed to the inevitable oxidation during testing or preparation.Consistent with our previous researches [45,47],the absence of the diffraction peaks of F-Ti3C2Txand E-F-Ti3C2Txmay be due to poor crystallization or restricted contents.In the highresolution transmission electron microscope(HRTEM)images(Fig.4),the appearance of small-sized F-Ti3C2Txand E-FTi3C2Txindicates that the structure of F-Ti3C2Txand E-FTi3C2Txcan be retained to a certain extent during ball milling process.This phenomenon is consistent with our previously reported results that the Ti3C2Txcould be always present during ball milling process and hydrogen ab/desorption tests[45,47].Besides,the contact between MgH2matrix and FTi3C2Txor E-F-Ti3C2Txcan also be found according to the corresponding HRTEM images.
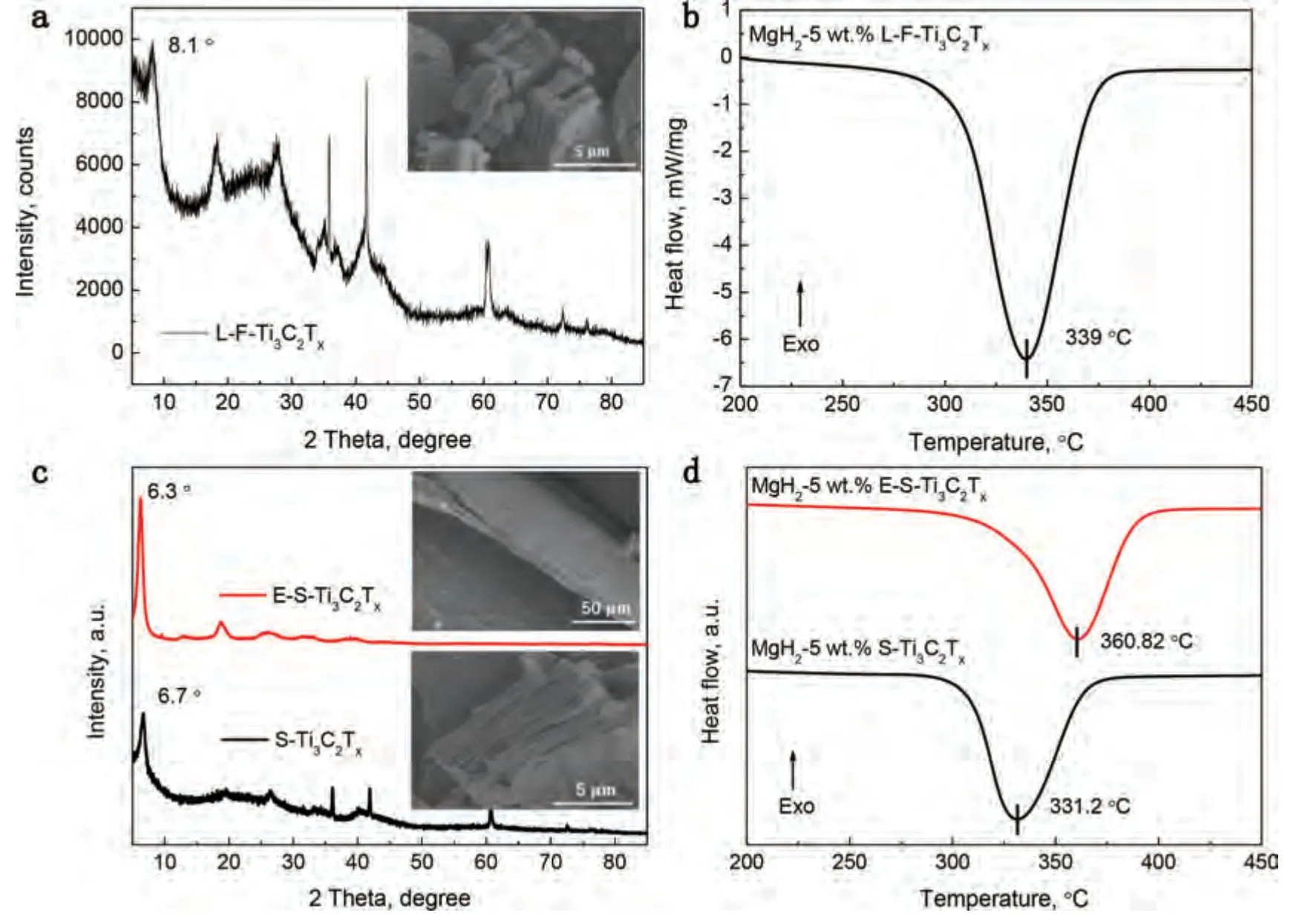
Fig.6.(a) XRD pattern of L-F-Ti3C2Tx (inset image is the corresponding SEM image).(b) DSC curve of MgH2-5 wt.% L-F-Ti3C2Tx.The heating rate is 10 °C/min.(c) XRD patterns of S-Ti3C2Tx and E-S-Ti3C2Tx (inset images are the corresponding SEM images).(d) DSC curves of MgH2-5 wt.% S-Ti3C2Tx and the MgH2-5 wt.% E-S-Ti3C2Tx.The heating rate is 10 °C/min.
The temperature-programmed desorption (TPD) and isothermal hydrogen ab/desorption kinetics tests were conducted to investigate the difference of catalytic activity between F-Ti3C2Txand E-F-Ti3C2Txon the hydrogen storage performance of MgH2Fig.3.b shows the TPD curves of MgH2-5 wt.% F-Ti3C2Txand MgH2-5 wt.% E-F-Ti3C2Tx.The onset dehydrogenation temperature for MgH2-5 wt.% FTi3C2Txand MgH2-5 wt.% E-F-Ti3C2Txare reduced to 199 and 255 °C,respectively,both lower than that of as-milled MgH2(300 °C) [31].The MgH2-5 wt.% F-Ti3C2Txshows the lowest onset dehydrogenation temperature,indicating that the catalytic activity of F-Ti3C2Txis better than that of EF-Ti3C2TxFig.3.c shows the isothermal dehydrogenation kinetics of MgH2-5 wt.% F-Ti3C2Txand MgH2-5 wt.% E-FTi3C2Txat different temperatures (225,250 and 275 °C).At 275°C,the faster dehydrogenation kinetics is obtained for the MgH2-5 wt.% F-Ti3C2Tx,releasing about 5.95 wt.% hydrogen within 1000 s,while only 4.97 wt.% hydrogen is released for the MgH2-5 wt.% E-F-Ti3C2Tx.Notably,a little hydrogen can be desorbed by as-milled MgH2within 7200 s at 275 °C,indicating the superb catalytic activity of F-Ti3C2Txand E-F-Ti3C2Tx[31].By calculating the tangent slope of the linear region of hydrogen desorption at 275 °C,the rate value of the MgH2-5 wt.% F-Ti3C2Tx(0.504 wt.%/min) is 1.52 times larger than that of the MgH2-5 wt.% E-F-Ti3C2Tx(0.331 wt.%/min).Similarly,the F-Ti3C2Txstill shows the better catalytic activity than the E-F-Ti3C2Txat 225 and 250 °C.The isothermal hydrogenation kinetics of dehydrogenated MgH2-5 wt.% F-Ti3C2Txand MgH2-5 wt.% E-FTi3C2Txat different temperatures (100,125 and 150 °C) are shown in Fig.3d.At 125 °C,the dehydrogenated MgH2-5 wt.% F-Ti3C2Txabsorbs approximately 4.57 wt.% hydrogen while only 3.46 wt.% hydrogen is stored by the dehydrogenated MgH2-5 wt.% E-F-Ti3C2Txwithin 1200 s.Notably,about 3 wt.% hydrogen can be stored by Mg within 1200 s even at 200 °C,indicating the superb catalytic activity of F-Ti3C2Txand E-F-Ti3C2Tx[31].Through calculating the tangent slope of the linear region of hydrogen absorption at 125 °C,the rate value of the dehydrogenated MgH2-5 wt.% F-Ti3C2Tx(0.559 wt.%/min) is 4.3 times faster than that of the dehydrogenated MgH2-5 wt.% E-F-Ti3C2Tx(0.130 wt.%/min).Compared with E-F-Ti3C2Tx,F-Ti3C2Txdisplays the better catalytic activity on the hydrogen absorption kinetics of Mg,which is also verified at 100 and 150 °C.To sum up,the remarkable improvement in the hydrogen ab/desorption kinetics is obtained by the MgH2-5 wt.% FTi3C2Tx,indicating the better catalytic activity of F-Ti3C2Tx,which may be due to the different exposed active facets of Ti3C2Tx.
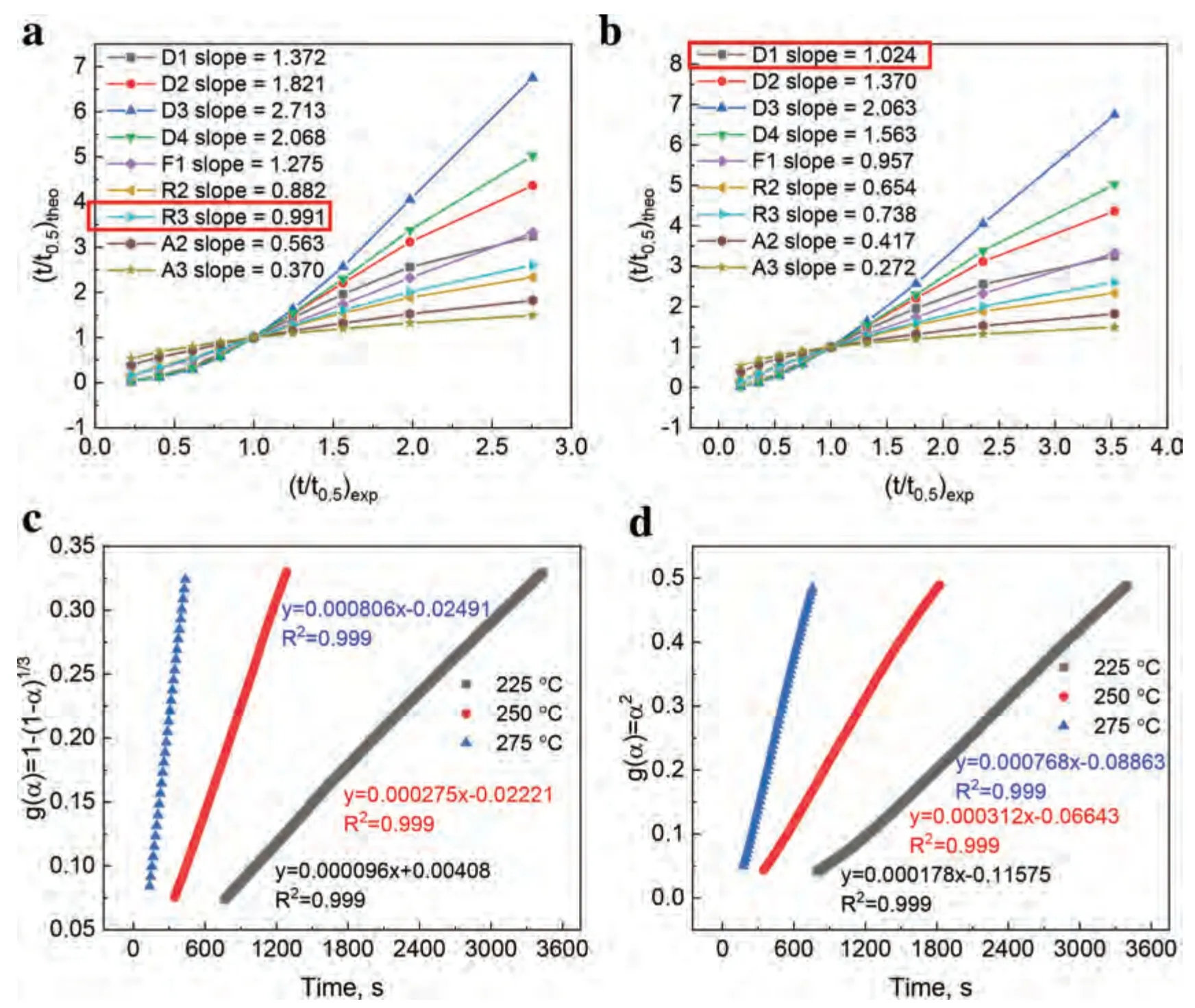
Fig.7.(t/t0.5)theo vs.(t/t0.5)exp of composites for various kinetic models: (a) MgH2-5 wt.% F-Ti3C2Tx and (b) MgH2-5 wt.% E-F-Ti3C2Tx at 250 °C.Time dependence of kinetic modeling equations g(α) for composites at different temperatures: (c) MgH2-5 wt.% F-Ti3C2Tx and (d) MgH2-5 wt.% E-F-Ti3C2Tx with 0.2<α<0.7.
The Kissinger method was used to calculate the activation energy (Ea) of the hydrogen desorption [25,53].The DSC curves and the corresponding Kissinger plots of the MgH2-5 wt.% F-Ti3C2Txand the MgH2-5 wt.% E-F-Ti3C2Txare shown in Fig.5.The apparent activation energies for the MgH2-5 wt.% F-Ti3C2Txand the MgH2-5 wt.% E-F-Ti3C2Txcan be delivered from the linear fitting of the data points to be 78.2 and 89.6 KJ mol-1,respectively,which are both lower than some of other catalysts doping MgH2system [4,28].The lower apparent activation energy of MgH2-5 wt.% F-Ti3C2Txsuggests higher catalytic efficiency for the F-Ti3C2Tx,which further verifies that the catalytic activity for enhancing the hydrogen storage performance of MgH2is influenced by the different exposed active facets of Ti3C2Tx.
In order to confirm that the different exposed active facets are the main point explaining the significant difference in catalytic activity between F-Ti3C2Txand E-F-Ti3C2Tx,other factors have also been explored in depth.The surface functional groups and interlayer space of Ti3C2Txare firstly excluded as the factors causing the significant difference of the catalytic activity between F-Ti3C2Txand E-F-Ti3C2Tx.As shown in Fig.6a,for F-Ti3C2Txwith larger interlayer space denoted as L-F-Ti3C2Tx,the peak of (002) shifts from 2θ=8.8° (Fig.2a) to 2θ=8.1° and the similar accordion-like morphology keeps stable after the expansion process [48].In Fig.6b,the peak dehydrogenation temperature of the MgH2-5 wt.% L-F-Ti3C2Txis 339 °C,which is similar to that of the MgH2-5 wt.% F-Ti3C2Tx(Fig.5a).According to references,further layer expansion and exfoliation of MXenes can lead to the increase of interlayer space and the change of surface functional groups [48,54].From F-Ti3C2Txto L-FTi3C2Txto E-F-Ti3C2Tx,the interlayer space and the ratio of-O/-F functional groups increase gradually.When Ti3C2Txis in accordion-like morphology (F-Ti3C2Txand L-F-Ti3C2Tx),the catalytic activity of Ti3C2Txbarely changes.Only when Ti3C2Txis in paper-like morphology (E-F-Ti3C2Tx),the substantial changes appear in the catalytic activity of Ti3C2Tx.These results indicate that the significant difference of the catalytic activity between F-Ti3C2Txand E-F-Ti3C2Txis dominated by the change of exposed active facets rather than the change of surface functional groups and interlayer space of Ti3C2Txhere.To avoid experimental contingency,another experiment was also carried out.The accordion-like and paperlike Ti3C2TxMXenes,which can be denoted as S-Ti3C2Txand E-S-Ti3C2Tx,respectively,were prepared through in situ HF etching (LiF and HCl as the precursor) and the same post-processing(ultrasonic exfoliation and filtration)methods.The difference of exposed facets (basal/edge ratio) between the S-Ti3C2Txand E-S-Ti3C2Txis the same as that between F-Ti3C2Txand E-F-Ti3C2Tx.Similar to the XRD curves of F-Ti3C2Txand E-F-Ti3C2Txin Fig.2a,the successful preparation of S-Ti3C2Txand E-S-Ti3C2Txare verified via the XRD curves as shown in Fig.6c.The accordion-like morphology of S-Ti3C2Txand paper-like morphology of E-S-Ti3C2Txare shown in the inset of Fig.6c.In Fig.6d,the peak dehydrogenation temperature of the MgH2-5 wt.% S-Ti3C2Tx(331.2 °C) is much lower than that of the MgH2-5 wt.% E-STi3C2Tx(360.82°C),indicating the higher catalytic activity of S-Ti3C2Tx.The same trend in two different preparation methods further indicates that the significant difference of the catalytic activity between F-Ti3C2Txand E-F-Ti3C2Txis mainly dominated by two different morphologies.The greater difference in catalytic activity between S-Ti3C2Txand E-S-Ti3C2Txmay be due to the more different exposed active facets.
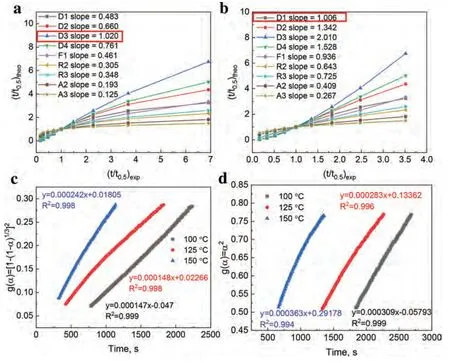
Fig.8.(t/t0.5)theo vs.(t/t0.5)exp of composites for various kinetic models: (a) MgH2-5 wt.% F-Ti3C2Tx and (b) MgH2-5 wt.% E-F-Ti3C2Tx at 125 °C.Time dependence of kinetic modeling equations g(α) for composites at different temperatures: (c) MgH2-5 wt.% F-Ti3C2Tx and (d) MgH2-5 wt.% E-F-Ti3C2Tx with 0.7<α<0.9.
To further explore the reasons behind the significant difference of the catalytic activity between F-Ti3C2Txand EF-Ti3C2Txcaused by the different exposed active facets,the analysis of solid-state reaction mechanism models,XPS measurements and DFT calculation were performed.According to the experimental results of isothermal de/hydrogenation kinetics at different temperatures,the corresponding solid-state reaction mechanism model and rate-controlling step can be identified.The general kinetics equation can be shown as follows [53]:
in Eq.(1),whereα,T,k(T) andf(α) represent the reaction extent,the reaction temperature,the reaction rate constant and the function depending on the specific kinetic mechanism,respectively.f(α) can also be described as the following equation [55]:

Fig.9.Ti 2p XPS spectra of (a) the F-Ti3C2Tx,MgH2-5 wt.% F-Ti3C2Tx and (b) E-F-Ti3C2Tx,MgH2-5 wt.% E-F-Ti3C2Tx.

Fig.10.The electrostatic potentials along c axis of Ti3C2Tx (001) and (010) slabs.The Ev and Ef denote the Fermi and vacuum energy levels,respectively.The red,black and blue balls represent oxygen,carbon and titanium atoms respectively.
in Eq.(2),whereA,t0.5represent the constant related to the kinetic mechanism,the time whenαequals 0.5,respectively.Through plotting the experimental values of t/t0.5against the theoretical values of t/t0.5for the composites from nine different kinetic models respectively,the corresponding reliable kinetic model can be obtained.The line with a slope closest to 1 is the reliable kinetic model.Based on the isothermal dehydrogenation curves of the MgH2-5 wt.% F-Ti3C2Txand the MgH2-5 wt.% E-F-Ti3C2Txat 250 °C (Fig.3c),nine curves derived from nine different kinetic models are shown in Fig.7a and 7b,respectively.The R3 (three-dimensional phase boundary) and D1 (one-dimensional diffusion) models are best matched to the MgH2-5 wt.% F-Ti3C2Txand the MgH2-5 wt.% E-F-Ti3C2Tx,respectively.As shown in Fig.7c and 7d,the R3 and D1 models are further verified through plotting the relatedf(α) against the reaction time at 225 and 275 °C (0.2<α<0.7),showing good linearity (R2>0.999) [56].Therefore,the dehydrogenation kinetics of MgH2-5 wt.% F-Ti3C2Txis influenced by the threedimensional phase boundary,while that of MgH2-5 wt.%E-F-Ti3C2Txis influenced by the one-dimensional diffusion process [53,55].The different dehydrogenation mechanism is consistent with different dehydrogenation performance,explaining the huge impact of the different exposed active faces between F-Ti3C2Txand E-F-Ti3C2Txon the dehydrogenation of MgH2.Similarly,the hydrogenation mechanism for the dehydrogenated MgH2-5 wt.% F-Ti3C2Txand MgH2-5 wt.% EF-Ti3C2Txis also explored via the same method.Based on the isothermal hydrogenation curves of the dehydrogenated MgH2-5 wt.% F-Ti3C2Txand MgH2-5 wt.% E-F-Ti3C2Txat 125 °C (Fig.3d),nine curves derived from nine different kinetic models are shown in Fig.8a and 8b,respectively.The hydrogenation process of the dehydrogenated MgH2-5 wt.%F-Ti3C2Txis controlled by the D3 (three-dimensional diffusion) model with a faster reaction rate while that of the dehydrogenated MgH2-5 wt.% E-F-Ti3C2Txis controlled by the D1 model with a slower reaction rate,which is further confirmed by the good linearity (R2>0.99) between the relatedf(α) and the reaction time at 100 and 150 °C (0.7<α<0.9)as shown in Fig.8c and 8d [25,56].The phenomenon is consistent with the performance of the experimentally obtained hydrogenation data,explaining the huge impact of the different exposed active facets between F-Ti3C2Txand E-F-Ti3C2Txon the hydrogenation of Mg.
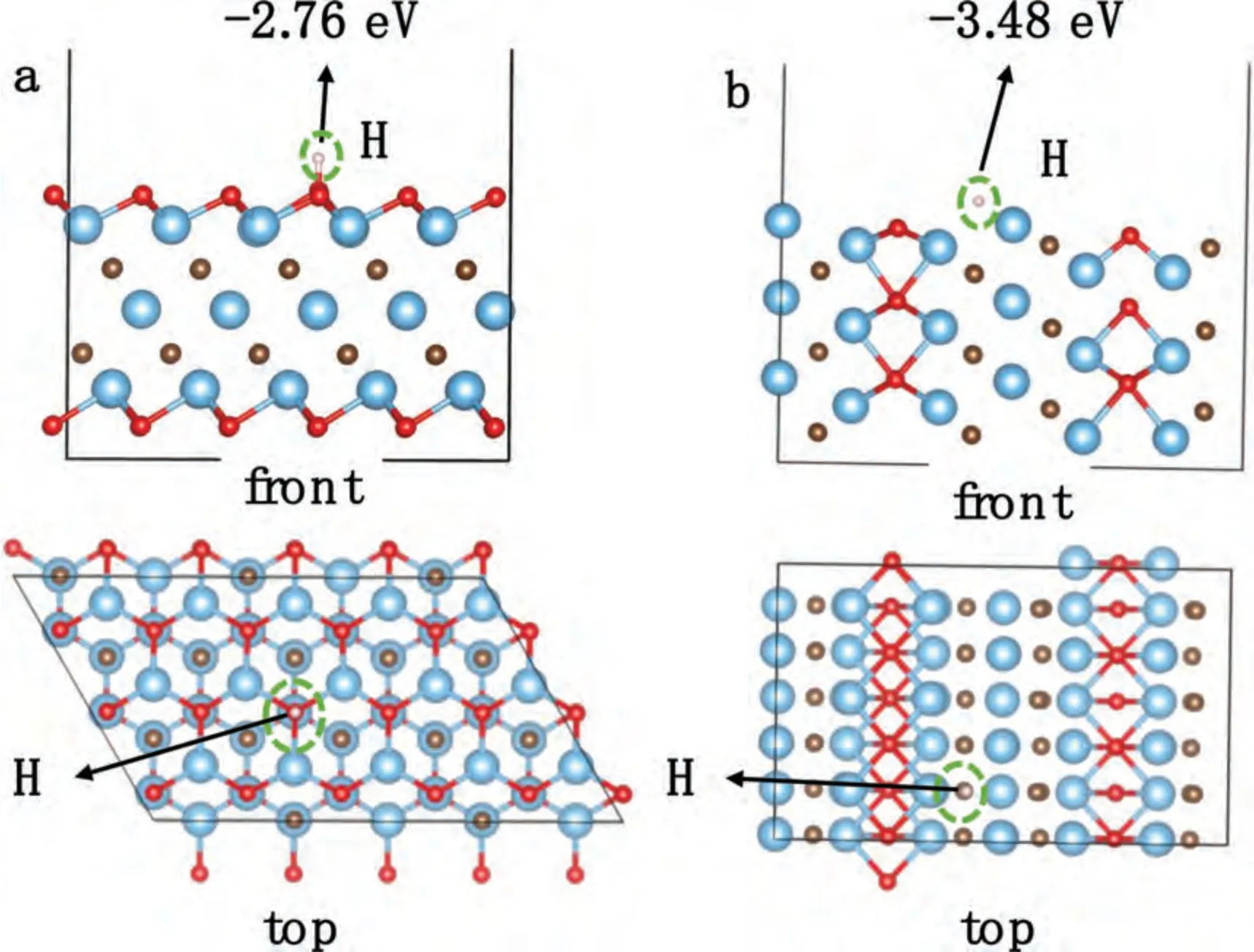
Fig.11.Optimized adsorption configuration of H atom over the (a) Ti3C2Tx (001) and (b) Ti3C2Tx (010) surface slabs.The red,brown and blue balls represent oxygen,carbon and titanium atoms respectively.
The valence state of Ti in F-Ti3C2Txand the as-milled MgH2-5 wt.% F-Ti3C2Tx,E-F-Ti3C2Txand the as-milled MgH2-5 wt.% E-F-Ti3C2Txwere measured by XPS analysis.As shown in Fig.9,the Ti 2p spectrum of F-Ti3C2Txand E-F-Ti3C2Txboth can be resolved into four sets of 2p1/2–2p3/2spin-orbit doublets at 457.9/463.3,457.0/462.4,455.9/461.5,and 454.9/460.6 eV which can be fitted to TiO2,Ti3+,Ti2+and Ti-C,separately [38,57].For MgH2-5 wt.% F-Ti3C2Txand MgH2-5 wt.% E-F-Ti3C2Tx,new peaks corresponding to Ti0(454.2/460.2) appear on the basis of the above peaks.The in situ formed metallic Ti may be due to the reduction of partial F-Ti3C2Txand E-F-Ti3C2Txby MgH2during ball milling process,which is consistent with previous reports[38,47].And the peaks of TiO2,Ti3+,Ti2+and Ti-C indicate the existence of partial F-Ti3C2Txand E-F-Ti3C2Tx,which is consistent with HRTEM results in Fig.4.Notably,the in situ formed metallic Ti and partial Ti3C2Txcould exist stably in the subsequent hydrogen ab/desorption tests according to our previous reports [45,47].The contents of various Ti components are listed in Table 1.The content of the in situ formedmetallic Ti in the as-milled MgH2-5 wt.% F-Ti3C2Tx(20.2%)is almost twice as much as that in the as-milled MgH2-5 wt.%E-F-Ti3C2Tx(11.3%),which may be ascribed to the different exposed active facets between F-Ti3C2Txand E-F-Ti3C2Tx.This phenomenon further demonstrates that the Ti atoms on the edge facets may be reduced more easily by MgH2than that on the basal facets.The in situ formed Ti can disintegrate and recombine hydrogen molecules on its surface more efficiently,and thus enhance the dehydrogenation kinetics of MgH2significantly [22,23].Therefore,the as-milled MgH2-5 wt.% F-Ti3C2Txwith more contents of the in situ formed metallic Ti should exhibit the faster dehydrogenation kinetics than the as-milled MgH2-5 wt.% E-F-Ti3C2Tx,which is consistent with experimental dehydrogenation kinetics data.

Table 1 The content of various Ti in the F-Ti3C2Tx,MgH2-5 wt.% F-Ti3C2Tx,E-FTi3C2Tx and MgH2-5 wt.%E-F-Ti3C2Tx according to the Ti 2p XPS spectra.
In order to simulate the different exposed facets between F-Ti3C2Txand E-F-Ti3C2Tx,the Ti3C2Tx(001) and Ti3C2Tx(010) slabs representing the basal facet and edge facet of Ti3C2Txwere built respectively.For Ti3C2TxMXenes,the basal facets cover with terminal groups while the edge facets expose middle Ti sites,which may affect the activity of two facets to some extent [58].To further confirm the differences in activity due to structure and composition,the work functions (Φ) of the two facets of Ti3C2Txwere calculated.As shown in Fig.10,the lowerΦvalue of edge facet (5.64 eV)than basal facet (5.86 eV) based on Fermi levels and vacuum energy levels,indicates that it is easier for an electron to escape from edge facet into the vacuum[45].This result verifies that the edge facet is more active than the basal facet,which is consistent with the XPS results and the literature [59].
The hydrogen adsorption energy (Ead) of Ti3C2Tx(001)and Ti3C2Tx(010) slabs was further compared via DFT calculations.Eadcan be calculated from the equation as follows[60]:
in Eq.(3),whereEslabH,EslabandEHrepresent the energy of the surface slab after hydrogen absorption,the energy of the surface slab and the energy of single hydrogen atom.Optimized adsorption configuration of H atom over the Ti3C2Tx(001) and Ti3C2Tx(010) surface slabs are shown in Fig.11.After calculation,the Eadof Ti3C2Tx(001)(-2.76 eV) is lower than that of Ti3C2Tx(010) (-3.48 eV),indicating that the edge facet of Ti3C2Txshows a better hydrogen affinity than the basal facet of Ti3C2Tx.The different catalytic activity between the edge and basal facets of Ti3C2Txwas also verified in the electrochemical nitrogen fixation[59].The calculation results above are consistent with the experimental results that the F-Ti3C2Txshows better catalytic activity than E-F-Ti3C2Txon the hydrogen ab/desorption kinetics of MgH2.
Combining the results above,the reasons behind the significant difference of the catalytic activity between F-Ti3C2Txand E-F-Ti3C2Txcan be uncovered.After combining with MgH2,the different exposed facets between F-Ti3C2Txand E-F-Ti3C2Txcause the different hydrogen ab/desorption kinetics mechanism and rate-controlling step of MgH2.The FTi3C2Txexposing more high active edge facets shows higher hydrogen affinity and facilitates the formation of metallic Ti,which can effectively accelerate the hydrogen ab/desorption kinetics of MgH2.
4.Conclusions
In this work,the Ti3C2TxMXenes exposing different facets are investigated about their catalytic activity on the hydrogen ab/desorption kinetics of MgH2.The F-Ti3C2Txexposing more edge facets shows better catalytic activity than E-FTi3C2Txexposing more basal facets.For example,the MgH2-5 wt.% F-Ti3C2Txshows the lower onset dehydrogenation temperature,the faster hydrogen ab/desorption kinetics and the lower dehydrogenation activation energies than the MgH2-5 wt.% E-F-Ti3C2Tx.Through investigating other structural parameters(interlayer space and functional groups)and experimental contingency,the different exposed facets are verified to be the dominant factor for affecting the catalytic activity of Ti3C2Tx.The F-Ti3C2Txexposing more high activity edge facets shows higher hydrogen affinity and facilitates the formation of metallic Ti.These two points can effectively accelerate the hydrogen ab/desorption kinetics of MgH2.Therefore,the different hydrogen ab/desorption kinetics mechanism and rate-controlling step for the MgH2-5 wt.% F-Ti3C2Txand MgH2-5 wt.% E-F-Ti3C2Txoccurs,due to the significant difference of the catalytic activity between F-Ti3C2Txand E-FTi3C2Tx.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51801100,51771092,21975125,51801099),Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJB430014),Six Talent Peaks Project in Jiangsu Province (2018,XNY-020),and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.We are grateful to the High-Performance Computing Center of Nanjing Tech University for supporting the computational resources.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Improvements in mechanical,corrosion,and biocompatibility properties of Mg–Zr–Sr–Dy alloys via extrusion for biodegradable implant applications
- Ultrasonic solidification mechanism and optimized application performances of ternary Mg71.5Zn26.1Y2.4 alloy
- Superplastic behavior of a fine-grained Mg-Gd-Y-Ag alloy processed by equal channel angular pressing
- GO/MgO/Mg interface mediated strengthening and electromagnetic interference shielding in AZ31 composite
- Experimental and theoretical studies on two-dimensional vanadium carbide hybrid nanomaterials derived from V4AlC3 as excellent catalyst for MgH2
- Electrochemical synthesis of boron-containing coatings on Mg alloy for thermal neutron shielding
