GO/MgO/Mg interface mediated strengthening and electromagnetic interference shielding in AZ31 composite
2023-12-27XuFngLiWngZhngTnWng
Z.Y.Xu ,C.F.Fng,∗ ,C.J.Li ,R.Wng ,X.P.Zhng ,J.Tn ,Y.M.Wng
a Key Laboratory of Solidification Control and Digital Preparation Technology (Liaoning Province), School of Materials Science and Engineering, Dalian University of Technology, Dalian 116024, China
b Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming 650093, China
c College of Materials Science and Engineering, Chongqing University, Chongqing 400045, China
d Key Laboratory of Materials Modification by Laser, Ion and Electron Beams (Ministry of Education), School of Materials Science and Engineering, Dalian University of Technology, Dalian 116023, China
Abstract More requirements of electromagnetic interference (EMI) shielding performance are put forward for lightweight structural materials due to the development of aerospace and 5G communications.Herein,graphene oxide (GO) decorated with SnO2 coating is introduced as reinforcement into AZ31 Mg alloy.During the smelting process,the MgO layer is in situ gernerated at interface between GO and the molten Mg alloy matrix by consuming SnO2.In the solid state,such kind of interface structure can improve the GO-Mg interface bonding intensity,also significantly generate stacking faults.The AZ31 composite reinfoced by trace modified GO (0.1 wt%) exhibits high ultimate strength and almost the same elongation with AZ31 alloy.Compared with AZ31 alloy,the yield strength and ultimate tensile strength of composite are increased by 33.5% and 23.7%,respectively.Meanwhile,the multi-level electromagnetic reflection from the multi-layer structure of GO and the interface polarization caused by the MgO mid-layer can significantly improve EMI shielding performance.The appropriate interface design strategy achieves the effect of “two birds with one stone”.
Keywords: Metal-matrix composites;Mechanical properties;EMI shielding;Microstructures.
1.Introduction
The advent of graphene brings new unlimited opportunities for the new materials development [1].Owing to its excellent mechanical properties,the graphene is considered as an ideal reinforcement additive into metal,polymer and ceramic matrices [2,3].In addition,its special 2D structure and physical properties,such as good electromagnetic absorption,also contribute to develop novel functional materials.A typical example is the graphene-containing polymer-based material for electromagnetic interference (EMI) shielding application [4,5].Due to poor conductivity of the polymer matrix,high-quality and high-content (usually ≥5 wt.%,even more than 20 wt.%) of graphene filler is always required to ensure the high electromagnetic shielding performance,such as the shielding effectiveness (SE) higher than 30 dB in the X-band[6–8].This undoubtedly significantly increases the production cost of the composites.Moreover,the polymer-based shielding materials have merits of lightweight,flexibility and low cost,however they are still inferior in terms of hardness,wear resistance,thermal stability and conductivity compared with metal-based counterparts.
Some metals or alloys,e.g.Cu,Ag,Fe,Ni and permalloy,exhibit excellent EMI shielding performance [9].However,their high density limits their applications in areas with light requirements,such as aerospace,portable communications and so on.This just provides space for the development of lightweight magnesium and its alloys with certain EMI shielding capability in this field.For a long time,improving the strength and ductility of magnesium and its alloys is the focus of development.Relatively speaking,the research on EMI shielding performance is still very little and not deep enough [10–12].In the limited research,the main idea to improve the EMI shielding performance of magnesium alloy is to improve the conductivity by texturing or desolving and introduce magnetic loss [10,13–18].It should be noted that texturing often means the reduction of material forming ability.More importantly,the improved conductivity will enhance the ability to reflect electromagnetic wave (EMW),but the excellent EMI shielding ability of the material is often determined by its ability to absorb EMW.The addition of magnetic powder is beneficial to the absorption of EMW,but the introduction of Fe or Ni elements will inevitably reduce the original lightweight advantages of Mg alloys and bring hidden dangers to corrosion resistance.
If graphene oxide (GO,a derivative of graphene),which has properties similar to graphene while being relatively inexpensive [19],is combined with magnesium alloys,its 2D multi-layer structure is expected to not only improve the mechanical properties,but also to introduce multi-level electromagnetic reflection inside the Mg-based electromagnetic shielding material.If this is the case,the above-mentioned problems would face a good solution.In order to prepare this kind of composite,the complex process methods such asin-situsynthesis [20–23],high-energy ball milling [24–27],flaky powder metallurgy [28–30],molecular level mixing[31–33] and so on can be used for reference to improve the dispersion of reinforcing phase and the interfacial bonding between phases.However,the simple stirring casting method that cannot only ensure the low cost of the process,but also make good use of the existing industrial production equipment for large-scale preparation is worthy of expectation.However,the poor wettability and density difference between graphene and metal melt determine the challenge of casting method[3,34].In addition,the agglomeration tendency of graphene or its derivatives is also a problem in the melting and casting process.Previous studies have shown that the conventional stirring operation in the casting process and the limited shear force in the subsequent deformation process of the ingot are not enough to break the nano-phase clusters [35].In this regard,the improvement approach is often to control the future interphase interface structure through the surface modification of nano-phase.MgO [36],ZnO [37] or Ni [38] coatings not only improve the wettability between graphene and metal matrix,but also improve the conductivity of the material.Unfortunately,the comprehensive effect of interphase interface structure on the mechanical and EMI shielding properties of magnesium matrix composites is rarely reported,and the relevant mechanism also needs to be clarified.
To this end,this paper provides an idea of coating GO with SnO2,and successfully realizes the preparation of Mg matrix composites containing GO by traditional stirring casting and hot rolling processes.Through the strategy of introduction of GO reinforcement with multi-layer structure andin-situMgO interface design,the effect of “two birds with one stone”in the GO-containing AZ31 composite is achieved:the mechanical and EMI shielding properties are simultaneously improved.This study provides a new insight for the functional development and application of magnesium-based structural materials.
2.Experimental
Fig.1 shows the preparation process of the Mg matrix composite reinforced by graphene oxide (GO),mainly including two steps: surface modification of GO and composite fabrication.
2.1.Surface modification of GO
The reagents used in this experiment were purchased from Sinopharm Chemical Reagent Co.,Ltd.,and no further purification was required.GO was provided by Jining Lite nanotechnology Co.,Ltd.In a typical synthesis (Fig.1a),GO of 0.6 g was added into 600 ml deionized water,following the addition of 2 ml of aqueous HCl solution (35 wt.%) under stirring.Then,a certain amount of SnCl2·2H2O was added into the solution.Ultrasonic treatment was carried out for 0.5 h at room temperature,resulting the solution into a black suspension.Then the liquid was filtered and washed to obtain the solid powders.Powders were vacuum dried at 90◦C for 10 h,The as-formed powders are SnO2coated GO (named GO-SnO2).
2.2.Fabrication of GO-SnO2/AZ31 composite
The preparation process of the 0.1 wt.% GO-SnO2/AZ31 composite is shown in Fig.1b.In an electric resistance furnace,AZ31 alloy was first melt with commercial pure Mg,Al and Zn as raw materials in a steel curcible protected by commercial anti-oxidizing flux (chlorine and fluoride salts,including MgCl2,KCl,CaF2,BaCl2).At 993 K,GO-SnO2powders were added into the AZ31 melt with a mechanical stirring (200 r/min) for 10 min.After standing for a while,the melt was finally cast into a steel mold preheated at 473 K.
For comparison,a AZ31 alloy as well as an uncoated GO reinforced AZ31 (GO/AZ31) composite was also prepared by using similar procedure.Table 1 summarizes the notations and chemical compositions of the materials measured by an optical spectrum analyzer(ARL 4460,Switzerland).The samples (80 × 90 × 18 mm) for cross-rolling were segmented from the three ingots,and then homogenized at 693 K for 12 h.After annealing at the rolling temperature for 0.5 h,the cross-rolling,in which each rolling direction changed by 90°,was conducted at 673 K.During the pass interval,the as-rolled samples were reheated up to the rolling temperature.Finally,a total plastic deformation of 70% was obtained and the sheets were then cooled in the air.
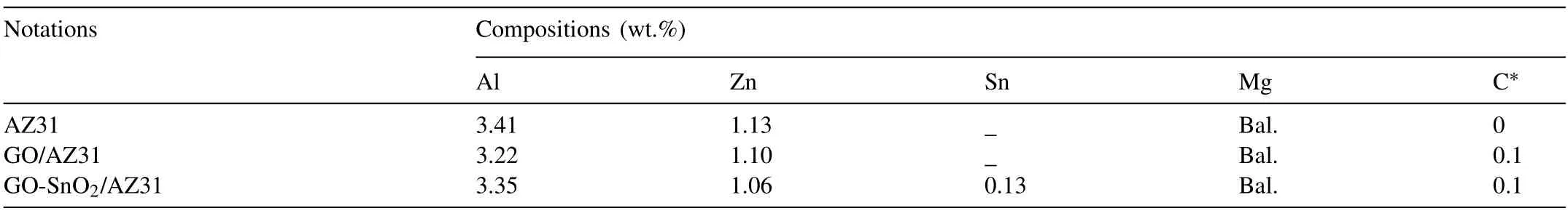
Table 1 Nominal and chemical compositions of the as-cast AZ31 alloy and its composites.
2.3.Materials characterizations
The microstructures of the GO,GO-SnO2,AZ31 alloy and composites were observed by an optical microscopy (OM)(P-3,Olympus,Japan),scanning electron microscopy (SEM)(ZEISS EV55,Germany) and transmission electron microscope (TEM) (Tecnai G220 S-Twin,USA).For the TEM observation of as-rolled samples,3 mm thin disks were firstly mechanical grinded to∼80 μm,and further thinned by the Gatan Precision Ion Polishing System operated at 5 kV.The electron backscatter diffraction (EBSD) analysis was performed by using a ZEISS EV55 SEM equipped with an HKL EBSD detector.EBSD samples were prepared by argon ion polishing after mechanical polishing.To evaluate the materials’ mechanical properties,uniaxial tensile testing was performed by Instron-1186 tension machine with a strain rate of 1 × 10-3s-1.The tensile specimens were cut along the RD1 with a gage length of 20 mm,a gage width of 3.5 mm and a thickness of 2 mm (for each test,three specimens were tested to obtain the average value).The shielding effectiveness(SE) of specimens in low-band frequency (200–1400 MHz)(Φ115 mm×2 mm (in thickness)) were tested through coaxial cable method according to ASTMD4935-2010.The shielding effectiveness of specimens in X-band frequency were tested through waveguide method.The samples were fabricated to rectangle plates of 22.9 mm ×10.2 mm ×2.0 mm to fit the waveguide WR 90.The conductivity of samples at 20◦C was tesed by the electromagnetic eddy current method (Sigmascope SMP10,Germany).Each conductivity datum was the average of ten testing results.
3.Results and discussion
3.1.Microstructures of the composites
Fig.2 shows the microstructure of raw GOs and GO-SnO2.The raw GOs exhibit typical flake structure with high flexibility and transparency (Fig.2a).Selected area electron diffraction (SAED) pattern (the inset in Fig.2a) shows good crystallization characteristic,indicating a high quality of GO.As shown in Fig.2b,the original GO edge is about 10 nm,indicating the original GO of 20–30 layers.After the coating process,numerous nanosized particles can be observed on the surface of GO (Fig.2c) (the lateral size of GO-SnO2after ultrasonic treatment was shown in Fig.S1,supplementary materials).Differing from the original GO,the SAED pattern shows a polycrystalline structure of the coating SnO2.Further high resolution TEM observation (Fig.2d) reveals the well crystallized nanoparticles with sizes of about 5 nm,as marked by white circles.The lattice fringes with a distance of 0.33 nm are clearly observed in the Fig.2e and f,which could be indexed as the (110) plane of SnO2.
The SEM image of as-rolled sample (Fig.3a) shows banded structure along the rolling direction.The corresponding EDS mappings indicate that C and O elements would be enriched in the bands (as shown in Fig.3(c,d)).This implies that the bands should be GO clusters distributed along the rolling direction.Fig.3(e-h) show the dispersion of GOs in the as-rolled GO/AZ31 and GO-SnO2/AZ31composites.The GO clusters in the GO-SnO2/AZ31 composite distribute more homogenously and exhibit smaller size than that of GO/AZ31 composite.There are two main reasons for the improvement of GO dispersion: (1) SnO2modified layer reduces the density difference between GO and AZ31 melt;(2) SnO2coating improved the wettability between GO and Mg matrix which is beneficial for the fixation of GO in the melt,and further avoids re-agglomeration during the melting process.
Fig.3i shows the Raman spectra of raw GOs,GO/AZ31 and GO-SnO2/AZ31 composites.The structural damage degree of GO can be semi-quantitatively characterized by the relative intensity between the D-band and G-band peaks(ID/IG) [39].In this work,the ID/IGvalue of raw GO,GO/AZ31 and GO-SnO2/AZ31 composites are 0.85,1.08 and 0.95,respectively.The higher ID/IGvalues mean the higher defect density in GO.The relatively low ID/IGvalue of GOSnO2/AZ31composite indicates that the SnO2coating could reduce the damage degree of GO during the preparetion process,which is very important to give full play to the excellent characteristics of GO.
The EBSD inverse pole figure (IPF) maps of the as-rolled specimen and corresponding statistical results for different types of grains are shown in Fig.4(a-f).All of the as-rolled specimens show typical basal texture characteristics and their maximum polar densities are almost the same,indicating that the introduction of graphene would have little effect on the texture type and strength of AZ31 alloy.The proportions of recrystallized grains in both composites are higher than the single AZ31 alloy,and the GO-SnO2/AZ31 composite presents the highest recrystallization ratio of 65% (as shown in Fig.4(d-f)).This indicates that the introduction of GOs would accelerate the recrystallization evolution during rolling.
Two reasons are suggested: (1) grain boundary nucleation,the addition of GOs refines the grain size of as-cast composites (as shown in Figs.S2 and S3,supplementary materials),which provide more nucleation sites for recrystallization.;(2) the promoting effect of GOs on recrystallization through particle stimulated nucleation(PSN)mechanism[40].The mechanism is rapid sub-boundary migration in the deformation zone that necessarily forms around large (>1 μm)hard particles during deformation (The lateral size of GOSnO2in the current study is 1.46 μm,as shown in Fig.S1,supplementary materials),leading to the creation of new highangle grain boundaries (HAGBs).The deformation zone is characterized by lattice rotation,and hence contains a misorientation gradient.For PSN to occur,the accumulation of misorientation by the rapid sub-boundary migration must be sufficient to generate the necessary HAGB.This forms a new grain nucleus,which grow to produce a recrystallized grain.The dispersion of GO in GO-SnO2/AZ31 composite is more uniform (as shown in Fig.3f and h),which is more favorable for the PSN of GO.The kernel average misorientation (KAM) map (Fig.S4,supplementary materials) indicates a larger orientation gradient within as-deformed regions than that in dynamic recrystallised (DRXed) grains.It is more probably caused by the high density of residual dislocations to accommodate the local lattice strains in un-DRXed grains.Due to more sufficient recrystallization in the composites,the number of residual dislocations in the composites is less.Relevant studies show that the increase of the number of residual dislocations can promote the transformation of
The average grain sizes of AZ31 alloy,GO/AZ31 composite and GO-SnO2/AZ31 composites were determined by the EBSD maps.The GO/AZ31 and GO-SnO2/AZ31 composites show the similar grain sizes (7.1 μm and 6.8 μm,respectively),which are slightly finer than that of AZ31 (8.5 μm)due to the pinning effect of GOs (as shown in Fig.4(g-i)).
3.2.Interfacial structures
In the GO/AZ31 and GO-SnO2/AZ31 composites,GOs distribute in the AZ31 matrix(as shown in Fig.5a and b),and no interfacial products is formed in the GO/AZ31 composite(as shown in Fig.5c).In the GO-SnO2/AZ31 composite,a considerable number of nanoparticles (5–10 nm) scatter on the surface of GO and the interface between GO and AZ31 matrix.By calibrating the polycrystalline rings in the illustration of Fig.5d,it can be determined that these nanoparticles are MgO nanoparticles,and the lattice fringe with a distance of 0.21 nm is indexed as the (200) plane of MgO [42],as show in Fig.5d.These MgO nanoparticles should be formed by anin-situreaction of magnesium melt with SnO2coating during melting,which is consistent with the EDS result in Fig.3d.
Thermodynamic could be used to analyze the interface reaction between the SnO2coating and Mg matrix.TheΔG of MgO formation is calculated as -616.2 kJ·mol-1at 973 K(the temperature melting) from the supplementary materials.Therefore,thein-situreaction between SnO2coating and Mg melt is fairly easy to occur during the smelting process.Fig.5e presents a schematic formation process ofin-situMgO.The SnO2on the surface of GO (Fig.2d) would be replaced by MgO,which is beneficial to improve the interfacial wettability of GOs in the Mg melt,further improve the dispersion of GOs during the smelting process.The unmodified GOs are easly aggregate due to being attracted to each other or being repelled by the melt,as shown in Fig.3e.The formation of interface product MgO makes GO easy to be fixed by Mg melt,which reduces its re-aggregation,as shown in Fig.3g.
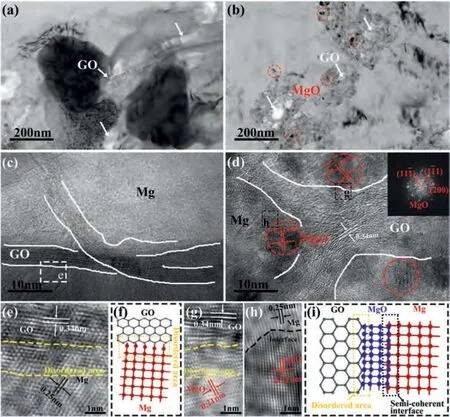
Fig.6.TEM images of as-rolled (a) GO/AZ31 composite,(b) GO-SnO2/AZ31 composite.HRTEM images of (c) GO/AZ31 composite,(d) GO-SnO2/AZ31 composite.(e) The interface between GO and Mg.(f) Schematic illustrate of the interface between GO and Mg observed in (e).(g) The interface between GO and MgO.(h) The interface between MgO and Mg.(i) Schematic illustrate of the GO-MgO and MgO-Mg interfaces observed in (g) and (h).
The edges of GOs with nanometer sized in the GO/AZ31 and GO-SnO2/AZ31 composites can be observed in Fig.6a and b (marked by white arrows),respectively.HRTEM image of GO/AZ31 composite (Fig.6c) shows that the GOs exhibit a curved multilayer structure.No interface product is found at the interface,indicating the simple mechanical bonding between GO and Mg (Fig.6c).The representative Inverse Fast Fourier transform(IFFT)image of the clean GO-Mg interface is shown in Fig.6e.A disordered area at the interface between GO and Mg is formed due to the difference of thermal expansion coefficient between GO and Mg [34,43].The schematic illusion corresponding to the GO-Mg interface is shown in Fig.6f.In Fig.6d,it can be seen that there are nanoparticles at the interface between GO and Mg matrix.The HRTEM and Fourier transform (FT) in Fig.6d can prove that these nanoparticles are MgO nanoparticles.The MgO nanoparticles with d-spacing values of 0.21 nm (corresponding to the(200) plane of MgO,marked as red line in Fig.6d) tightly contact with the GO in the GO-SnO2/AZ31 composite.The disordered area is also observed at the GO-MgO interface,as marked with the yellow dotted line in Fig.6g.The (002)plane of GO at the disordered area are screwy,indicating a strong internal stress.Fig.6h shows a clear and tight interface between MgO and Mg,which is semi-coherent one with strong interface adhesion [42,44].Based on the above TEM analysis,the schematic illustration of the GO-MgO-Mg interface is established in Fig.6i.These results indicate that thein-situMgO nanoparticles could tightly connect the GO and Mg matrix,thus effectively enhance the interfacial bonding strength and load-transfer efficiency.
3.3.Mechanical properties and strengthening mechanism
Fig.7a shows the representative engineering stress-strain curves (the loading direction parallel to the RD1) of AZ31alloy,GO/AZ31 and GO-SnO2/AZ31 composites.The values of yield stress (YS),ultimate tensile strength (UTS),elongation (EL) are listed in Table 2.The GO decorated with SnO2nanoparticles can significantly improve the mechanical properties of the AZ31 composites.Compared to the monolithic AZ31 matrix (YS=197 MPa,UTS=249 MPa),the YS and UTS for GO-SnO2/AZ31 composites are 263 and 308 MPa,which have been enhanced by 33.5% and 23.7%,respectively.The YS and UTS values of the GO-SnO2/AZ31 composite are 17.9%and 8.1%higher than those of GO/AZ31 composites (YS=223 MPa and UTS=285 MPa),revealing that the surface modification of GO would significantly improve its strengthening ability.It is well known that obtaining high strength and ductility at the same time is an expectation for research and development of new materials.However,the traditional preparation methods of GO (or graphene) reinforced metal matrix composites often improve the strength with the sacrifice of plasticity [34,45-47].The composites in current work not only improve the strength,but also maintain the elongation similiar to the AZ31 alloy(the EL of AZ31,GO/AZ31 and GO-SnO2/AZ31 composite are 13.0%,13.4% and 12.9%,respectively).This implies a good balance between strength and ductility.According to Considere’s criterion,larger uniform elongation means higher strain-hardening capacity,which would delay the onset of necking [48,49].Fig.7b shows the true stress-strain curves.Fig.c shows the strain-hardening rate (dσ/dε,whereσandεare the true stress and the true strain,respectively) vs true strain curves.Meanwhile,according to the true stress-strain curve,we calculated the strain hardening exponent of AZ31 alloy,GO/AZ31,and GO-SnO2/AZ31 composite,which are 0.141,0.182 and 0.193,respectively.The GO-SnO2/AZ31 composite exhibits a higher strain-hardening rate than that of GO/AZ31 and AZ31 throughout the entire probed strain range.
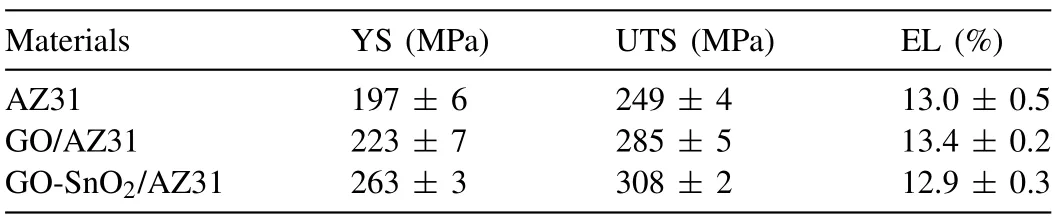
Table 2 Mechanical properties of AZ31,GO/AZ31 and GO-SnO2/AZ31 composites.
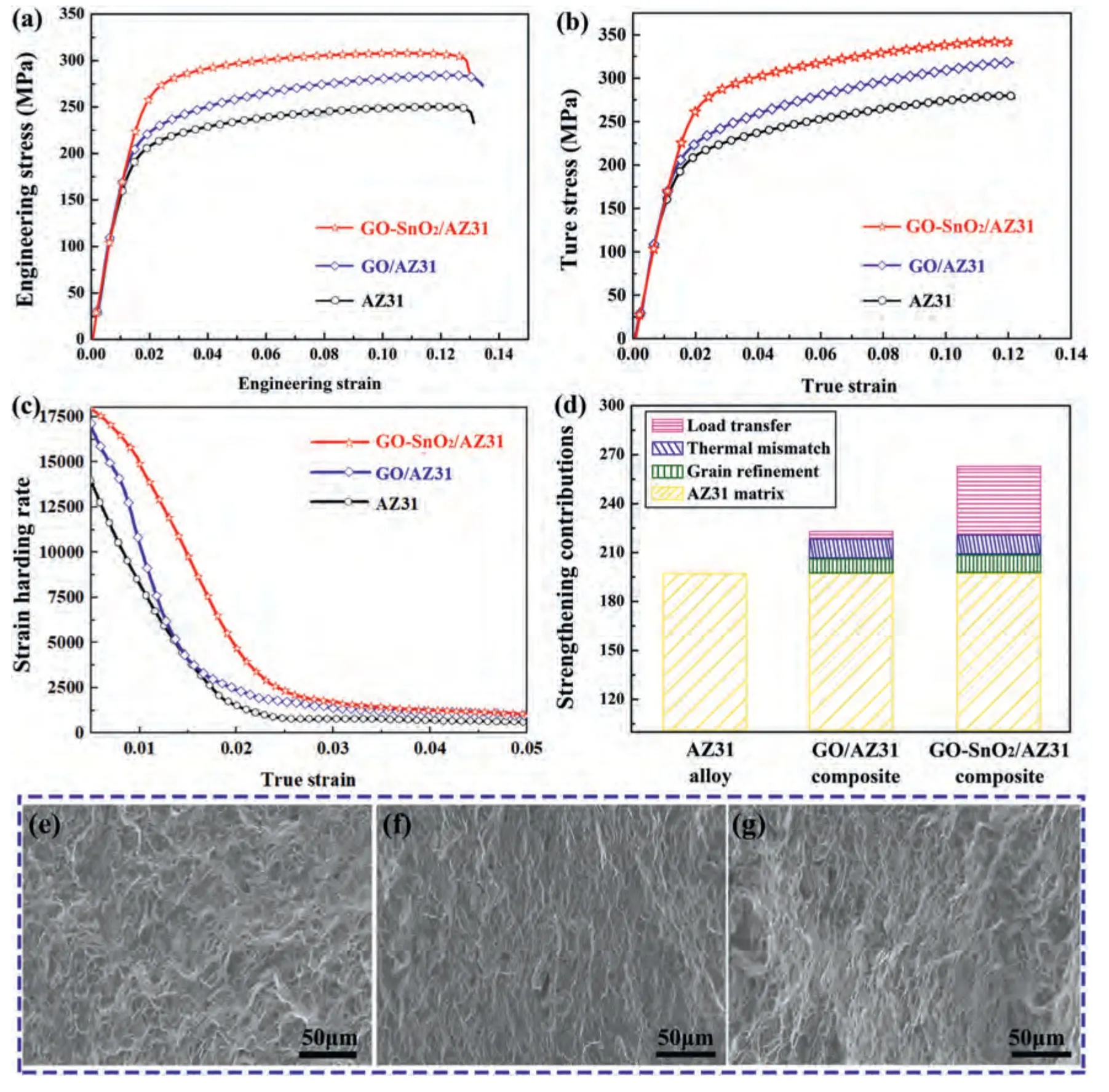
Fig.7.(a) Engineering stress-strain curves of AZ31,GO/AZ31,and GO-SnO2/AZ31 composites.(b) Ture stress-strain curves of AZ31,GO/AZ31,and GOSnO2/AZ31 composites.(c) Strain hardening rate vs true strain curves of AZ31,GO/AZ31 and GO-SnO2/AZ31 composite.(d) Theoretical calculations of yield strength contributions from each strengthening mechanism for AZ31,GO/AZ31 and GO-SnO2/AZ31 composit.Sample tensile fracture: (e) AZ31,(f)GO/AZ31,(g) GO-SnO2/AZ31.
A quantitative analysis is performed on the underlying strengthening mechanisms for yield strength of the composites(the calculation details are given in supplementary materials).The merits of different strengthening mechanisms are summarized in Fig.7d.The improvement of yield strength mainly benefits from the load transfer,which increases from 5 MPa for the GO/AZ31 to the 43 MPa for the GO-SnO2/AZ31 composite.This high load transfer should be ascribed to the significantly improved interfacial bonding due to thein situformation of MgO.
The fracture surfaces of the as-rolled samples are shown in Fig.7(e-g),from which typical plastic fracture features can be observed.All three samples exhibit numerous dimples on the fracture surface,and the size of these dimples is smaller and more uniform than that of the AZ31 alloy due to the refinement of grain size in the composite.
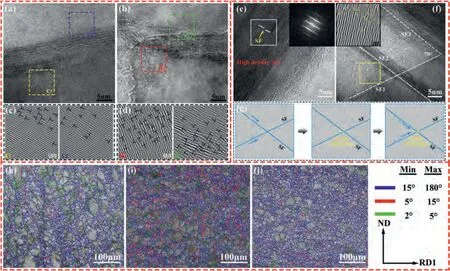
Fig.8.HRTEM images of (a) GO/AZ31 composite,(b) GO-SnO2/AZ31 composite.(c) and (d) are the corresponding IFFT image of the box in (a) and (b),respectively.(e) and (f) TEM micrograph shows the SFs in GO-SnO2/AZ31 composite.(g) Schematics showing SF interception and the sessile dislocation(at the intersection) block the glide of dislocations.Image quality (IQ) mappings with information on grain boundary misorientation angles of (h) AZ31,(i)GO/AZ31 composite,(j) GO-SnO2/AZ31 composite obtained from EBSD.
The good elongation of composite is related to its higher strain hardening rate,which is mainly resulted from dislocation accumulation [49–51].TEM examinations was carried out on the post-loading samples to observe the dislocation accumulation in the composites.The IFFT images from the four regions (marked by squares of c1,c2,and d1,d2) show the presence of lots of dislocations near the GO-Mg interface.The dislocation density near the GO-Mg interface in GO-SnO2/AZ31 composite (Fig.8b and d) is higher than that in GO/AZ31 composite (Fig.8a and c).Due to the improved interfacial bonding of GO-SnO2/AZ31 composite,the grain roation near the interface becomes harder.Hence,deformation of the Mg matrix around the GO is difficult to be coordinated by the grain rotation.Furthermore,the dislocations would accumulate at the interface [52].In addition,TEM image of GO-SnO2/AZ31 composite (Fig.8e and f) shows high-density stacking fault (SF) bands (the inserted FFT image shows superlattice types of diffraction spots arising from high-density SFs),which intersect to form a network structure with an angle of 70° (Fig.8f).This is because when SnO2coating reacts with molten Mg,it will not only generate MgO at the GO-Mg interface,but also generate elemental Sn.The solid solution of Sn atoms reduces the stacking fault energy of AZ31 matrix,resulting in SFs in the tensile process [53].The inset of Fig.8f shows the IFFT of the yellow rectangular region,revealing the existence of high density dislocations.During the deformation process,when a dislocation moves to the intersection of SF ribbons,it would transform to a sessile dislocation,as schematically illustrated in Fig.8g.With increasing deformation,the dislocations would pile up here and form a high-density dislocation region [53,54].The large angle GBs (with misorientation angles of larger than 15°,blue lines in Fig.8h-j) in the composites are more common than AZ31 alloy.Especially,the GO-SnO2/AZ31 composite has the most large angle GBs among the three samples.Large angle GBs are favor of the dislocation storage [55],so dislocations are easy to pile up in the GO-SnO2/AZ31 composite during the deformation process,which would result in high strain hardening rate (as shown in Fig.7c).
3.4.Electromagnetic interference shielding properties and mechanism
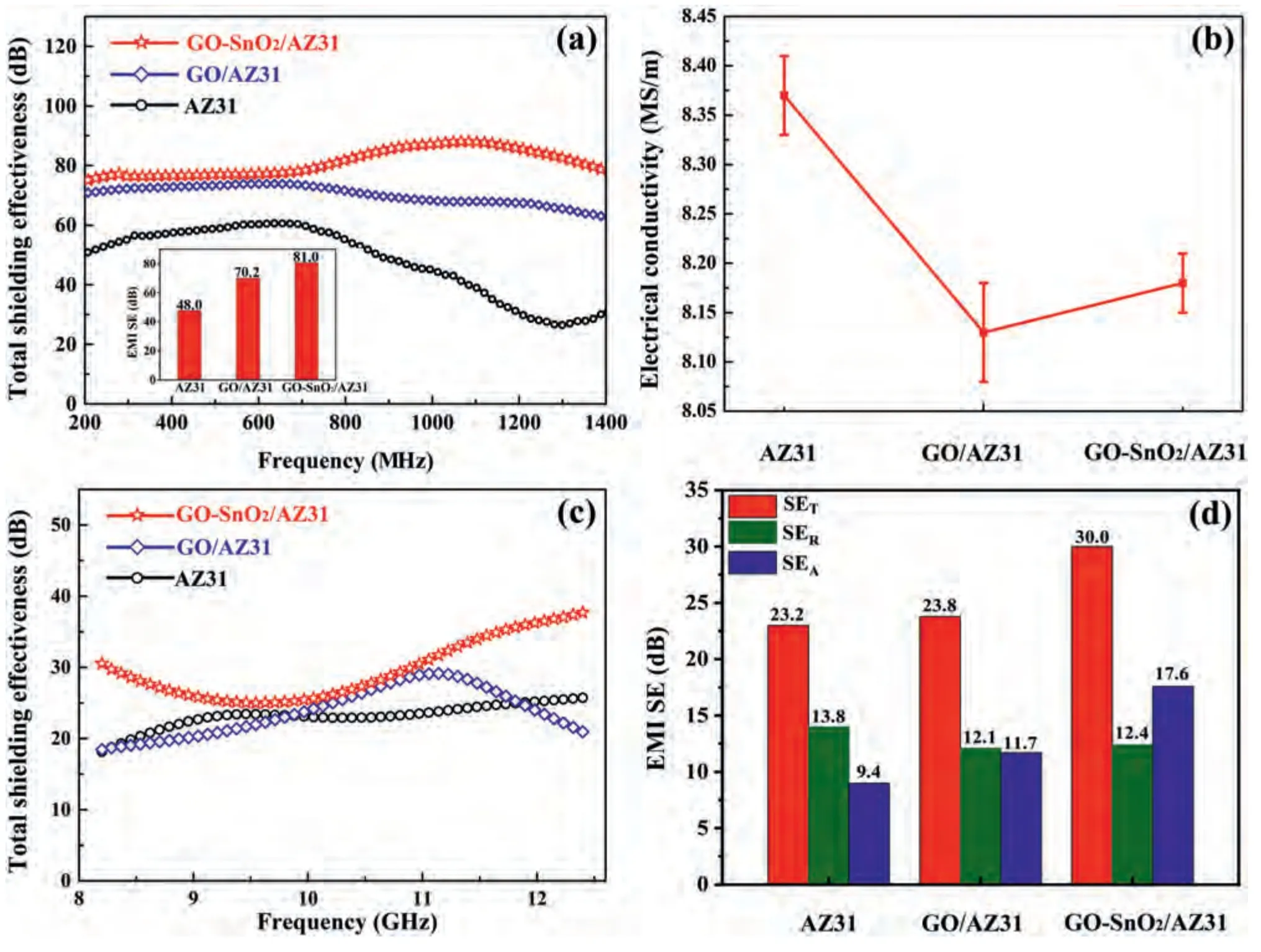
Fig.9.(a) Electromagnetic interference shielding properties of AZ31 and composites at 200–1400 MHz.(b) Electrical conductivity of AZ31 and composites.(c) Electromagnetic interference shielding properties of AZ31 and composites at X-band.(d) Average SET,SEA and SER values in the X-band of AZ31 and composites.
Fig.9a show the electromagnetic interference (EMI)shielding performances of AZ31 alloy and its composites in frequency range of 200∼1400 MHz.The total shielding effectiveness (SET) of shielding materials is sum of the reflection loss (SER) and absorption loss (SEA),the multiple reflection loss (SEMR) is included in the SEA[56].As shown in Fig.9a,in the range of 200∼1400 MHz,the composite shows better EMI shielding performance than AZ31 alloy,especially GOSnO2/AZ31 composite.At the same time,the introduction of GO also changed the trend of sample shielding effectiveness decreasing with the increase of frequency.The illustration in Fig.9a shows the average EMI SETvalues of samples.The EMI shielding efficiency of AZ31 alloy can be increased from 48.0 dB to 81.0 dB by adding a small amount of GO-SnO2(0.1 wt.%).It is reported that strong basal texture will improve the conductivity of Mg matrix and will help to improve the SETof materials by increasing SER.However,in the current work,the texture type and strength of alloy and composite are similar (Fig.4a-c),so there is also no obvious difference in conductivity (Fig.9b).This means that SERis not sensitive to the introduction of GO.In other words,the improvement of EMI shielding effectiveness of composite should be attributed to the absorption loss of composites to EMW.
In the X-band,the EMI shielding effectiveness of rolled samples (Fig.9c) shows that the GO-SnO2/AZ31 composite,similar to the results in the MHz band,still shows the best EMI shielding performance.Fig.9d shows the average SET,SERand SEAof the three samples in the X-band.The SERvalues of the three samples are greater than 10 dB,which means that more than 90% of the incident EMW is lost by reflection.From the perspective of power efficiency,the three samples are EMI shielding mechanisms based on reflection.However,the excellent EMI shielding performance of a metal material is determined by the SEAvalue (or SEA/SET) [57].After the introduction of GO,the SEAand SEA/SETvalues of AZ31 alloy increased from 9.4 dB,40.5%,11.7 dB,49.2%,to 17.6 dB,58%.Thus,the EMW incident into the material is greatly lost through absorption.Compared with reported magnesium-based composites,the GO-SnO2/AZ31 composite in this work has lower shielding performance in the X-band than the composite based on dual-phase Mg-Li alloy.However,as far as we know,rolled Mg–9Li alloys have an excellent SE but a relatively low ultimate tensile strength (UTS) of<170 MPa [11,13],which is insufficient to meet the demands of high-end devices.In addition,compared with the reported graphene reinforced magnesium matrixed composites,the SE value per unit thickness of the GO-SnO2/AZ31 composite in the current work shows certain advantages [58].Therefore,it can be demonstrated that the regulation of the interface structure is an effective means to improve the electromagnetic shielding performance of magnesium matrixed composites.
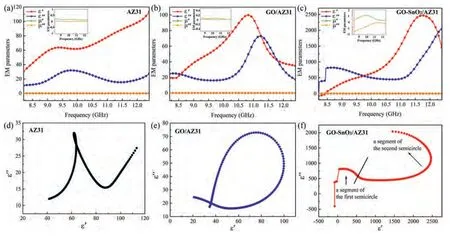
Fig.10.Complex permittivity and complex permeability of (a) AZ31,(b) GO/AZ31,(c) GO-SnO2/AZ31.Cole–Cole plots of (d) AZ31,(e) GO/AZ31,(f)GO-SnO2/AZ31.
The relative complex permittivity (εr=ε´-jε") and relative complex permeability (μr=μ´-jμ") are shown in Fig.10(a–c).In general,the real parts and imaginary parts of electromagnetic parameters are on behalf of the EMW energy shortage and dissipation,respectively [59–61].For the AZ31 and two composites,the real parts (μ´) and the imaginary parts (μ") of permeability keep a low value (about 0)over the whole measured frequency range,reflecting the nonmagnetic nature.Therefore,the EMI shielding performance of all samples are mainly related to dielectric properties.In the GO/AZ31 composite,the value ofε′is similar to that of AZ31 alloy,whileε′′is improved in the test range,which indicates that the GO/AZ31 composite would have stronger EMW dissipation.Surprisingly,the GO-SnO2/AZ31 composite exhibits one order of magnitude higherε′andε′′than the AZ31 alloy and GO/AZ31 composite.We think this significant difference should be caused by the change of interface structure.Largeε′andε′′in the GO-SnO2/AZ31 composite are ascribed to the dipole orientation polarization and interfacial polarization.The defects and functional groups in GO would strengthen the dipole orientation polarization [62].Thein-situMgO nanoparticles improve the interfacial bonding between GO and Mg and provide abundant phase interface to enhance the interfacial polarization [63].
According to the Debye theory,the plot ofε′versusε′′would form either one or several semicircles,generally referred to as Cole–Cole semicircles,with each semicircle associated with one relaxation process [64].Fig.10(d-f) shows Cole-Cole plots of AZ31,GO/AZ31 and GO-SnO2/AZ31 composite,respectively.No obvious relaxation process is found for the AZ31 alloy.One semicircle segment is shown in the Cole-Cole plots of GO/AZ31 composite,indicating only one relaxation process (Fig.10b).While,there are two semicircular segments in the Cole–Cole plots of GO-SnO2/AZ31 composite,which indicates that there would be two relaxation processes (Fig.10f).The relaxation in GO/AZ31 composite should be caused by the dipole orientation polarization of defects or functional groups in GO,and the two relaxation processes in GO-SnO2/AZ31 composite should be caused by dipole orientation polarization and interface polarization respectively.Since both dipole orientation polarization and interface polarization usually occur at higher frequencies,the EMI shielding performance of the GO-SnO2/AZ31 composite would exhibit significant improvement at high frequencies,as shown in Fig.9a and c.
Crystal defects,such as dislocations,grain boundaries,electronic defects and phase boundaries,can contribute to the interfacial polarization [59].The formation of nano MgO increases the dislocation density at the interface of the composites (as shown in Fig.8b and d).Also,it provides more abundant phase interfaces.In order to obtain more detailed defect information,geometric phase analysis (GPA) has been applied to qualitatively characterize the lattice strain fields from HRTEM images(Fig.11a-f)[65,66].The corresponding strain maps exhibit many spots with different color contrasts,which are ascribed to lattice distortion in the composites.Compared with those of GO/AZ31 composite (Fig.11a-c),the strain maps of GO-SnO2/AZ31 composite show more spot numbers and stronger spot brightness and contrast(Fig.11d-f).It could be inferred that the existence of the GO-MgO-Mg heterogeneous interface induces more intensive lattice distortion cores to generate more lattice defects.These defects or lattice distortions could produce more sufficient carriers to strengthen the uneven distribution and accumulation of spatial charges in the heterojunction contact,so as to promote interface polarization and consume EM energy [67].
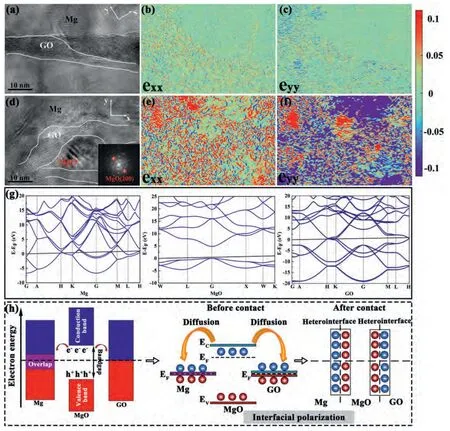
Fig.11.HRTEM images of (a) GO/AZ31,(d) GO-SnO2/AZ31 and the corresponding dislocation strain maps of (b,c) GO/AZ31,(e,f) GO-SnO2/AZ31.(g)band structure of Mg,MgO and GO.(h) charge transfer schematic diagram of the three substances at the GO-MgO-Mg heterojunction.
In the GO-SnO2/AZ31 composite,the presence of heterogeneous interfaces could effectively encourage the accumulation and uneven distribution of charges in the phase boundary of Mg,MgO and GO.To further confirm the uneven distribution of positive and negative charges,first-principles calculations of Mg,MgO and GO were carried out based on density functional theory (DFT).Fig.11g presents the band structure of Mg,MgO and GO,which shows a significant difference in the band structure of the three substances.Heterojunction will be formed when two substances contact each other [68].Furthermore,the difference of energy band would promote the migration and diffusion of the carrier from one substance to another [69],resulting in spatial separation of positive and negative charges.Fig.11h shows the charge transfer diagram of the three substances at the GO-MgO-Mg heterojunction,where the positive charge accumulates on one side of MgO and the corresponding negative charge tends to gather on the Mg and GO side.Therefore,EM energy could be effectively consumed in the dynamic balance of the polarization process caused by the alternating EM field [65].
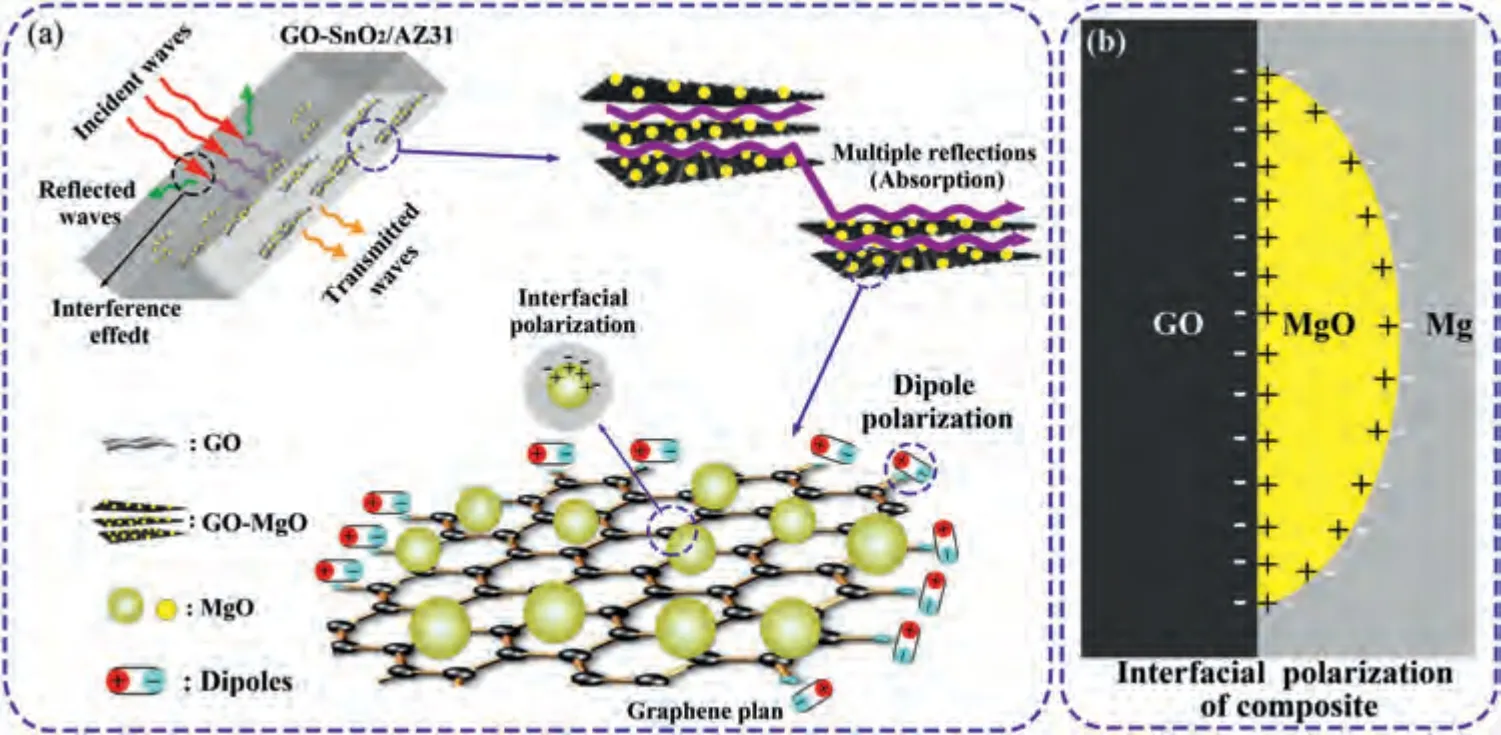
Fig.12.(a) Schematic diagram of EMI shielding mechanism of GO-SnO2/AZ31 composites.(b) Interface polarization diagram of GO-SnO2/AZ31 composites.
As a summary of all above analysis,the EMI shielding mechanism of GO-SnO2/AZ31 composite is illustrated in Fig.12a.Firstly,the incident EMW would be reflected on the surface of the composite,and the amount of reflected EMW would be mainly influenced by the conductivity.Secondly,due to the large surface area and ordered alignment of the GO,prominent absorptions are expected due to the multiple internal reflections of the EMW between the massive parallel interfaces inside the composites.Thirdly,the defects and functional groups in GO would cause dipole orientation polarization and consume EMW.More importantly,interfacial polarization would occur when charges accumulate at the GOMgO-Mg boundary.Charges transfer and accumulation are expected on the GO-MgO and MgO-Mg interfaces as shown Fig.12b.The assembled movements of collective interfacial dipoles at these interfaces would amplify the response to the incoming electromagnetic field,furthermore enhance EMW absorption performance.
4.Conclusions
In summary,the mechanical properties and EMI shielding properties of Mg matrix composites are simultaneously improved through an interface design strategy.GO reinforced Mg matrix composites were prepared by a simple stirring casting combined with hot rolling.The GO renforcement was modified by pre-coated SnO2.Thein-situMgO nanoparticles were formed at the Mg-GO interface through the reaction between the SnO2on GO and molten Mg,and the Sn was disolved in the Mg alloy matrix,The interface bonding between GO reinforcement and AZ31 matrix was significant improved due to thein-situMgO nanoparticls.Lots of SFs were generated during the deformation due to the solid solution of Sn.The dislocation storage ability in Mg grains is considerably improved through this interface design strategy,leading to a remarkably enhanced strain-hardening capacity.In addition,the directional arrangement and the huge specific surface area of GO increase the multiple reflection of electromagnetic wave in the composite,and nano MgO at the interface promoting the interface polarization.Thus,the GO-SnO2/AZ31 composite exhibits outstanding EMI shielding performance.
Declaration of competing interest
J.Tan is an editorial board member for Journal of Magnesium and Alloys and was not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Acknowledgments
The authors gratefully acknowledge financial support provided by the National Natural Science Foundation of China(No.52174357),Fundamental Research Funds for the Central Universities (No.DUT21LAB132).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2023.07.001.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Magnesium and its alloys for better future
——The 10th anniversary of journal of magnesium and alloys - Magnesium alloys in tumor treatment: Current research status,challenges and future prospects
- Twin-solute,twin-dislocation and twin-twin interactions in magnesium
- Recent advances on grain refinement of magnesium rare-earth alloys during the whole casting processes: A review
- Magnesium research in Canada: Highlights of the last two decades
- Structure-function integrated magnesium alloys and their composites
