Experimental and theoretical studies on two-dimensional vanadium carbide hybrid nanomaterials derived from V4AlC3 as excellent catalyst for MgH2
2023-12-27ZhiqingLnHuirenLingXiobinWenJiyngHuHuNingLingZengHizhenLiuJunTnrgenEckertJinGuo
Zhiqing Ln ,Huiren Ling ,Xiobin Wen ,Jiyng Hu ,Hu Ning ,Ling Zeng ,Hizhen Liu,Jun Tn,Jürgen Eckert,Jin Guo,∗
a Guangxi Novel Battery Materials Research Center of Engineering Technology, Guangxi Colleges and Universities Key Laboratory of Novel Energy Materials and Related Technology, School of Physical Science and Technology, Guangxi University, Nanning 530004, PR China
b College of mathematics and Physics, Guangxi Minzu University, Nanning 530006, PR China
c College of Materials Science and Engineering, Chongqing University, Chongqing 400044, PR China
d Erich Schmid Institute of Materials Science, Austrian Academy of Sciences, Jahnstraße 12, Leoben A-8700, Austria
e Department Materials Physics, Montanuniversität Leoben, Jahnstraße 12, Leoben A-8700, Austria
fNational University of Science and Technology «MISiS», LeninskyProsp., 4, Moscow 119049, Russia
Abstract Hydrogen is considered one of the most ideal future energy carriers.The safe storage and convenient transportation of hydrogen are key factors for the utilization of hydrogen energy.In the current investigation,two-dimensional vanadium carbide (VC) was prepared by an etching method using V4AlC3 as a precursor and then employed to enhance the hydrogen storage properties of MgH2.The studied results indicate that VC-doped MgH2 can absorb hydrogen at room temperature and release hydrogen at 170 °C.Moreover,it absorbs 5.0 wt.%of H2 within 9.8 min at 100 °C and desorbs 5.0 wt.% of H2 within 3.2 min at 300 °C.The dehydrogenation apparent activation energy of VC-doped MgH2 is 89.3 ± 2.8 kJ/mol,which is far lower than that of additive-free MgH2 (138.5 ± 2.4 kJ/mol),respectively.Ab-initio simulations showed that VC can stretch Mg-H bonds and make the Mg-H bonds easier to break,which is responsible for the decrease of dehydrogenation temperature and conducive to accelerating the diffusion rate of hydrogen atoms,thus,the hydrogen storage properties of MgH2 are remarkable improved through addition of VC.
Keywords: MgH2;Two-dimensional;Hydrogen storage material;Density functional theory.
1.Introduction
With the increasing demand for energy and the need to decrease the use of conventional energy sources,the development and utilization of renewable energy becomes extremely important.Another aspect is the environmental pollution due to the use of traditional energy sources,so it is urgent to develop clean and efficient energy systems.Hydrogen is one kind of efficient and clean energy carriers,which has attracted extensive worldwide attention in recent years [1–3].One of the key technologies for hydrogen energy development and utilization is how to storage hydrogen safely.Magnesium hydride(MgH2)is one of the most suitable candidates for hydrogen storage materials because of its high gravimetric energy density.Unfortunately,the relatively high operating temperature and the slow de/hydrogenation rate hinder its extensive industrial application.Therefore,the hydrogen storage properties of MgH2need to be further improved to overcome these drawbacks.
One of the effective ways for reducing the de/hydrogenation temperature and improving the de/hydrogenation rate of MgH2is the introduction of catalysts,such as transition metals [4–9],intermetallic compounds[10–12],transition metals oxides [13–18],and carbon materials [19–26].Zhang et al.[4] introduced Fe into MgH2by wet-chemical ball milling and found that the onset temperature of desorption and the apparent activation energy of dehydrogenation of MgH2+5 wt.% Fe were 182.1 °C and 40.7 ± 1.0 kJ/mol,respectively,thus being far lower than the values for pure MgH2.Korablov et al.[6] reported that the activation energy of Ti-doped MgH2is 53.6 kJ/mol and the investigated 0.75Mg-0.25Ti composite can absorb hydrogen at room temperature.Yang et al.[10] used FeCo nanosheets to enhance the hydrogen storage properties of MgH2and observed that FeCo-containing MgH2can rapidly uptake 6.7 wt.% of H2within one minute at 300 °C.The hydrogenation and dehydrogenation activation energies of FeCo-containing MgH2were reduced to 65.3 ± 4.7 kJ/mol and 53.4 ± 1.0 kJ/mol,respectively.Transition metal oxides have also been proved to be promising alternatives for improving the hydrogen storage properties of MgH2.Wang et al.[14] doped 9.0 wt.% of V2O3@C in MgH2by mechanical milling and demonstrated that the presence of V weakens the strength of Mg–H bonds in MgH2;thus,the hydrogenation and dehydrogenation temperatures of V-catalyzed MgH2are strongly reduced.Valentoni et al.[17] reported that VNbO5-doped MgH2can adsorb more than 5 wt.% of H2within five minutes at 160 °C and its hydrogen storage capacity does not decline even after 70 hydrogen absorption-desorption cycles.It has also been shown that carbon materials can significantly improve the thermodynamic and kinetic properties of MgH2.For example,Liu et al.[21] introduced one-dimensional bamboo-shaped carbon nanotubes (BCNTs)with a high specific surface area to improve the integrated hydrogen storage properties of MgH2and found that the dehydrogenation activation energy (97.97 kJ/mol) and enthalpy (68.92 kJ/mol) of MgH2@BCNTs were reduced by 111.24 kJ/mol and 6.07 kJ/mol as compared to those of pristine MgH2,respectively.Zhang et al.[25] noticed that the hydrogen absorption/desorption rates of TiO2@C-containing MgH2are significantly faster than those of pristine MgH2and the Mg-H bond strength weakens under the catalytic action of TiO2@C.The above results imply that although the operating temperature and hydrogen storage kinetics of MgH2can be significantly improved by adding catalysts,this approach still cannot meet the requirements of practical applications.
In recent years,two-dimensional (2D) materials were used as catalysts to improve the hydrogen storage properties of MgH2.Liu et al.[27] used two-dimensional Nb4C3Txto enhance the hydrogen storage properties of MgH2and asserted that the hydrogen storage kinetics and thermodynamics of MgH2are improved by the unique layered structure ofin-situ-formed NbHx.Wang et al.[28] prepared NbTi nanocrystals using a NbTiC solid-solution as precursor and revealed that NbTi-containing MgH2starts to release H2at 195 °C and desorbs about 5.8 wt.% of H2within 30 min at 250 °C.Liu et al.[29] found that 5 wt.%Ti3C2-containing MgH2has excellent hydrogen storage kinetics and can uptake 6.1 wt.% of H2within 30 s at 150 °C.Furthermore,the hydrogenated samples can absorb hydrogen at room temperature.In the periodic table,V and Ti are neighboring elements;thus,they have similar electronic structures and also manifest some similar catalytic effects on the hydrogen storage performance of MgH2.Theoretical calculations have revealed that the heat of formation for dehydrogenated V-containing MgH2is -43.42 kJ/mol,which is 7.85 kJ/mol lower than that of Ti-containing MgH2[30].da Conceição et.al.[31] studied results indicated that VC could enhance the hydrogen absorption and desorption kinetics properties of Mg,and a desorption rate of 1.0 × 10-2wt.%s-1at 300°C was obtained for VC-catalyzed Mg system.In our previous study [32],it was found that the hydrogen storage properties of V-doped Mg-Al alloys were better than those of the Ti-doped samples.It was also reported that the catalytic effect of V-based compounds on the hydrogen storage performance of Mg-based alloys is superior to that of single V [33].Therefore,it can be speculated that V-based 2D materials might have some unexpected catalytic effects on the hydrogen storage properties of MgH2.Hence,in this research,VC was synthesized using V4AlC3as a precursor and employed to improve the hydrogen storage performance of MgH2.
2.Experimental section
2.1.Synthesis of VC and MgH2-VC composites
V4AlC3(1.5 g) (purity≥98%,Beijing Beke New Material Technology Co.,LTD) was slowly poured into 40 ml of 40% HF (purity≥99%,Aladdin) under magnetic stirring for 96 h at 55 °C.The resulting product was washed with deionized water more than three times until the pH value became≥6.Finally,VC was obtained by drying the washed sample for 24 h in a frozen drying oven.Subsequently,10 wt.% of VC was incorporated into commercial MgH2(purity≥98%,Langfang Bede Trading Co.,LTD) by milling a mixture of MgH2and 10 wt.% of VC for five hours at a milling speed of 500 rpm (ball-to-power weight ratio=40:1) in an Ar atmosphere;the product was named MgH2-VC.
2.2.Characterization
The phase compositions of the samples were characterized by X-ray diffraction (XRD;Miniflex 600,Rigaku) under Cu-Kαradiation at 40 kV and 200 mA with a scanning step size of 5°/min.The micromorphologies of the samples were examined by field-emission scanning electron microscopy(FE-SEM;SU8020,HITACHI) and transmission electron microscopy (TEM;FEI Tecnai G2,f20 s-twin 200 kV).The distributions of V and C in the VC-doped samples were characterized by energy-dispersive X-ray spectrometry (EDS)coupled with SEM and TEM.The surface chemical bonding structures of the samples were characterized by an Xray photoelectron spectroscope (XPS;ESCALAB 250Xi Microprobe),and binding energy spectra were fitted via XPSPEAK41 software.The de/hydrogenation performances of the samples were analyzed by a Sievert-type device.The activated samples were heated from room temperature to 380 °C at a rate of 1 °C·min-1under a hydrogen pressure of 6 MPa during hydrogenation and at a rate of 0.5 °C·min-1under a hydrogen pressure of 0.01 MPa during dehydrogenation.The dehydrogenation and hydrogenation kinetics of the samples were determined at different temperatures under a hydrogen pressure of 6 MPa and a vacuum pressure of 0.01 bar,respectively.
2.3.Computational methods
Dehydrogenation simulations were performed with the Vienna Ab Initio Simulation Package (VASP) [34–36].The interaction between electrons and ions and the exchangecorrelation effect were analyzed using the projector augmented wave (PAW) method of Blöchl [37] and the Perdew-Burke-Ernzerhof (PBE) functional [38] under generalized gradient approximation (GGA) [39],respectively.The cutoff energy of the plane wave basis set was set to 500 eV.The convergence criteria for the Hellmann–Feynman force and the total energy were 1 × 10-2eV/˚A and 1 × 10-4eV/atom,respectively.The dipole correction along the surface normal was also considered.The Monkhorst-Pack method with 5 × 5 × 1 k-point meshes was employed for the dehydrogenation of Mg4H8clusters on the VC (100) surface.
3.Results and discussion
Fig.1 displays a XRD pattern and a SEM image of VC,and SEM-EDS images of VC-doped MgH2.It is noticeable that Al in V4AlC3was corroded in 40% HF (Fig.1a),implying the successful synthesis of VC.The sharp diffraction peaks at 2θ=37.4°,43.4°,63.1°,75.6°,and 79.7° (Fig.1a)correspond to the (111),(200),(220),(311),and (222) lattice planes of VC (JCPDS card no.74–1220).An additional diffraction peak appears at low-angles stemming from the(002) lattice plane of the graphite nitrate (JCSDS,card No.742330).The high-resolution V 2p and C 1 s XPS spectra of VC are presented in Fig.1b and 1c,respectively.The binding energies of 520.7 eV (V 2p3/2) and 513.3 eV (V 2p1/2)with an energy gap of 7.4 eV can be assigned to V-C bonds,and the peak at 522.4 eV (V 2p1/2) and 514.9 eV (V 2p3/2)correspond to the V-Txbond (Tx=–O,OH,and–F) [40,41].The peaks at 282.3 eV,284.8 eV,286.2 eV,and 288.6 eV are due to C-V,C-C,C-O and O-C=O bonds (Fig.1d),respectively [41,42].The microtopography of VC is presented in Fig.1(d–f).Apparently,VC possesses an accordion-like layered structure.These results suggest that VC was successfully synthesized using V4AlC3as a precursor.After VC was introduced into MgH2sample,numerous small particles attached to the large particles in VC-doped MgH2composite(Fig.1g),and EDS mapping image indicates that VC had been dispersed in MgH2after ball milling (Fig.1h).
The microstructure of 2D VC was further examined by TEM,HRTEM,SAED,and EDS.The TEM image in Fig.2a confirms the synthesis of nanoscale VC.The distance between crystal faces in VC was calculated as 0.208 nm (Fig.2b),which corresponds to the (200) lattice plane of VC.The SAED pattern in Fig.2c reveals diffraction rings from the(111),(200),(220),(311),and (222) lattice planes of VC.The EDS mapping image indicates that V and C are homogeneously distributed in VC.The above results suggest that VC was successfully prepared by the etching method using V4AlC3as a precursor.Moreover,Fig.2g reveals that many small particles clustered around the large particles in VCdoped MgH2composites,which is consistent with the observation from Fig.1g.In Fig.1g,the size of the large particles is in the range of 1.0 μm∼10.0 μm,the small ones are at the submicron level.In Fig.2g,the grains are about 200 nm in size,and the small ones are about 50 nm.The distances between crystal faces of 0.219 nm and 0.210 nm can be assigned to the(111)plane of MgH2and the(200)plane of VC,respectively.In addition,diffraction rings associated with the(002),(101),(212) planes of MgH2and the (200) plane of VC are visible in Fig.2i.Therefore,the SEM-EDS,HRTEM and SAED results are in good agreement with the XRD observations,suggesting that VC and MgH2were well mixed during the preparation process.
Fig.3(a,b) display the isothermal and non-isothermal hydrogenation and dehydrogenation curves of MgH2and VCdoped MgH2.MgH2and VC-doped MgH2can absorb approximately 6.98 wt.% and 6.42 wt.% of hydrogen,respectively,when heated from room temperature to 350 °C.Undoped MgH2can hardly absorb hydrogen until the temperature reaches 125 °C.However,dehydrogenated VC-doped MgH2can absorb hydrogen even at room temperature,and its hydrogen absorption capacity is about 5.0 wt.% when the temperature reaches 150°C.It was found that undoped MgH2and VC-doped MgH2release about 6.95 wt.% and 5.75 wt.%of hydrogen,respectively,when heated from room temperature to 400 °C.However,the initial dehydrogenation temperatures of these two samples are different.Undoped MgH2cannot release hydrogen until the temperature reaches 320°C.The onset dehydrogenation temperature of VC-doped MgH2significantly decreases after the incorporation of VC,and it starts to release hydrogen at 170 °C,which is 150 °C lower than for undoped MgH2.The operating temperature of VC-doped MgH2is also found to be lower than those of TiO2@C-doped MgH2[43],FeB@CNTs-doped MgH2[44],SrTiO3-doped MgH2[45],and other materials [46,47].To investigate the catalytic effect of VC on the hydrogen absorption/desorption kinetics of MgH2,the isothermal hydrogen absorption/desorption kinetics of undoped and VC-doped MgH2were analyzed at various temperatures (Fig.3(c–f)).Undoped MgH2can absorb approximately 4.0 wt.% of H2within 180 min at 175 °C and 6.0 wt.% of H2within 180 min at 200 °C,respectively,and its hydrogen absorption capacity increases to about 6.2 wt.% for temperatures beyond 225 °C.In contrast,VC-doped MgH2exhibits excellent hydrogenation properties and can absorb about 3.0 wt.% of H2at 25 °C within 180 min,and its hydrogen absorption capacity increases as the temperature increases.It can absorb approximately 4.0 wt.% of H2within 180 min at 50 °C,and the hydrogen absorption capacity increases to about 5.5 wt.%when the temperature increases above 100 °C.In particular,VC-doped MgH2can absorb 5.0 wt.% of H2within 9.8 min at 100 °C.Hence,the catalytic effect of VC on the hydrogen absorption performance of MgH2is superior to that of Ni-V [48],NiS [49],Ni@rGO [5],Fe3S4[50],and Co@C[51].For example,Mg-Ni-V [48],Mg-5 wt.%NiS [49],and MgH2–Co@C [51] can only absorb 1.0 wt.%,3.5 wt.%,and 2.71 wt.% of hydrogen within 10 min at 100 °C,respectively.During dehydrogenation,undoped MgH2can desorb approximately 7.0 wt.% of hydrogen at 375 °C at a hydrogen desorption rate of 1.06 ± 0.02 wt.%/min,and the hydrogen desorption rate decreases to 0.42 ± 0.04 wt.%/min and 0.13 ± 0.01 wt.%/min at 350 °C and 325 °C,respectively.VC-doped MgH2can release approximately 6.0 wt.% of hydrogen at 325 °C at a hydrogen desorption rate of 2.18±0.03 wt.%/min,which is 16.8 times faster than what was found for undoped MgH2under the same conditions.The dehydrogenation rates of VC-doped MgH2at 300 °C,275 °C,and 250°C are 1.32±0.02 wt.%/min,0.54±0.02 wt.%/min,and 0.26 ± 0.04 wt.%/min,respectively;thus,desorption proceeds even faster than for undoped MgH2at 325 °C(0.13 ± 0.01 wt.%/min).At 300 °C,VC-doped MgH2can desorb 5.0 wt% of H2within only 3.2 min.The dehydrogenation kinetics of VC-doped MgH2are also better than those of Mg-Ni-V [48],Mg-5 wt.%NiS [49],and MgH2-Co@C [51].Therefore,both the hydrogenation/dehydrogenation properties and hydrogen absorption/desorption kinetics of MgH2are significantly improved after the addition of VC.
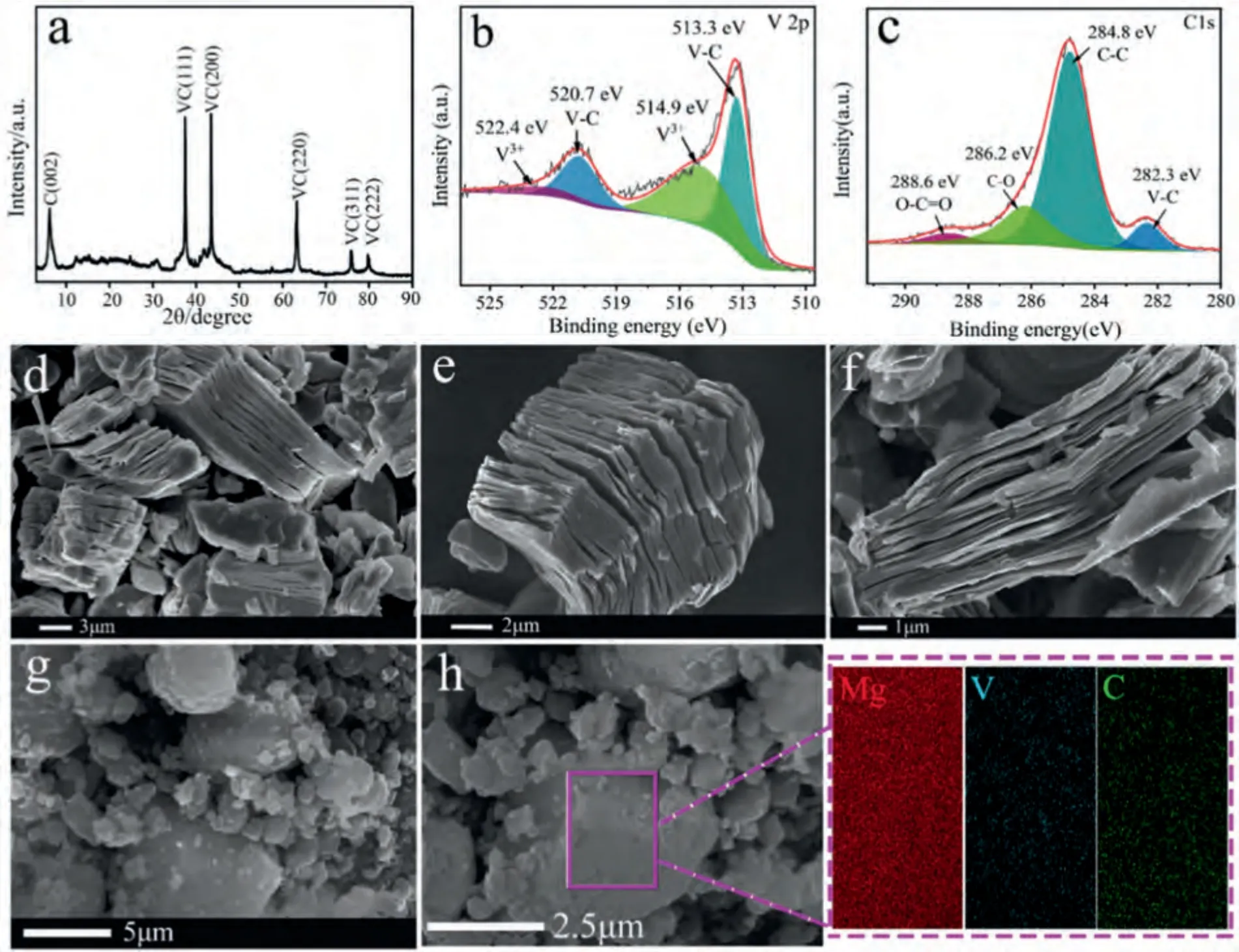
Fig.1.(a) XRD pattern,(b) V 2p XPS spectra,(c) C 1 s XPS spectra,and (d∼f) FE-SEM image of VC,and (g) FE-SEM image,(h) SEM-EDS image of VC-doped MgH2.
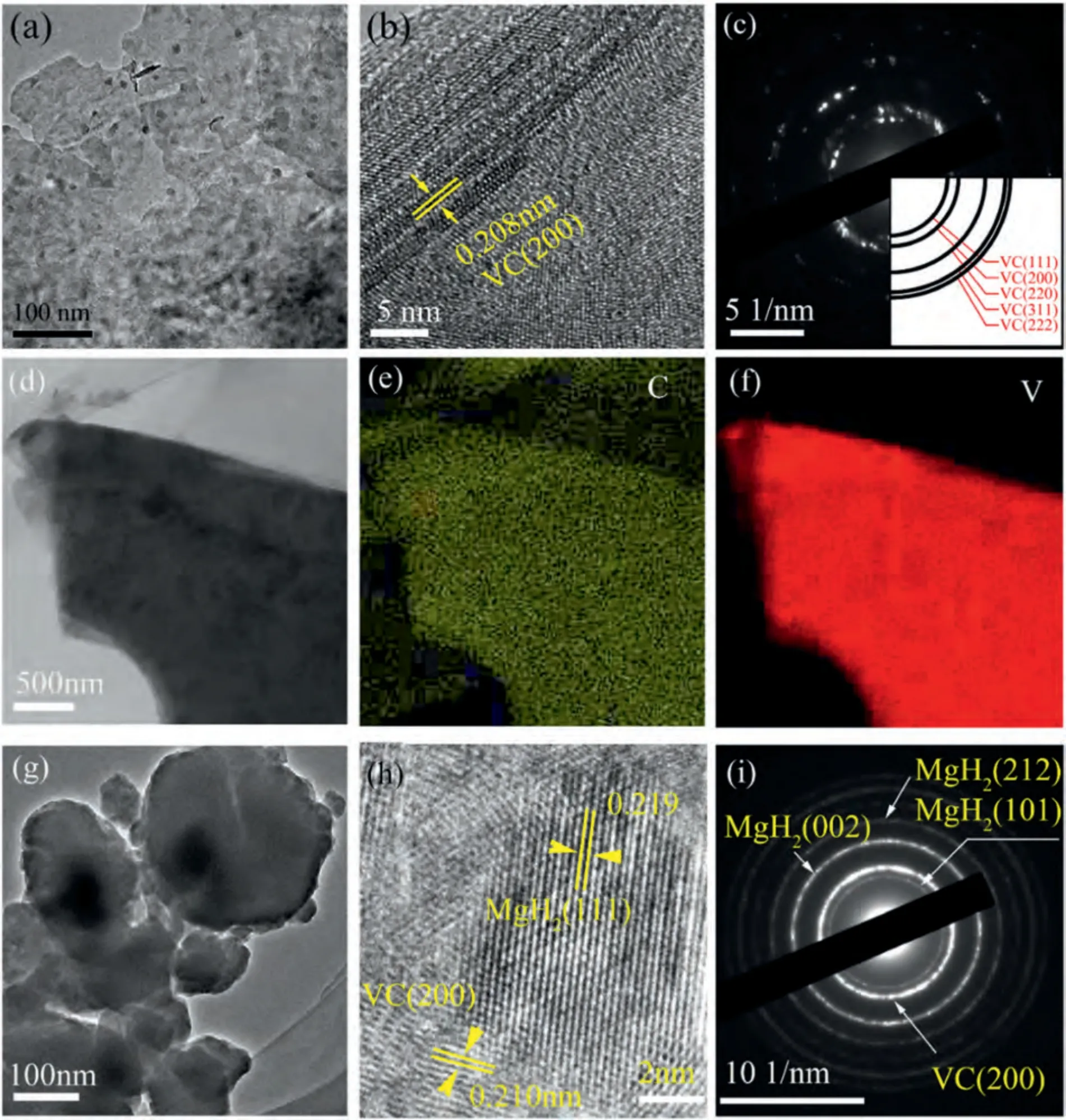
Fig.2.(a) TEM image,(b) HRTEM image,(c) SAED pattern,and (d–f) EDS patterns of VC,(g) TEM image,(h) HRTEM image,and (i) SAED pattern of VC-doped MgH2.
The apparent activation energy is an important parameter to evaluate the hydrogen adsorption and desorption kinetics of materials.It can be calculated by the Johnson-Mehl-Avrami (JMA) equation (ln [-ln(1-α)]=nlnk+nlnt,whereα,k,tandnare the phase transformation fraction,a temperature-dependent kinetic parameter,the reaction time and the order of the reaction,respectively [52]).According to the slopes of the lnk∼1000/T plots shown in Fig.3(g,h),the hydrogenation apparent activation energy (Eabs) values of VC-doped MgH2and undoped MgH2were calculated as 42.4 ± 1.4 kJ/mol and 77.3 ± 3.0 kJ/mol,respectively.In addition,the dehydrogenation apparent activation energy (Edes)values of VC-doped MgH2(89.3 ± 2.8 kJ/mol) and undoped MgH2(138.5 ± 2.4 kJ/mol) were also obtained from the linear relationship of the lnkversus 1000/T plots,revealing that the apparent activation energy for hydrogenation and dehydrogenation of MgH2is significantly reduced after the addition of VC.Thus,VC is an efficient catalyst to improve the hydrogen absorption/desorption properties of MgH2.Table 1 represents the empirical dehydrogenation apparent activation energies of catalyzed common MgH2systems.It can be seen from the table that the dehydrogenation activation energy of oxides or Nb-based compounds as catalysts was higher than that of VC as catalysts.For example,the dehydrogenation activation energy of VNbO5-catalyzed (99.0 kJ/mol) [17],KNbO3-catalyzed (93.6 kJ/mol) [54],NbN-catalyzed (113.9 kJ/mol)[55],MnFe2O4-catalyzed (108.4 kJ/mol) [59] MgH2was 9.7 kJ/mol,4.3 kJ/mol,24.6 kJ/mol and 19.1 kJ/mol lower than that of VC-catalyzed MgH2.In addition,the dehydrogenation activation energy of VC-catalyzed MgH2system also lower than those of K2SiF6-catalyzed,Ni@pCNF-catalyzed,HfCl4-catalyzed and some others catalyzed MgH2systems listing in Table 1.Obviously,the introduction of VC into MgH2remarkably lowered the hydrogen desorption energy barrier.

Fig.3.(a,b) Non-isothermal and (c–f) isothermal hydrogenation and dehydrogenation curves,and (g,h) 1000/T∼lnK plots of MgH2 and VC-doped MgH2,respectively.
To further analyze the hydrogen storage properties of MgH2and VC-doped MgH2,pressure-composition-isotherm(PCI) measurements were performed at various temperatures (Fig.4a and c).The hydrogen absorption and desorption plateau increases with the increasing temperature.Undoped MgH2causes completely reversible hydrogen absorption/desorption at 350,375,and 400 °C;however,it cannot undergo a reversible hydrogen storage process at 325 °C.The reversible hydrogen storage capacity of undoped MgH2is approximately 6.7 wt%.After doping with 10 wt.% of VC,although the reversible hydrogen storage capacity of VC-doped MgH2is reduced to about 5.8 wt.%,completely reversible hydrogenation/dehydrogenation is observed at 300,325,350,and 375 °C.Moreover,the reversible hydrogenation/dehydrogenation temperatures for MgH2remarkably decrease after the addition of VC.The hydrogenation/dehydrogenation enthalpies of undoped and VC-doped MgH2samples were estimated by the Van’t Hoff equation (lnP=-,wherePis the hydrogenation/dehydrogenation plateau pressure,Ris the universal gas constant,ΔHis the reaction enthalpy,ΔSis the reaction entropy,andTis the hydrogenation/dehydrogenation temperature).The linear relationships between lnPand 1/Tfor the hydrogenation and dehydrogenation processes are plotted in Fig.4b and 4d.The hydrogenation and dehydrogenation enthalpies of VC-doped MgH2were calculated as 71.6 ± 2.8 kJ/mol H2and 74.7 ± 0.8 kJ/mol H2,respectively.These values are about 2.1 kJ/mol and 2.4 kJ/mol lower than those of undoped MgH2(73.7 ± 2.7 kJ/mol and 77.1 ± 5.3 kJ/mol),respectively.The decrease of reaction enthalpy is responsible for the decrease of the hydrogenation and dehydrogenation temperature of MgH2,indicating that the addition of VC dramatically improves the hydrogen storage properties of MgH2.

Fig.4.(a,c) Pressure-composition-isotherm (PCI) curves and (b,d) 1000/T versus lnP curves of MgH2 and VC-doped MgH2.

Table 1 Empirical dehydrogenation activation energies of some catalyzed MgH2 systems.

Fig.5.XRD patterns of (a) MgH2,(b) MgH2-VC,(c) dehydrogenated MgH2-VC,and (d) rehydrogenated MgH2-VC.
To investigate the role of VC in the improvement of the hydrogen storage performance of MgH2,XRD characterization of hydrogenated/dehydrogenated VC-doped MgH2specimens was performed.For comparison,the XRD characterization results for VC-doped MgH2and undoped MgH2are also presented in Fig.5.The diffraction peaks of rehydrogenated MgH2can be assigned to MgH2(Fig.5a).The crystal structure of VC does not change during milling and hydrogen absorption and desorption processes,implying that VC remains stable and only acts as a catalyst during these processes (Fig.5(b–d)).In addition,the diffraction peaks of VCdoped MgH2became sharp after dehydrogenation,indicating an increment in the crystallization.
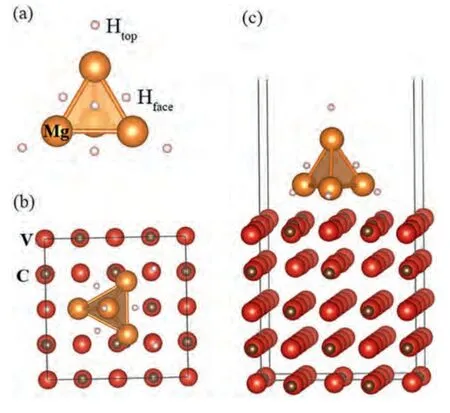
Fig.6.(a) Mg4H8 cluster and (b,c) its top and side views on the VC (100)surface,respectively.
It has been proved that VC acts as a catalyst during the dehydrogenation of MgH2.Moreover,the reflections of the VC (200) lattice planes can be clearly detected from its XRD Fig.1a) and SAED (Fig.2b) patterns.Hence,in the present study,the VC (100) surface consisting of 80 atoms with five atomic layers was constructed and a vacuum layer of 15 ˚A was used between the slabs.An adsorption model of an Mg4H8cluster on the VC (100) surface was built to simulate the role of VC in the dehydrogenation process of MgH2(Fig.6).The geometries of the adsorbate and VC layers were completely relaxed,except for the bottom three layers,during DFT calculations.To reveal the dehydrogenation behavior of VC-doped MgH2,the dehydrogenation enthalpy and electronic structure of MgH2on the VC (100) surface were studied.It should be noted that dehydrogenation energy affects the dehydrogenation rate and dehydrogenation temperature of hydrogen storage materials.To analyze the catalytic effect of VC on the dehydrogenation properties of MgH2,the dehydrogenation energy of the pure Mg4H8cluster and a Mg4H8cluster on the VC (100) surface were calculated by Eqs.(1) and ((2),respectively.In the Mg4H8cluster,Mg atoms were arranged in a tetrahedron structure,that is,two kinds of H atoms bonded with one Mg atom and three Mg atoms each,which are labeled as Htop and Hface (Fig.1),respectively:

Fig.7.Dehydrogenation energy for the desorption of one H atom (Htop or Hface),two H atoms,and eight H atoms in the Mg4H8 cluster and the Mg4H8 cluster on the VC (100) surface.
Here,E(Mg4H8) andE(VC+Mg4H8) are the total energies of the Mg4H8cluster and of the Mg4H8cluster on the VC (100) surface,respectively,E(Mg4H8-x) andE(VC+Mg4H8-x) are the total energies of the Mg4H8cluster and of the Mg4H8cluster on the VC(100)surface with the desorption ofxnumber (x=1,2,8)of H atoms,respectively,andE(H2) is the total energy of gaseous H2.
Fig.7 presents the dehydrogenation energy for the desorption of one H atom (Htopor Hface),two H atoms,and eight H atoms in the Mg4H8cluster and the Mg4H8cluster on the VC (100) surface.It was found that in all cases,the dehydrogenation energy of Mg4H8is improved by VC,causing a decrease in the dehydrogenation temperature of VC-doped MgH2.The catalytic effects of the Mg4H8cluster on the VC(100) surface were also revealed.For the desorption of one H atom,Htopatoms require a lower dehydrogenation energy than Hfaceatoms.For the desorption of two H atoms,Hfaceand Htopatoms require the lowest dehydrogenation energy.This finding confirmed that the addition of VC is beneficial to weaken interaction between Mg and H.Further,to reveal the interactions between MgH2and the VC (100) surface,the total density of state (DOS),the partial density of states(PDOS),and the electron density difference of Mg4H8on the VC (100) surface were calculated (Fig.7).It is evident from the DOS shown in Fig.7a that the s orbitals of Mg are located at -4.0 eV below the Fermi level and are hybridized with thedorbitals of V atoms and theporbitals of C,indicating a strong interaction between Mg and VC.The yellow (blue)areas in Fig.8(b) indicate the increase (decrease) of electron density.It is discernible from Fig.8(b) that mass charge depletion areas appear around Mg atoms,whereas charge accumulation areas are found between Mg4H8and the VC(100)surface,indicating that electrons of thesandporbitals of Mg are transferred to the VC (100) surface,weakening the bonding between Mg and H atoms.The lengths of Mg-Htopand Mg-Hfacebonds in Mg4H8on the VC (100) surface are longer than those in Mg4H8.In the pure Mg4H8cluster,the lengths of Mg-Htopand Mg-Hfacebonds are 1.70 ˚A and 1.99 ˚A,respectively,and the corresponding values for Mg4H8on the VC (100) surface increase to 1.82 ˚A and 2.07 ˚A,respectively.These findings indicate that the elongation of Mg-H bonds promotes the dehydrogenation of MgH2.

Fig.8.(a) DOS and (b) electron density difference of Mg4H8 on the VC (100) surface.
4.Conclusions
VC was successfully synthesized by an etching method and employed to improve the de/rehydrogenation of MgH2.VC imparts superior catalytic effects on the hydrogen storage thermodynamics and kinetics of MgH2.VC-doped MgH2can absorb hydrogen at room temperature and release hydrogen at 170 °C.Non-isothermal hydrogenation tests revealed that undoped MgH2can hardly absorb hydrogen until the temperature reaches 125 °C,which is far higher than for the VCdoped samples.Isothermal re/hydrogenation measurements indicate that VC-doped MgH2can absorb 5.0 wt.%of H2within 9.8 min at 100 °C and desorb 5.0 wt.% of H2within 3.2 min at 300 °C.At 325 °C,VC-doped MgH2can release approximately 6.0 wt.% of H2with a hydrogen desorption rate of 2.18 ± 0.03 wt.%/min,which is 16.8 times faster than for undoped MgH2under the same conditions.TheEaof VCdoped MgH2is 89.3±2.8 kJ/mol,which is about 49.2 kJ/mol lower than that of undoped MgH2.Mass charge depletion areas were detected around Mg atoms,whereas charge accumulation areas were found between Mg4H8and the VC (100)surface,weakening the bonding between Mg and H atoms.The Mg-H bond length on the VC (100) surface is significantly longer than that in MgH2.The elongation of Mg-H bonds promotes the dehydrogenation of MgH2;thus,the hydrogen storage properties of MgH2are remarkable improved through addition of VC.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos.52261038 and 51861002),the Natural Science Foundation of Guangxi Province (Grant No.2018GXNSFAA294125),and the Innovation-driven Development Foundation of Guangxi Province (Grant No.AA17204063).J.E.acknowledges additional support by the Ministry of Science and Higher Education of the Russian Federation in the framework of the Increase Competitiveness Program of NUST "MISiS" (grant number K2-2020-046).We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Improvements in mechanical,corrosion,and biocompatibility properties of Mg–Zr–Sr–Dy alloys via extrusion for biodegradable implant applications
- Ultrasonic solidification mechanism and optimized application performances of ternary Mg71.5Zn26.1Y2.4 alloy
- Superplastic behavior of a fine-grained Mg-Gd-Y-Ag alloy processed by equal channel angular pressing
- GO/MgO/Mg interface mediated strengthening and electromagnetic interference shielding in AZ31 composite
- Electrochemical synthesis of boron-containing coatings on Mg alloy for thermal neutron shielding
- Achieving high-strain-rate and low-temperature superplasticity in an ECAP-processed Mg-Y-Er-Zn alloy via Ag addition
