Improvements in mechanical,corrosion,and biocompatibility properties of Mg–Zr–Sr–Dy alloys via extrusion for biodegradable implant applications
2023-12-27FislKiniJixingLinKhurrmMunirCuieWenYuncngLi
Fisl Kini ,Jixing Lin ,Khurrm Munir ,Cuie Wen ,Yuncng Li,∗
a School of Engineering, RMIT University, Melbourne, Victoria 3001, Australia
b School and Hospital of Stomatology, Wenzhou Medical University, Wenzhou 325027, China
Abstract In this study,extrusion was performed on Mg–Zr–Sr–Dy alloys for improving their mechanical,corrosion,and biocompatibility properties.Effects of extrusion and alloying elements on the microstructural characteristics,tensile and compressive strengths,corrosion behavior,and biocompatibility were investigated.The Mg–Zr–Sr–Dy alloys were composed of an α-Mg matrix containing {102} extension twins and secondary phases of intermetallic compounds Mg17Sr2 and Mg2Dy.Evolution of basal and rare earth (RE) textures was observed in the extruded alloys and an increase in Dy content to 2 wt.% resulted in texture randomization and strengthening of the RE component,mainly due to particle-stimulated nucleation and a change from discontinuous dynamic recrystallization to continuous dynamic recrystallization,which also led to an improved tension–compression yield asymmetry of 0.87.Extrusion of the alloys significantly enhanced their tensile and compressive properties due to improved distribution of alloying elements and formation of textures.Corrosion rates tested by hydrogen evolution testing,potentiodynamic polarization,and electrical impedance spectroscopy showed similar trends for each composition,and the lowest corrosion rate of 3.37 mmy-1 was observed for the Mg-1Zr-0.5Sr-1Dy in the potentiodynamic polarization testing.Dy2O3 was observed in the inner layers of the Mg(OH)2 protective films,whose protective efficacy was confirmed by charge-transfer and film resistances.A comparison among the minimum CRs observed in this study and previously studied as-cast Mg–Zr–Sr–Dy and extruded Mg–Zr–Sr alloys,demonstrates that both the extrusion process and addition of Dy in Mg–Zr–Sr improved the CR.Similarly,extruded Mg–Zr–Sr–Dy alloys showed improved cell viability and adhesion of human osteoblast–like SaOS2 cells due to increased corrosion resistance and enhanced Sr distribution within the Mg matrix.
Keywords: Corrosion;Microstructure mechanical properties;Cytotoxicity;Mg–Zr–Sr–Dy alloy.
1.Introduction
High-purity magnesium (Mg) has been known for its biomedical properties and its potential as a biodegradable implant material since the late 19th century.The major shortcomings experienced during those early studies were its inferior mechanical properties and high corrosion rate (CR) bothin vitroandin vivo[1].Based on the biocompatibility,mechanical,and corrosion property requirements,only a limited number of alloying elements were suggested for use in Mg alloys [2].Novel Mg–Zr–Sr and Mg–Zr–Sr–Dy alloys were developed which exhibited superior mechanical and corrosion properties along with excellent biocompatibility [3,4].
Strontium (Sr) is considered an osteoconductive element and showed improved bone mineralization,formation of Srsubstituted hydroxyapatite (HA),and enhanced bone growth around the implant after implantation in mice femurs and in dog femoral arteries [5,6].Sr also promoted osteoblast maturation and improved vertebral bone density [7].Formation of new bone directly adjacent to an Mg-1Zr-2Sr implant was reported 3 months after implantation in rabbits [3] and radiography of the newly formed bone showed high mineral density due to the osteointegration properties of Sr.
Rare earth (RE) addition to Mg has been found to improve the mechanical and corrosion properties [8–10].In vivotests of RE-containing Mg alloys showed an acceptable host response and low CR [11].A comprehensive biocompatibility evaluation of RE elements showed that dysprosium (Dy) is one of the most suitable alloying elements for Mg [12].Addition of Dy to as-cast Mg–Zr–Sr led to significant improvements in corrosion resistance and mechanical properties along with excellent biocompatibility in a SaOS2 cell line [4].A recent study on the extrusion of Mg–Zr–Sr showed substantial increases of 26.9% in ultimate compressive stress (σUCS) and 100% in compressive yield stress (σCYS) as compared to ascast conditions.Moreover,the CR significantly reduces due to favorable accumulation of the Mg17Sr2phase at the grain boundaries (GBs).
Generally,alloying,heat treatment,and plastic deformation are the most common techniques used to improve the mechanical and corrosion properties of Mg alloys [13,14].The main underlying mechanism on which these techniques rely is the suitable microstructure modification in Mg alloys.Alloying is considered one of the most effective techniques to improve the mechanical and corrosion properties of Mg alloys.However,due to the stringent biocompatibility requirements for medical applications,the choice of the alloying elements and their concentrations in Mg alloys is limited [12].Nonetheless,solid solution strengthening,grain refinement,and precipitation strengthening are the dominant mechanisms for improving the mechanical properties of Mg alloys [15].On the other hand,the addition of some elements acts to refine the grain size of Mg alloys and form a second phase that continuously surrounds theα-Mg matrix,leading to a decrease in their corrosion rate,whereas others may reduce the precipitation of the second phase at grain boundaries (GBs),thereby balancing the potential difference between theα-Mg matrix and the second phase,thus decreasing the micro-galvanic corrosion [16].Furthermore,alloying elements may also promote the formation of a compact oxidation film on the surface of Mg-based implants,improving the CR [17].
Heat treatment utilizes the fact that the solubility of certain alloying elements changes with the temperature.The solution treatment reduces or eliminates the secondary-phase particles around the Mg matrix,which may induce solid solution strengthening and decrease the galvanic corrosion in Mg alloys.Furthermore,suitable aging treatments could precipitate the homogeneously distributed phases at GBs and interior of the grains,whereas an annealing could reduce intrinsic defects and relieve stresses in the Mg matrix.As a result,precipitation-strengthening and uniform corrosion behavior could be attained [13,18].
A significant enhancement in mechanical and corrosion properties in the Mg-Zn alloy was reported through ECAP and post-water annealing,resulting in the formation of a more compact Mg(OH)2surface layer [19].The severe plastic deformation(SPD)decreases the grain size of Mg matrix,which promotes grain boundary strengthening [20].This is accompanied by an increase in high-angle GBs,which improves strength because they are more effective in blocking dislocations [21].In addition,refinement of the secondary-phase particles during SPD may also contribute to the strength via the Orowan mechanism,where the critical stress for dislocation bowing around particles increases as the particle size decreases [22].On the other hand,an enhanced strain and uniform plasticity may be attained in ultrafine grains by activation of non-basal slip system in Mg alloys [23].Furthermore,although an increase in corrosion rate is expected due to the presence of high dislocation density and other crystal defects from SPD,however,finer grains and secondary particles exhibit more uniform corrosion behavior [24].
To examine the benefits of extrusion,in this study as-cast Mg–Zr–Sr–Dy alloys were extruded and their mechanical,corrosion,and biocompatibility properties were studied.Their mechanical properties were evaluated by tensile and compression tests,and the dependence of these properties on the microstructure and crystallographic texture was determined.Hydrogen evolution (HE) testing,potentiodynamic polarization(PDP) testing,and electrical impedance spectroscopy (EIS)were used to determine the corrosion behavior,while human osteoblast–like SaOS2 cells were used to assess the biocompatibility of the Mg alloys.Based on the results,the effects of extrusion and alloying elements on the microstructural characteristics and material properties have been determined.
2.Materials and methods
2.1.Casting and extrusion of Mg–Zr–Sr–Dy alloys
As-cast Mg–Zr–Sr–Dy samples were prepared under similar conditions to those reported in our previous study [4].Briefly,pure Mg and Mg-30Zr,Mg-30Sr,and Mg-10 Dy alloys (Hunan Rare Earth Metal and Material Institute,China)were used as the master alloys in this study.To prevent iron(Fe) contamination in the molten Mg,a coated steel crucible was used during the casting.The Mg alloys were melted under an atmosphere of high-purity argon.The melt was maintained by stirring for 30 min and was cast at 700 °C into cylindrical steel dies with an inner diameter of 22 mm which were preheated to 250 °C.The 22 mm diameter Mg cylindrical alloy ingots were machined to remove the outer layer to obtain cylindrical bars with a diameter of 20 mm and a length of 40 mm.The as-cast samples were not subjected to any post-heat treatment before extrusion.
The actual chemical compositions of the Mg–Zr–Sr alloys were measured using inductively coupled plasma atomic emission spectroscopy (ICP-AES) (service provided by Spectrometer Services Pty Ltd,Australia).Composition analysis was conducted on three samples for each alloy to obtain the average values.Variations between actual and nominal compositions were observed.Chemical compositions of the extruded Mg–Zr–Sr–Dy alloys are given in Table 1.
A schematic of the extrusion apparatus used in this study is shown in Fig.S1.The ram was powered using an Instron 500 kN machine under compression.A flat die with a halfangle of 90° was used for the extrusion [25].The bore of the container was 30 mm in diameter.The as-cast cylindricalbars of 20 mm in diameter and 40 mm in length were used as the billets.The temperature of the billets was measured using a dummy sample with the same composition and size as those of the experimental billets and embedded with a thermocouple.Initially,the container was heated to an extrusion temperature of 400 °C at a heating rate of∼60 °C/min and maintained for approximately 3 h.Subsequently,the die and spacer were inserted,and the temperature was maintained at 400 °C for 30 min.The billets were then placed inside the container on top of the die and held for 10 min to homogenize the temperature.Extrusion was conducted at a constant temperature of 400 °C,a stem speed of 1 mm/s,and an extrusion ratio(ER)of 36:1.Finally,extruded rods of 5 mm in diameter were obtained.After extrusion,the samples were air-cooled to room temperature (water quenching was not used) and were not subjected to any post-heat treatment.An equivalent plastic strain (∈eqv) of 4.181 was calculated using the formula derived from the incremental changes in the configuration of a body during the deformation process (see supplementary information) [26–29].Test samples for microstructural characterization,mechanical and corrosion testing,andin vitrobiocompatibility assessment were cut from the extruded rods using electrical discharge machining (EDM).
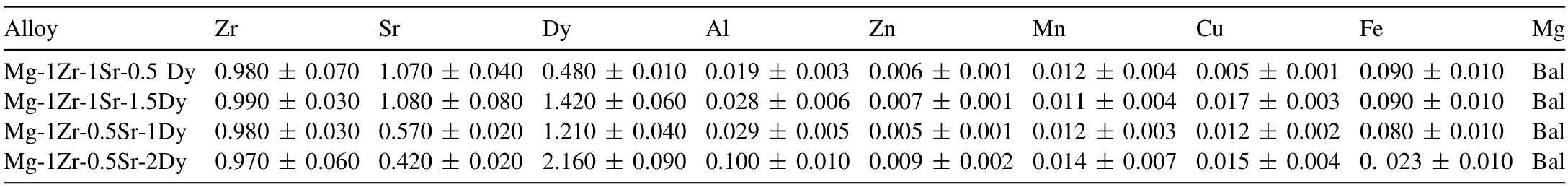
Table 1 Chemical compositions of Mg–Zr–Sr–Dy alloys measured using ICP-AES.All compositions are given in wt.%.
2.2.Microstructural characterization
Samples for microstructural characterization were prepared with their surfaces perpendicular to the extrusion direction(ED).The phase constituents of the Mg–Zr–Sr–Dy alloys were characterized by X-ray diffractometry (XRD;Bruker AXS D4 Endeavor).For XRD analysis,samples were scanned over an angular range of 5–90° at a step size of 0.02° using Cu–Kαradiation (λ=0.154 nm).The International centre for Diffraction Data (ICDD PDF4) database was used for indexing the phases.For electron backscatter diffraction (EBSD),Mg alloy samples were cold-mounted in epoxy resin and the exposed surfaces were progressively ground for 1 min each using 800,1200,2400,and 4000 grit silicon carbide (SiC) grinding papers lubricated with the diamondpolishing (DP) Lubricant Brown.This was followed by the standard Struers polishing procedure for the Mg alloys,using 0.04 μm colloidal silica–based oxide polishing suspension (OP-U NonDry) as a final polish (details of the polishing steps are given in the supplementary information).EBSD analysis was done using AZtecCrystal 5.1 software from Oxford Instruments.For SEM/optical microscopy,the polished samples were sonicated in ethanol and then etched using a picral reagent (formulation: 3.0 ml picric acid,5 ml acetic acid,50 ml ethanol,and 10 ml distilled water).Optical microscopy(Leica DM2500M with 3.1 MP CCD)and SEM(JEOL 7200F Schottky Field Emission SEM) along with energy-dispersive X-ray spectroscopy (EDS) were used to study the microstructure of the etched samples and the fractured surfaces of the samples after tensile testing.The grain size (GS) was measured using the linear-intercept method according to ASTM E122–12 [30].The average GS was estimated by counting the number of grains intercepted by five straight lines long enough to yield at least 50 intercepts.SEM was also used to study the corroded surfaces after immersion in simulated body fluid (SBF) for 24 h.
2.3.Mechanical testing
Compression test samples with a length/diameter (L/D) ratio of 2 were prepared.Dogbone-shaped tensile test samples with a 4 mm gage diameter and 18 mm gage length were machined from the extruded rods by EDM.All samples prepared for mechanical testing were polished with SiC grinding paper (800 grit) to remove surface defects.
Tensile and compression testing was undertaken in accordance with ASTM B557M-15,ASTM E8/E8M-16a,and ASTM E9–09 (2018) [31–34].Both compression and tensile tests were conducted at room temperature using a uniaxial Instron testing machine (Instron 5569).The tests were conducted at a loading rate of 1 mm/min.The stress–strain curves obtained from compression and tensile testing were used to calculate the respective yield strength,ultimate strength,and maximum strain at failure.The yield strength was calculated using the 0.2% offset method.Five samples from each alloy composition were tested to determine the average values.
2.4.Corrosion behavior characterization
Disk samples of 5 mm in diameter and 3 mm in thickness were prepared for corrosion testing.The sample surfaces were mechanically ground with 1200 grit SiC paper,polished using a 9 μm diamond suspension with felt nap mats,washed with acetone,ethanol,and distilled water,and dried in a cool airstream.The corrosion behavior of the Mg alloys was assessed using HE,PDP,and EIS tests in SBF at 37 °C.For corrosion tests,the ratio of SBF volume to sample surface area was maintained at 381.97 ml/cm2as defined by ASTM G31–72 [35,36].The preparation of SBF involved the addition of reagents to 1 liter of pure water in the following order:NaCl (5.403 g),NaHCO3(0.504 g),Na2CO3(0.426 g),KCl(0.225 g),K2HPO43H2O (0.230 g),MgCl26H2O (0.311 g),0.2 M NaOH(100 ml),HEPES(17.892 g),Na2SO4(0.072 g),and 1.0 M NaOH (15 ml).For immersion tests,the disk samples were immersed in SBF for 24 h.The Mg and Sr ion concentrations in SBF were then analyzed via microwave plasma atomic emission spectroscopy,while ICP-MS was utilized for measuring the Zr ion concentration [37].The CR was determined through HE,PDP,and EIS tests.Three samples for each test and from each composition were used.The PDP and EIS tests were carried out using a three-electrode cell system with a saturated calomel electrode (SCE) as the reference electrode,a platinum electrode (1.5 × 1.5 cm2) as the counter electrode,and the alloy samples as the working electrode.The samples were connected to a copper wire and then cold-mounted in epoxy resin leaving an exposed area of 0.785 cm2.The sample surfaces were mechanically ground with 4000 grit SiC paper,polished using a 9 μm diamond suspension with felt nap mats,washed with acetone,ethanol,and distilled water,and dried in a cool airstream.The PDP curves were obtained with a scan scope of ±500 mV against open circuit potential (OCP) at a scan rate of 1.5 mV s-1using an electrochemical workstation (VSP-300,BioLogic,France).EIS using ZsimpWin software was measured at a frequency range from 10 MHz to 100 kHz at an amplitude of 10 mV.The samples were immersed in SBF for 1 h to stabilize the OCP.The CRiwas calculated from the PDP curves using the equation given in ASTM G102–89 [38]:
where CRiis the corrosion rate (mmy-1),icoris the corrosion current density (μAcm-2),ρis the density of the sample(gcm-3),K1is a constant (3.27 × 10-3),and EW is the alloy equivalent weight (dimensionless).
All HE tests were conducted in SBF at 37 °C using an inverted funnel as described in previous studies [3,39].
Corrosion product characterization was carried out via XPS analysis utilizing Thermo Scientific K-Alpha XPS instrument.The X-rays used were monochromatic Al–Kαphotons (1486.7 eV) and photo-emitted electrons were analyzed with a hemispherical energy analyzer.Wide survey scans were collected from 0 to 1350 eV at a pass energy of 200 eV to determine the overall elemental composition.The C1s peak at 284.8 eV was set as binding energy reference to compensate the influence of charge shift.The specimens for XPS analysis were immersed in SBF for 24 h at 37 °C.Surface compositions were calculated by measuring peak areas of the primary core levels for all elements present and normalizing the peak areas using tabulated sensitivity factors.It may be noted that XPS can only provide information of the corrosion products at/near to the surface,as the detection limit of XPS in Mg alloys is limited to a depth of 10 nm [40].
2.5.In vitro biocompatibility assessment
The biocompatibility of the Mg alloys was assessedin vitrousing osteoblast-like cells,i.e.,the human SaOS2 osteosarcoma cell line exhibiting osteoblastic properties [41].
The SaOS2 cells were cultured in a modified minimal essential medium (MMEM) at 37 °C in a humidified atmosphere composed of 5% CO2in air for 72 h.Biocompatibility tests were carried out in accordance with the indirect-contact method as described in previous studies [3,42].Briefly,extracts were prepared of eachSc-containing Mg alloy specimen using MMEM with a surface area/MMEM ratio of 0.8 cm2ml-1in a humidified atmosphere of 5% CO2at 37 °C for 72 h.The extracts were then collected and filtersterilized with a 0.22 μm filter (Falcon,BD Biosciences,USA).Control extracts were prepared as described above by incubation of MMEM without the addition of the Mg alloy samples.All Mg alloy extracts and the controls were supplemented with 10% fetal bovine serum (FBS) (Bovogen Biologicals,Australia) and 1% penicillin–streptomycin(Gibco,Life Technologies,Australia).The SaOS2 cells were seeded into a 48-well plate at a density of 10,000 cells per well with 400 μL of supplemented MMEM and incubated for 24 h to allow cell attachment on the plate.Then the supplemented MMEM was replaced with 400 μL of the respective specimen extracts and controls supplemented with 10% FBS and 1% penicillin–streptomycin.After cell culturing for 3 and 5 d,the cytotoxicity of theSc-containing Mg alloys was assessed using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium(MTS) (CellTiter® Aqueous One Solution,Wisconsin,USA).In brief,the supplemented extracts and controls in each well were removed and the wells were washed three times with phosphate-buffered saline (PBS).Then 300 μL phenol red–free RPMI 1640 Medium (Gibco,Life Technologies,Australia) was added to each well,then 100 μL MTS/PBS solution was added to each well and the plate was incubated for 1 h at 37 °C.After incubation,100 μL of solution from each well was transferred into a 96-well plate.The optical absorbance was measured using a microplate reader(FlexStation 3 Microplate Reader,Waltham,CA,USA) at 490 nm.The viable cell counts were calculated according to the optical absorbance.Three samples from each alloy were used for cytotoxicity assessment.
2.6.Statistical analysis
Experimental data are expressed as mean ± standard deviation.A one-way analysis of variance (ANOVA) was used for the statistical analysis using SPSS software (SPSS Inc.,USA) and statistically significant differences between the two groups were accepted at p<0.05.
3.Results and discussion
3.1.Chemical compositions and XRD patterns of extruded Mg–Zr–Sr–Dy alloys
Two distinct group of compositions were selected i.e.,Mg-1Zr-1Sr-xDy (x=0.5 or 1.5 wt.%) and Mg-1Zr-0.5Sr-xDy(x=1 or 2 wt.%) to evaluate the effects of Sr and Dy on the mechanical and corrosion properties of these alloys (Table 1).
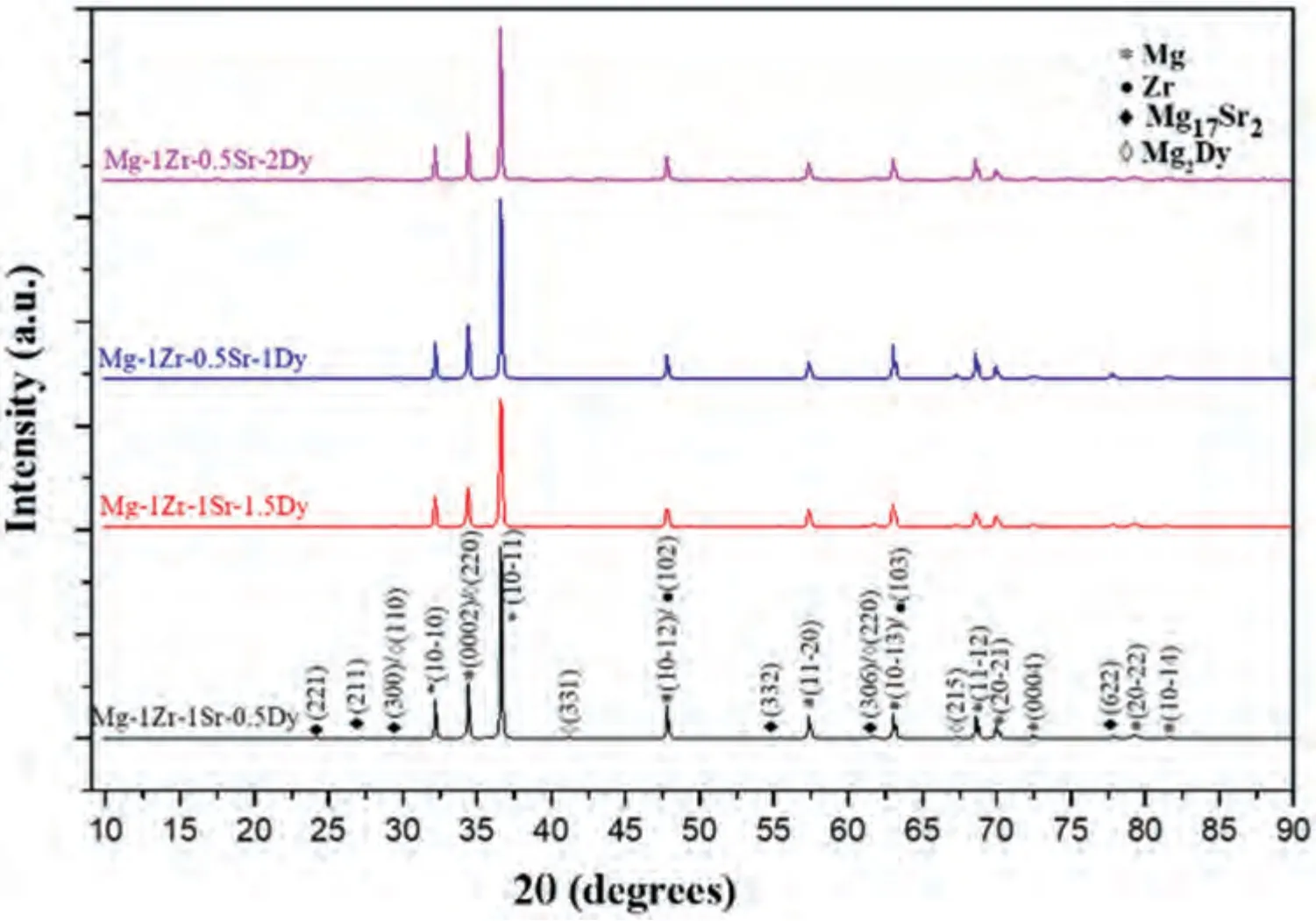
Fig.1.XRD patterns of extruded Mg–Zr–Sr–Dy alloys.
The concentrations of aluminum (Al),manganese (Mn),zinc(Zn),and copper(Cu)impurities were observed to be well below the threshold values beyond which deleterious effects on the CR were observed in binary Mg alloys [43].The contamination limit of Fe in Mg is generally reported as 0.017 wt.%(170 ppm) [44].However,the threshold concentration cannot be specified exactly as it is strongly dependent upon Zr and Mn content and the presence of other functional elements in Mg alloys [45].Zr and Fe exhibit mutual solubility in molten Mg and readily form Fe–Zr intermetallic phases which normally settle down in the melt [46].Nonetheless,complete removal of the Fe and Fe–Zr phases was not likely as stirring was conducted in a coated steel crucible prior to casting in order to obtain homogenous distribution of the undissolved Zr particles.
XRD patterns of the extruded Mg–Zr–Sr–Dy alloys are shown in Fig.1.As per the binary phase diagrams of Mg–Zr,Mg–Sr,and Mg–Dy,anα-Mg solid solution withα-Zr precipitates and Mg17Sr2and Mg2Dy intermetallic compounds are the phases expected to be formed in Mg–Zr–Sr–Dy alloys [47–50].The peak intensities of the Mg2Dy phase in all the alloys were relatively low (Fig.1).Zr has a limited solubility of 3.8 wt.% [51] and also does not form intermetallic compounds with Mg;instead,the presence of Zr-rich halo structures containing pure Zr particles has been reported in Mg–Zr alloys [52,53].Both Mg and Zr have hexagonal close packed (HCP) structures and therefore exhibit overlapping of peaks in their XRD patterns [54].
On the other hand,Sr is almost insoluble in Mg with a solubility of only 0.11 wt.% [51] and Mg17Sr2is the predominant intermetallic compound reported in Mg–Sr alloys up to 30.8 wt.% of Sr [6,55].In addition,previous studies of as-cast Mg–Zr–Sr alloys have reported the precipitation of an Mg17Sr2phase at GBs [56].
As suggested by the Hume–Rothery rules,the atomic radius difference between the rare earth elements (REEs) and Mg is ≤15%,which is considered favorable for the formation of substitutional solid solutions in Mg alloys [51,57].Furthermore,a study on the solubility of lanthanide group elements in Mg–RE systems showed that Dy,which belongs to the yttrium (Y) subgroup,exhibited much higher solubility than cerium (Ce) subgroup elements in spite of their similar atomic radii.This difference in solubility was attributed to the changes in atomic radii of dissolved REEs and the interplanar distances of the Mg solid solution [58].Among the alloying elements in the Mg–Zr–Sr–Dy system,Dy showed the highest solubility of 25.8 wt.% [51].As per the binary Mg–Dy phase diagram,up to 37.3 wt.% of Dy,Mg24Dy5is the intermetallic phase expected to form in equilibrium with the Mg solid solution [49,59].However,except Mg2Dy (with an HCP),all the MgxDy compositions form a cubic lattice,as per the ICCD PDF4 database,hence explaining the formation of Mg2Dy.
3.2.Microscopy of extruded Mg–Zr–Sr–Dy alloys
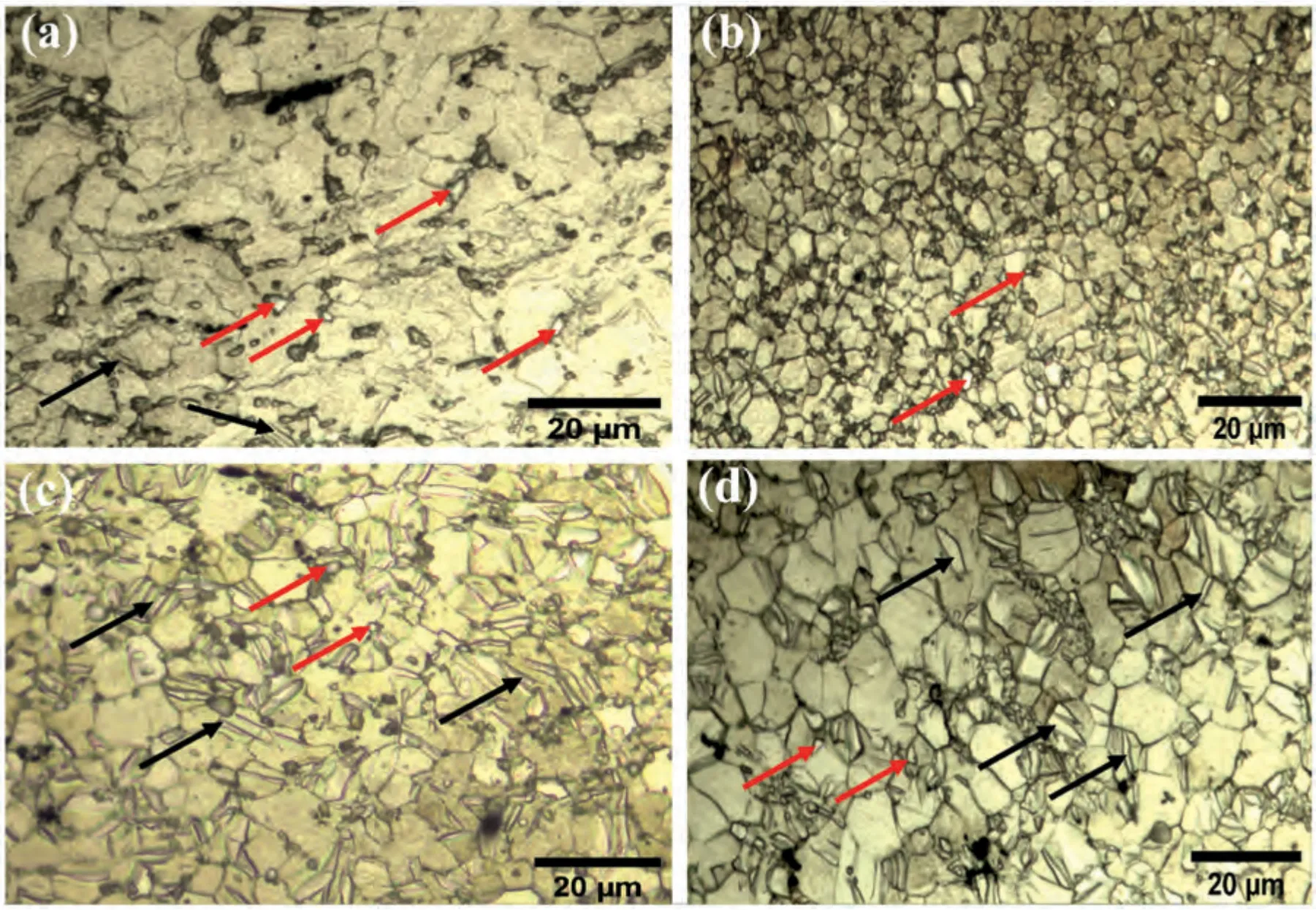
Fig.2.Optical micrographs of extruded Mg–Zr–Sr–Dy alloys under polarized light: (a) Mg-1Zr-1Sr-0.5Dy;(b) Mg-1Zr-1Sr-1.5Dy,(c) Mg-1Zr-0.5Sr-1Dy;and (d) Mg-1Zr-0.5Sr-2Dy.The surfaces shown are perpendicular to the ED.
The micrographs obtained from optical microscopy and SEM including EDS maps are shown in Figs.2 and 3,respectively.A decrease in GS was observed with the addition of Dy only in the Mg-1Zr-1Sr-xDy (x=0.5 or 1.5 wt.%)alloys.No significant effect on the GS was observed for the alloys with low Sr compositions,i.e.,Mg-1Zr-0.5SrxDy (x=1 or 2 wt.%).The GS of the extruded Mg-1Zr-1Sr-0.5Dy,Mg-1Zr-1Sr-1.5Dy,Mg-1Zr-0.5Sr-1Dy,and Mg-1Zr-0.5Sr-2Dy was measured as 10.02±1.22,4.12±0.49,6.92±0.82,and 7.31±0.79 μm,respectively,whereas the earlier reported GS of as-cast Mg-1Zr-2Sr-xDy(x=1,1.63,2.08)was 20.98±6.17,24.41±6.84,and 65.31±19.39 μm,respectively.This clearly shows that extrusion of Mg–Zr–Sr–Dy alloys significantly reduces the GS.Almost all of the secondphase particles were located at the GBs.The micrographs reveal two distinct color contrasts in the second-phase particles,which indicates the presence of two separate phases;small,dark-colored Mg17Sr2particles and a light-colored Mg2Dy phase (indicated by red arrows in Fig.2 and evident from BSE images in Fig.3c and d).The Mg17Sr2particles were mainly observed in the GBs,whereas elemental Sr was also observed within the Mg matrix.As evident from the optical micrographs,twinning was prominent in all compositions except for Mg-1Zr-1Sr-1.5Dy (twins are indicated by black arrows in Fig.2),whereas,within the respective compositions,an increase in Dy ≥1.5%significantly reduces twinning.This is evident from the micrographs,where Mg-1Zr-1Sr-1.5Dy(Fig.2b)and Mg-1Zr-0.5Sr-2Dy(Fig.2d)exhibit significantly reduced twins as compared to the Mg-1Zr-1Sr-0.5Dy(Fig.2a)and Mg-1Zr-0.5Sr-1Dy (Fig.2c),respectively.All the twins present in these alloys were observed to terminate at the GBs.The Mg-1Zr-1Sr-1.5Dy alloy showed equiaxed grains and only a few twins,unlike the other compositions,which showed irregular shapes and elongated grains.On the other hand,EDS maps of Sr in Fig.3 reveal that the concentration of Sr-rich phase was higher in the extruded Mg-1Zr-1SrxDy (x=0.5 or 1.5 wt.%) than the Mg-1Zr-0.5Sr-xDy (x=1 or 2 wt.%) alloys.Also,the EDS maps of Dy showed high solubility of Dy in Mg matrix at concentrations ≤1.5 wt.%(Fig.3).The elemental compositions of the selected particles with different color contrasts in the backscattered electron images are shown in Fig.3b.
The Mg-1Zr-1Sr-1.5Dy showed a finer GS of 4.1 μm and a higher fraction of recrystallized and undeformed equiaxed grains compared to the other compositions (Fig.2b).Most of these recrystallized grains were observed around Mg17Sr2stringers,similar to the observation of Borkar et al.[60].The Mg17Sr2elongated-stringer morphology may not be obvious from the micrographs in Fig.2,probably due to the low Sr content and as the surfaces shown were perpendicular to the ED [60].Nucleation of recrystallized grains promoted by Sr-rich particles through PSN is a well reported mechanism [61,62].It was reported that PSN is dominant around the Mg–Sr stringers with a particle size of ≥1 μm and Sr content of ≥1 wt.% Furthermore,the presence of a RE phase in Mg–RE alloys also promotes PSN [64].In the extruded Mg-1Zr-1Sr-1.5Dy,a significant fraction of PSN grains was observed around the Mg17Sr2particles (Fig.2b).It may be noted that nucleation of new grains from static recrystallization at the die exit can be ignored due to the fast convection cooling of a small rod (5 mm diameter),which can be represented with a one-dimensional heat conduction model,and thus there is a negligible temperature gradient across the sample diameter [60].The low population of Mg17Sr2particles in the extruded Mg-1Zr-0.5Sr-xDy (x=1 or 2 wt.%) alloys is attributed to the low Sr content (EDS maps in Fig.3c and d).The accumulation of intermetallic Mg17Sr2at the GBs in Mg–Sr based alloys has been widely reported [4,5,56,65,66].Intermetallic Mg17Sr2is a brittle phase and breaks into fragments during extrusion.Subsequently,in addition to forming stringers,Mg17Sr2particles also showed boundary pinning effect that restricted grain growth and eventually accumulated on the GBs [60,67].In addition,the nucleation of new grains with diverse orientations due to solute segregation of the REphase was also reported in Mg–RE alloys [68].Consequently,the decrease in GS may be attributed to the dynamic recrystallization (DRX) from PSN by the Mg17Sr2particles and nucleation due to solute segregation of Mg2Dy,and restricted grain growth from the secondary-phase particles [69,70].
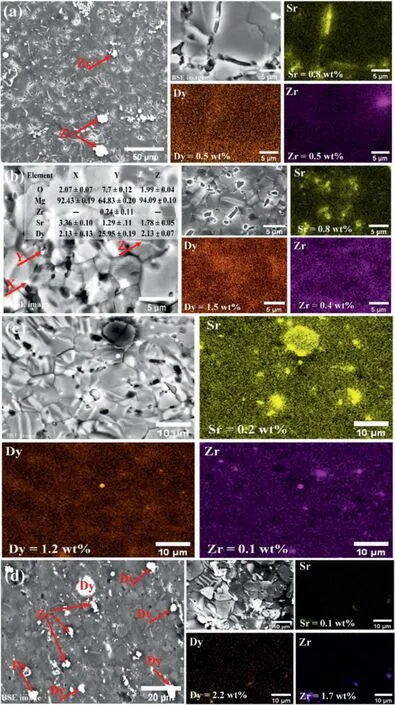
Fig.3.SEM micrographs and EDS maps of extruded Mg–Zr–Sr–Dy alloys: (a) Mg-1Zr-1Sr-0.5Dy;(b) Mg-1Zr-1Sr-1.5Dy;(c) Mg-1Zr-0.5Sr-1Dy;and (d)Mg-1Zr-0.5Sr-2Dy.Respective elemental compositions calculated using EDS are given in the maps.Surfaces shown were perpendicular to the ED.
During the early stage of extrusion,Mg alloys undergo plastic deformation by the activation of extension {102} and contraction {101} twins [71].However,limited {101} twininduced discontinuous dynamic recrystallization (DDRX) was observed with a non-basal orientation [72].Depending upon the loading direction,twinning planes with the highest Schmid factor (m) are more likely to become active and may promote deformation by slip due to favorable reorientation of the basal planes during twinning [73,74].Nonetheless,the{102} extension twin was reported to facilitate continuous dynamic recrystallization (CDRX) in an AZ31 alloy [72].As a result,the grains with twins were mostly observed to have irregular and non-equiaxed shapes (Fig.2a,c,d).
Except for the Mg-1Zr-1Sr-1.5Dy,significant twinning was observed in all other compositions extruded at 400 °C.This indicates that the DRX was limited in these compositions and deformation by twinning was the prevalent mode.The formation of twinned microstructure upon extrusion and without post-heat treatments at 400 °C was reported in the Mg–Gd and Mg-Y alloys,as RE elements retards recrystallisation due to solute drag on the GBs [68,75,76].On the other hand,an RE addition in Mg was found to increase the internally stored deformation energy of the Mg alloy due to partitioning of RE elements to dislocations during deformation,thus inhibiting the dynamic recovery/restoration processes [68,77].Furthermore,the flow stress is found to be greatly influenced with the RE content.For example,increasing the Gd content from 0.5 to 1.5 wt.% caused a drastic increase in the deformation resistance in Mg–Dy alloys [78].In Mg–RE alloys,the internally stored deformation energies initiate DRX [79].Concurrently,the presence of a RE-containing phase during such recrystallizations may promote PSN [64].It is therefore deduced that the presence of the twinned microstructures in Mg–Zr–Sr–Dy alloys extruded at 400 °C is attributed to the retardation of dynamic recrystallization due to RE elements and additionally,the non-conduct of the post-heat treatments after extrusion.Furthermore,the reduction in twinning with Dy ≥1.5 wt.% as observed in Mg-1Zr-1Sr-1.5Dy and Mg-1Zr-0.5Sr-2Dy is attributed to the dynamic recrystallization that is actuated by the internally stored deformation energy which promotes PSN.
The solubility of the alloying elements in Mg is ranked:Dy (25.80) ˃Zr (3.80) ˃Sr (0.11) (wt.%) [51].High solubility of REs in Mg is attributed to their atomic radius differences.Rokhlin et al.[80] suggested a direct correlation between the atomic radius and the solubility of RE metals in Mg.An increase in solubility was observed with a decrease in atomic radius and an increase in interplanar distance in the Mg–RE system [58].The Mg–Dy phase diagram exhibits an HCP structure in the solubility region up to 25.8 wt.% of Dy [81],whereas the intermetallic phases display cubic lattices except Mg2Dy,which has an HCP structure,as per the ICDD PDF4.On the other hand,due to the low solubility of Sr,the HCP Mg17Sr2phase was readily formed [48].As a solid solution,Dy was homogenously distributed within the Mg matrix.However,with an increase in Dy content,segregation of Dy within the matrix around the GBs was observed.As a result,in comparison to the Zr-or Sr-rich phases,only a few Dy-rich Mg2Dy particles were observed in alloys with Dy≤1.5 wt.%,due to its high solubility in Mg.The low presence of the Mg2Dy phase is also evident in the XRD spectrum,showing low peak intensities (Fig.1).Contrarily,the number of Mg17Sr2phase particles were higher and exhibited a dark contrast due to their low average atomic number Z (Fig.3b).
3.3.EBSD crystallographic characteristics of extruded Mg–Zr–Sr–Dy alloys
EBSD micrographs along within situEDS maps of the extruded Mg–Zr–Sr–Dy alloys are shown in Fig.4.The magnified images of the selected grains exhibiting a CDRX are shown in Fig.4b and d and a PSN behavior in Fig.4d.In addition,GB misorientation angle chart of extruded Mg-1Zr-0.5Sr-1Dy is shown in Fig.4c.The unresolved EBSD Kikuchi pattern showing black regions in the EBSD inverse pole figure (IPF) maps corresponds to the intermetallic Mg17Sr2and Mg2Dy particles.A bimodal GS distribution was observed with small,equiaxed crystals at the GBs and relatively large twinned and twin-free grains.A large fraction of these grains was considered recrystallized grains,as they were twin-free and showed an internal misorientation spread of less than 3° i.e.,were strain-free when measured from the grain orientation spread [61].A large number of {102} extension twins were observed (indicated by white arrows in Fig.4a and c) that showed a misorientation angle of 86° and rotation axis of 〈110〉 between the twin and the parent grain[72].This was confirmed from the GB misorientation angle chart which showed a clear spike at 86° and measurement of the misorientation angles across the selected twin boundaries in Fig.4c.Moreover,all compositions had more than 89.5%high-angle grain boundaries (HAGBs) (≥15°),lineated with the black colored boundaries in Fig.4.CDRX was observed in which low-angle grain boundaries (LAGBs) transformed into HAGBs due to grain rotation under strain,indicated with red circles in Fig.4a and d.A magnified image of a selected deformed grain in Fig.4b exhibits the misorientation angle of sub-grain boundaries of 30°,which indicates the presence of a CDRX mechanism [82].Similarly,the transformation of LAGBs to HAGBs due to CDRX can be seen in the enlarged image at Fig.4d.Moreover,as DDRX occurred due to the GB bulging,therefore it was normally observed at GB triple junctions and were easily identified (marked with the black circles in Fig.4).Extensive PSN was observed in the Mg-1Zr-0.5Sr-2Dy and a few such locations are highlighted with white circles in Fig.4d,including an enlarged image of a selected site which clearly exhibits a PSN nucleation.No apparent twin-induced recrystallization or nucleation inside the twins was observed.All compositions except the Mg-1Zr-0.5Sr-2Dy showed a 〈100〉 Mg-fiber texture,i.e.,(0001)aligned near-perpendicular to the ND;in other words,the basal plane tilted approximately 20° toward the ED/extrusion radial direction (ERD) and there was a prismatic 〈100〉 fiber component (PF) as seen in Fig.4.However,the Mg-1Zr-0.5Sr-2Dy showed an entirely randomized basal texture with a weak prismatic〈100〉fiber.Comparison of the inverse pole figures (IPFs) in the ED showed that with the addition of Dy in Mg-1Zr-1Sr-xDy (x=0.5 to 1.5 wt.%) and Mg-1Zr-0.5SrxDy (x=1 to 2 wt.%),the texture peaks shifted from 〈100〉to a position between 〈110〉 and 〈0001〉 i.e.,〈111〉 parallel to the ED,termed an RE texture [48].
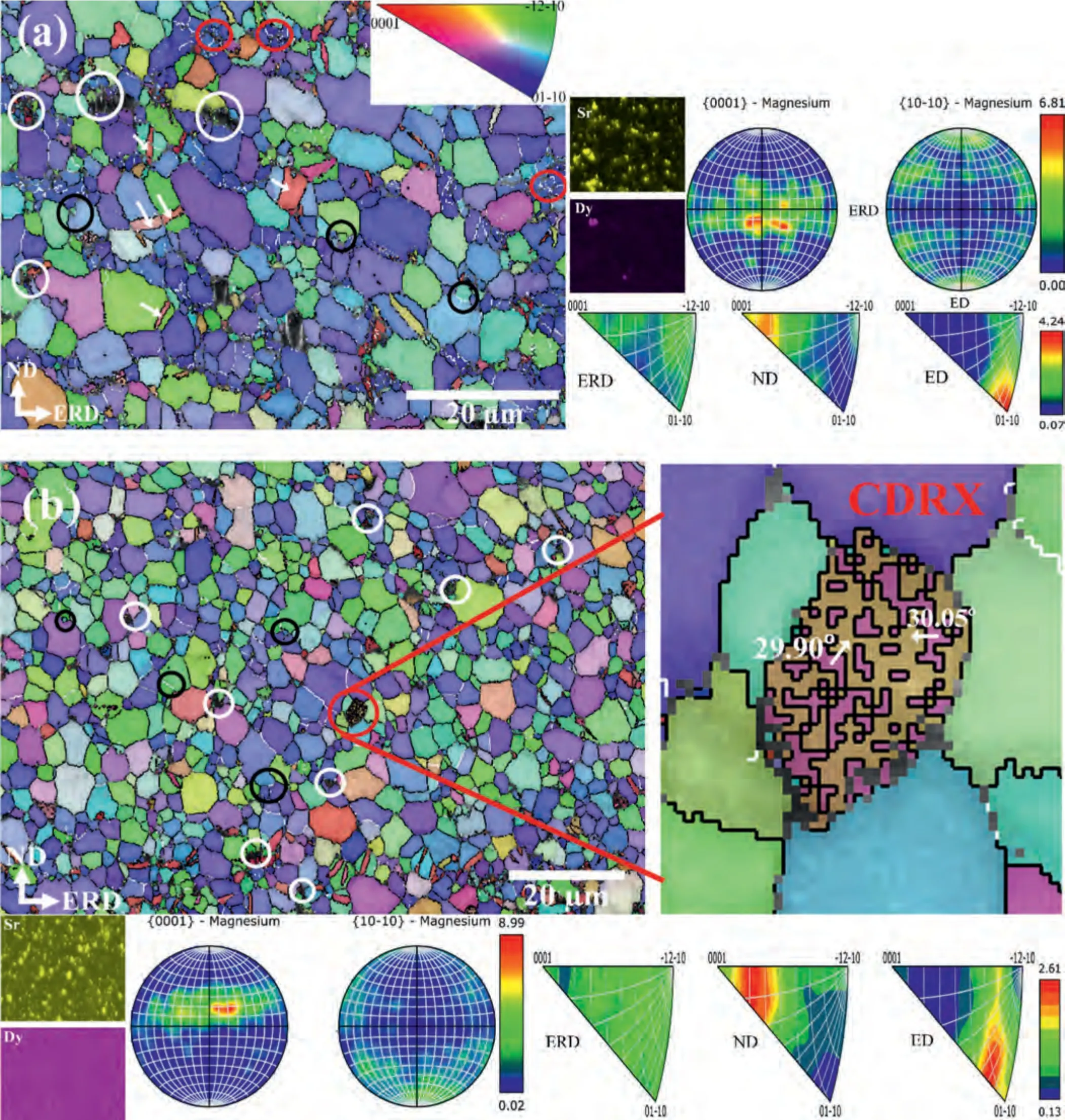
Fig.4.Orientation maps,pole figures,and IPFs of extruded alloys: (a) Mg-1Zr-1Sr-0.5Dy;(b) Mg-1Zr-1Sr-1.5Dy;(c) Mg-1Zr-0.5Sr-1Dy;and (d) Mg-1Zr-0.5Sr-2Dy.Locations of crystal misorientations measured between parent grain and twin,i.e.,across twin boundaries,are indicated with white arrows in Fig.4a and 4c.Boundaries marked with white indicate LAGBs (2–15°),whereas black boundaries signify HAGBs ≥15°.Selected CDRX,DDRX,and PSN locations are marked with red,black,and white circles,respectively.IPF maps of the surfaces shown are perpendicular to the ED.NB: ERD is the extrusion radial direction and ND is the normal direction which is perpendicular to both ED and ERD.
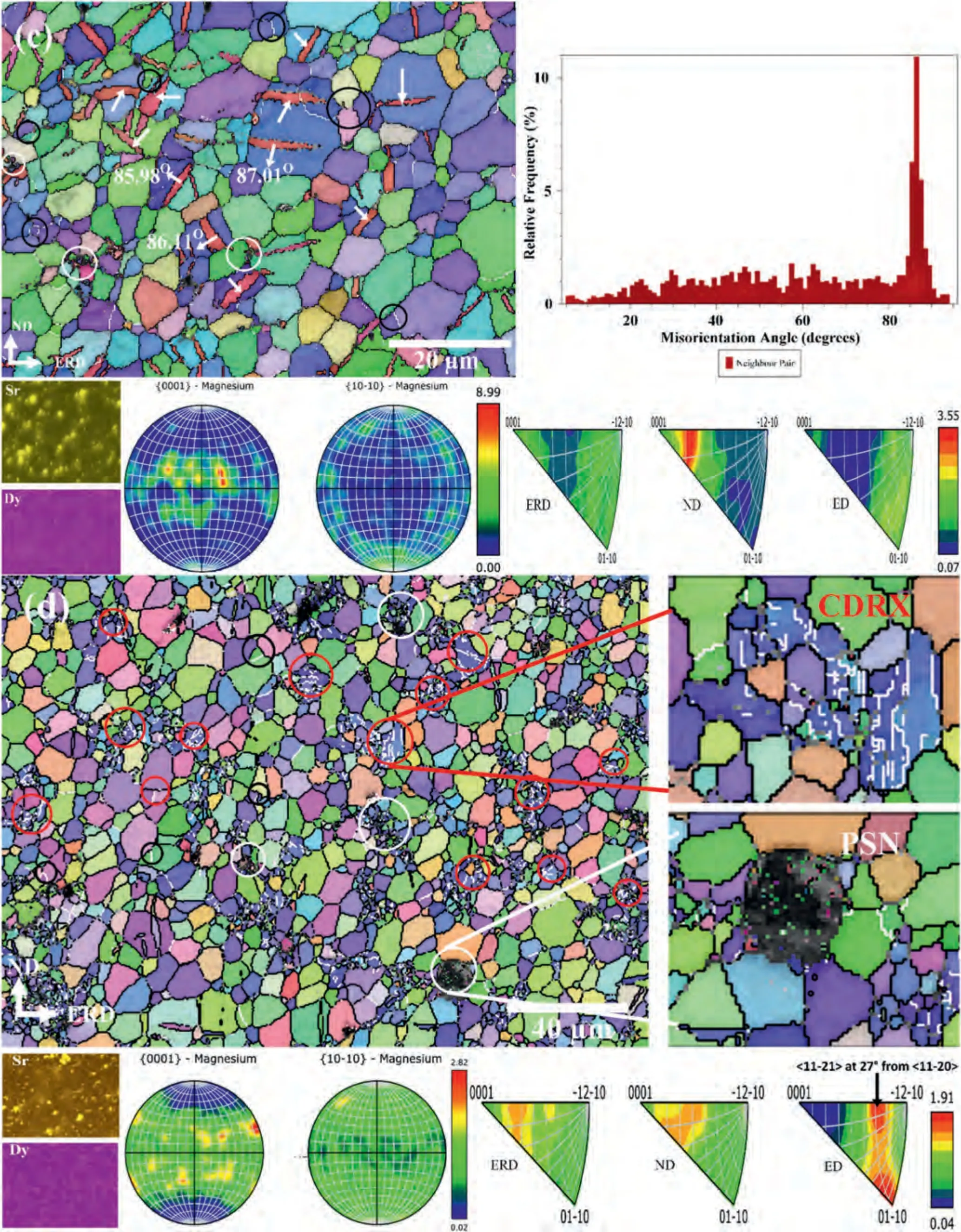
Fig.4.Continued
The deformation mechanism in Mg alloys is dependent upon the CRSS andmvalues,which in turn depend upon the extrusion processing conditions such as temperature,effective strain,strain rate,and orientation between the loading direction and the parent grains.Consequently,due to the low strain in early stages of extrusion,deformation by twinning was found to be dominant in Mg alloys [72].Only {102}extension twins were observed,which are characterized by 86° of misorientation between parent grain and twin,and are indicated by white arrows in Fig.4a and c [83].These extension twins exhibit basal poles parallel to the ED (red color in the pole figures of Fig.4a and c) [84].It has been reported that extension twins are predominant in Mg alloys due to their low CRSS and are readily observed during deformation[85,86].This mode of twinning is more capable of growth and was found to be responsible for producing the 〈100〉fiber texture in Mg alloys [72].It is noted that {101} contraction twins were not observed in this study and this may be attributed to the weakening of texture during extrusion by RE addition to Mg,as also reported in Mg–La and Mg–Gd alloys[71].
The textural evolution in the extruded Mg–Zr–Sr–Dy alloys was evaluated in terms of the contributions of DDRX,CDRX,and PSN grains.It is generally considered that CDRX grains exhibit random orientations and weaken texture,whereas DDRX grains exhibit similar orientations to those of the deformed parent grains in Mg alloys.In contrast,PSN is a characteristic feature of a purely randomized texture [83,87–90].As shown in Fig.4,DDRX grains (black circles) were dominant in the extruded Mg–Zr–Sr–Dy alloys,although an increase in CDRX grains (red circles) was observed in the Mg-1Zr-0.5Sr-2Dy (Fig.4d).At temperatures ≥350 °C,DDRX is the predominant mechanism in Mg alloys due to activation of secondary slip systems,high dynamic recovery,and GB mobility [61,91–93].It was reported that CDRX promoted the shift of prismatic 〈100〉 toward a 〈110〉 fiber texture with basal orientations near the parent deformed grains in Mg–RE alloys [94,95],whereas DDRX splits or weakens the basal textures [48,83,84].Imandoust et al.[96] proposed that the RE texture during extrusion is mainly developed via the DDRX mechanism,whereas CDRX only contributes to the formation of bands of deformed grains which form stable interfaces for subsequent recrystallization by bulging.Nonetheless,almost all studies have reported the role of CDRX at low processing temperatures,in either the formation or sharpening of the RE texture in Mg alloys [87,88,96,97].
The textural modification in Mg–RE alloys depends strongly on the deformation conditions,i.e.,the processing method,temperature,strain and strain rate,and the choice of REEs [85,87,89].The formation of an RE texture,i.e.,〈111〉 parallel to the ED where peak intensity is located at 27° from 〈110〉 and has a maximum intensity of 3° from the edge of the IPF [71],was observed in the Mg-1Zr-0.5Sr-2Dy (Fig.4d).Moreover,a slightly weakened RE texture was observed in the Mg-1Zr-1Sr-1.5Dy (Fig.4b).However,Mg-1Zr-1Sr-0.5Dy and Mg-1Zr-0.5Sr-1Dy did not display such a texture,which could be attributed to the low concentrations of Dy i.e.≤1.5 wt.%,as development of an RE texture has been found to be dependent on the concentration of REEs in Mg alloys and stored deformation energy [90,98,99].The addition of REEs has been reported to cause solute segregation at the GBs and interdendritic regions within the matrix which altered the DRX mode [9,68,76,89,100–102].The segregation of Dy at higher content and the presence of a few CDRX locations (red circles) were observed in the Mg-1Zr-0.5Sr-2Dy(Fig.4d and corresponding EDS map).
Thein situEDS mapping of Sr in Fig.4 clearly shows the presence of a large number of Mg17Sr2particles localized at the GBs with a size greater than 1 μm (Fig.2).In contrast,Mg2Dy particles were rarely observed at the GBs,while the Dy element was mostly observed to be distributed within the Mg matrix as a solid solution.Such distributions of Sr-and Dy-rich particles in the EDS maps are obvious due to their respective solubility in Mg.PSN around the Mg17Sr2particles at the GBs is evident in the IPF maps in Fig.4.Increases in PSN and CDRX regions were observed with the addition of Dy at 2 wt.% to the Mg-1Zr-0.5Sr-2Dy (Fig.4d;a smaller magnification micrograph is shown to highlight the increased presence of PSN).In Mg–Sr alloys,PSN is largely initiated by the Sr-rich phase ≥1 μm [61,63,103],whereas a contribution by the RE particles in Mg–RE has also been reported [74,104–108].In addition,it has been reported that the addition of RE elements causes the suppression of DDRX due to the solute drag effect and thus promotes the CDRX mechanism in Mg alloys [88,97].This was corroborated by the unexpected presence of CDRX at a high extrusion temperature of 460 °C upon addition of Y to an Mg–Zn–Y–Nd alloy [109].
The appearance of more CDRX locations (red circles in Fig.4d) is considered to be due to the solute segregation of Mg2Dy at the GBs and apparent clustering within the interdendritic regions (EDS map of Dy).Furthermore,due to the Mg2Dy particles at the GBs,PSN is likely to have occurred due to the increased number and size of the Mg2Dy particles,as evident from the EDS maps in Fig.3d.Consequently,a completely randomized basal texture with a strong RE component was developed in the Mg-1Zr-0.5Sr-2Dy(Fig.4d),which is attributed to PSN by the Mg17Sr2and Mg2Dy particles at the GBs and initiation of the CDRX mode by RE addition.
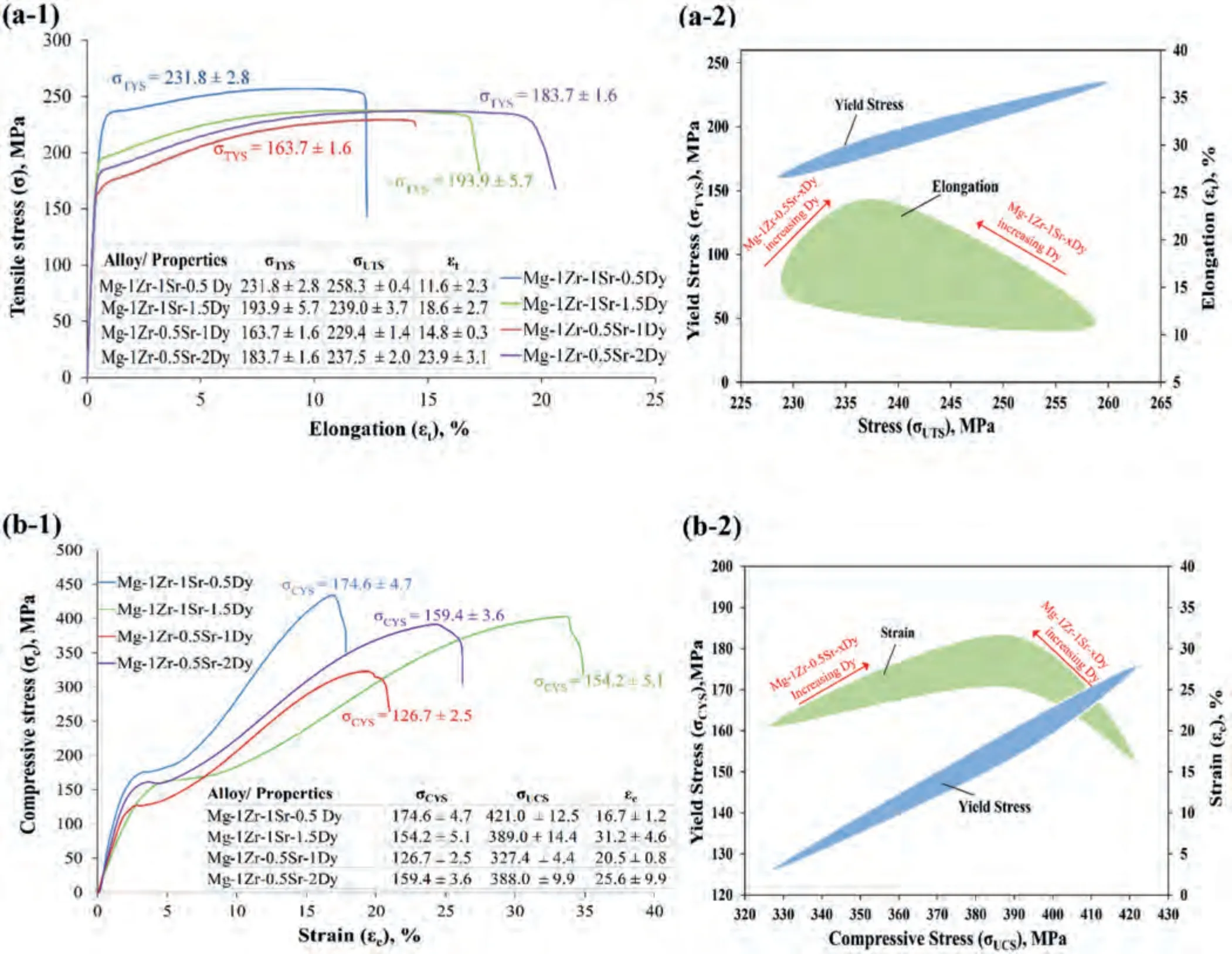
Fig.5.Mechanical properties of extruded Mg–Zr–Sr–Dy alloys: (a) tensile stress–strain graphs and Ashby plot (σUTS: ultimate tensile strength (MPa), σTYS:0.2% tensile yield strength (MPa),and εt: tensile elongation (%));and (b) compressive stress–strain graphs and Ashby plot (σUCS: ultimate compressive strength (MPa), σCYS: 0.2% compressive yield strength (MPa),and εc: compressive strain (%)).NB: ED and loading axis coincide with specimen longitudinal axis.
3.4.Mechanical properties of extruded Mg–Zr–Sr–Dy alloys
Fig.5 shows tensile and compressive stress-strain curves,as well as tabulated mechanical properties and Ashby plots of the extruded Mg–Zr–Sr–Dy alloys.The stress–strain curves show significant strain hardening during compression in contrast to tensile loading.As evident from the Ashby plots in Fig.5(a-2)and (b-2)that an increase in the Dy content within their respective compositions enhanced the elongation(εt)and compressive strain (εc).The Mg-1Zr-1Sr-1.5Dy and Mg-1Zr-0.5Sr-2Dy exhibited extraordinarily highεtof 18.6±2.7%and 23.9±3.1%,and ultrahighεcof 31.2±4.6% and 25.6±9.9%,respectively.The effect of Sr content on the tensile yield strength (σTYS) was observed because an increase in Dy from 0.5 to 1.5 wt.% in the Mg-1Zr-1Sr-xDy showed a decrease inσTYSof 16.3%,while an increase in Dy from 1.0 to 2.0 wt.% in the Mg-1Zr-0.5Sr-xDy showed an increase inσTYSof 12.2%.A similar trend was observed in the compression yield strength (σCYS),where a decrease inσCYSof 11.7% for Mg-1Zr-1Sr-xDy (x=0.5 to 1.5 wt.%) and an increase inσCYSof 25.8% for Mg-1Zr-0.5Sr-xDy (x=1.0 to 2.0 wt.%) were observed.The Mg-1Zr-0.5Sr-2Dy showed an improved tension–compression yield asymmetry (σCYS/σTYS)of 0.87,whereas theσCYS/σTYSratio for the other compositions ranged from 0.75 to 0.79.
As a comparison,the addition of 2.0 wt.%Dy to previously studied extruded Mg-1Zr-0.5Sr [70] exhibited a significant increase inεtof 181.2% at the expense of a moderate decrease inσTYSandσUTSof 12.5% and 8.3%,respectively.However,the compressive properties of extruded Mg-1Zr-0.5Sr-2Dy showed an increase inεc,σCYS,andσUCSby 74.2%,15.5%,and 34.2%,respectively.Furthermore,comparing the compressive properties between extruded and as-cast Mg–Zr–Sr–Dy alloys,exhibits similarεcbut significant increases of 191.0% and 50.4% inσCYSandσUCS,respectively.It may be noted that the pure extruded Mg exhibitedσTYS,σUTS,andεtof 84 MPa,189 MPa,and 12%,respectively [110].On the other hand,an AZ31 alloy extruded in similar conditions to those of this study,i.e.,at 350 °C and 450 °C with an extrusion ratio of 25:1,showed a higher average GS of 12.1 μm with slightly enhanced tensile properties but lower compressive strength than the extruded Mg–Zr–Sr–Dy alloys.As a result,a weakened tension–compression yield asymmetry (σCYS/σTYS) of 0.64 was observed in contrast to 0.87 in the extruded Mg–Zr–Sr–Dy alloys [111].
Improvement in mechanical properties from extrusion and RE addition to Mg alloys has been extensively reported[106,112,113].The pure Mg exhibits low ductility due to limited slip systems.This was overcome by the RE addition which activated the non-basal slip systems through reducing thec/aratio or increasing the stacking fault energy(SFE) [114,115].The RE addition has also shown promising results in improving the formability of Mg through weakening the texture [90].Further,the formation of hard eutectic from RE addition was reported to increase the strength of Mg alloys [116].Generally,the strengthening by RE addition in Mg alloys is via solid solution strengthening and a precipitation hardening mechanism [117].Thus,the benefits of an RE addition in Mg alloys can be exploited for automotive applications [118].Besides,good mechanical and corrosion properties of the Mg–RE alloys,the Mg–Dy alloy was also found suitable for the bone fixture application due to the good biocompatibility of Dy and its high solubility in Mg [9,116].
The strengthening mechanism in magnesium alloys is mainly attributed to grain refinement strengthening (Δσys),solid solution strengthening (Δσss),dislocation strengthening(Δσd),and precipitation strengthening (ΔσOrowan).The governing relationships are given by [119–121]:
whereΔσysis the increment in yield stress,kis the H–P coefficient,dis the average GS,τCRSSis the critical resolved shear stress,mis the Schmid factor,Mis the Taylor factor(the inverse of the Schmid factor),Gis the shear modulus,bis the Burgers vector of gliding dislocation,λis the effective inter-particle spacing,νis the Poisson ratio,Dpis the mean diameter of particles,rois the core radius of dislocation,Cis the binary alloy strengthening rate,Xis the atomic fraction of the solute,αis the Taylor constant andρis the dislocation density.Firstly,the role of GS strengthening given by the H–P relationship was disregarded,as the Mg-1Zr-0.5Sr-1Dy and Mg-1Zr-0.5Sr-2Dy exhibited similar GS but differentσys.Likewise,previously studied extruded Mg–Zr–Sr alloys did not show the dependence ofσTYSon the GS [70].Secondly,theσTYSandσCYSof the Mg-1Zr-1Sr-xDy (x=0.5 or 1.5 wt.%) were observed to be higher than those of the Mg-1Zr-0.5Sr-xDy (x=1 or 2 wt.%),i.e.,an increase in Sr-rich particles enhanced theσysas per the Orowan relationship (the volume fraction of the second-phase particles is denoted by the parameterλ).Thirdly,as per Eqs.(2) and (3),the Schmid factor (m) and Taylor factor (M),which influence the texture,may also affect theσys.Cáceres et al.[122] reported a Taylor factor of about 4.5 for random polycrystals in pure Mg,decreasing to about 2.1∼2.5 for polycrystals with textures that inhibited basal and prismatic slip while favoring pyramidal slip,whereas a Taylor factor of 6.5 was suggested for basal slip at low stress[123].Eq.(2)indicates that a decrease inσyscan be expected with an increase inm.
Based on the loading axis parallel to the ED,the averagemwas calculated for basal slip using EBSD software.The extruded Mg-1Zr-1Sr-0.5Dy,Mg-1Zr-1Sr-1.5Dy,Mg-1Zr-0.5Sr-1Dy,and Mg-1Zr-0.5Sr-2Dy alloys exhibited an averagemof 0.22,0.28,0.25,and 0.31,respectively.Consequently,a decrease inσyswith the addition of Dy to the Mg-1Zr-1Sr-xDy(x=0.5 to 1.5 wt.%) was solely attributed to the increase inmfrom 0.22 to 0.28.This was corroborated by the fact that as per Eq.(2),the ratios of≈ 1.2.Notwithstanding the above,in spite of an increase inmfrom 0.25 to 0.31 in the Mg-1Zr-0.5Sr-xDy (x=1 to 2 wt.%),an increase inσyswas considered to be due to the substantial precipitation of Dy-rich particles (EDS maps in Figs.3 and 4).This shows that the precipitation strengthening was more pronounced and effective than the texture contribution in the extruded Mg–Zr–Sr–Dy alloys [124].
On the other hand,based on both atomic size and shear modulus misfit models,the solid solution strengthening arises from the elastic interactions between the strain fields of a solute and dislocation [125,126].As the solute atoms have different shear modulus and sizes from the solvent atoms,additional strain fields on the lattice of the surrounding matrix can be imposed and restrict the dislocation motion in the local lattice,resulting to the solid solution strengthening [127].Whereas,in Mg–Realloys,the effect of valency on solid solution strengthening was also found critical[128].Conversely,addition of RE elements may also augment the solid solution ductilizing along with the strengthening.It was reported that Gd addition in Mg–Gd alloy up to 4 wt.% tends to reduce the difference in slip resistance between the prismatic and basal slips,thus increasing the strength and ductility simultaneously [129].Nonetheless,in the case of Mg–RE alloys,the Dy element was found to contribute most to the solid solution strengthening because of its high solubility in Mg matrix at low temperatures (∼10 wt.% at 200 °C) [130].
The increase in the yield strength from dislocation strengthening (Δσd) is primarily due to the interactions among dislocations that were developed during the extrusion.For example,
Stanford et al.[135] reported improved ductility with RE addition in an Mg–Gd alloy due to weakening of the texture.Similarly,a significant improvement in ductility was observed in a rolled Mg–Y alloy with a weakened texture and activation of pyramidal 〈c+a〉 slip [136–138].Consequently,large increases inεtandεcin the Mg–Zr–Sr–Dy alloys with Dy ≥1.5 wt.% can be attributed to the weakened texture,as is clearly evident from the pole figures in Fig.4b and d.The deformation mechanisms involved in thermomechanically processed alloys are mainly dependent upon the process parameters and the direction of the loading axis with respect to the crystallographic orientation.Tension along the ED where the basal planes are generally aligned parallel will cause deformation through both prismatic and basal slips.On the other hand,compression along the ED will cause the formation of a significant amount of extension twins {102},which reorients thecaxis toward the compression loading direction and enables pyramidal 〈c+a〉and basal slips as the deformation modes [83,139,140].The tensile stress–strain curves exhibit a ‘concave-down’ shape,indicative of crystallographic slip and slight strain hardening,whereas the compressive curves show a ‘concave-up’shape,signifying the presence of twins along with significant strain hardening until fracture [139].The difference in the stress–strain behavior clearly signifies different deformation mechanisms during tension and compression.The predominance of crystallographic slip in tension and {102} twinning followed by rapid S-shaped strain hardening in compression are considered to be responsible for such distinct behavior,thereby leading to the tension–compression yield asymmetry[105,141,142].The low level of strain-hardening behavior in the tensile stress–strain graph (Fig.5a) is considered to be due to the lack of {102} twin formation during tension in the ED [143].Moreover,the improved tension–compression yield asymmetry of 0.87 in the Mg-1Zr-0.5Sr-2Dy is solely attributed to the texture randomization,as evident in Fig.4d.
3.5.Tensile fracture analysis of extruded Mg–Zr–Sr–Dy alloys
Micrographs of the tensile fractured surfaces of the extruded Mg–Zr–Sr–Dy alloys are given in Fig.6.The ductility features,i.e.,the presence of dimples,is observed in all micrographs and these are mainly embedded with the Dy-rich particles,as shown in Fig.6c and d-2.Transgranular cracks are observed in the dimple-free regions with high Dy content of ≥10 wt.% (blue ellipses in Fig.6d-2).Similarly,regions with high Sr content also exhibited transgranular cracks,as indicated by the location Y in Fig.6a.On the other hand,low Dy content of ≤5 wt.% regions as measured by EDS showed dimples (Fig.6d-1).Cracks were also observed inside the dimples,probably initiated by the Dy-rich particles(blue ellipses in Fig.6b and d-1).The number and size of the Dy-rich particles were increased in the Mg-1Zr-0.5Sr-2Dy as compared to the rest of the compositions.The maximum size of the Dy-rich particles was observed to be around 6.5 μm(measured sizes of selected Dy-rich particles are shown in Fig.6d-2).
Overall,the presence of dimples in all the micrographs indicates a typical characteristic of ductile fracturing [144].The extruded Mg-1Zr-0.5Sr-2Dy showed a relatively high number of dimples with small microvoids which conformed to its maximum elongation of 23.9±3.1% (Fig.5a).A substantial difference in the atomic radii between Mg (141 pm) and Dy(228 pm) [9] would cause lattice distortion at the Mg and Mg2Dy interface and hence a higher dislocation density is expected at these interfaces.In Mg–RE alloys,under excess stress micro-cracks were generated at the interfaces,resulting in crack propagation across the grains and particles,and formation of dimples at the locations of the Mg–RE particles [145].It has been reported that REEs tend to segregate at GBs due to anisotropic diffusion behavior,i.e.,diffusion coefficients along theaaxis exhibit higher values than those of thecaxis in Mg alloys [101,146].Moreover,clusters of REEs show different atomic arrangements to normal Mg–RE precipitates found in the Mg system [147].Furthermore,RE segregation was found to improve the cohesion bond of GBs and thus promoted the transgranular failure mode [148].The cracks in regions enriched with Dy and Sr content were found to be oxidized and probably contained impurities as well as the Zr-rich phase.These sites were considered highly prone to cracking [149].
The deformation mechanism in extruded Mg alloys is strongly dependent on the pre-loading texture of the sample.Based on the CRSS,the tensile loading in the ED on a sample with a basal texture is generally expected to deform through prismatic 〈a〉 and pyramidal 〈c+a〉 slips with the lowest contribution from tensile twinning[150,151].However,Chakkedath et al.[152] demonstrated in extruded Mg-1Mn alloy that during tension in the ED,contraction twins were formed,which greatly influenced the deformation such that these twins evolved into {101} -{102} double twins and enhanced the basal slip activity due to the reoriented lattice within the twin volume,thereby turning the twin volume into a ‘soft spot’ for localized shear and void initiation.In addition to double twins,crack-induced twins were also found,promoting the nucleation of voids during tensile deformation[144].Consequently,it can be deduced that the randomized texture in the Mg-1Zr-0.5Sr-2Dy (Fig.4d) minimized the formation of such twins during tension and hence contributed to its ultrahigh ductility.
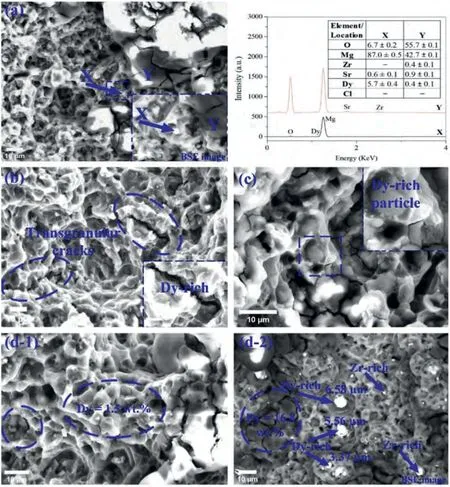
Fig.6.SEM micrographs of tensile fractured surfaces of extruded Mg–Zr–Sr–Dy alloys: (a) Mg-1Zr-1Sr-0.5Dy;(b) Mg-1Zr-1Sr-1.5Dy,(c) Mg-1Zr-0.5Sr-1Dy;and (d) Mg-1Zr-0.5Sr-2Dy.Elemental compositions via EDS are given in wt.%.Backscatter electron images are highlighted as BSE images.Explanation of highlighted regions is given in the text.
3.6.HE of extruded Mg–Zr–Sr–Dy alloys
HE evolution curves and the corresponding average CR(CRh) (mmy-1) of the extruded Mg–Zr–Sr–Dy alloys are given in Fig.7.The graph of each curve shows two distinct regions with different rates of hydrogen production,highlighted with black arrows.All the curves except that for Mg-1Zr-0.5Sr-1Dy exhibit similar initial slopes but different final rates.Moreover,the deviation from the initial slope of each alloy occurred at different time intervals.The minimum CRhof 15.14 mmy-1,followed by 17.48 mmy-1,was measured for the Mg-1Zr-0.5Sr-1Dy and Mg-1Zr-1Sr-1.5Dy,respectively.As clearly evident from Fig.7,no deviation from the initial slope was observed in the Mg-1Zr-0.5Sr-1Dy at completion of the test.
The instantaneous hydrogen evolution rateVH(ml cm-2d-1) is given by the slope of the volume of evolved hydrogen versus time and it directly relates to the instantaneous CRh[153]:
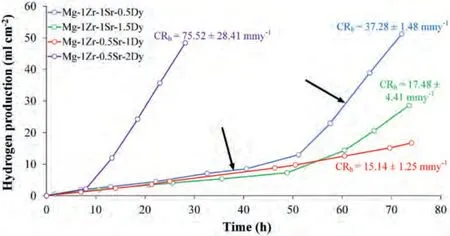
Fig.7.HE curves of extruded Mg–Zr–Sr–Dy alloys in SBF at 37 °C.Two regions exhibiting different slopes in curves are highlighted with black arrows.Average CRh of each alloy,calculated from the volume of hydrogen gas evolved,are also shown.
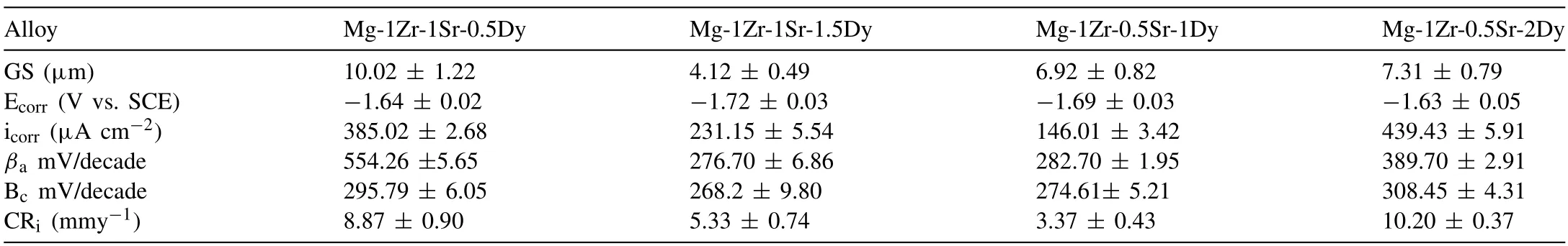
Table 2 Corrosion properties of extruded Mg–Zr–Sr–Dy alloys obtained from PDP curves.
The initial slope of the curve represents the general corrosion mechanism,i.e.,the anodic dissolution of the Mg matrix and the regeneration of a protective Mg(OH)2film in the presence of chloride salts [154],whereas a subsequent drastic increase in the slope indicates pitting corrosion,i.e.,localized corrosion as the dominant mechanism [155].As evident in Fig.7,early initiation of pitting corrosion after 8 h of testing caused the maximum CRhof 75.52 mmy-1in Mg-1Zr-0.5Sr-2Dy.Similarly,the pitting that initiated in Mg-1Zr-1Sr-0.5Dy and Mg-1Zr-1Sr-1.5Dy after 40 and 48 h exhibited CRhof 37.28 and 17.48 mmy-1,respectively.The lower initial and final slopes for the Mg-1Zr-1Sr-1.5Dy suggest that the alloy had higher corrosion resistance and the protective film was more effective than for the Mg-1Zr-1Sr-0.5Dy.It can be deduced that the addition of RE to the Mg alloys improved the CRh.However,in the Mg-1Zr-0.5Sr-xDy(x=1 or 2 wt.%)the opposite behavior was observed.It was reported that until the precipitation of the Mg–RE phase,addition of an RE to Mg alloys showed substantial improvement in the CRhand the alloy surfaces were well protected by an Mg(OH)2layer [156].As discussed earlier,significant precipitation of the Dy-rich phase was observed only in the Mg-1Zr-0.5Sr-2Dy (EDS map of Dy in Fig.3).
3.7.PDP curves of extruded Mg–Zr–Sr–Dy alloys
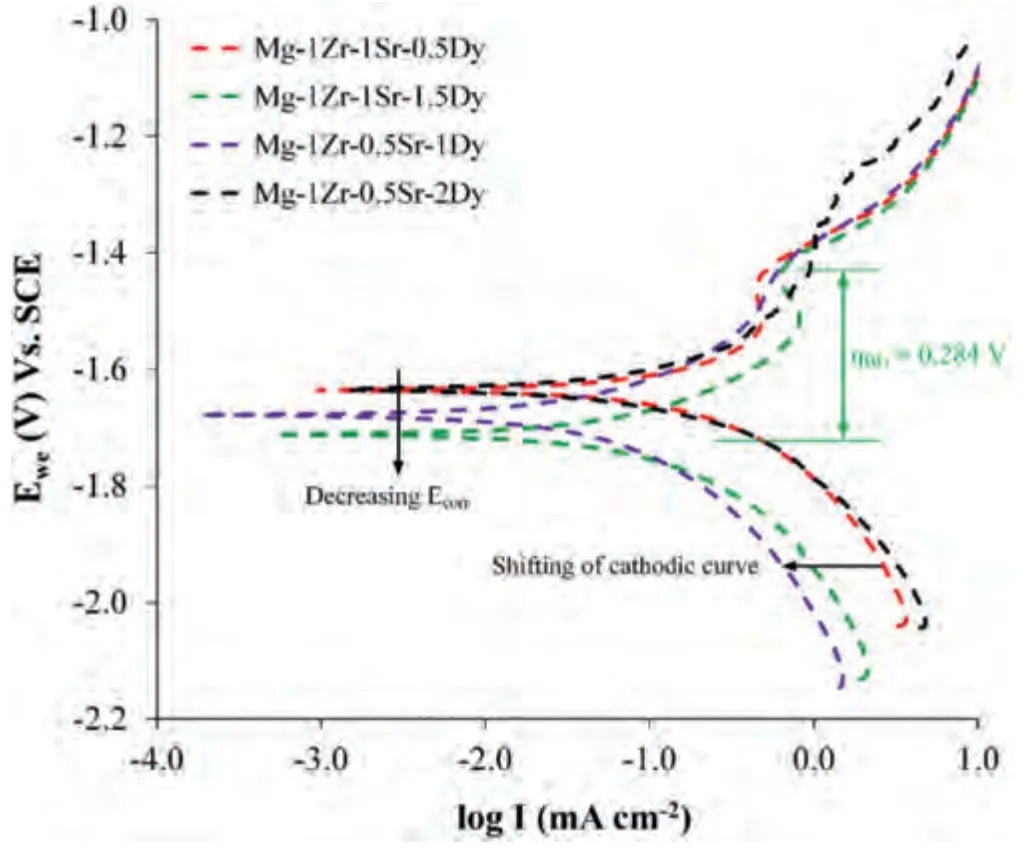
Fig.8.PDP curves of extruded Mg–Zr–Sr–Dy alloys.Maximum breakdown potential (ηBD) measured for Mg-1Zr-1Sr-1.5Dy is shown.Black arrows indicate shifting of cathodic curves and decrease in Ecorr.
PDP curves and relevant Tafel parameters of the extruded Mg–Zr–Sr–Dy alloys are given in Fig.8 and Table 2.The CR calculated from PDP is denoted CRi(mmy-1).The minimum CRiof 3.37 followed by 5.33 mmy-1were observed for the Mg-1Zr-0.5Sr-1Dy and Mg-1Zr-1Sr-1.5Dy,respectively.The corrosion potential (Ecorr) of the alloys ranged between -1.63 and -1.72 V vs.SCE.Both Sr and Dy showed influence on Ecorrsuch that,in the Mg-1Zr-1Sr-xDy (x=0.5 or 1.5 wt.%),an increase in Dy shifted the Ecorrto more negative potential,while the Mg-1Zr-0.5Sr-xDy (x=1 or 2 wt.%) showed the opposite trend.Both the Mg-1Zr-0.5Sr-1Dy and Mg-1Zr-1Sr-1.5Dy,having lower CRi,exhibited a shift in their cathodic regions toward the left (highlighted with a black arrow in Fig.8).In the anodic region,distinct humps representing the pitting potential (Epit) in the curve were observed at-1.45 V for all compositions,except the Mg-1Zr-0.5Sr-2Dy at -1.30 V.The maximum difference between the Ecorrand Epit,given by the breakdown potential (ηBD),was measured as 0.284 V in the Mg-1Zr-1Sr-1.5Dy alloy.The extruded Mg-Zr-Sr-Dy showed minimum CRiof 3.37 mmy-1in SBF,compared to 2.6 mmy-1for extruded pure Mg [157].
The composition and compositional distribution of the secondary phases in Mg alloys are crucial to the overall corrosion performance of the alloys [158].The electrochemical potential of Dy was reported to be -2.35 V,close to that of Mg(-2.37 V) [130].This implies that within the solid solution range,addition of Dy will not significantly impact on the Ecorrof the Mg matrix.Furthermore,a study of Mg–RE alloys showed that the corrosion potential of the RE-rich phases was more positive than that of Mg and hence the RE-rich phases generally acted as a cathode during galvanic corrosion[8,159].On the contrary,second phases in GW93 (an Mg-9Gd-3Y) alloy showed more negative potentials than the Mg matrix and were therefore preferentially corroded in the initial stages [160].Ding et al.[4] conducted polarization tests of Mg17Sr2and Mg2Dy phases,showing Ecorrvalues of around-1.52 and -1.54 V,respectively.These results indicate that Mg17Sr2is nobler than the Mg2Dy phase.Since corrosion is dependent on thermodynamics and both the anodic and cathodic kinetics,separate effects on the Ecorrcan be expected due to the presence of an REE,either as a dissolved constituent in a solid solution or as an intermetallic second phase within the Mg matrix [161–163].
Consequently,the decrease of Ecorrin the Mg-1Zr-1Sr-xDy(x=0.5 or 1.5 wt.%) was attributed to the increase of the dissolved Dy within a solid solution without any significant precipitation of the Mg2Dy phase (EDS maps of Dy in Fig.3).This caused a decrease in the cathodic kinetics.Similarly,the opposite trend in the Mg-1Zr-0.5Sr-xDy (x=1 or 2 wt.%)was considered to be due to the greater precipitation of intermetallic Mg2Dy phase which increased the cathodic activity and hence shifted the curve toward the positive potential[164].Overall,both the Mg-1Zr-0.5Sr-1Dy and Mg-1Zr-1Sr-1.5Dy,having low CR,showed more negative Ecorrthan the other two compositions,i.e.,decreased cathodic activity and increased film passivation in the anodic region (Fig.8).
It was reported that precipitation of the Mg24Dy5phase increased the CR due to the galvanic effect in Mg–Dy alloys[9].On the other hand,an increase in RE within a solid solution range in the Mg matrix decreased the CR until precipitation occurred [165].A shift in the cathodic region toward the left implied a reduction in the cathodic kinetics and may have led to a lower Icorr[8].As evident from the SEM images and EDS maps in Figs.3 and 4,considerable precipitation of the Dy-rich phase was only observed in the Mg-1Zr-0.5Sr-2Dy alloy.It can therefore be deduced that the addition of Dy up to 1.5 wt.% to the Mg-1Zr-xSr-yDy suppressed the cathodic kinetics and reduced the CRi,while with the addition of 2 wt.% Dy to the Mg-1Zr-0.5Sr-2Dy a substantial increase in CRiwas observed due to the precipitation of the Mg2Dy phase.
Benefits of RE alloying in Mg alloys included removal of impurities (i.e.,the ‘scavenger effect’) and formation of a stabilized surface layer [8].Improvement in the corrosion resistance due to the formation of RE oxides on Mg alloy surfaces has been reported in several studies[146,152–154].Brar et al.[166] demonstrated that RE oxides are thermodynamically stable and may be incorporated into existing Mg(OH)2layers or form RE(OH)x.The anodic regions on all compositions except the Mg-1Zr-0.5Sr-2Dy clearly revealed the passive tendency at Epitof -1.54 V,beyond which pitting corrosion is likely to occur.The maximum breakdown potential (ηBD) of the Mg-1Zr-1Sr-1.5Dy showed that within the solid solution range,an increase in Dy up to 1.5 wt.% facilitated the formation of a stabilized and effective passive layer[160].
3.8.EIS of extruded Mg–Zr–Sr–Dy alloys
EIS plots of the extruded Mg–Zr–Sr–Dy alloys and the equivalent electric circuit model used for fitting the curves are given in Fig.9.The relevant electrochemical parameters and CReisof extruded Mg–Zr–Sr–Dy alloys are given in Table 3.Corrosion currents (ieis) and CReiswere calculated from the Stern–Geary equation,which included the Tafel slopes (βaandβc) and polarization resistance (Rp) obtained from the PDP and EIS experiments,respectively [153,167]:
The equivalent electric circuit model is mainly represented by the two impedance circuits in series,comprised of the intrinsic charge transfer resistance(Rct)and film resistance(Rf),which were connected in parallel to the constant phase elements.Depending on the corrosion mechanism,an inductor element may cause a decrease in resistance at low frequencies which corresponds to the polarization resistance (Rp) in EIS studies [167,168].As per the circuit configuration of the equivalent model,CReisis governed by the net impedance of the circuit in series,thus revealing the combined effect and individual importance of the Faradic reaction kinetics (Rct)and surface protection films (Rf).
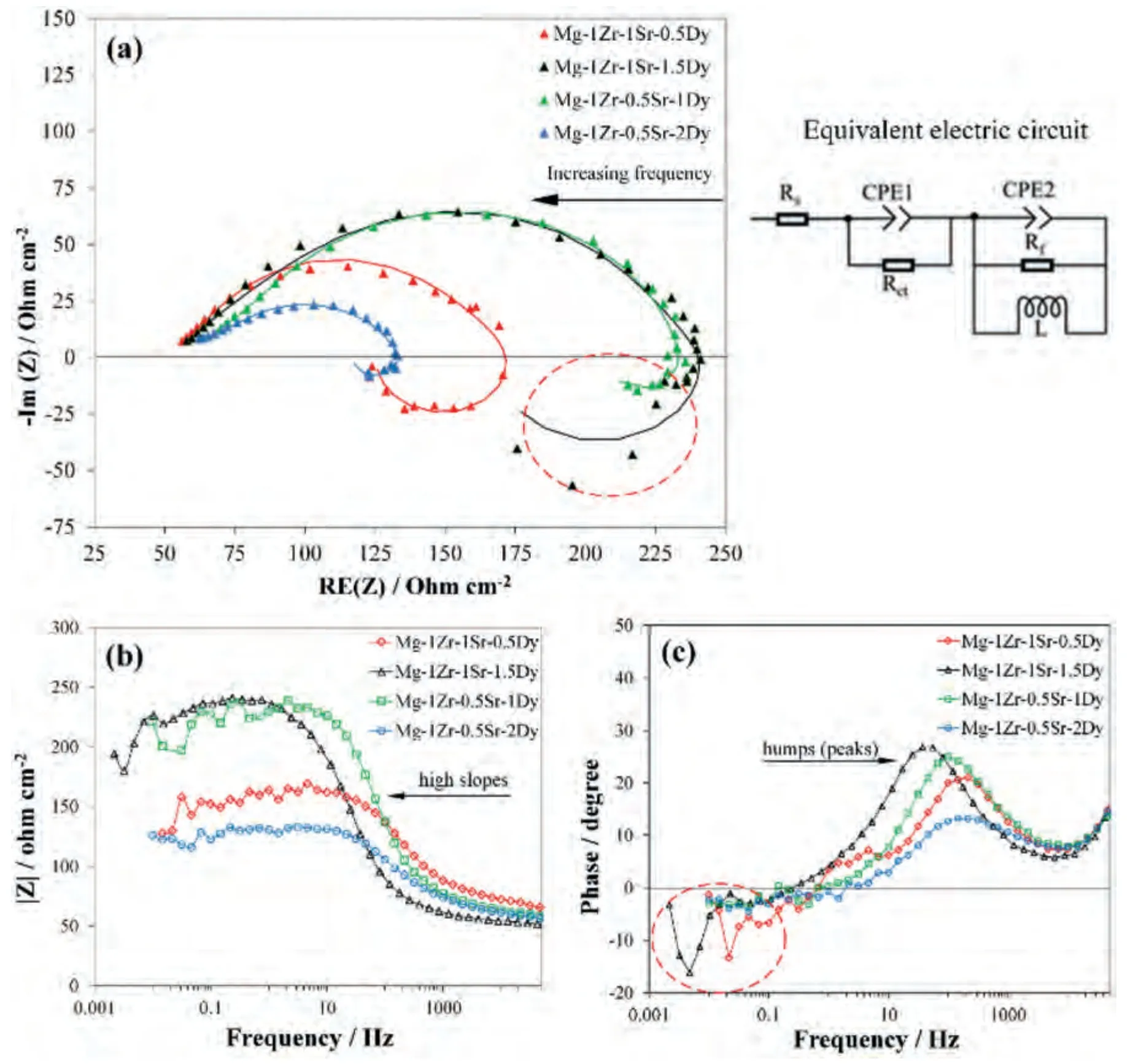
Fig.9.EIS plots of extruded Mg–Zr-Sr-Dy alloys in SBF at 37 °C: (a) Nyquist plot;(b) and (c) Bode plots.
Clearly,the semicircles observed in the Nyquist plot are depressed and elongated (Fig.9a).These elongated semicircles are actually composed of two overlapping capacitive loops and are also seen in the equivalent circuit model[169,170].The diameter of a capacitive loop generally corresponds to the corrosion resistance [171].All curves exhibited inductor behavior at low frequencies,which indicates the occurrence of pitting,dissolution of protective films,or presence of adsorbed species on the surface such as Mg(OH)+ads,Mg+,etc.[172,173].The capacitive loops of the Mg-1Zr-0.5Sr-1Dy and Mg-1Zr-1Sr-1.5Dy were similar and exhibited large resistances which conformed to their low CRi(Fig.9a).The enhanced corrosion resistance of these alloys is also observed in the Bode plots,which show large slopes(Fig.9b).However,a difference in the inductive behavior,i.e.,a relatively large inductive loop in the Mg-1Zr-1Sr-1.5Dy(red circles in Fig.9a and b) caused a decrease in Rctfrom the maximum 217.7 to 130.7Ωcm2(Table 3).In addition to Rct,the Mg-1Zr-0.5Sr-1Dy also showed a maximum Rfof 196.8Ωcm2,which corresponds to the formation of a strong surface protection film with the least pitting,as shown by its minimum inductance of only 6.5 H cm2[56].As expected,the second highest Rfof 94.1Ωcm2was observed for the Mg-1Zr-1Sr-1.5Dy,but with a high inductance value.An increase in Dy in the Mg-1Zr-0.5Sr-xDy (from 1 to 2 wt.%)caused a drastic reduction in Rct,which is attributed to an increase in micro-galvanic corrosion due to Mg2Dy precipitation [168,172].Consequently,it is deduced that addition of Dy within the solid solution range facilitated the formation of a stabilized protective layer,enhanced Rct,and thus reduced CReis.Moreover,precipitation of the Mg2Dy phase was found to be unfavorable to the corrosion resistance in these extruded Mg–Zr–Sr–DY alloys.
3.9.Corrosion morphology of extruded Mg–Zr–Sr–Dy alloys
XRD and XPS spectra of the corroded surfaces of the extruded Mg–Zr–Sr–Dy alloys after 24 h of immersion in SBF at 37 °C are given in Fig.10.However,the low-magnification SEM micrographs reveal nonhomogeneous corrosion along with the presence of pit holes in all compositions except the Mg-1Zr-0.5Sr-1Dy.Moreover,the extent of the corrosion and pitting observed in the micrographs is in accordance with their respective CR as measured in these alloys(Fig.10a–d).However,the corroded surface of the Mg-1Zr-0.5Sr-1Dy shows completely homogenous corrosion with no pitting.The XRD of alloys with ≥1 wt.% Dy show peaks of Dy2O3(Fig.10e),while Mg(OH)2,Sr2P2O7,and Zr2O3can be seen in both XRD and XPS spectra (red circle in Fig.10f).The absence of Dy2O3in the XPS spectrum indicates that Dy2O3was not present within 12 nm of the surface depth [173].
Further evaluation of the surface composition and distribution of the corrosion products was conducted through EDS and is given in Fig.11.EDS analysis of the corroded Mg-1Zr-1Sr-0.5Dy did not show the presence of Dy on the surface.The surface layer was mainly composed of Mg(OH)2;however,the presence of Dy2O3was observed below the surface layer (location X in Fig.11a-2).Moreover,at a few locations localized presence of hydroxyapatite (Ca10(PO4)6OH2)[174] and needle-like MgCl2were noted (locations 3 and 4 in Fig.11a-1).Furthermore,with an increase in Dy to 1.5 wt.%in the Mg-1Zr-1Sr-xDy,varying content of Dy was observed at all locations.The localized regions with the highest Dy content formed tall islands of corrosion products and did not contain any pit holes(location X in Fig.11).This implies that Dy was segregated within the matrix and the Dy-rich regions acted as a cathode in relation to theα-Mg matrix.Similar surface morphology was observed for the Mg-1Zr-0.5Sr-2Dy(Fig.11d),but,with an increase in pitting.However,in the Mg-1Zr-0.5Sr-1Dy the distribution of Dy within the Mg matrix was fairly homogenous with negligible pitting (Fig.11c).The entire surface was homogenously covered with a protective film and exhibited good stability even after 24 h immersion in SBF (Fig.10).
Mg(OH)2is a typical product of an electrochemical reaction of Mg with water and is considered to be a partially protective film [175].Azzeddine et al.[173] reported the formation of Dy2O3in the inner layers of corrosion products whereas the surface was mainly comprised of Mg(OH)2.A Pilling–Bedworth ratio of approximately 1.25 was calculated for the Dy2O3,which was well-above 0.81 for MgO and closer to that of the Mg(OH)2reported as 1.780 [176,177].This suggests that internal stresses were reduced during the growth of the passive film and that the Dy2O3and Mg(OH)2could coexist [178].The segregation of the second phase at the GBs was found to have two major effects on the corrosion in Mg alloys,namely,inducing a galvanic couple and modifying the cathode-to-anode area ratio [179].An increase in CR due to the segregated areas at the GBs and interdendritic regions has also been reported in Mg–RE alloys [180,181].As evident from the EDS maps of Dy in Fig.3,segregation of the Dy became pronounced at ≥1.5 wt.% and would therefore have promoted pitting in these localized regions.Overall,contributions to the corrosion resistance by Sr2P2O7and Zr2O3are considered to be limited due to their low solubilities.Additionally,the precipitated Mg17Sr2and Zr-rich particles are known to act as cathodes in relation toα-Mg.Consequently,formation of most of the Sr2P2O7and Zr2O3during the anodic dissolution was expected due to their dissolved constituents in the Mg matrix.Nonetheless,the presence of Sr-incorporated hydroxyapatite was observed in the present study and has been reported to improve the CR and biocompatibility [6,70].
3.10.Corrosion mechanism of extruded Mg–Zr–Sr–Dy alloys
A comparison of the CRs obtained from the HE,PDP,and EIS methods applied in this study is given in Fig.12.The CRs showed similar trends for each composition.The differences in CR among these respective techniques has been examined previously in various studies [158,167,168,182].A comparison of minimum CRs between the present extruded and previously studied as-cast Mg–Zr–Sr–Dy alloys [4],revealed a decrease of 52.9% in the extruded samples.Whereas,with Dy addition,a decrease of 26.2% in the CR was measured between the previously studied extruded Mg–Zr–Sr [70] and present Mg–Zr–Sr–Dy alloys.Thus,it is strongly suggested that both extrusion process and addition of Dy improved the CR.
In view of the findings discussed above,a corrosion mechanism is proposed for the extruded Mg-1Zr-0.5Sr-1Dy alloy,which showed the lowest CR among all the alloys.The microstructure revealed the presence of Mg17Sr2 and Mg2Dy phases at the GBs and extensive twinning within the grains.Moreover,it is noted that both the Mg17Sr2and Mg2Dy acted as cathodes during the corrosion of the extruded Mg–Zr–Sr–Dy alloys.The addition of RE within the solid solution range caused a decrease in the corrosion potential (Ecorr),hence reducing the cathodic activity in the Mg–Zr–Sr–Dy alloys.Furthermore,an increase in Dy content enhanced the formation of Dy2O3,which was mainly observed in the inner layers(below 5 nm) of the passive film.However,at higher content of Dy ≥1 wt.% in solid solution,pronounced segregation of the Dy element was observed at the GBs and interdendritic regions,which induced a galvanic effect and promoted nonhomogenous corrosion along with the formation of pit holes on the surface.During the initial stage,anodic dissolution of theα-Mg matrix in SBF took place and a protective film of Mg(OH)2,mainly embedded with the evenly distributed Dy2O3along with minor quantities of Sr2P2O7and Zr2O3,was formed on the surface.The uniform dissolution ofα-Mg and even distribution of Dy2O3in the passive film were mainly due to the homogenous dispersal of Dy in the Mg matrix,with a negligible segregation effect and without any Mg2Dy precipitation.Consequently,a stabilized passive film exhibiting high charge-transfer and film resistances was observed in the extruded Mg-1Zr-0.5Sr-1Dy alloy.The presence of a strong passivation film was corroborated by the maximum breakdown potential (ηBD) of 0.284 V vs.SCE measured in the Mg-1Zr-0.5Sr-1Dy alloy.In addition,the formation of hydroxyapatite due to the presence of Sr was observed at a few locations,which could have improved the corrosion resistance.Notwithstanding the above,contributions by a strong basal texture and large number of tension twins in the Mg-1Zr-0.5Sr-1Dy alloy cannot be ruled out.Based on the proposed mechanism,the following corrosion reactions are suggested [160,183,184].
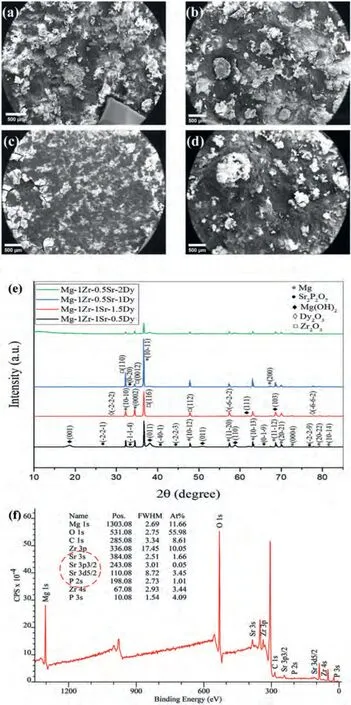
Fig.10.SEM micrographs,XRD and XPS spectra of corroded surfaces after immersion in SBF for 24 h at 37 °C: (a–d) SEM images of Mg-1Zr-1Sr-0.5Dy,Mg-1Zr-1Sr-1.5Dy,Mg-1Zr-0.5Sr-1Dy,and Mg-1Zr-0.5Sr-2Dy,respectively;(e) XRD spectra;and (f) XPS spectrum.
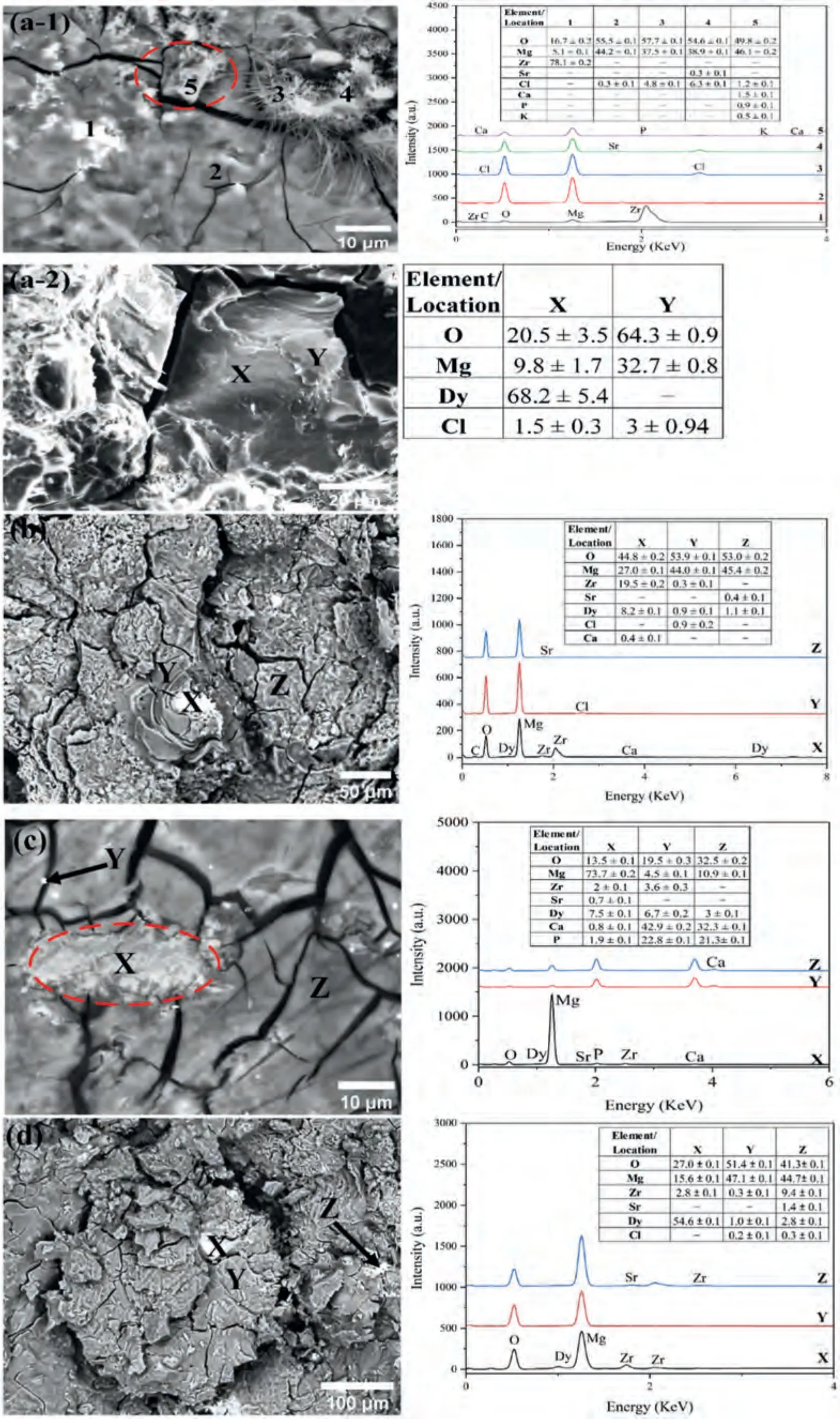
Fig.11.SEM images and EDS spectra of corroded surfaces of extruded Mg–Zr–Sr–Dy after 24 h of immersion in SBF at 37 °C: (a-1) Mg-1Zr-1Sr-0.5Dy;(a-2) Mg-1Zr-1Sr-0.5Dy;(b) Mg-1Zr-1Sr-1.5Dy;(c) Mg-1Zr-0.5Sr-1Dy;and (d) Mg-1Zr-0.5Sr-2Dy.Red circles indicate regions with presence of calcium hydroxyapatite.Elemental compositions from EDS are in wt.%.
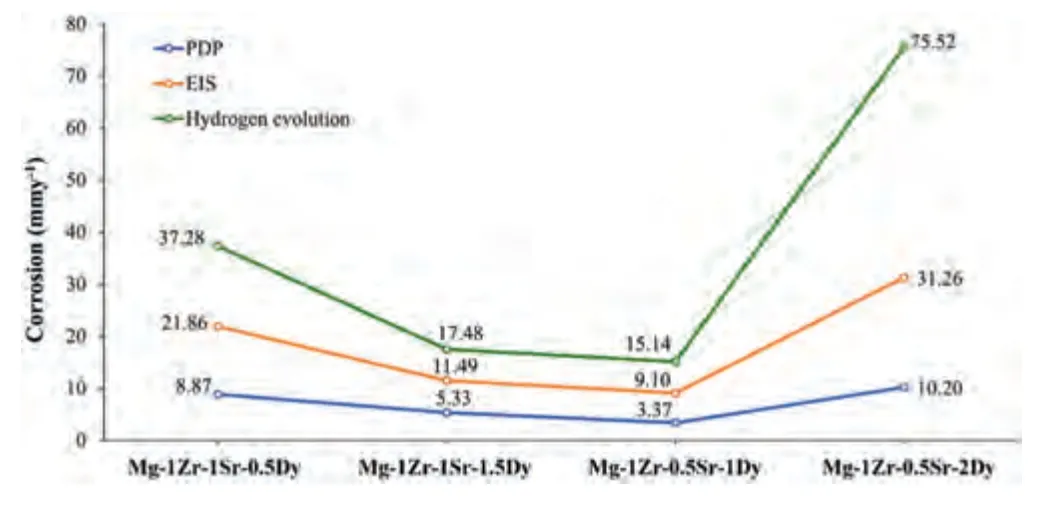
Fig.12.Comparison of CRs obtained from HE,PDP,and EIS methods in extruded Mg–Zr–Sr–DY alloys.
The corrosion of Mg converts metallic Mg to the stable ion,Mg2+in two electrochemical steps.These anodic reactions Eqs.(10) and (11),are balanced by the cathodic partial reaction Eq.(12).The uni-positive ion,Mg+is highly reactive and can react with water.Thus,a fraction,k,of the uni-positive Mg ion reacts electrochemically to Mg2+(Eq.(11)),and the complement reacts chemically via Eq.(13).The overall reaction is given by Eq.(14).
Anodic dissolution of dissolved Dy and Sr in the Mg matrix in accordance with Eqs.(15) and (16).
In the meantime,surface hydroxide film is formed accompanying the dissolution reactions.The newly formed Sr(OH)2is readily dissolved in the solution due to its high-water solubility,havingKsp=3.2 × 10-4.Consequently,formation of the strontium phosphate is facilitated in SBF solution[185,186].
Dysprosium hydroxide is insoluble in water withKsp=1.4 × 10-22and thus may form oxides [187].
3.11.In vitro cytotoxicity and cell adhesion of extruded Mg–Zr–Sr–Dy alloys
Thein vitrocytotoxicity of the extruded Mg–Zr–Sr–Dy alloys was evaluated by measuring the viability of the SaOS2 cells after 3 d of exposure in a cell-culturing medium.Thereafter,cell adhesion on the surface of the cultured samples was investigated under SEM.A graph of the cell viability (CV)and corresponding SEM images of the cultured samples are given in Fig.13.The CV measured with respect to the control(i.e.,media only)showed a substantial improvement (p<0.05)with Sr addition to the extruded Mg–Zr–Sr–Dy alloys.A maximum CV of 102.3% followed by 94.51% was measured for the Mg-1Zr-1Sr-0.5Dy and Mg-1Zr-1Sr-1.5Dy,respectively.However,with Dy addition a slight decrease in CV of 7.6%was observed in the Mg-1Zr-1Sr-1.5Dy.Furthermore,the effect of Dy addition to the Mg-1Zr-0.5Sr-xDy(x=1 or 2 wt.%)was found to be statistically insignificant.The SaOS2 cell adhesion and morphology observed via SEM were found to be in accordance with the respective CV of the samples,given in Fig.13a.The Mg-1Zr-1Sr-xDy (x=0.5 or 1.5 wt.%) surfaces showed well-spread lamellae (flattened cells) with extended lamellipodia and long filopodia with drumstick-shaped ends(red arrow and circle in Fig.13c),thereby exhibiting good cell adhesion [188–190].Aggregation of the cells was also observed at a few locations,as indicated with a red circle in Fig.13b-1.The globular structures observed at the edge of a lamellipodium in Fig.13b-2 were probably due to the retracted cell processes [190].In addition to the flattened cells,a few cells showed rounded morphology with shortened filopodia in the Mg-1Zr-0.5Sr-xDy (x=1 or 2 wt.%),as evident in Fig.13 (d and e).This may be attributed to slow growth kinetics or as a result of the retraction processes[189,191].
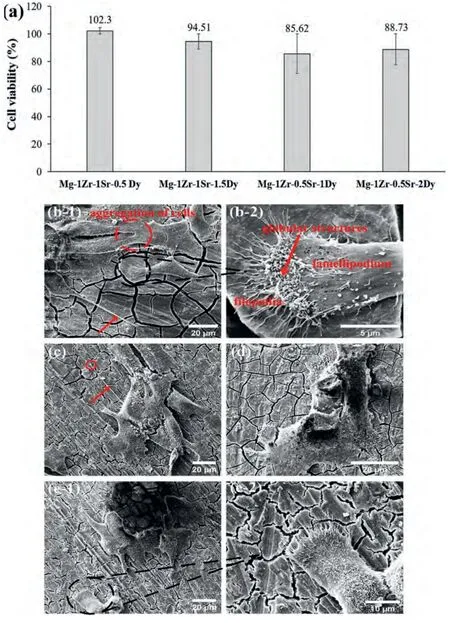
Fig.13.Cytotoxicity and cell adhesion of extruded Mg–Zr–Sr–Dy alloys: (a) cell viability of SaOS2 cells after culturing for 3 d and SEM micrographs of SaOS2 cells on: (b) Mg-1Zr-1Sr-0.5Dy;(c) Mg-1Zr-1Sr-1.5Dy;(d) Mg-1Zr-0.5Sr-2Dy;and (e) Mg-1Zr-0.5Sr-1Dy.Long extended filopodia are highlighted with red arrows in Fig.13b-1 and 13c.
In vitroandin vivostudies have shown direct correspondence of the CR with the biocompatibility of Mg alloys [192,193].Similarly,a decrease in CR with RE addition showed enhanced biocompatibility as the Mg–RE alloys possessed significantly better capability to sustain endothelial cell attachment and growth with no adverse cytotoxicity in human aorta endothelial cells for 7 days [194].Furthermore,cytotoxic effects of REs were observed beyond concentrations where localized corrosion occurred [195].A comprehensive biocompatibility evaluation of the Mg-10Dy revealed that Dy did not induce cytotoxic effects and the decrease in CV was mostly due to the Mg2+ions released during corrosion [196].Moreover,Dy2O3showed acceptable CV up to a concentration of 1000 μg/mL and was a potential constituent of the contrast agent in high-field magnetic resonance imaging (MRI) [197].The addition of Sr to Mg has been observed to increase the alkaline phosphatase activity of MG63 cells and enhance the bone density around implants [3,5,198,199].Li et al.[200] demonstrated that the addition of Sr at 2 wt.% to Mg-1Ca-xSr(x=0.2 to 2 wt.%) induced higher CV,protein adsorption,alkaline phosphatase activity,extracellular mineralization,and osteogenesis via the extracellular signal-regulated kinase pathway.
To study the effect of extrusion on cytotoxicity,the results for CV observed in the extruded Mg–Zr–Sr–Dy alloys were compared with those of similar as-cast compositions reported earlier [3,4].In spite of the higher Sr content of 2 wt.%,the CV of the as-cast Mg-1Zr-2Sr and Mg-1Zr-2Sr-1Dy was similar to that of the extruded Mg-1Zr-1Sr-0.5Dy alloy.This shows that cytotoxicity was reduced upon extrusion of the Mg–Zr–Sr–Dy alloys.Another salient difference in the microstructure was the distribution of Sr within the matrix.The extruded alloy exhibited considerable presence of elemental Sr within the matrix and at the GBs as the Mg17Sr2phase(EDS maps of Sr in Figs.3 and 4),whereas in the as-cast Mg–Zr–Sr–Dy,Sr content was observed to be relatively low in the Mg matrix [4].Consequently,the enhanced biocompatibility of the extruded Mg–Zr–Sr–Dy alloys is attributed to their decreased CR and increased Sr distribution within the Mg matrix.
4.Conclusion
A comprehensive evaluation of the mechanical,corrosion,and biocompatibility properties of the extruded Mg–Zr–Sr–Dy alloys was performed.These properties of the extruded Mg–Zr–Sr–Dy alloys have not been reported on earlier.The effects of alloying elements and textures on the microstructure,tensile and compressive properties,corrosion behavior,and biocompatibility were investigated.In addition,based on the findings a corrosion mechanism was proposed.The main conclusions are as follows:
1.Alloys with Dy content ≥1.5 wt.% showed REE textural components in the EBSD inverse pole figures.Moreover,all compositions exhibited a basal texture except Mg-1Zr-0.5Sr-2Dy,which showed a randomized texture with a strong RE component due to PSN and a change in the recrystallization mode from discontinuous to continuous dynamic recrystallisation,leading to an improved tension–compression yield asymmetry (σCYS/σTYS) of 0.87.
2.A significant increase in ductility was observed with an increase in Dy content.The highest tensile elongation of 23.9% and maximum strain of 31.2% were observed for the Mg-1Zr-0.5Sr-2Dy and Mg-1Zr-1Sr-1.5Dy,respectively.
3.CRs calculated via HE,PDP,and EIS methods showed similar trends for each composition.The minimum CR of 3.37 mmy-1and breakdown potential of 0.284 V were observed for the Mg-1Zr-0.5Sr-1Dy in PDP testing.Furthermore,EIS study confirmed the presence of a strong passive layer with a maximum charge-transfer resistance of 217.7Ωcm2,film resistance of 196.8Ωcm2,and minimum inductance of only 6.5 H cm2.
4.XRD patterns and XPS spectra of the corroded surface revealed the presence of an Mg(OH)2film embedded with Dy2O3in the inner layers.Pilling–Bedworth ratios of 1.25 and 1.780 were calculated for the Dy2O3and Mg(OH)2,respectively.This suggests the presence of a reduced stress within the film as compared to that of the MgO,hence led to the formation of a more stabilized passive layer.
5.The corrosion mechanism suggests that both the Mg17Sr2and Mg2Dy phases acted as cathodes in the Mg–Zr–Sr–Dy alloys.Moreover,the corrosion was greatly influenced by the segregation of Dy within the matrix and the precipitation of the Mg2Dy phase.
6.Extrusion of the Mg–Zr–Sr–Dy alloys improved the CV and cell adhesion of SaOS2 cells due to a decrease in CR and enhanced Sr distribution within the Mg matrix.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the financial support for this research by the Australian Research Council(ARC)through the Future Fellowship (FT160100252) and the Discovery Project(DP170102557).The authors also acknowledge the scientific and technical assistance of RMIT University’s Microscopy and Microanalysis Facility,a linked laboratory of the Australian Microscopy &Microanalysis Research Facility.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2023.09.031.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Ultrasonic solidification mechanism and optimized application performances of ternary Mg71.5Zn26.1Y2.4 alloy
- Superplastic behavior of a fine-grained Mg-Gd-Y-Ag alloy processed by equal channel angular pressing
- GO/MgO/Mg interface mediated strengthening and electromagnetic interference shielding in AZ31 composite
- Experimental and theoretical studies on two-dimensional vanadium carbide hybrid nanomaterials derived from V4AlC3 as excellent catalyst for MgH2
- Electrochemical synthesis of boron-containing coatings on Mg alloy for thermal neutron shielding
- Achieving high-strain-rate and low-temperature superplasticity in an ECAP-processed Mg-Y-Er-Zn alloy via Ag addition
