Reaction characteristics of magnesium production under argon flow by silicothermic reduction and numerical simulation of argon entrainment process
2023-12-27ShimingZhangChaoZhangGengpengMaiJianxunSongaYusiCheaJilinHea
Shiming Zhang ,Chao Zhang ,Gengpeng Mai ,Jianxun Songa, ,Yusi Chea,,∗ ,Jilin Hea,
aSchool of Material Science and Engineering, Zhengzhou University, Zhengzhou 450001, China
b Henan Province Industrial Technology Research Institute of Resources and Materials, Zhengzhou University, Zhengzhou 450001, China
cSchool of Thermal Engineering, Shandong Jianzhu University, Jinan 250101, China
Abstract In this study,the reaction characteristics of reduction of calcined dolomite with ferrosilicon under argon flow to produce magnesium were studied by conducting experiments Pidgeon pellets were used to study the effect of reduced temperature,argon flow,and reduced time on the conversion of calcined dolomite reduction by ferrosilicon.The results show that the conversion significantly increases with the increase in the reduction temperature and reduction time.The conversion first increases and then decreases with the increase in argon flow.The highest conversion was obtained when the argon flow rate was 3 L·min-1,and a nearly spherical shape,nanoscale magnesium powder was obtained.Then the characters of the circulating argon entrainment process were numerically studied by ANSYS Fluent 17.A physical model of multilayer pellet arrangement was established,and a numerical calculation model of chemical reaction,radiation,heat conduction,and convection heat transfer was constructed.This confirms that high-temperature argon can effectively strengthen the heat exchange between pellets,improve the heat transfer efficiency,and facilitate the pellets to react quickly.When the conversion is 80%,the production efficiency increased by about 28.6%.In addition,the magnesium production efficiency showed an increase tendency with the increase of the argon inlet flow rate.
Keywords: Silicothermic reduction of magnesium;Enhanced heat transfer;Convection heat transfer;Numerical simulation;Argon flow.
1.Introduction
Magnesium and magnesium alloys are mainly used as additives and structural materials [1,2];they are widely used in automotive[3],aerospace[4],military equipment[5],medical[6],3D products [7],and other fields [8–11],known as “21st Century Green Engineering Materials [12,13];.China has the largest magnesium reserves,and China is also the largest producer and exporter of magnesium.Since the 1990s,because of the advantages of fast construction,high purity,simple operation,and low investment cost,the Pidgeon process for magnesium production has been rapidly developed in China[14,15].After 2007,80% of the primary magnesium in the world is produced in China,and the primary magnesium in China is mainly produced by the Pidgeon process [16].In the recent decades,significant progress has been made on the Pidgeon process in China,but there are still some problems such as high energy consumption,heavy pollution,high labor intensity,and high production cost [17–19].Therefore,it is essential to develop low-cost green magnesium production technologies to boost the rapid development of magnesium industry.
It is an important research direction to use low-cost carbon reducing agent instead of expensive ferrosilicon for magnesium production.Professor Yang of Kunming University of science and technology and his group studied the thermodynamics and dynamics of magnesium production by carbothermal process [20],which established a solid theoretical foundation.They also investigated the behavior of CaF2and magnesium and carbon monoxide vapor in reduction process in vacuum [21,22].Li et al [23].and Xie et al [24].studied the kinetics mechanism of carbothermic reduction of magnesia and calcined dolomite respectively.In order to inhibit the reaction between magnesium and carbon monoxide,the CSIRO[25,26] used Laval nozzle to coagulate the supersonic magnesium vapor in the lab.Fu et al[27,28].studied the kinetics and mechanisms of magnesium production using Aluminothermic reduction process and found that its reduction efficiency was higher than that of the silicothermic method.Professor Zhang of Northeastern University and his team [29–31] proposed a novel short process for magnesium production by silicothermic reduction of pre-prepared dolomite pellets,in which the calcination and reduction were performed in one retort.They also studied the isothermal kinetic analysis of extracting magnesium from prefabricated pellets in flowing argon [32],and the nucleation and condensation of Magnesium vapor carried by argon [33].Morsi and Ali [34],Wulandari et al [35].,Kazhikenov et al[36].,and Barua and Wynnyckyj[37]studied the kinetic of silicothermic reduction of magnesium in argon flowing by isothermal method.In addition,the raw materials of silicothermic process are mainly oxides,and the pellets are pressed with a low thermal conductivity and high thermal resistance [38,39],which leads to low production efficiency.Our research group investigated the kinetic of silicothermic reduction of magnesium in argon flowing by non-isothermal method and the numerical simulation study of heat transfer enhancement of single-layer pellet by argon [40,41].It was found that the kinetics model of the best statistical fit base on experimental data was two consecutives probably and the heat transfer efficiency between pellets could be enhanced by circulating argon.
In this study,the effect of reduction temperature,reduction time,and argon flow rate on the conversion was studied through the experimental platform.Based on the singlelayer pellet data simulation,a physical and numerical model of multilayer pellet was established,and the effect of argon characteristics on the enhanced heat transfer efficiency and conversion of pellet was studied using a numerical simulation method.
2.Materials and experiments
2.1.Raw material composition and pellet preparation
The pellets used in this study are industrial pellets of Pidgeon process from Dongyi Magnesium Industry Co.,Ltd.in Shanxi Province.The forming process of pellets is shown in Fig.1,and the composition of raw materials is shown in Table 1.The dolomite ore was calcined for 2.5 h at the temperature of 1150 °C in a rotary kiln,then it was cooled down to the room temperature for weighing and mixed with the ferrosilicon and fluorite.Next,the mixed raw materials are ground into fine powder less than 30 μm by ball mill,and mixed powder was compressed into pellets like peach pit by twin-roller machine with the size of 47 × 25 × 20 mm.
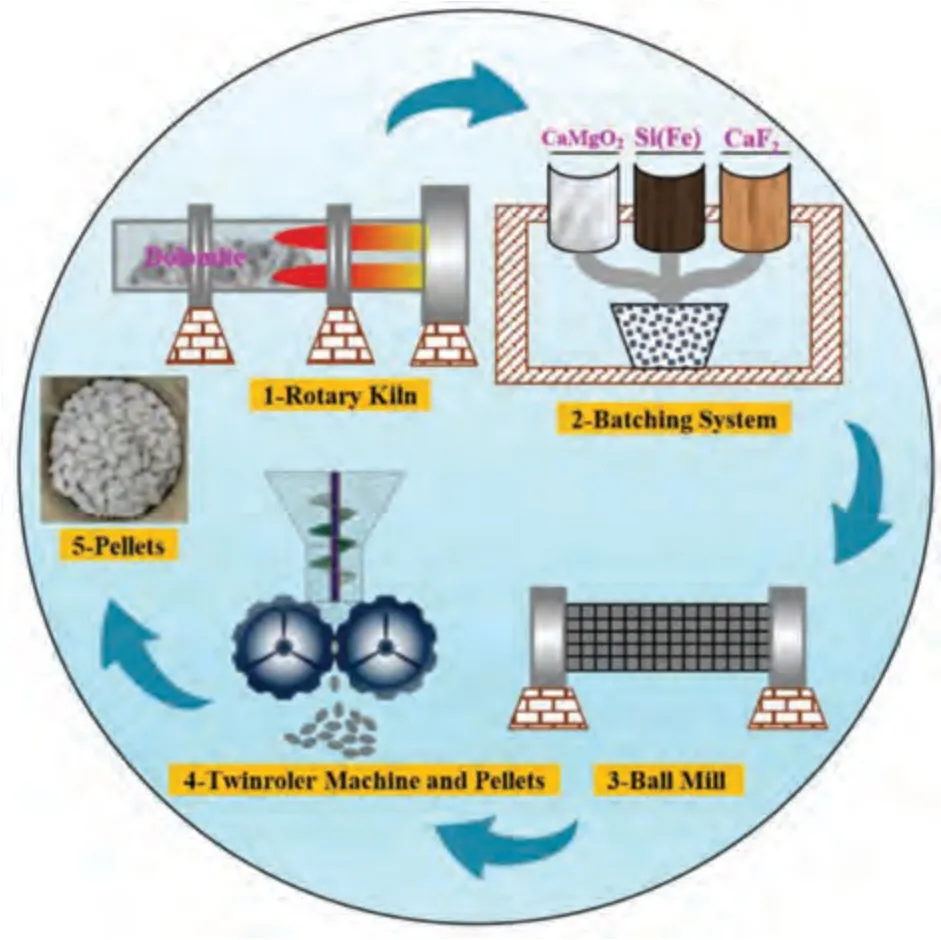
Fig.1.Technological process of pellet preparation.
Calcined dolomite was used as a raw material;75% ferrosilicon was used as a reducing agent;3% fluorite was used as a mineralizer;the molar ratio of silica to MgO (Si/2MgO)is 1.2;and the forming pressure is∼150 MPa.
2.2.Experimental device and principle
The experimental principle is shown in Fig.2.In a shaft furnace heated using a silicon molybdenum rod,the pellet was placed in the constant-temperature heating area of corundum tube,which is filled with silicon carbide particles of a height of 300 mm and a size of 2 mm to support the pellet and preheat the normal-temperature argon from the outside.Silicon carbide was placed on a tray with an argon distribution channel,and the argon distribution channel at the bottom helped the argon to enter the heat storage layer evenly.A circulating water condenser was installed at the upper part of the ball material to collect the generated magnesium vapor,and a heat baffle was set at the lower end of the condenser to adjust the temperature of the lower part of the condenser.Argon was heated via the regenerator to form high-temperature argon,and then the argon flowed through the pellet and carried magnesium vapor into the condenser.When the magnesium vapor was cooled,crystalline magnesium was obtained.
The main chemical reaction of raw materials occurs at a high temperature,as shown in formula (1).
where the subscript s represents solid state;g represents gaseous state;and x is a specific stoichiometric coefficient.
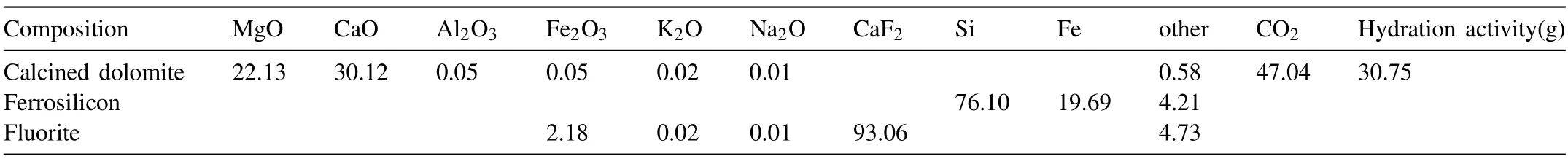
Table 1 The composition and calcining index of calcined dolomite (wt%).
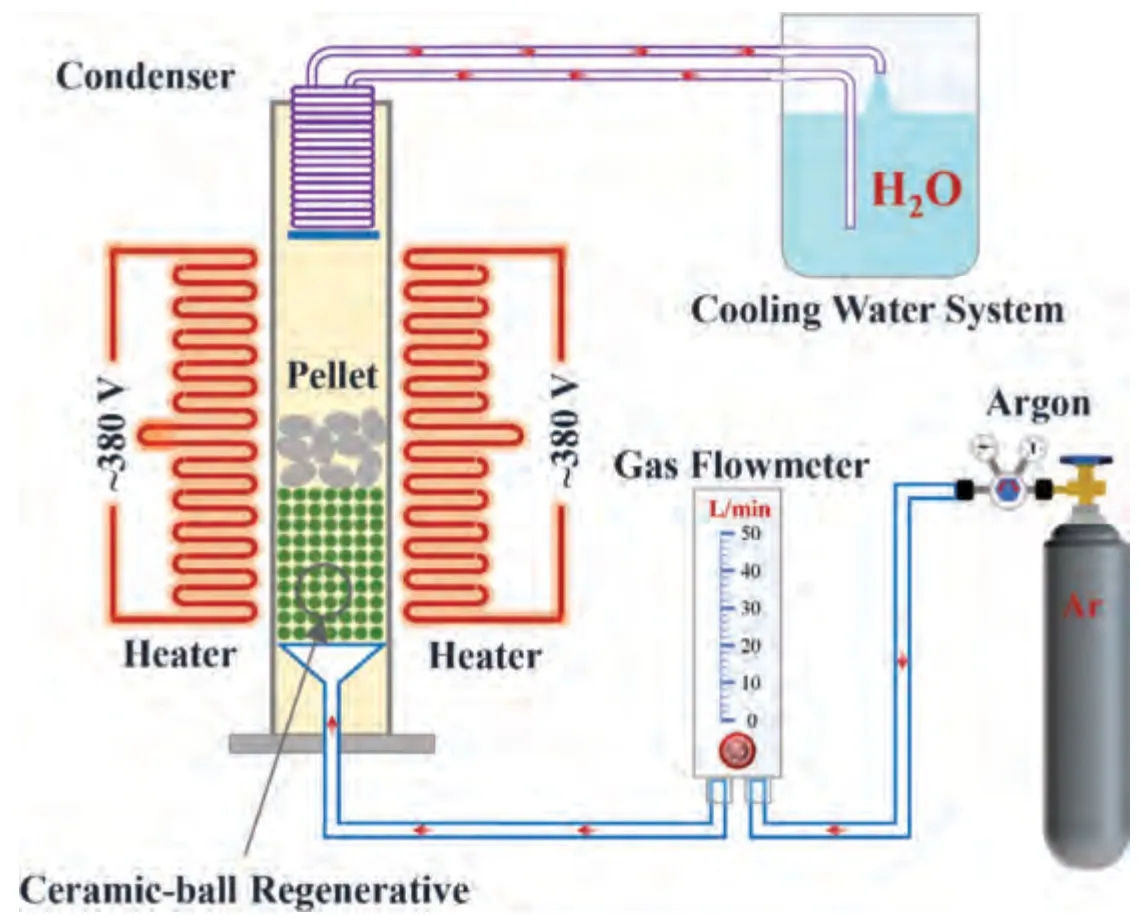
Fig.2.Experimental principle of extracting metal by silicothermic reduction in argon flow.
Formula (1) shows that only the magnesium vapor is gaseous in the system before and after the reaction,so the conversion of pellets can be expressed by the weight loss rate of pellets,as shown in Formula (2).
whereηis the conversion;Mbeforeis the mass of pellets before reaction;Mafteris the mass of pellets after reaction;andω% is the theoretical magnesium content in pellets,which is 19.83%.
3.Results and discussion
3.1.Reaction characteristics of silicothermic reduction of calcined dolomite in argon flow
3.1.1.Effect of main technological parameters on conversion
To determine the effect of reduction temperature,reduction time,and argon flow rate on the conversion during the reaction,an orthogonal test was designed to further determine the optimal process parameters.
Reduction temperature: 1200 °C,1250 °C,1300 °C,1350°C,1400 °C;
Argon flow rate: 1 L·min-1,2 L·min-1,3 L·min-1,4 L·min-1,5 L·min-1;
Restore time: 30 min,60 min,90 min,150 min,180 min;
According to the process parameters,three factors and five levels of orthogonal test were designed,as shown in Table 2.
The results of mean value and range of the orthogonal test shown in Table 2 indicate that reduction temperature and reduction time are positively correlated with conversion,consistent with the trend of silicothermic reduction under traditional vacuum conditions.Among the three factors,the effect of reaction temperature on conversion is more obvious.The effect trend of these three factors on conversion is shown in Fig.3.
Fig.3 shows that the effect of reduction temperature and reduction time on conversion is linear in the determined test range.With the increase in temperature and time,the conversion significantly increases,and the effect of temperature on conversion is more significant.With the increase of argon flow rate,the conversion first increases and then decreases.
The effect of reduction temperature and time on conversion has been extensively studied [42].Here,the effect of argon flow rate on conversion was further studied.According to the results of orthogonal experiment shown in Fig.3,the higher the reduction temperature and the longer the reduction time,the higher the conversion of pellets.Therefore,the effect of different argon flow rates on the conversion was evaluated under a reduction temperature of 1400 °C and reduction time of 180 min by means of experiments,and the results are shown in Fig.4.
Fig.4 shows that when the argon flow rate increased from 1 L · min-1to 3 L · min-1,the conversion increased,exhibiting a linear growth relationship.When the argon flowrate increased from 3 L · min-1to 4 L · min-1,the conversion decreased slowly.When the argon flow rate increased from 4 L · min-1to 5 L · min-1,the conversion decreased rapidly.According to the previous analysis,the larger the argon flow rate,the faster the argon flow through the pellet,and the larger the local pressure head.This situation will decrease the partial pressure of magnesium gas and increase the heat transfer efficiency of pellet.The reduction reaction will be faster,and the conversion will increase.However,this is quite different from the experimental results.After an in-depth analysis,it is inferred that the ability of preheating argon in the regenerative layer is limited and fixed,while the flow rate of argon is gradually increasing.This makes the argon flowing through the SiC particles contact the pellets without preheating to the reaction temperature.Instead,the temperature of pellet surface is reduced,and the effect of reaction temperature on the conversion is greater than that of argon flow rate,so the increase of argon flow rate reduces the conversion of pellets.However,this rule is only valid for this experimental platform.If the heat storage capacity of argon is enough,the relationship between conversion and argon flow rate should also be in a positive proportion.
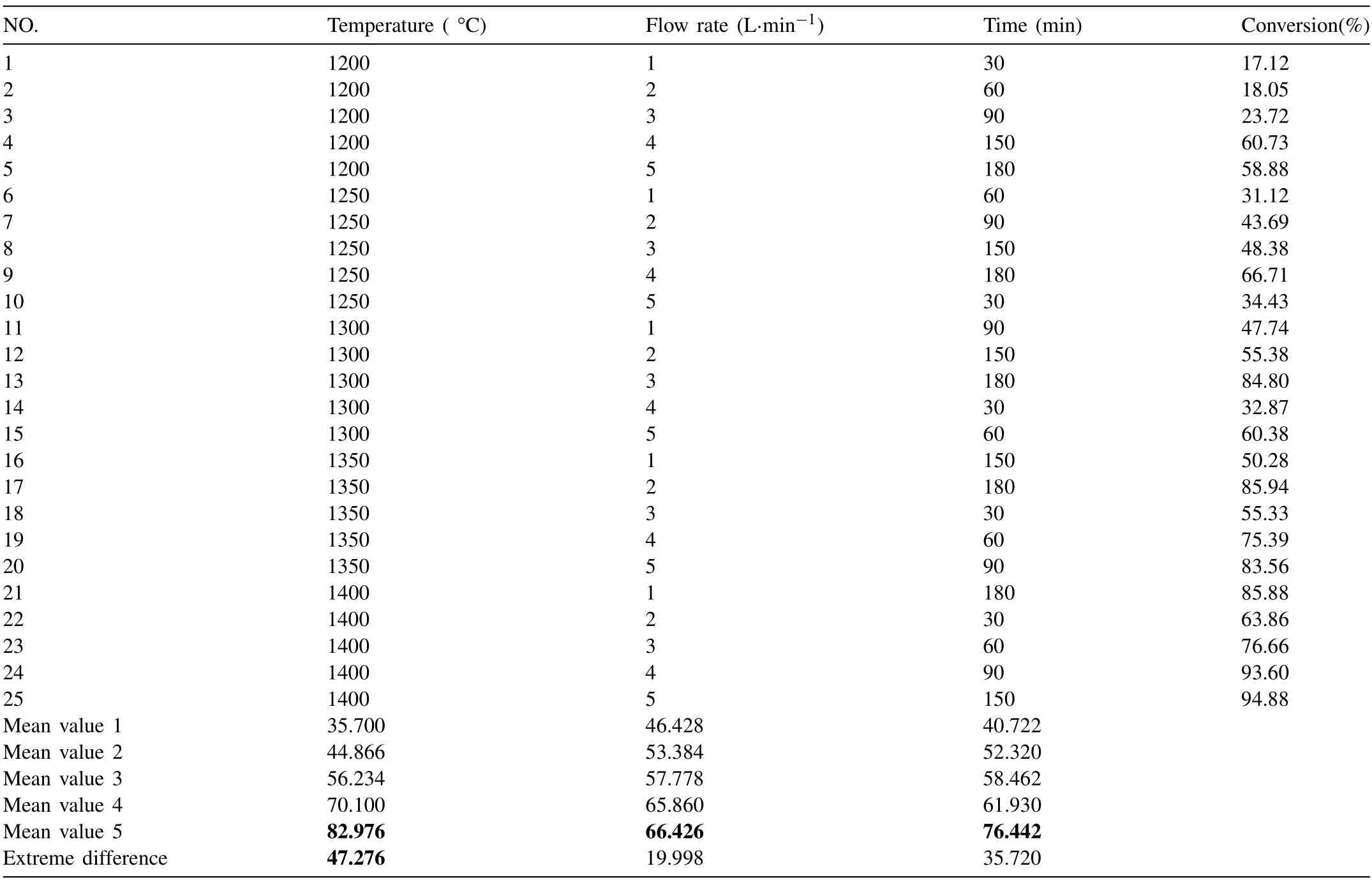
Table 2 Orthogonal test table of the effect of main process parameters on conversion.
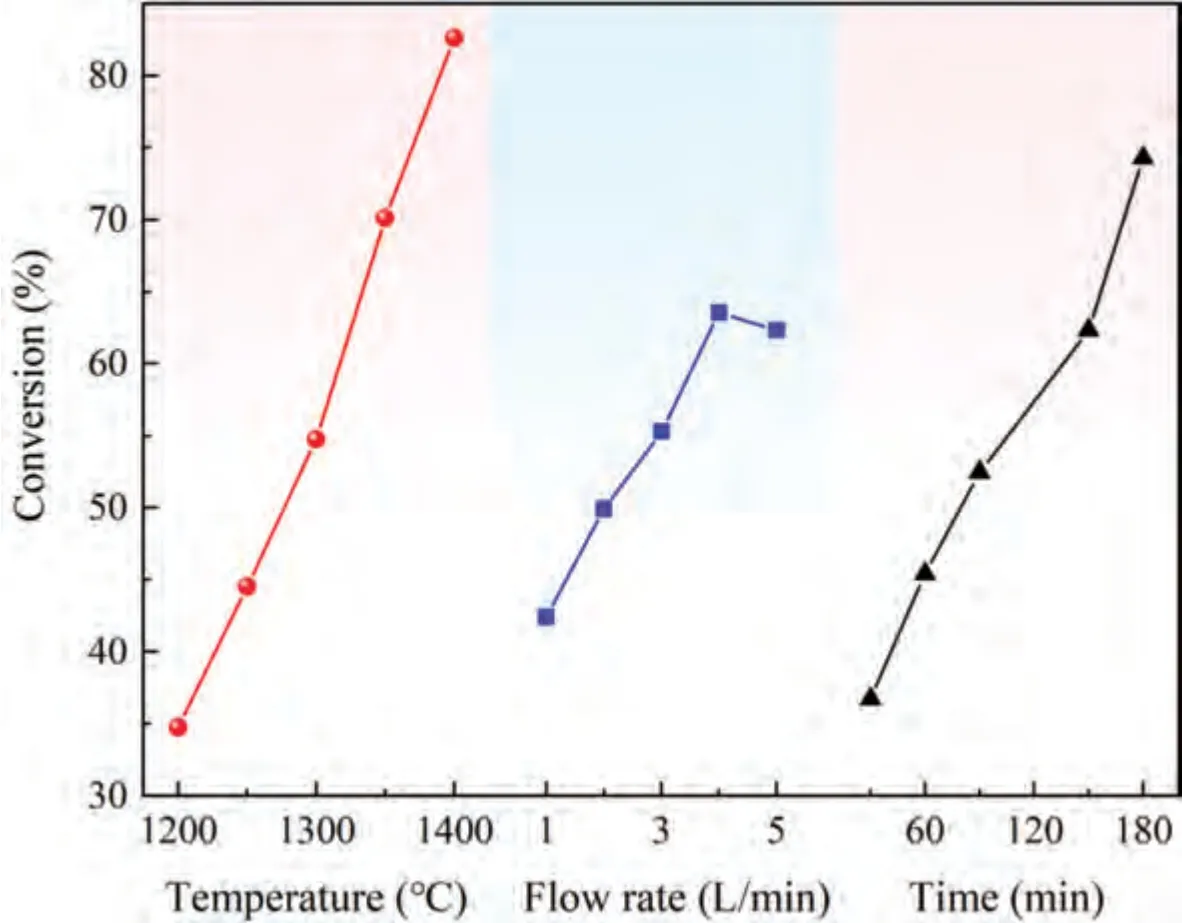
Fig.3.Effect of main process parameters on conversion.
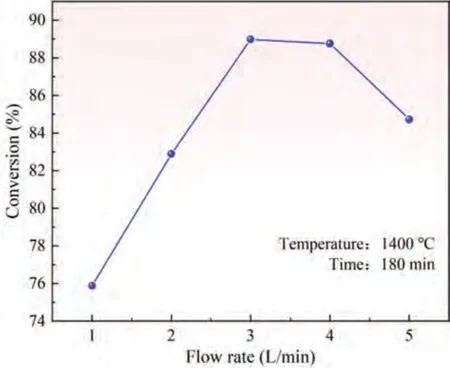
Fig.4.Effect of different argon flow rate on conversion.
3.1.2.Characterization and analysis of reducing slag and crystalline magnesium
After the reduction reaction,a layer of powder was attached to the condenser on the upper part of the reaction device.After cleaning and collecting the powder,it was characterized by XRD.The results are shown in Fig.5.
Fig.5 shows a layer of light gray powder on the condenser,which can be easily cleaned from the surface of the condenser using a brush.Clearly,the powder is very fine.The XRD results show that only magnesium is present in the sample,indicating that the powder on the condenser is magnesium powder.
Fig.6 shows the real photographs before and after the pellet reaction and the XRD results of the powder residue.The pellets reacted according to the shrinking core model,and the size of pellets before and after the reaction significantly decreased.The length of pellets decreased from 50 mm(Fig.6(b)) to 20 mm (Fig.6(d)).According to the XRD characterization results of the powder residue shown in Fig.6(a),the residue is mainly composed of Ca2SiO4and Fe,also consistent with the product of reaction (1).
Fig.7 shows the micromorphology of magnesium powder collected on the condenser characterized by SEM.The morphology of magnesium powder is relatively regular,mostly in the form of spherical or regular polyhedron.The particle size distribution of powder on the electron micrograph is statistically characterized using Nano Measurer software.The average particle size D50 of magnesium powder is 510 nm,and the morphology of most powders is almost spherical.According to the literature [29],when the condensation temperature and vacuum degree are adjusted properly,magnesium powder and crystalline magnesium can be produced.Because of the low temperature of condenser,the magnesium vapor carried by argon directly turns into magnesium powder.If the condensing capacity of the condenser is not enough in the experiment,the magnesium vapor will not have enough time to condense and flow out of the condenser with argon.During the experiment,white smoke was also observed at the argon outlet,which may be magnesium vapor or magnesium oxide oxidized by air.
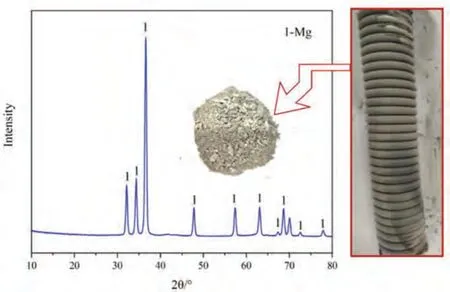
Fig.5.Characterization of crystallization products on condenser.
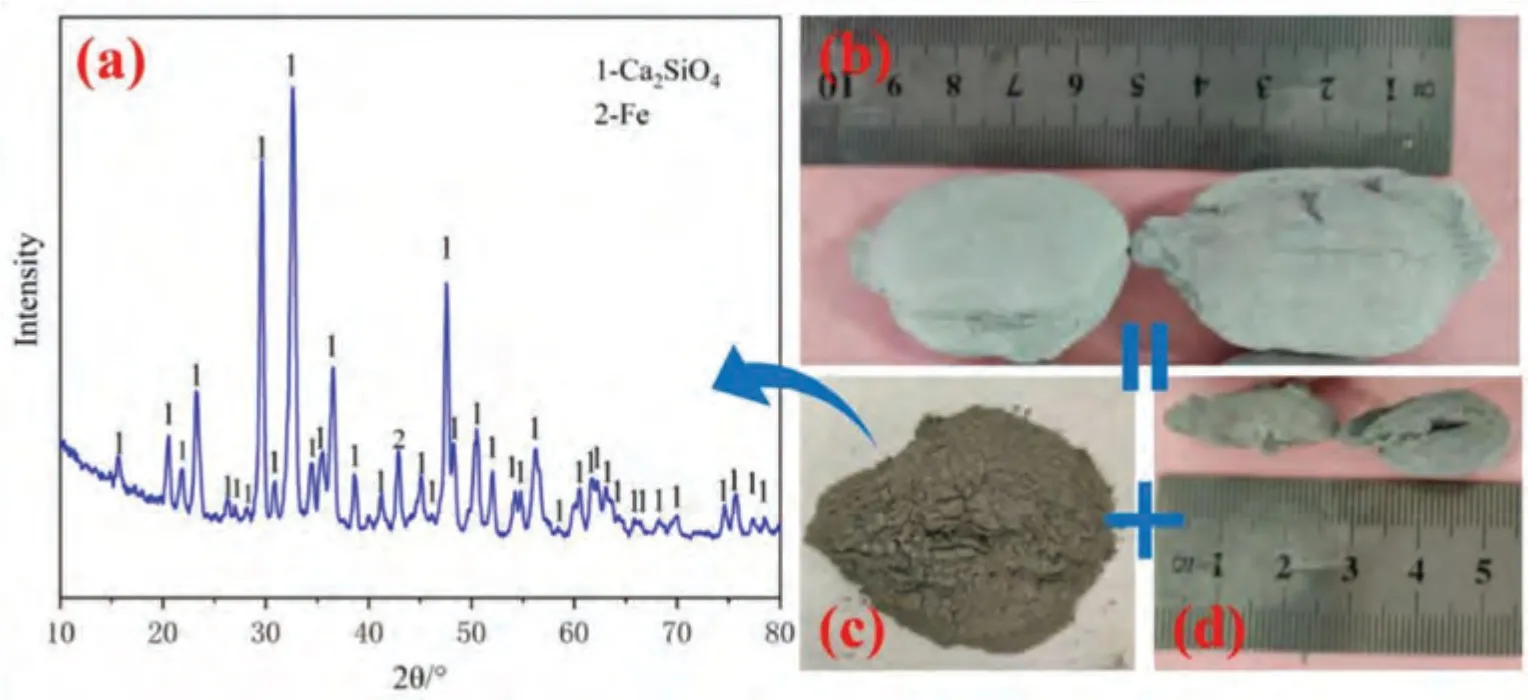
Fig.6.characterization of the residual powder and pellets: (a) XRD results of the powder residue;(b) Pellets before the reaction;(c) The magnesium powder residue;(d) Pellets after the reaction.

Fig.7.Particle size and distribution of magnesium powder on the condenser.
The formation of magnesium powder is mainly related to the partial pressure and condensation temperature of magnesium vapor.Crystalline magnesium can be formed only when the partial pressure of magnesium vapor in the condenser is greater than the saturated vapor pressure at the condenser temperature.The higher the temperature of the crystallization zone and the lower the residual pressure,the denser the crystalline magnesium is.In the experiment,less raw materials are used,the partial pressure of magnesium vapor is small,and the temperature of condenser is low,so it is more inclined to form magnesium powder.
3.2.Process development and numerical simulation of magnesium production by argon entrainment process
Numerical simulation has gradually become an important method on magnesium production in recent years,especially in pilot-scale experimental research and industrial production research.Li et al [43,44].established the conventional coal combustion model to describe the process of magnesium production in a coal-fired furnace.Yu et al [45].developed a mathematical model to simulate the phenomenon of the heat transfer occurring during a new magnesium reduction process and to predict the temperature distributions,the heating curves,and the total process time.Zhang et al [46].developed a three-dimensional unsteady numerical approach which verified by industrial production data and they [47] also studied on a one-step method for magnesium production using numerical simulation method.
3.2.1.Development of argon entrainment process
In this paper,based on the kinetic study of silicothermic reduction of calcined dolomite in argon flow and the numerical simulation study of heat transfer enhancement of single-layer pellet by argon,a production process of magnesium carried by circulating argon flow was developed,as shown in Fig.8.
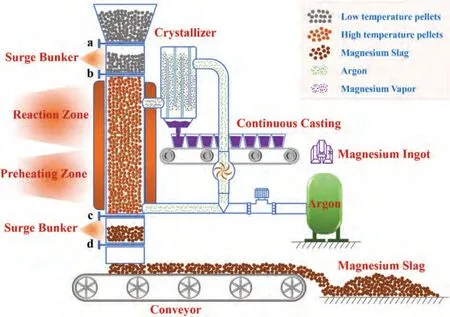
Fig.8.Production process of magnesium by argon entrainment process.
The pellets enter the upper buffer bin from the feeding bin and then enter the reaction zone;heating is carried out by the double heat exchange of heater and high-temperature argon.When the temperature rises to the reaction temperature,the pellets undergo a chemical reaction,and the generated magnesium vapor will flow into the condenser together with argon for heat exchange.After the heat exchange,the magnesium vapor will become liquid magnesium and flow into the bottom of the condenser,which will be refined and cast into magnesium ingot.After the reaction,the pellets slowly drop in the reactor,enter the lower buffer bin,then enter the conveyor,and finally transported to the slag yard.Argon is recycled in the entire process.First,it enters the reactor from the slag discharge area.In the slag discharge area,argon is preheated to a high temperature by the pellet temperature and then flows into the reaction area.It enters the condenser together with the magnesium vapor generated in the reaction area.The cooled magnesium vapor turns into liquid magnesium,and the cooled argon continues to flow forward along the pipeline.Then,it flows into the reactor again after passing through the pressure pump.An argon storage tank supplements the argon consumed in the system,thus maintaining the balance of the system.
3.2.2.Establishment of physical and numerical models
The core reaction area in the argon entrainment process was selected as the research object,as shown in Fig.9(a).In the circular reactor with inner diameter D and height H,the pellets were randomly stacked in the tank,and the crosssection is shown in Fig.9(b).The wall temperature of reactor isTconst.Argon enters the reaction zone of material layer from the bottom of the reactor and leaves from the top of the reactor.The inlet temperature isTAr.Unlike the Pidgeon process,the entire reaction is carried out in argon flow without vacuum environment;therefore,heat-resistant steel with a short service life should not be selected as the material of reactor.
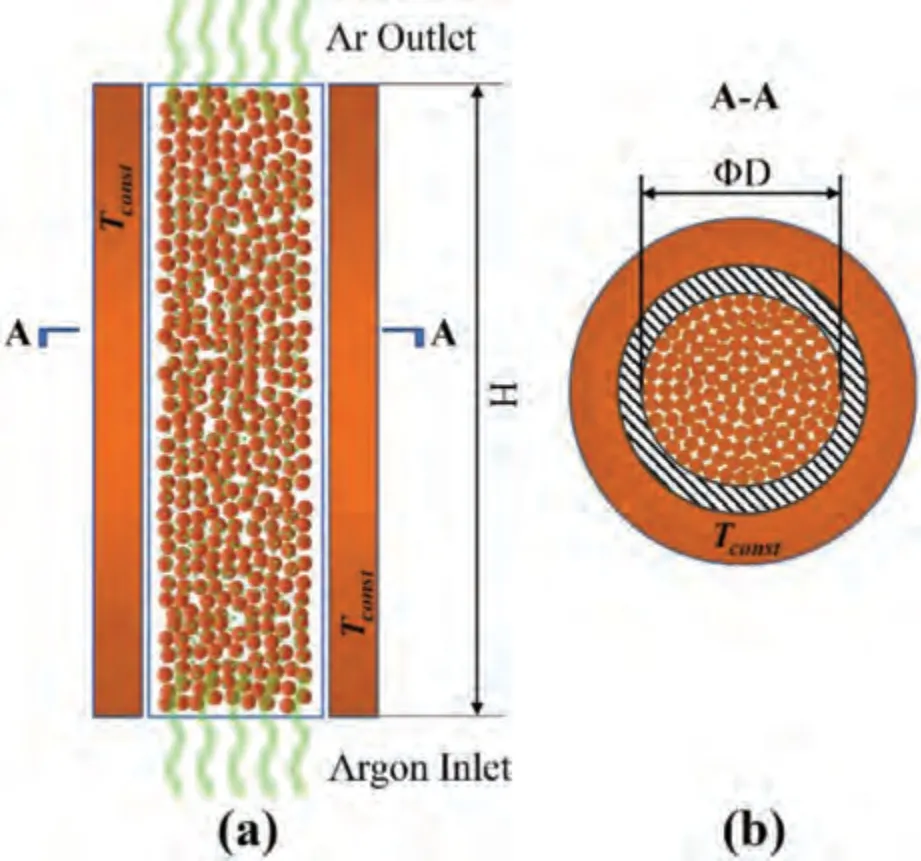
Fig.9.Establishment of physical model for argon entrainment process: (a)The main view of the core reaction area;(b) The cross-section of the core reaction area.

Fig.10.(a) Traditional reduction tank size;(b) Pellet filling layer for industrial production;(c) Arrangement of single layer pellets in reduction tank.
Establishment of physical model.The reduction tank used in the Pidgeon process is generally a cylinder with an outer diameter of 330 mm,wall thickness of 30 mm,and length of 3000 mm,as shown in Fig.10(a).The reduction tank is filled with walnut or ellipsoid pellets,as shown in Fig.10(b).Here,to facilitate the establishment and calculation of the model,the ellipsoidal raw material was simplified as a sphere.The volume of pellets is closely related to the amount of heat absorbed in the reduction process,ensuring the accuracy of heat transfer calculation.Therefore,the equivalent volume method was used to simplify the formula.
where A,B,and C represent the length of the three axes of ellipsoid;D is the diameter of sphere.
The size of industrial pellet is about 50 mm × 30 mm ×20 mm.After equivalent volume calculation,the diameter of spherical material ball is about 31 mm.The spheres are tightly arranged in the reduction tank.1/8 circumferential geometry was obtained according to the geometrical symmetry.The arrangement of single pellets is shown in Fig.10(c).
Because the high-temperature argon flows from one end of the reduction tank to the other end in the argon entrainment process,the pellet in the tank is heated during the flow,so the difference in pellet temperature and conversion in the axial direction should be considered.Using a single-layer pellet,it is difficult to reflect the changes intuitively and accurately in process parameters in the axial direction,so it is necessary to establish a three-dimensional (3D) solid physical model of multilayer pellet.However,as the length of traditional reduction tank is close to 3000 mm,it is difficult to model and mesh all the pellets in the tank,so the height of 10 layers of pellets in the axial direction is taken as the calculation area to study the effect of high-temperature argon flow on pellet temperature and conversion in the axial direction.A 3D solid physical model was established,as shown in Fig.11.

Fig.11.Establishment of 3D physical model.
Numerical models and governing equations.The heat transfer process in the traditional Pidgeon process reduction tank includes the following: 1○heat transfer from the outside to the inside of the reduction tank;2○radiation heat transfer between the inner wall of reduction tank and pellet surface and between different pellet surfaces;3○heat transfer inside the pellet;and 4○chemical heat absorption during the reduction reaction of pellet.The argon entrainment process proposed in this study not only includes the above mentioned four heat transfer processes,but also includes the convective heat transfer between the high-temperature argon flowing in the tank and the pellet surface,which is carried out at the same time.Therefore,the interaction mechanism of heat transfer and chemical reaction involved in the argon entrainment process is more complex,so it is necessary to accurately calculate the relevant control equations and select the accurate physical parameters.To investigate the detail characters of the circulating argon entrainment process,the software ANSYS Fluent 17 was implemented to solve all the equations.The chemical reaction heat which was added as source term was calculated in the numerical model through a user-defined function compiled by Language C.Because the thermal conductivity of the material of reduction tank is much higher than that of the pellet and the thickness of the tank wall is small,the temperature of inner and outer walls of the tank can be approximately the same,i.e.,in the numerical calculation model,the wall of the reduction tank is replaced with the wall ignoring the thickness,and the wall temperature is consistent with the temperature in the reduction furnace.
(a) Heat conduction model
The chemical reaction in the pellet is an endothermic process,so the heat transfer process and the endothermic process of the reduction reaction affect each other.The endothermic amount of the reduction reaction in the pellet can be treated as a source term and substituted into the differential equation of heat conduction in the pellet.The governing equation for the three-dimensional numerical model in a polar coordinate system can be described as follows,
whereρbis the density of briquette,kg·m-3.cbis specific heat capacity of briquette,J·kg-1.λbis the thermal conductivity of briquette,W·m-1·K-1.Tis the temperature of briquette,K.Sbis the chemical reaction heat source,W·m-3,which could be calculated by Eq.(5),
WhereMbis the maximum magnesium production per unit volume and the value is 12,587.9 mol·m-3according the ratio of reactants.ϕbis the thermal energy required per mole of magnesium in the reduction reaction,J·mol-1,and the expression ofϕbcould be given as follows:
(a) Convective heat transfer model
The convective heat transfer between high temperature argon and pellet can be calculated by Newton’s cooling formula as follows.
WhereAbis the surface area of the briquette,m2.TAris the temperature of argon,K.hconis the convection heat transfer coefficient calculated from the Eq.(8).
WhereNuis the Nusselt number,dbis the diameter of briquette which was assumed to remain constant while the density of briquette changed during the reduction process,m.λAris the thermal conductivity of argon,W·m-1·K-1.Re is the Reynolds number based on the briquette diameter and relative velocity.Pr is the Prandtl number of argon.
(a) Radiation heat transfer model
The Discrete Transfer radiation model (DTRM) in Fluent was suitable to describe the model which was the radiative heat transfer between briquettes,briquettes and pot wall.The change in radiant intensity,dI,along a path,ds,can be written as:
Whereα,σ,I,andTare absorption coefficient,Stefan-Boltzmann constant,total hemispherical intensity,and local temperature respectively.The refractive index is assumed to be unity and the scattering effects are neglected;thus,I(s)can be represented as:
WhereI0is radiant intensity at the start of the incremental path,which is determined by the appropriate boundary condition.
(a) Kinetic model of chemical reaction
According to the chemical kinetics model is reported in previous work [40],dα/dτin Eq.(5) is the change of conversion with time can be expressed as:
(a) Thermophysical properties and boundary conditions
During the magnesium production by using the argon entrainment process,the pellet temperature significantly changes with reaction time,and the reduction state of pellets at different positions is also different.Therefore,the change in physical parameters with temperature or conversion should be considered in the numerical calculation model,and the relevant physical parameters are shown in Table 3.
The calculation domain of this numerical model is 1/8 reduction tank in the circumferential direction and 10-layer pellet height in the length direction,so the boundary condition of the calculation model can be expressed as follows:
where D0Pis the inner diameter of reduction tank,m;Tconstis the wall temperature of reduction tank,K;νAris the inlet velocity of high-temperature argon,m·s-1;His the calculated domain height,m;P0is the ambient pressure,Pa;T0is ambient temperature,K.In the actual industrial production process,to ensure that the magnesium vapor flows out in the form of a gas at the outlet,the outlet temperature will be higher than the condensation temperature of magnesium vapor,924 K.To simplify the calculation,the outlet boundary condition was set at 1000 K.

Table 3 Thermophysical properties in numerical calculation.
Because of the unsteady heat transfer process,the initial conditions should be set.It can be considered that at the beginning,the pellet temperature is 300 K at room temperature.
3.2.3.Analysis and discussion of numerical simulation results Model validation and comparison with traditional Pidgeon process.Although there is a large difference in the production process between the traditional Pidgeon process and the circulating argon entrainment process proposed in this study,the main difference in the numerical calculation is whether the high-temperature argon enters the reduction tank to heat the pellets.Therefore,in the validation of numerical calculation model,the flow rate of high-temperature argon was set to zero,i.e.,no high-temperature argon was allowed to enter.At this time,the calculation conditions are basically consistent with the traditional Pidgeon process,and the production data of Pidgeon process can be used to verify the numerical calculation model.
When verifying the process conditions,the wall temperature of reduction tank was set as 1473 K,and the argon flow rate was zero.In this case,the variation of conversion with time was compared with the industrial production data of Pidgeon process,as shown in Fig.12.
Fig.12 shows that the numerical calculation model established in this study is consistent with the traditional Pidgeon process when the high-temperature argon is not accessed,and the calculation data is consistent with the industrial production data.Therefore,it has high reliability for the follow-up study of high-temperature argon.
In the argon entrainment process,the introduction of hightemperature argon can effectively heat the pellet,allow the pellet to rise to the required temperature for the reduction reaction,provide enough heat to make the pellet produce magnesium quickly,reduce the reduction cycle,and improve the production efficiency.
According to the results of previous single-layer pellet simulation calculations,the high-temperature argon flow rate of 0.25 m·s-1was selected as the representative velocity for numerical calculation and compared with the Pidgeon process.
Fig.12 shows that high-temperature argon can significantly improve the efficiency of magnesium production by comparing the two processes.When the reaction time is 2 h,the magnesium production rate of Pidgeon process is only 26.16%,while that of argon entrainment process is 43.65%.It takes 7 h for Pidgeon process to produce 80% magnesium,while the argon entrainment process only takes 5 h,i.e.,the production cycle of magnesium carried by circulating argon flow under this condition is 5 h,which is 2 h less than that of Pidgeon process.This significantly improves the production efficiency.
Fig.13 shows a comparative diagram of the conversion distribution of the argon entrainment process under an argon flow rate of 0.25 m·s-1and Pidgeon process.In the production process of traditional Pidgeon process,the heat source is only the constant-temperature tank wall,so the pellet shows a layer-by-layer reaction from the tank wall to the center.For the production process of magnesium carried by circulating gas,besides the wall of constant-temperature tank,hightemperature argon is available as the heat source.The hightemperature argon continuously enters the reduction tank at a certain flow rate to heat the pellet,so the pellet also undergoes layer-by-layer reaction along the flow direction of hightemperature argon.The superposition of the two makes the argon entrainment process significantly shorten the reduction cycle.
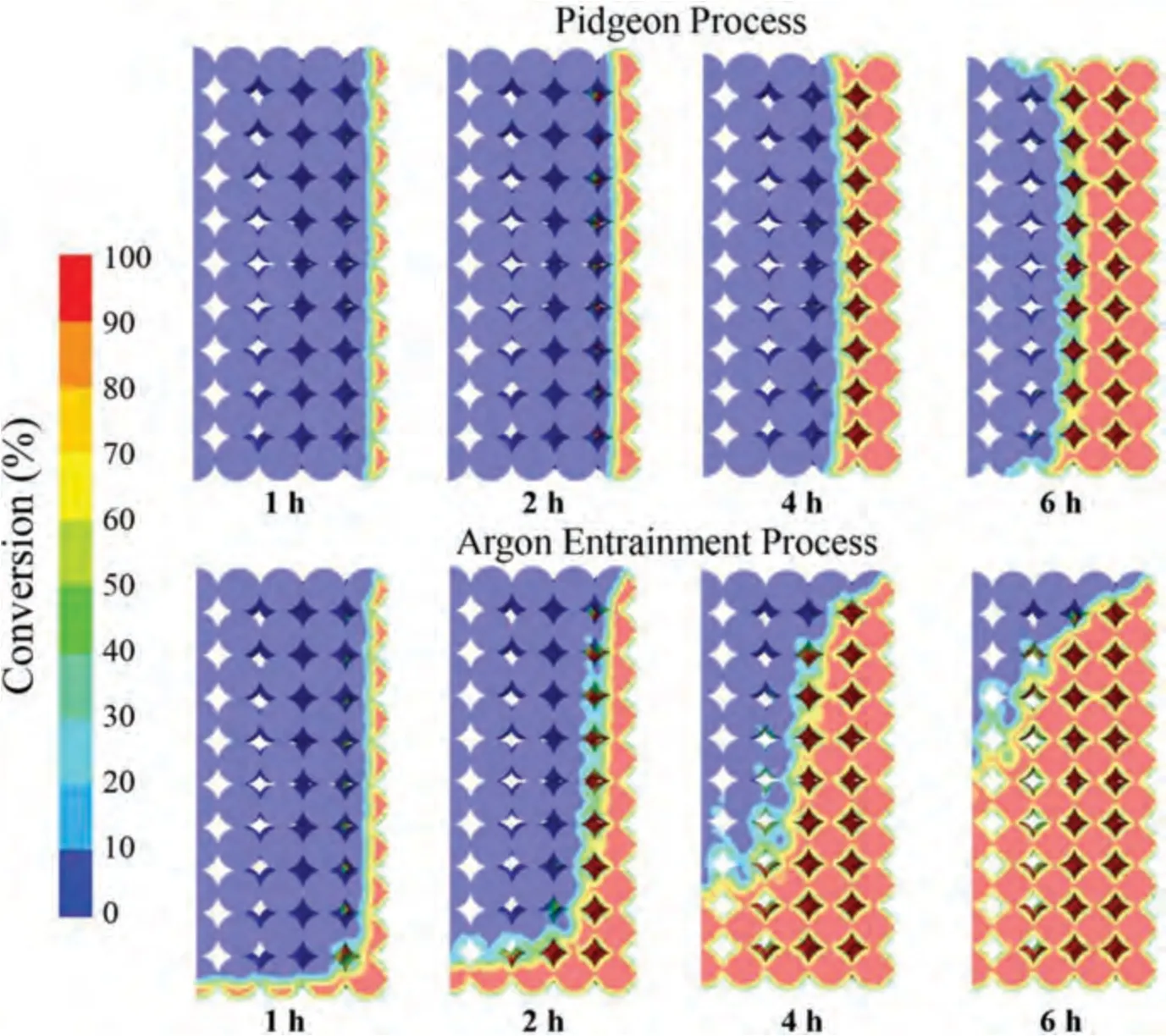
Fig.13.Comparison of the conversion distribution between the argon entrainment process with argon flow rate of 0.25 m s-1 and Pidgeon process.
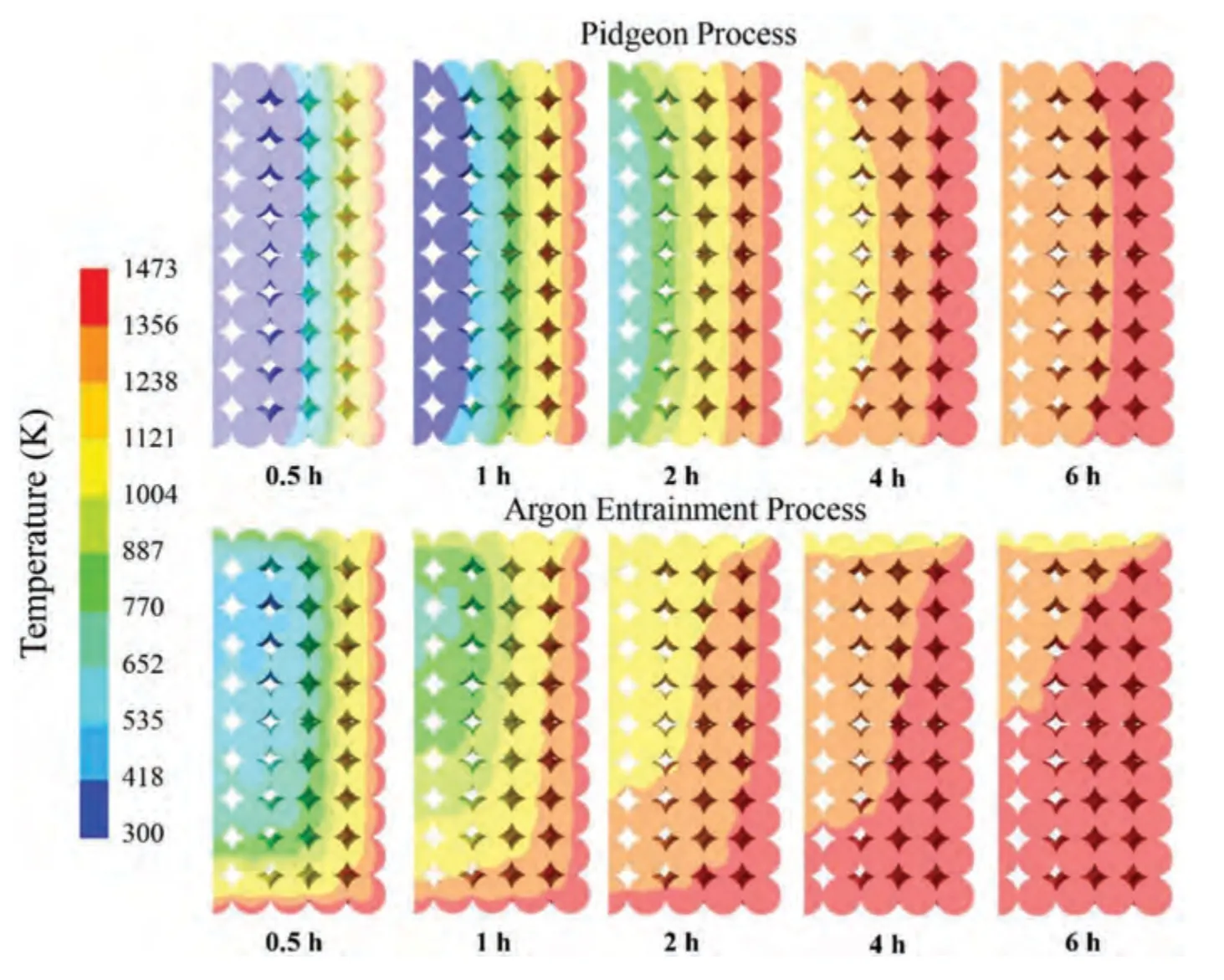
Fig.14.Comparison of temperature distribution between Pidgeon process and 0.25 m/s argon flow rate in the argon entrainment process.
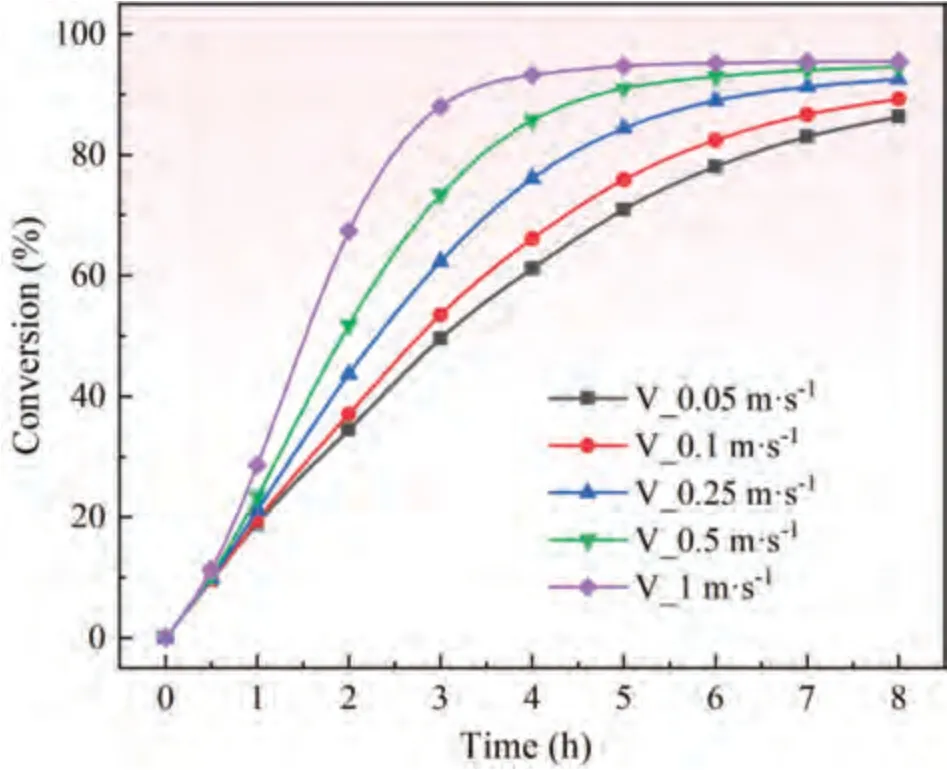
Fig.15.Variation of conversion with time under different argon inlet velocity.
The reason why the argon entrainment process can improve the production efficiency is that the pellet temperature can be quickly heated to the temperature required by the reduction reaction with the introduction of high-temperature argon.A comparison of temperature distribution of pellets under the two processes is shown in Fig.14.
In the production process of circulating argon carrying magnesium,the pellets near the tank wall and argon inlet are heated rapidly,and the double heat superposition effect makes the production efficiency of the argon entrainment process significantly higher than that of the traditional Pidgeon process.Because the temperature at the argon outlet used in the numerical calculation is set at a constant temperature of 1000 K,there may be a slight deviation from the actual situation,but it has slight effect on the calculation domain.Therefore,the calculation model established in this study can be used to predict some characteristics of the argon entrainment process.
Effect of argon flow rate on conversion.On the premise of constant argon inlet temperature,a change in the argon flow rate will affect the convective heat transfer between argon and pellet.The variation in conversion with time under different argon flow rates was simulated by numerical simulation,which is shown in Fig.15.
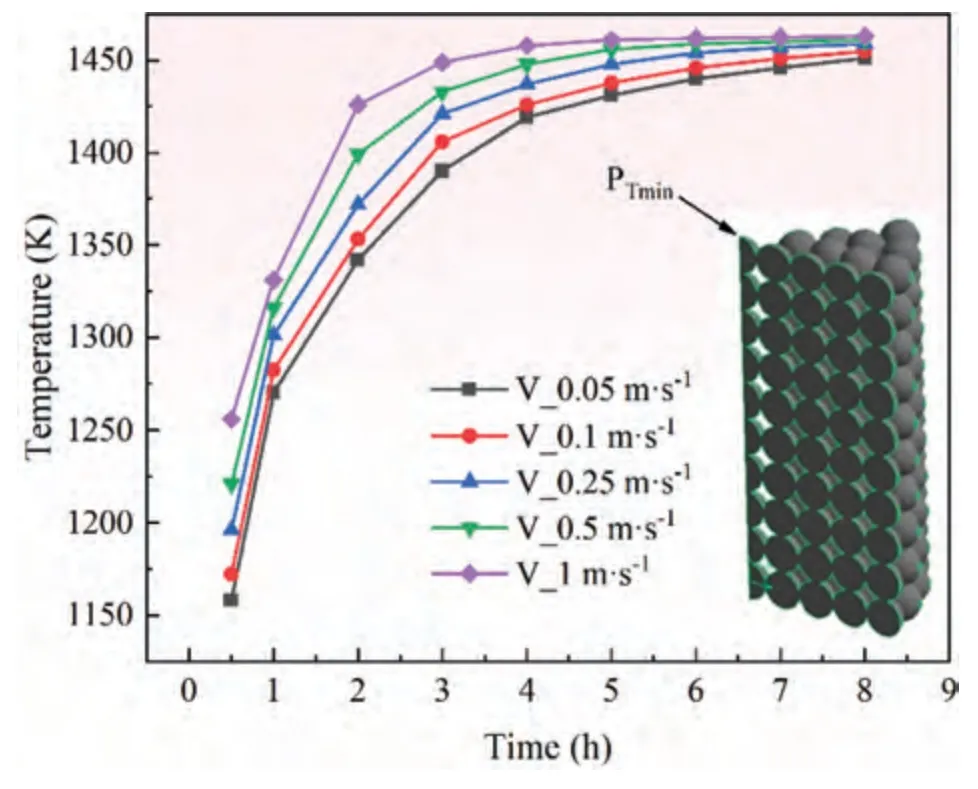
Fig.17.Variation of temperature with time at the slowest temperature rise under different argon flow rates.
Fig.15 shows that an increase in the argon inlet flow rate can significantly improve the magnesium production efficiency.Under the same reduction time,the conversion increases with the increase of argon flow rate.However,the increase of argon flow rate will definitely increase the related costs.Therefore,according to the market situation of magnesium,it is necessary to accurately calculate the economic relationship between the cost of increasing argon flow rate and the production efficiency to determine a more appropriate argon economic flow rate.
A reaction time of 2 h was selected as a representative reaction time.The conversion distribution under different argon flow rates was also simulated by numerical simulation,which is shown in Fig.16.The distribution of conversion at different argon flow rates is consistent with Fig.15 when the reaction time is 2 h.Under the same reaction time,the reduction degree is higher when the argon flow rate is high.
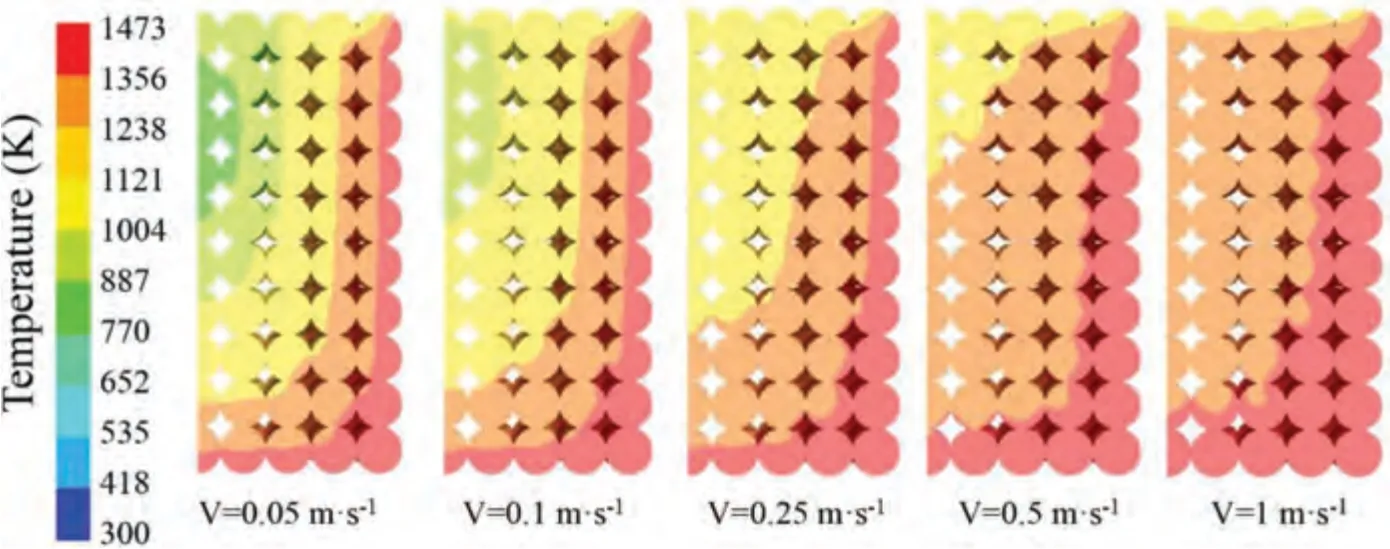
Fig.18.Temperature distribution at different argon flow rates for reaction time of 2 h.
Effect of argon flow rate on temperature.Select a point PTminin the upper left corner of the calculation domain to monitor the temperature,and compare the temperature variation with time under different argon flow rates,as shown in Fig.17.The temperature at the slowest temperature rise also increases with the increase in argon flow rate.At the same heating time,the temperature rise at this point is proportional to the argon flow rate.
A reaction time of 2 h was selected as the representative reaction time.The temperature distribution under different argon flow rates is shown in Fig.18.The temperature distribution shown in the figure is consistent with that shown in Fig.17.Under the same reaction time,the high-temperature area of high argon flow rate is larger.
4.Conclusion
In this study,the effects of argon flow rate,temperature,and reduction time on the yield of ferrosilicon reduction of calcined dolomite were studied by conducting experiments,and the argon entrainment process was explored by numerical simulation.The novel green process is carried out under recycled argon flowing without vacuum,which leads to the continuous magnesium production.And the reduction efficiency is greatly improved by enhanced convective heat transfer between pellets.The effect of reduction temperature,argon flow rate,and reduction time on the reduction of calcined dolomite by ferrosilicon was studied by orthogonal tests.With the increase in reduction temperature,the conversion significantly increased,and with the increase in reduction time,the conversion also significantly increased.With the increase in argon flow rate,the conversion first increased and then decreased,and the conversion reached the maximum when the argon flow rate was 3 L·min-1.Argon is recycled and preheated by using the residual heat of high temperature reducing slag.High-temperature argon flows between pellets,which not only changes the partial pressure of magnesium vapor on the surface of pellets to shift the equilibrium of chemical reaction,but also achieves enhanced convective heat transfer between pellets.A physical model of 10-layer pellet arrangement was established,and a numerical calculation model of chemical reaction,radiation,heat conduction,and convection heat transfer was established.The results show that high-temperature argon can effectively heat the pellets,promote rapid production reaction of pellets,reduce the reduction cycle,and improve the production efficiency.When the conversion is 80%,the production efficiency is increased by about 28.6%.
Declaration of competing interest
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed.We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication,including the timing of publication,with respect to intellectual property.In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office).He is responsible for communicating with the other authors about progress,submissions of revisions,and final approval of proofs.We confirm that we have provided a current,correct email address which is accessible by the Corresponding Author.
Acknowledgment
The study was supported by Key Program of the National Natural Science Foundation of China (Grant No.92062223)and the National Natural Science Foundation of China (Grant No.51804277) and Anhui University Natural Science Research Project (KJ20190048).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Improvements in mechanical,corrosion,and biocompatibility properties of Mg–Zr–Sr–Dy alloys via extrusion for biodegradable implant applications
- Ultrasonic solidification mechanism and optimized application performances of ternary Mg71.5Zn26.1Y2.4 alloy
- Superplastic behavior of a fine-grained Mg-Gd-Y-Ag alloy processed by equal channel angular pressing
- GO/MgO/Mg interface mediated strengthening and electromagnetic interference shielding in AZ31 composite
- Experimental and theoretical studies on two-dimensional vanadium carbide hybrid nanomaterials derived from V4AlC3 as excellent catalyst for MgH2
- Electrochemical synthesis of boron-containing coatings on Mg alloy for thermal neutron shielding
