Recent progress in self-repairing coatings for corrosion protection on magnesium alloys and perspective of porous solids as novel carrier and barrier
2023-12-27YjieYngYufeiWngMeiXunLiTinshuiWngDweiWngChengWngMinZhHuiYunWng
Yjie Yng ,Yufei Wng ,Mei-Xun Li,∗ ,Tinshui Wng ,Dwei Wng ,Cheng Wng ,Min Zh,c,Hui-Yun Wng,b,c,∗
a Key Laboratory of Automobile Materials of Ministry of Education &School of Materials Science and Engineering, Jilin University, Changchun 130022,China
b School of Materials Science and Engineering, Hebei University of Technology, Tianjin 300130, China
cInternational Center of Future Science, Jilin University, Changchun 130012, China
Abstract Featuring low density and high specific strength,magnesium (Mg) alloys have attracted wide interests in the fields of portable devices and automotive industry.However,the active chemical and electrochemical properties make them susceptible to corrosion in humid,seawater,soil,and chemical medium.Various strategies have revealed certain merits of protecting Mg alloys.Therein,engineering self-repairing coatings is considered as an effective strategy,because they can enable the timely repair for damaged areas,which brings about long-term protection for Mg alloys.In this review,self-repairing coatings on Mg alloys are summarized from two aspects,namely shape restoring coatings and function restoring coatings.Shape restoring coatings benefit for swelling,shrinking,or reassociating reversible chemical bonds to return to the original state and morphology when coatings broken;function self-repairing coatings depend on the release of inhibitors to generate new passive layers on the damaged areas.With the advancement of coating research and to fulfill the demanding requirements of applications,it is an inevitable trend to develop coatings that can integrate multiple functions (such as stimulus response,self-repairing,corrosion warning,and so on).As a novel carrier and barrier,porous solids,especially covalent organic frameworks (COFs),have been respected as the future development of self-repairing coatings on Mg alloys,due to their unique,diverse structures and adjustable functions.
Keywords: Mg alloys;Coatings;Self-repairing;Corrosion protection;Porous solids.
1.Introduction
Magnesium alloys are considered to be one of the most promising green engineering materials in the 21st century with the advantages of low density,high specific strength and stiffness,high damping capacity,and good biocompatibility[1,2].These characteristics make Mg alloys show excellent application prospects in a broad range of fields,especially in 3C products (computer,consumer electronic products,and communication),high-speed rail,automobiles,aerospace,architectural decoration,and medical rehabilitation equipment,where the demand for Mg alloys is increasing year by year[3–5].
In the last two decades,considerable efforts have been devoted to improving mechanical properties of Mg alloys either through alloying,advanced processing technologies or the combination of both,which demonstrates a beneficial effect in achieving enhanced strength and ductility [4].However,most of the commercial and newly developed high-performance Mg alloys suffer from poor corrosion resistance[4–7].It is mainly because the standard electrode potential of Mg is very low(-2.37 V vs.standard hydrogen electrode),and its chemical and electrochemical activities are extremely high.Therefore,Mg alloys are exceedingly susceptible to corrosion [8,9].Galvanic and localized corrosion are common corrosion modes in Mg alloys,originating from poor design (alloy elements),microstructures (second phase and defect),unstable surface films,and harmful impurities(Fe,Ni,Cu,Pb,and Si)[10–12].In a humid environment and/or solution medium,Mg alloys rapidly react with water to form loose and porous Mg(OH)2layers with poor protectiveness,accompanied by the generation of hydrogen (Eq.(1)) [9].
As the reaction progresses,the outer layer comprises Mg(OH)2,and the inner layer is mainly MgO which gradually transforms into Mg(OH)2.Especially,when Cl-,NO3-,SO42-,Cr2O72-,MnO4-or ClO3-ions exist in the external environment,the corrosion reaction of Mg alloys will be accelerated.In the corrosive medium,the corrosion rate of Mg alloys increases with the decrease of pH value [13,14].
All of these factors limit the wide application of Mg alloys in humid,salt,acid,and oxidizing environments [15,16].As a consequence,improving the corrosion resistance of Mg alloys is extraordinarily critical to prolong service life and expand their applications.
Alloying is one of the main strategies to enhance the anticorrosion property of Mg alloys [17,18].The alloying method is conducted by adding other metal elements such as aluminum (Al),zinc (Zn),and rare earth elements (e.g.,lanthanum (La),cerium (Ce),and neodymium (Nd)) into metallic Mg[19],which changes the type of second phases[20,21],refines the grain size [22],reduces the influence of harmful impurities [23],and improves the stability of corrosion product films,thus leading to improved corrosion resistance.
Nevertheless,the aforementioned method to modify microstructures by adding alloy elements can only slow down the corrosion rate of Mg alloys,which are incapable to completely solve the inherent corrosion conundrum [24].At the same time,corrosion-resistant Mg alloys are highly demanded in national defense,aerospace,deep-sea and extreme conditions (high salt,high temperature or high humidity),which encourages us to seek various avenues to design and prepare novel corrosion-resistant Mg alloys [25].
Since the corrosion reaction of Mg alloys normally begins on surface,the preparation of covering layer plays a key role in blocking Mg alloys from contacting with outside environment [26,27].Recently,more and more researchers have devoted to the surface treatment of Mg alloys to produce environmentally friendly and efficient anti-corrosion coatings[28,29].Basically,surface coatings can be divided into two categories—physical barrier coating and self-repairing coatings.
Physical barrier coatings refer to metallic coatings,paint coatings,and other coatings that only play a role of physical insulation and act as a contact barrier to protect Mg alloys from being eroded by corrosive media [30,31].However,in the process of transportation and use,physical coatings inevitably suffer from mechanical damage.Besides,the dents,aging,and cracks caused by light,heat,oxygen,humidity,and ions seriously influence the function of coatings.The corrosive media (H2O,H+,Cl-,NO3-,SO42-,Cr2O72-,MnO4-or ClO3-) could quickly interact with the Mg substrate at the defect sites,resulting in structural destroy [32].Manual repair and replacement of the coatings are complicated and costly.In particular,it is difficult to manually repair damaged coatings on Mg alloys used in spacecraft or orthopedic implantation[25].
As a consequence,it is urgent for researchers to develop facile,safe,reliable,and low-cost self-repairing coatings for Mg alloys [33,34].Featured with sustainable working capability,long service life,and cost-effectiveness,self-repairing coatings are deemed to be a feasible and effective approach to improve the long-term corrosion resistance of Mg alloys[35].The main techniques for fabricating self-repairing coatings include chemical conversion,plasma electrolytic oxidation (PEO),layer-by-layer assembly technology,ion implantation,spin,chemical modification,etc.[11].According to the composition,the self-repairing coatings can mainly be divided into metal,metal hydroxide,silane sol-gel,polymer,and other coatings [36,37].
The self-repairing of the coatings can be realized from the perspective of restoring shape or function.Shape restoring coatings can repair the morphology and structure of coatings via the regulation of reversible reactions,recombination of chemical bonds,and shape memory effect of polymers [38,39].Different from shape restoring coatings,function restoring coatings restore the protective capability of the coating by loading inhibitors[40,41].As defects appear in the coating,the inhibitors are released and react with the metal ions or the corrosive medium,forming new passive layers at the cracks and covering the surface of Mg alloys,thus avoiding corrosion propagation [42].
The function restoring coatings are mainly divided into three categories,namely,chemical conversion coatings (metal oxides,metal hydroxides,and organic acids),coatings directly loaded with inhibitors (epoxy resin and micro-arc oxidation),and inhibitors encapsulated into nano-containers(layered double hydroxides,nanotube,biological containers and porous materials).Typically,the inhibitors are loaded into the carriersthrough various approaches and interactions,for example,physical mixing,weak interaction (vander Waals forces,hydrogen bond,or coordination bond),strong interaction(chemical bond),and capsule embedding [43].Generally,the inhibitors are classified as follows:inorganic corrosion inhibitor,organic corrosion inhibitor and polymer inhibitor[44].Table 1 presents the common inhibitors and their main anti-corrosion mechanisms.
Apart from inhibitors,carriers also play an important role in function restoring coatings [45].Only when carriers can load the inhibitors in the early stage and release them when necessary,can the effects of self-repair and anti-corrosion bebetter achieved.Therefore,excellent carriers should have the following characteristics: large load capacity,functional diversity,transfer channel,and extreme condition applicability[46].

Table 1 Common corrosion inhibitors for Mg alloys and their classification,as well as the main anti-corrosion mechanisms.
Carriers mainly include inorganic,organic and biological carriers [47].Though inorganic carriers are cheap and have excellent wear resistance,it is not easy to modify their structures to load organic corrosion inhibitors [48].Various biological carriers can contain organic and polymer corrosion inhibitors,but under extreme conditions (acid,alkali,salt or organic solvent,they have poor stability [49].The organic carriers with functional groups (such as -OH,-COOH,-SH,-NH2) have the ability to load inorganic,organic or polymer inhibitors [50,51].However,traditional organic carriers are mostly flexible chain structures with low utilization of internal space,resulting in poor inhibitor loading capacity [52].Therefore,the carriers with suitable internal space and rigid skeleton structure are excellent candidates for function restoring coatings.Because of the large surface area,wide internal space and rich functional groups,the organic skeleton carriers can improve the capacity of inhibitors,and provide stimulus response and corrosion early warning functions for coatings via targeted design [53,54].Therefore,the organic skeleton carriers are excellent candidates for function restoring coatings.
In recent years,several excellent reviews illustrated the self-repairing coatings on Mg alloys from different perspectives.In 2021,Liu et al.[55].presented the development of Mg alloy protection from physical coatings to self-repairing coatings.The work shows remarkable advances made in recent years on Mg alloy protection.However,they have emphasized more on physical coatings,while relatively few descriptions of self-repairing coatings have been provided.In 2022,Vaghefinazari et al.systematically summarized and introduced chromate-free corrosion protection for Mg alloys:“part I—pre-treatment and conversion coating [56],part II—PEO and anodizing[57]and part III—corrosion inhibitors and combining them with other protection strategies” [58].These three parts of the work introduced Mg alloy self-repairing coatings such as conversion coatings and encapsulation coatings,with a focus on the preparation strategies of these coatings.In 2023,Peng et al.[59].comprehensively summarized the types of self-repairing coatings for Mg alloys and proposed the problems and future directions that should be paid attention to in self-repairing coatings on Mg alloy.
Different from the previous works,this review illustrates the developments from shape restoring coatings to function restoring coatings on Mg alloys,and focuses on the advantages and prospects of the different carriers in function restoring coatings,including chemical components,structure characteristics,and how to select suitable carriers to improve selfrepairing performance.We also provide a perspective on the design and application of porous solids,especially covalent organic frameworks (COFs),which are expected to be enhanced in terms of increasing the capacity of corrosion inhibitors,accelerating the release rate,and modifying multifunctional groups,and thus improve the overall corrosion resistance and intelligence of coatings in the future.The shape restoring coatings are mainly linear synthetic polymers.For function coatings,chemical conversion coatings(metal oxides,metal hydroxides,and organic acids),coatings directly loaded with inhibitors (epoxy resin and micro-arc oxidation) and inhibitors encapsulated into nano-containers(layered double hydroxides,nanotube,biological containers and porous materials) are reviewed,as shown in Fig.1 and Table 2.Based on the corrosion potential (Ecorr) and corrosion current density (Icorr),the corrosion resistance of various self-repairing coatings is summarized in Table 3.

Fig.1.Schematic representation of diverse self-repairing coatings on magnesium alloys in this review.
2.Shape restoring coatings
Nowadays,the shape restoring coatings for Mg alloys that have been extensively studied are mainly composed of linear synthetic polymers,including poly (ethylene imine) (PEI),poly (acrylic acid) (PAA),perfluorodecyl polysiloxane (PFPOS),shape memory polymers (SMP),polymethyltrimethoxysilane (PMTMS),etc.[60–63],which have excellent plastic deformation ability when triggered by external conditions.Once the shape restoring coatings are damaged,these polymers will swell,shrink,or reassociate reversible chemical bonds,so as to return to the original state and morphology,and cover the crack or damaged part again under the action of light,heat,or solvent [25].
Fan et al.[60]developed a self-repairing composite coating consisting of a cerium-based layer,a graphene oxide layer,and a branched PEI/ PAA on magnesium alloy (AZ31),in which graphene oxide was used as a barrier layer by stopping the penetration of corrosive electrolytes.The PEI/PAA multilayers were deposited on the top,which optimized the density of crosslinking through electrostatic interactions,and the swelling ability of the PEI/PAA multi-layers in water provides the coating with a self-repairing capability [60].
Zhang et al.[61]combined a heat stimuli-responsive epoxy resin (SHEP) with perfluorodecyl polysiloxane modified silica (PFPOS@silica) to produce a superamphiphobic coating on AZ31B Mg alloy.Through electrochemical tests,the corrosion current densities (Icorr) of the uncoated Mg alloy and Mg alloy with respective PFPOS@silica,SHEP,and PFPOS@silica/SHEP coatings were calculated to be 7.85 × 10-5,7.87 × 10-11,2.02 × 10-11,and 5.97 × 10-12A/cm2,respectively,which revealed theIcorranti-corrosion performance was decreased by 7 orders of magnitude after loading with the superamphiphobic coating.After the coating suffered physical scratched (width of∼27 μm),the coating recovered the original state through a two-step heating process(65 and 80 °C,respectively) in 30 min [61].
More recently,Zhang et al.[64] investigated another superamphiphobic coating SMP-BTA/fluoroATP composed of SMP with 1,2,3-benzotriazole (BTA) and attapulgite (ATP)on AZ31B Mg alloy.The superamphiphobic fluoroATP layer provided an air cushion between corrosive media and coatings.The thermoresponsive SMPs recovered the surface morphology and bulk integrity after temporary deformation by physically closing.As for the unbonded crevice remained at the crack,BTA molecules were released and adsorbed on the AZ31B Mg alloy surface serving as an inhibitor [65].The self-repairing performance of the coating mainly depends on the shape memory effect of the SMP,but the complete restoration of anti-corrosion performance is attributed to the synergistic effect of the SMP,BTA,and fluoroATP(Fig.2).Based on this triple protection,the coating demonstrated excellent protective properties and played a longterm protective role for the Mg substrate.Note that pure AZ31B Mg alloy appeared obvious corrosion spots after 2 days in salt spray.In contrast,the SMP-BTA/fluoroATP coated Mg alloy maintained the structural integrity in 55-day test [64].
SMP can also be combined with micro-arc oxidation (MAO) layer to obtain composite coating.Jun Liang’ s group [66] developed a self-repairing coating on AZ31B Mg alloy,which combined dual actions of the MAO layer with the corrosion inhibitor 1-(3-((N-n–butyl)aminecarboxamido)propyl)-3-hexadecyl imidazolidin bromide (M-16) and self-healing polyurethane (PU)layer with disulfide bonds.Once the coating suffered physical damage,M-16 molecules would be released from the MAO layer to form a new protective layer on the surface of the Mg substrate.At the same time,the PU’s bulk integrity and surface morphology could be restored from temporary deformation through the disulfide exchange reaction and shapememory effect after heat treatment,thereby showing excellent recovery and corrosion resistance [66].
Similarly,the hybrid MAO/PMTMS self-repair coating on AZ31 Mg alloy with a thickness of 13.65 ± 0.85 μm was prepared by Cui et al.[62] via MAO processing and subsequent alkaline and PMTMS treatment.The PMTMS film sealed the pores from MAO film and acted as a physical barrier to block the penetration of corrosive medium.In addition,driven by water swelling and hydrolytic polycondensation,thePMTMS film possesses self-repairing capability to realize the long-time protection for AZ31 Mg alloy [62].

Table 2 Common self-repairing coatings on Mg alloys and main self-repairing mechanisms,as well as the characteristics.

Fig.2.Schematic illustration of the self-repairing mechanism of the SMP-BTA/fluoroATP coatings [64].
The PMTMS also can combine other layers to realize biological applications.Zeng at al.[63] prepared corrosionresistant coatings (PMTMS/(AgNPs/PEI)5) with self-healing and antibacterial properties on AZ31 Mg alloys through layer-by-layer (LbL) assembly.The PMTMS/(AgNPs/PEI)5coating was composed of polyethyleneimine (PEI),Ag nanoparticles,and PMTMS.TheIcorrvalue was determined to be 9.73 × 10-8A cm-2through electrochemical tests,which was much lower than that of the bare AZ31 Mg alloy (1.62 × 10-5A/cm2) and Mg/(AgNPs/PEI)(9.87 × 10-7A/cm2).Besides,the antibacterial efficacy of PMTMS/(AgNPs/PEI)5film was 98.40% compared with the blank,attributing to the destruction of cell membrane via the adsorption of released Ag+ions by cells [63].
In short,linear synthetic polymers based self-repairing coatings mainly rely on the physical interactions to help broken coating to return to the original state and morphology and avoid further corrosion reactions.The advantage is that it canrestore the original morphology,but it depends on external conditions such as light and heat.

Table 3 Summary of corrosion resistance (evaluated by Tafel tests) of different self-repairing coatings on Mg alloys.

Table 3 (continued)
3.Function restoring coatings
The function restoring coatings mainly repair the anticorrosion function of the coating.When the function restoring coating is scratched,the released inhibitor can form a precipitation film with metal ions (e.g.Mg2+,Ca2+,and Zn2+) or form a passive film with anions(e.g.Cl-,CO32-,and SO42-)in the corrosive medium to cover the surface of cracked coating,thereby restoring the anti-corrosion function of the coating [67].The inhibitor does not need a high concentration to realize the function of corrosion resistance [68,69],however,it need to interact properly with the coating to avoid failure of the anti-corrosion function caused by the premature and rapid release of inhibitors.At present,loading modes generally involves two routines—one is to directly dope the inhibitors into the coatings,and the other is to encapsulate the inhibitors into some nano-containers with different sizes first and then dope them into coatings [70].
3.1.Chemical conversion coatings
Chemical conversion coatings (CCCs) usually release highly active reagents whose valence/structure states are markedly transformed into a passive matter by chemical or electrochemical treatments [71,72].Subsequently,these passive species adhere to the Mg-based substrate to form a film of metallic oxide/other compounds,chemically bonded to the surface.Chemical conversion coatings mainly include inorganic/organic conversion coatings [73].
Inorganic conversion coating mainly contains metal oxides or hydroxides,which are formed on the surface of Mg matrix after being treated in the metal salt solution.In a corrosive medium,the coating dissolves and the resulting metal ions precipitate to form a new passive layer.
Early studies on inorganic conversion coatings mainly focused on Cr (Ⅵ),which can form a protective oxide film at the crack[74].The main components of the film after Cr(Ⅵ)passivation are Cr (Ⅵ) and Cr (Ⅲ),which protect the alloy through a synergistic effect.Cr (Ⅲ) is indissolvable in water,and its adhesion will not reduce due to moisture dissolution in the environment,so it acts as the skeleton of the passive film,while Cr (Ⅵ) is soluble in water and exists in the skeleton of Cr (Ⅲ) through adsorption,inclusion,and chemical bonds.After the passive layer is scratched in a humid environment,Cr(Ⅵ)could dissolve and flow to the scratches,and then passive the matrix again by producing new Cr (Ⅲ),so that the film can be repaired and restored to a continuous and dense state [75].This is the special self-healing function of Cr (Ⅵ)which has been the best passive function so far.However,because of the high toxicity,the utilization of Cr (Ⅵ) ions has been gradually prohibited in the area of coatings [76].
Therefore,Cr (Ⅵ)-free coatings have been widely studied.Up to now,Cr (Ⅵ)-free inorganic conversion coatings mainly include stannate (Sn)-based [77,78],Cr (III)-based,vanadium(V)-based [79,80],and rare earth element (RE)-based conversion coatings [35,81–84].The self-repairing performance of these coatings with diverse components depends on the dissolution and reprecipitation effect of active reagents [85,86].
Zhang et al.[87] synthesized a novel CCC on the AZ31B alloy with the dual function of superhydrophobicity and selfrepairing from a Cr(III) containing deep eutectic solvent.The superhydrophobicity (water contact angle of 157°) is resulted from the hierarchically porous structure of modified stearic acid,preventing initial contact of corrosive medium from the AZ31B alloy.It can be seen from the scratch test that the coating can repair itself within 60 min.This self-repairing behavior is achieved by the formation of Cr-based oxides or compounds when the coating breaks or the corrosive medium enters the coating [87].After soaking in 3.5% NaCl solution for 120 min,the corrosion current density (Icorr) of hydrophobic coating is 2.92 × 10-5A cm-2(Table 3).
In addition to Cr(III)-based coatings,Hamdy et al.[88]designed a vanadium (V)-based self-repairing coatings on ZE41 Mg alloy.The alloy substrate was protected from corrosion in the environment containing chloride due to the formation of vanadium-rich oxide film over the pitting areas.After one week of immersion in 3.5% NaCl solution,the uncoated ZE41 Mg alloy showed a surface resistance of approximately 1.3 × 103Ωcm2,while the ZE41 Mg alloy with vanadiumbased coatings showed a double value (2.6 × 103Ωcm2).Similarly,the application of vanadium conversion coating could be expanded to the AZ31 Mg alloy for rendering the self-healing capability[89].The corrosion rate(0.3096 mm/y)of the AZ31 HP–O alloy treated with 50 g/L vanadium solution at pH 7 is obviously slower than that (0.7996 mm/y) of the untreated sample [89].
Apart from the above inorganic conversion coatings,organic conversion coatings have also been extensively developed,which is mainly composed of organic framework or small organic molecules,such as polymers,chitosan,and organic acid(e.g.phytic acid,vanillic acid[90],tannic[91],and gallic acid) [92–97],whose abundant surface active groups can not only chelate with Mg matrix/surface hydroxide to enhance adhesion,but also contribute to further modification to get more functions.
The use of phytic acid (PA) to prepare organic conversion coatings is a typical example.The nontoxic and biocompatible PA molecule contains a large number of hydroxyl and carboxyl groups which can chelate with Mg [98],Cu [99],Zn [100],and other metals.Wan et al.[101] adopted an alkaline pre-treatment process to covalently immobilize PA on the Mg substrate,as shown in Fig.3,and the PA layer has been successfully loaded on Mg surfaces.Compared with the untreated as-extruded bare Mg (3.35 × 10-2μg cm-2s-1),the Mg sample alkaline-pretreated for 24 h and coated with PA shows a decreased corrosion rate of 3.93×10-4μg cm-2s-1in the phosphate buffered saline (PBS) solution.It is owing to the dense and homogenous PA layer providing better corrosion protection by preventing electrolyte diffusion.When the coating is broken,the PA layer can be chelated with Mg2+to form a compact passive layer to complete the self-repair process [101].
Furthermore,the organic acid conversion layer can combine with other components,such as aluminum oxide [102],hydroxyapatite [103],and organic matters [104],to prepare composite coatings with a self-repairing effect.For example,Zhu et al.[91] reported the AZ31 Mg alloy with hydroxyapatite/tannic acid (HA/TA) conversion layers,illustrating a significantly reducedIcorrwith a value of 5.6494 × 10-8A cm-2in simulated body fluid (SBF) relative to the bare alloy (4.8978 × 10-6A cm-2).Moreover,the results of the MC3T3-E1 cell proliferation assays and morphology observations show the HA/TA coating is nontoxic to the cells [91].
3.2.Coatings directly loaded with inhibitors
The coatings directly loaded with inhibitors are mainly fabricated by physical doping,which possesses characteristics of high efficiency,simple preparation process,and low cost.Gnedenkov et al.[99] prepared PEO coatings loaded with inhibitors (8-hydroxyquinoline) (8-HQ) on the surface of the MA8 Mg alloy (1.5–2.5 wt.% Mn;0.15–0.35 wt.% Ce;balance–Mg).The self-repairing effect of the coating is realized through the chelation of the Mg2+with 8-HQ,and the 8-HQcontaining PEO coating exhibits excellent protective ability.The specimen with self-repairing coating shows higher value of the polarization resistance (RP=420 kΩcm2) as compared to the uncoated MA8 alloy (RP=1.8 kΩcm2).The corrosion current density (Icorr) is 8.6 × 10-8A/cm2for selfrepairing coating in the corrosion media,which is by three orders of magnitude lower than that for the uncoated MA8 alloy (Icorr=5.3 × 10-5A/cm2) [70].
The PEO can not only be prepared on the surface of Mg matrix alone,but also be compounded with other film-forming substances to play a self-repairing role.A PEO layer impregnated with inhibitor (3-methysalicylate) was prepared on the surface of pure Mg,and then sealed by the epoxy resin to obtain a self-repairing coating [105].The porous PEO layer acted as a carrier for storing and releasing inhibitors,which can play a key role in self-repairing and protection via physical/chemical adsorption and formation of precipitates with detrimental impurities.Chen et al.[106] incorporated and immobilized three inhibitors (sodium salts of glycolic,4-aminosalicylic,and 2,6-pyridinedicarboxylic acids) into the porous PEO coating by sealing with a thin sol-gel layer on the AZ91 Mg alloy.These three coatings all show admirable corrosion protection with little reduction in corrosion resistance after immersion in 0.5 wt.% NaCl solution for two weeks.For the coating loaded with inhibitor,two mechanisms can explain the superior corrosion resistance.On one hand,the active functional groups (like carboxyl,hydroxyl,etc.)in the inhibitors can decelerate Mg dissolution by adsorbing on the Mg matrix to decrease the anodic area.On the other hand,inhibitors can avoid the re-deposition of detrimental impurities (like Fe) via chelating with them to form stable complexes.
The loaded inhibitors can be released when encountering corrosion medium.However,if the release rate can be adjusted through the change of external conditions,Mg alloys can be protected more accurately.So some researchers employed pH sensitive inhibitors to achieve excellent corrosion protection effect.The tri(bis(2-ethylhexyl)phosphate)(Ce(DEHP)3) as a novel inhibitor in an epoxy-silane coating applied on the AZ31 Mg alloy was reported for the first time by Calado et al.[107].The self-repair process was observed by Localized Electrochemical Impedance Spectroscopy– LEIS,Scanning Vibrating Electrode Technique– SVET,and Scanning Ion-Selective Electrode Technique–SIET.The Ce(DEHP)3-modified hybrid coating showed superior anti-corrosion performance via forming Ce-and Pcontaining species to avoid further corrosion spread.After 600 days of immersion in NaCl (0.05 M),the coating showed low-frequency impedance modulus (at 0.01 Hz) in the order of 109Ω•cm2,being 3 orders of magnitude above the impedance modulus of the uncoated Mg alloy after 70 days of immersion,showing excellent long-term corrosion resistance for AZ31 Mg alloy [107].

Fig.3.Surface morphology of the bare Mg before and after being loaded with PA layer: (a) optical images,(b) SEM photos,and (c) AFM images [101].
Different from commercial small molecule inhibitors,the researchers,in order to achieve a better effect,directionally synthesized corrosion inhibitors with higher molecular weight,and prepared coatings by loading them on Mg alloys.Li et al.[108] synthesized a new composite anti-corrosion coating for the AZ31 Mg alloy,basing on the synergistic effect of an organic/inorganic composite coating with micro-and nanoporous MAO membrane as the container and the longchain ionic compound (1-(3-((N-n–butyl) aminecarboxamido)propyl)-3-hexadecyl imidazolidine bromide) (M-16) as the inhibitor.A thin epoxy resin layer is covered on the top surface to get the MAO/M-16/epoxy composite coating.When the damaged coating encounters the corrosive medium,the M-16 can be released and react with Mg2+ions to form a chelated layer,which covers the surface of the Mg matrix[108].

Fig.4.(a) The salt spray photos for AZ31,MAO,MAO/N-16 and MAO/N-16/wax film.(b) The wax/N-16/MAO composite coating and mechanism diagram of self-repairing [109].

Fig.5.The surface morphology of the coating samples after wet adhesion test and dry/wet adhesion strength diagrams of different coating systems [111].
Afterwards,they further synthesized another inhibitor(N-(5-hydroxypent-3-yn-1-yl)-N,N-dimethylhexadecan-1-aminium bromide) (N-16) and employed a hydrophobic wax layer as the top sealing film to get hydrophobic MAO/N-16/wax composite coating,which can effectively avoid the contact between the substrate materials with the corrosive medium[109].Through 15 days of comparative experiments,it is found that the MAO/N-16/wax composite coating has a good protective effect on AZ31 Mg alloy,as shown in Fig.4a.When the coating is damaged,the inhibitor can combine with Mg2+to form a new covering layer,thus reconstructing the protection function of the coating (Fig.4b).TheIcorr(5.764 × 10-9A/cm2) and protection efficiency (99.7%) of the MAO/N-16/wax composite coating is similar with those of MAO/M-16/epoxy coating (9.7 × 10-9A/cm2and 99.3%)after immersion in 3.5 wt.% NaCl solution.It is possibly due to their similar coating structure and self-repairing mechanism[109].
In addition,some polymers can also be used as inhibitors due to their unique properties.For example,the poly hydroxyquinoline (HQ) layer containing graphene oxide (GO)nanoparticles can achieve a better self-repairing and anticorrosion effect after the AZ31 Mg alloy is subjected to a pre-oxidation or phosphating treatment [110].Among them,GO serves as an inhibitor,whose carbon-oxygen bonds and the honeycomb-like structure play an important role in corrosion protection.The function of self-repairing is attributed to the swelling and mixing of charged groups in the GO particles.
The inorganic inhibitor and the organic inhibitor can be simultaneously loaded in the coating to achieve synergistic corrosion resistance.Aliofkhazraei et al.[111] prepared the PEO/silane/epoxy triplex coating system with inorganic inhibitor (Ce(NO3)3) in the PEO layer as well as organic inhibitors (8-hydroxyquinoline (8-HQ),indole-3-carbaldehyde (I3C),2-mercaptobenzoxazole (MBO),and sodium diethyldithiocarbamate (DDTC)) in the silane layer.In the corrosive environment,the inorganic inhibitor and the organic inhibitor can be released at the same time to form stable precipitation film,covering the Mg matrix to achieve the corrosion inhibition effect.As shown in Fig.5,the presence or absence of inhibitors has no effect on dry adhesion strength,and coating systems have comparable adhesion properties,but for the wet adhesion test,triplex-Ce-HQ shows the highest corrosion resistance,resulting from the formation of Ce(OH)3and Mg(HQ)2[111].
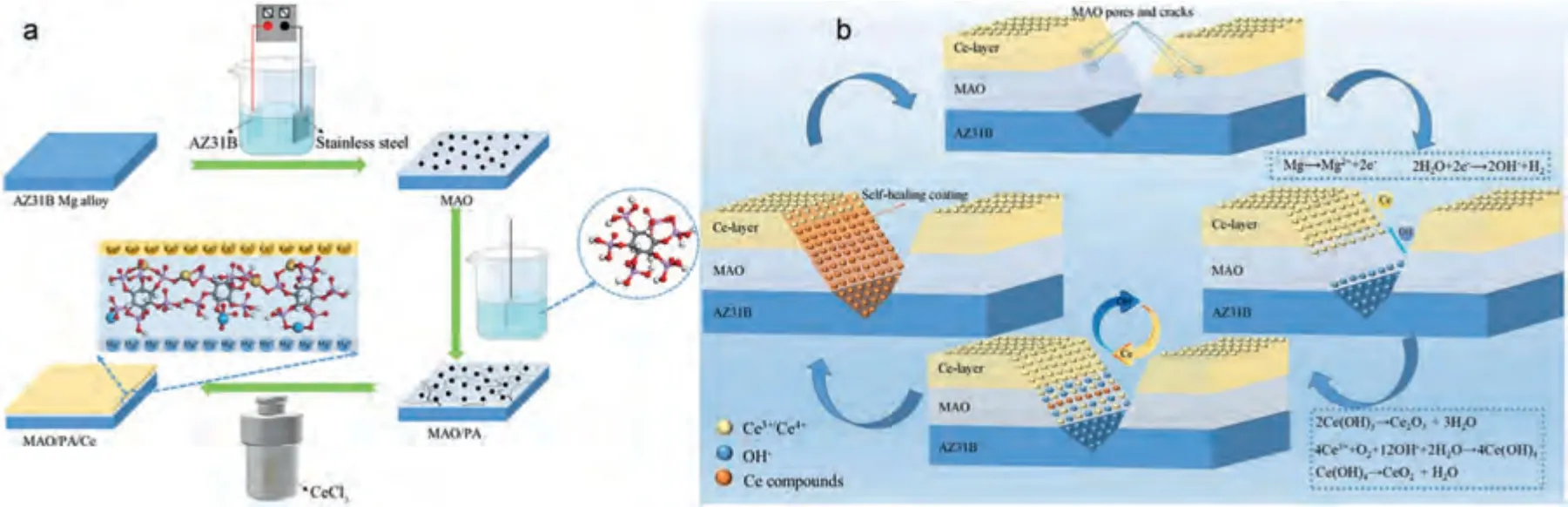
Fig.6.Schematic illustration of (a) the preparation process and (b) the self-repairing mechanism of MAO/PA/Ce composite coating on AZ31B Mg alloy[113].
Similarly,a duplex self-repairing coating,with the MAO layer loaded with inorganic inhibitor (Na3PO4) and the top paint layer loaded with the organic inhibitor (2-mercaptobenzothiazole (MBT)),was prepared on the surface of the AM60 Mg alloy by Liu et al.[112] The self-repairing property is greatly improved in comparison with addition of each inhibitor alone,which is due to the synergistic effect of the inorganic phosphate ions and organic MBT.
Cerium salt is a kind of good inhibitors,and the evolved CeO2,which possesses a physical blocking effect of the electrolyte diffusion,can significantly impede corrosion propagation in self-repairing coatings [86].A composite coating(MAO/PA/Ce) with a thickness of about 5 μm was prepared on the surface of the AZ31B Mg alloy (Fig.6a).[113] Phytic acid (PA) as an intermediate chelating agent enhances the bonding strength between the Ce particles and the MAO layer.When the coating is scratched,the Mg matrix is exposed to the corrosive medium,and OH-is released because of the reductive reaction of H2O.Then Ce compound in the coating is dissolved simultaneously and reacts with OH-.Meanwhile Ce can switch reversibly between Ce4+and Ce3+,so the Ce3+diffused to the surface of the AZ31B alloy can be partially oxidized to Ce4+.Finally,the newly formed Ce oxide/hydroxide precipitates efficiently cover the defects in the coating and thus hindering further corrosion (Fig.6b).TheIcorrandRPof the MAO/PA/Ce sample are 1.24 × 10-7A cm-2and 3.26 × 105Ω•cm2,respectively,while the corresponding values of the uncoated AZ31B alloy are 7.90 × 10-5A cm-2and 3.13 × 102Ω•cm2,respectively,illustrating the excellent anti-corrosion property of the MAO/PA/Ce coating [113].
Furthermore,in addition to improving the corrosion resistance of the coating,the mechanical strength is also an important factor.Loading nanoparticles in the coating is beneficial to enhance the mechanical strength and the friction resistance of the coating [114,115].Calado et al.[116] prepared a hybrid siloxane-based coating modified with CeO2nanoparticles on AZ31.The CeO2-modified coating exhibits excellent selfrepairing anti-corrosion performance,which can be attributed to the adsorption of aggressive species such as Cl-and stabilization of Mg corrosion products that block electrolyte access to the substrate.Compared with the blank coating (∼-100 μA cm-2),the CeO2-modified coating reduced the cathodic activity by about 20 times (∼-5 μA cm-2) when the coated samples were immersed in 0.05 M NaCl solution [116].
Similarly,two kinds of nanoparticles can be combined to improve corrosion resistance.Zeng’s group [117] reported a novel spin-spray layer-by-layer assembly technique to construct a multilayer film composed of SiO2and CeO2nanoparticles on the AZ31 Mg alloy.In the multilayer film,SiO2acts as the physical barrier and CeO2reacts with corrosive medium in damaged parts to regenerate a CeO2passive film,thereby realizing the self-repair of the coating [117].
3.3.Inhibitors encapsulated into nano-containers
The direct incorporation of inhibitors in the coatings is facile and can make the coating self-repairing for improved corrosion resistance.Another way is encapsulating inhibitors into containers to achieve controllable release.Among them,micro-nano containers and microcapsules are relatively wellstudied.Micro-nano containers are a kind of hollow materials with large surface area,which can store and release the target inhibitors and are favored by researchers because of their simple preparation process and low cost.Generally,they mainly include layered double hydroxides (LDHs) [118–120],nanotubes,biological containers and porous materials [121].
3.3.1.Layered double hydroxides(LDHs)
The LDHs refer to a class of compounds with layered structures,interlayer ions,and ion exchange ability[122–125].because metal ion layers of LDH have different affinities for different kinds of anions,the corrosive ions can be captured in the middle layer.The embedded anions will be released and act as inhibitors to enhance the corrosion resistance of LDH coatings.At the same time,LDH are also conversion coatings,the released anions may react with Mg2+ions to form dense and stable precipitates,which can fill the defects caused by corrosion,thus imparting self-repairing ability to the coatings and reducing the erosion from the environment[126].Besides,a variety of inorganic salts and small organic molecules can be loaded as inhibitors into LDHs,playing a very important role in the corrosion resistance of coatings[127–135].

Fig.7.3D current density maps over the artificial defects of PEO,PEO-LDH and PEO-LDH-SLIPS during immersion in 0.05 M NaCl solution [137].
Tang et al.[128] prepared ZnAl-LDHs coatings with different intercalated anions (Cl-,VO43-,PO43-,and MoO42-)on the AZ31 Mg alloy by hydrothermal method.Polarization curves and EIS results illustrate that the corrosion resistance of ZnAl-LDHs films with different anions is in the decreasing order as follows: ZnAlVO43--LDHs>ZnAl-MoO42--LDHs>ZnAl-PO43--LDHs>ZnAlCl--LDHs>ZnAlNO3--LDHs.This is because the ZnAlVO43--LDHs has the strongest ability to release anions and absorb corrosive Cl-ions.In short,the ZnAl-LDHs films intercalated with different anions show a good potential for protecting AZ31 Mg alloy in 3.5 wt.% NaCl [128].
As an excellent container,LDH can also be loaded with organic inhibitors in addition to inorganic inhibitors.A MgAl-LDH coating modified with a new synthesized thiophene derivative inhibitor,i.e.N-alkyl-N,Ndimethyl-N-(3-thienylmethylene) ammonium bromides (NTA),was produced on the AZ31 Mg alloy by Li et al.[136] via the hydrothermal method and subsequent immersion step.In 3.5 wt.% NaCl medium,theIcorrof the AZ31 Mg alloy is 5.888 × 10-6A cm-2,which decreases by five orders of magnitude to 4 × 10-11A cm-2for NTA@MgAl-LDH coating.The exceptional corrosion resistance of NTA@MgAl-LDH coating is mainly attributed to the fact that Mg2+ions can form a precipitation film with CO32-from MgAl-LDH,and NTA can be adsorbed on the surface of Mg (0001) crystal plane [136].
Besides,LDH could combine with PEO to produce a sandwich-structure coating.Dong et al.[137] prepared a selfrepairing coating on the surface of the AZ91 Mg alloy.The bottom PEO film grownin situon Mg alloy plays the role of a physical barrier,and the top LDH layer integrates ternary roles involving sealing the holes of the PEO film,loading inhibitor (molybdate),and providing substantial spaces for the perfluoropolyether oil infusion to generate a slippery surface.Immersion tests indicated that the slippery surface has a better water-repellent durability than the superhydrophobic surface.Furthermore,the LDH layer can adsorb Cl-and release molybdate through ion exchange to achieve good corrosion resistance.From Fig.7,compared with pure PEO coating,PEO-LDH and PEO-LDH-SLIPS coatings show excellent self-repairing abilities [137].
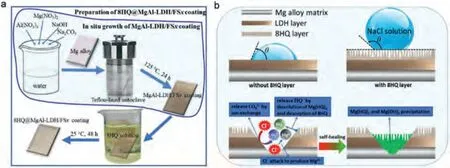
Fig.8.(a) The schematic diagram for in-situ preparation of 8HQ@MgAl-LDH/FSx coating on AZ31 Mg alloy.(b) Schematic representation of self-repairing effect and corrosion protection mechanism of 8HQ@MgAl-LDH/FSx coating [140].
Similarly,the synergistic effect of the sandwich structure coatings can achieve better self-repair anti-corrosion performance.Zhang et al.[138] fabricated a composite coating on the AZ31 Mg alloy via (i) PEO with Ce salt sealing,where LDHs were deposited via a hydrothermal treatment,and (ii) then modified by PA via an ion-exchange reaction.The self-healing ability and corrosion resistance of the four coatings (PEO,PEO-Ce,PEO-Ce-LDH,and PEO-Ce-LDHPA) are compared.The results show that the PEO layer can only provide a passive corrosion protection.Although the corrosion products of PEO-Ce could block the cathodic zones and the released Ce cations could precipitate in the cathodic areas to heal scratches,the PEO-Ce layer exhibits a limited active corrosion protection.It is worth noting that PA has strong chelation with metal cations.Mg2+and Al3+from the Mg substrate or/and Mg and Al oxides/hydroxides can chelate with PA to form deposition products which prevent further corrosion reaction.On account of the synergistic effect between Ce species and phosphate,the best self-healing and corrosion resistance are shown in the PEO-Ce-LDH-PA[138].
The NiAl-LDH coating was preparedin situon AZ31 Mg alloy using different nickel salts,(i.e.,carbonate,nitrate,and sulfate salts) by Xie et al.[139] which exhibited remarkable enhancement in corrosion protection.It is significantly important for industrial applications to establish feeding standards for preparation of LDH coatings.Wang et al.[140] investigated a MgAl-LDH coating on the AZ31 Mg alloy (Fig.8a).It was found that the MgAl-LDH layer can protect the AZ31 Mg alloy from corrosion,because MgAl-LDH can exchange with anions in the corrosive medium,such as Cl-and CO32-.After loading 8-HQ,the layer shows better corrosion inhibition capability.This is mainly attributed to the fact that the hydrophobicity of the coating is enhanced after loaded with 8-HQ,which can form a precipitate with Mg2+to cover the Mg matrix at the crack to prevent the occurrence of further corrosion reactions (Fig.8b).After 30 days of immersion in sodium chloride solution,the surface morphology of the 8HQ@MgAl-LDH/FSxdoes not change significantly,and it still has excellent corrosion resistance [140].
In addition,LDH can also be used to build super hydrophobic coatings.Wang et al.[141] synthesized Mg-Al LDH on AZ31 Mg alloy and then sealed it with sodium stearate (SS),lauric acid (LA),and myristic acid (MA) to make the surface energy go down,finally getting the super-hydrophobic coating(water contact angle>139°).When MoO42-from MA was exchanged into LDH by ion exchange,the coating exhibited the best self-healing properties and corrosion resistance.TheIcorrwas only 1.7 × 10-9A cm-2,which is four orders of magnitude lower than that of pure AZ31 Mg(1.529×10-5A cm-2) in 3.5 wt.% NaCl solution,attributed to the synergistic effect of molybdate and LA [141].
If the inhibitors loaded in the coating can be released intelligently with the change of environment,the corrosion of Mg alloys can be more accurately prevented.Recently,many nanocontainers are developed,which have the abilities to responses to various external and internal stimuli such as pH,light and temperature.Ouyang et al.[142] incorporated a pH-responsive nanocontainer (MSN-MBT@LDH,MM@L) into self-assembled nanophase particles (SNAP) to produce a smart self-repairing coating (SNAP-MM@L) on the AZ31 Mg alloy.The nanocontainer consists of mesoporous silica nanoparticle (MSN) core loaded with inhibitor(2-mercaptobenzothiazole,MBT) and LDH nanosheet shell serving as gatekeepers.The MBT molecules can be loaded in the nanopores and interiors of the MSNs via weak noncovalent interactions (physical adsorption,electrostatic interaction,hydrogen bonding,andπ-πstacking).The release of MBT from MSN can be influenced by the pH change.When pH decreases,the LDH can be dissolved,resulting in the release of MBT which reacts with Mg2+to produce precipitation films (Fig.9) [142].
3.3.2.Nanotubes
Researchers take advantage of the large internal space of nanotubes,which can accommodate a variety of substances,to apply them in the field of self-repairing anti-corrosion coatings [143].Halloysite nanotubes (HNTs) are hollow tubular structures with an inner diameter of about 10–40 nm.Featuring small size,high strength,and low density,HNTs have been widely used as nanocontainers,catalysts,adsorbents,and so on.

Fig.9.Schematic diagram of the corrosion protection mechanism: (a)nanocontainers incorporated hybrid coating with local damage,(b and c)self-repairing process,and (d) SNAP coating after self-repairing [142].
Sun et al.[144] successfully incorporated HNTs in the PEO coatings on AM50 alloy by a simple single-step galvanostatic process.The benzotriazole (BTA) inhibitor was loaded into HNT containers to obtain the self-healing functionality in PEO coatings.When the pH increases at corrosion sites,BTA in HNTs could mediate the formation of a dense Mg(OH)2film,hindering further corrosion and showing the self-healing functionality.Similarly,magnesium silicate nanotubes are also a kind of nanocontainers for inhibitors.Self-repairing coating (MS-TNs(2-MBI)/DTMS) was constructed on AZ31B Mg alloy by Liu et al.[145] via using the magnesium silicate nanotubes (MS-TNs) containing 2-mercaptabenzimidazole (2-MBI) inhibitor as the first layer and MS-TNs modified with dodecyltrimethoxysilane (DTMS)as the second layer.MS-TNs (2-MBI) layer has the ability of self-healing,and MS-TNs/DTMS contributes to the superhydrophobicity (water contact angle at about 155◦) and anticorrosion.The superhydrophobicity of the coating reduces the contact area between the coating and corrosive medium.At the same time,after long-time immersion or surface damage,the inhibitor 2-MBI is released to form a new protective layer on the surface of Mg matrix,so as to endow the coating with self-repairing ability.
3.3.3.Biological containers

Fig.10.The preparation process and the structure of M-CSCe hybrid coating[147].
There are also some natural polymers that can be used to carry inhibitors.In particular,some biomolecules,such as chitosan and proteins,are similar to synthetic polymers,have deformation ability and often have stimulus-response ability,which is a great feature for the design of self-repair coatings[146].Jia et al.[147] developed a composite coating (MCSCe coating),whose inner layer is composed of MAO layer and outermost layer is chitosan (CS) multilayers containing inhibitor (nanosized Ce oxides) (Fig.10).The pH-dependent charge behavior (swelling or shrinking) and the mobility of chitosan endow the hybrid multilayer coating with intrinsic potential for self-repairing [147].Due to the response to the stimulation of pH,Ce oxides are released and dissolved,producing a protective layer (cerium oxides/hydroxides) by reprecipitation,so the coating exhibits an excellent protective ability for the Mg–1Ca alloy.Compared with the bare alloy,the alloy covered by this coating shows the superior cytocompatibility,which is confirmed byin vitroculture via MC3T3-E1 preosteoblasts.So the CS container is suitable for biomedical applications.
Furthermore,proteins can also be used as containers to load more kinds of corrosion inhibitors owing to their flexible structures and abundant active groups.At the same time,they are responsive to acid-base changes,which is more conducive to the controllable release of inhibitor.Xiong et al.[148,149] fabricated self-healing coatings with a pH response effect on Mg-1Ca alloy surface,where the first layer is MgF2precoating,the middle layer is a silk fibroin loaded with inhibitors (K3PO4or phytic acid),and the top is a pure silk fibroin layer.When the local pH rises,the silk fibroin can release the inhibitors to form a new layer with metal ions,covering the surface of the body to achieve the purpose of self-repairing.Moreover,thein-vitrocell responses (cell adhesion,cell proliferation,and differentiation) confirm these coatings can display excellent biocompatibility and great osteogenic activity.
3.3.4.Porous materials

Fig.11.(a) Structure of F@MSNs and self-repairing mechanism of coating.(b) Metallographic images of Mg alloy substrate: with or without F@MSNs after immersion in 0.35% NaCl solution for 24 h [158].
Porous materials have a broad inner surface,large porosity,and open functional windows,and therefore are widely used in adsorption,separation,catalysis,energy,and optoelectronics,covering many important fields such as materials science,engineering,mechanics,earth science,and life science [150–153].For example,porous catalysts can replace highly toxic chlorine-containing catalysts,whichgreatly improves the selectivity and yield of the reaction and prolongs the overall life of the catalytic system.The above excellent properties of porous materials have aroused wide interests and concerns of researchers,thus stimulating a revolution in porous material architecture,which aims at developing and preparing novel topological structures and "targeting" functions [154–157].Porous materials have also been extensively investigated in the field of anti-corrosion and self-repairing coatings for Mg alloys.According to the different structural components,porous materials can be divided into inorganic porous materials (such as zeolite),inorganic-organic hybrid porous materials (such as metal-organic framework),and organic porous materials (such as covalent organic framework and porous aromatic framework).
3.3.4.1.Zeolites.Zeolites are a class of porous materials with uniform pore structure and large internal surface area,which are widely used in many fields because of their high adsorption capacity,strong selectivity,and excellent high temperature resistance.Moreover,the zeolites play an important role in the field of self-repairing coatings for Mg alloys,especially the MCM family with homogeneous mesoporous structures,which is generally selected to load inhibitors.
For example,Xie et al.[158] developed a Ni coating containing uniform MCM-41 type mesoporous silica nanocontainers (MSNs) loaded with anion F-on the AZ31 Mg alloy (Fig.11a).In the corrosive medium,pure Ni coating and the Ni coating incorporated with silicon nanoparticles can produce corrosive products,while the Ni coating incorporated with F@MSNs can produce a protective film (a compact MgF2layer) to cover the surface of Mg matrix at cracks.Ni coating incorporated with F@MSNs can release F-to react with Mg2+,thus enhancing the corrosion resistance (Fig.11b).Compared with the uncoated Mg alloy(Icorr=7.18 × 10-7A cm-2),theIcorrwas 1.10 × 10-10A cm-2for the alloy covered with F@MSNs,which was reduced by three orders of magnitude [158].
Similarly,the MCM-22 loaded with cation Ce3+was dispersed in the epoxy coating to prepare a self-repairing coating (epoxy coating with Ce-MCM-22) on the surface of the Mg-Li alloy by Wang et al.[159] Compared with the single epoxy coating or MCM-22,the epoxy coating with Ce-MCM-22 has a better self-repairing anti-corrosion function,because Ce-MCM-22 can release ions through ion exchange to form a new protective layer with the corrosive medium.
Likewise,Zhang et al.[160] first prepared a PEO film on the surface of AZ31 Mg alloy,and then coated an epoxy resin layer dispersed with zeolite microparticles serving as nanocontainers to load Ce3+ions.Mg2+and Na+in the corrosive medium can stimulate the release of Ce3+to produce protective films.Moreover,the pH of solution varying from 4 to 10 would not affect Ce release.Therefore,the coating exhibits good self-repairing and corrosion resistance for the AZ31 Mg alloy.
All the above works take advantage of the large internal space and ion exchange capacity of zeolites.It demonstrates that zeolites are suitable carriers for corrosion inhibitors and show great potential in self-healing coatings.
3.3.4.2.Metal organic frameworks(MOFs).Metal organic frameworks (MOFs) are periodic crystalline lattice polymers that are formed by self-assembly of metal ions or clusters (as coordination centers) and organic ligands (as bridged bodies)[161,162].MOFs have ultra-high specific surface area (from 800 to 10,000 m2g-1) and permanent porosity.By adjusting the composition of metals/organics and reaction conditions,MOFs with various structures can be prepared.MOFs are excellent candidates for self-repairing corrosion-resistant coatings due to their abilities of metal-chelating,ion-exchange,and guest molecule loading.Generally,MOFs can be dispersed in the organic coating as containers and actively repair the defects induced by corrosion[163].In addition,MOFs can also act as inhibitors in the self-repairing coatings.
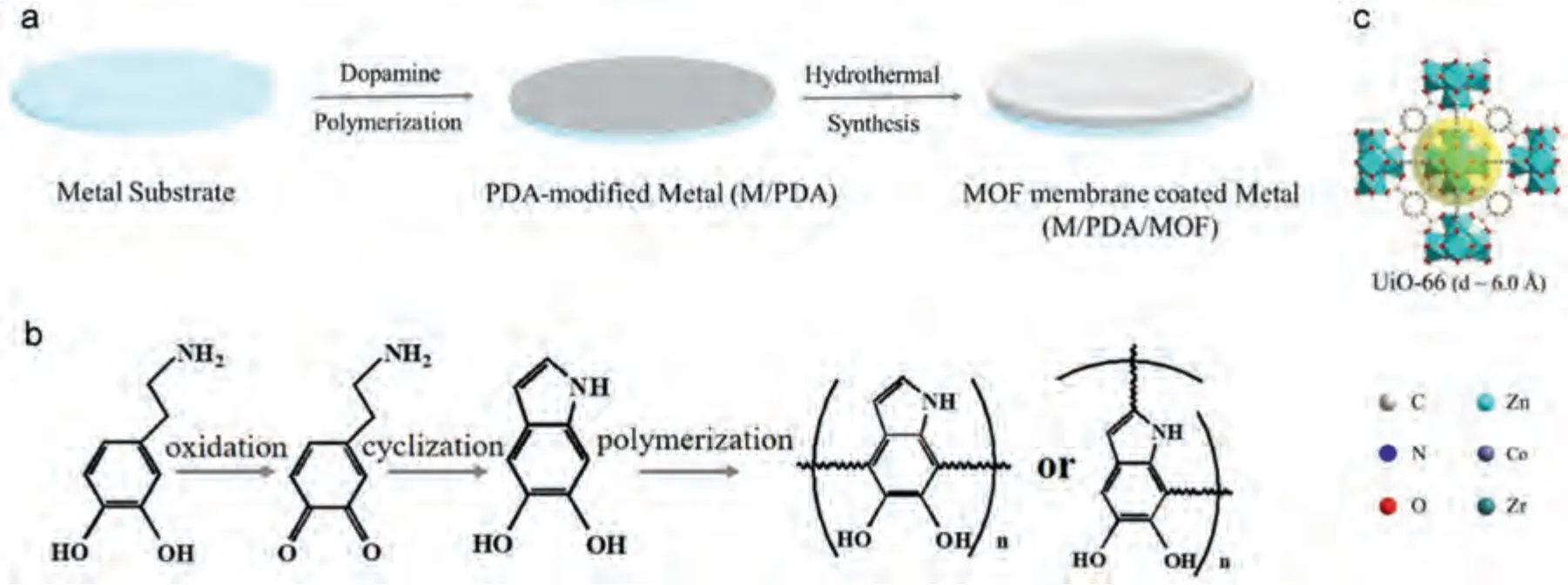
Fig.12.(a) Scheme of preparation of MOF membranes onto metal substrates;(b) Reaction mechanism for dopamine polymerization (c) Topological crystal structures of UiO-66 [164].
Liu et al.[164] polymerized dopamine to the polydopamine (PDA) on the surface of pure Mg (99.99 wt.%),and then prepared a zirconium-1,4 carboxybenzene (UiO-66)layer.UIO-66 is loaded on the top layer and has strong adhesion to PDA (Fig.12).Since the UiO-66 possesses a compact structure,hydrophobicity,and the ability to effectively reject corrosive ions,the PDA/UiO-66 coating shows a good effect of anti-corrosion and self-repairing.TheIcorr(2.2 × 10-5A cm-2) of Mg/PDA/UiO-66 electrode has dropped about six-fold in 3.5 wt.% NaCl solution,compared with bare Mg(1.46 × 10-4A cm-2) [164].
Surprisingly,MOF materials can also be biocompatible.Zheng et al.[165] constructed a hybrid coating composed of polycaprolactone (PCL) and copper-based MOF (HKUST-1 consisting of Cu centers and 1,3,5-benzenetricarboxylic acid linkers)modified with folic acid (FA) on AZ31 Mg alloy.The hydrogen bonds between PCL and MOFs make the PCL/MOF coating compact.PCL is biodegradable and could degrade when it encounters PBS.However,when PBS contacts MOF,the MOF can degrade and form holes to contain PBS,thus slowing down the degradation rate and improving the protection of Mg alloy for longer time.TheIcorris reduced from 7.18 ± 3.243 × 10-7A cm-2(bare Mg alloy) to 1.10 ± 0.937 × 10-10A cm-2(Mg-PCL-MOF) monitored by the electrochemical tests in SBF [165].
MOFs themselves are composed of coordination bonds,which can serve as containers or as inhibitors through the destruction of coordination bonds.In a word,they are good candidates for self-repairing and anti-corrosion coatings,and can also provide us with new ideas for self-repairing coatings.
3.3.4.3.Covalent organic frameworks(COFs).Covalent organic frameworks(COFs)are novel crystalline porous organic frameworks (POFs) formed by the covalent bonding of organic molecules,which endows COFs with structural stability.The skeletons of COFs are mainly composed of light elements.Because of many favorable properties,including high specific surface area,controllable pore size,and excellent modifiability,COFs can provide active sites and internal spaces for guest molecules.Therefore,by adjusting the pore sizes and modifying the binding sites,one or more guest substances can be loaded and assembled onto a variety of carrier surfaces,showing broad application prospects in the fields of ion capture [166],catalysis [167],and coatings [168,169].COFs as a kind of porous materials,which possess adjustable structures,customized functional sites,and regular pore structures (Fig.13) [170],so COFs can be prepared on the surface of Mg matrix to act as excellent candidates for carriers.Although the COFs have pores,the penetration of corrosive media can be hindered by changing the pore size,polarity,hydrophobicity,etc.In addition,the pores of COFs also provide abundant space for the loading of inhibitors.Up to now,there is only one case of anti-corrosion coating based on COFs for Mg alloy [53].
Liu et al.[53] produced a TiO2/TpBD (a COF) composite coating on the surface of AZ31 Mg alloy modified by nickel (Fig.14).As a photoanode,TiO2/TpBD provides direct protection for the nickel interlayer and indirect protection for the Mg matrix via photoelectrochemical cathodic protection (PECCP).Because of the well-matched conduction and valence bands between the TiO2and TpBD,the charge transfer process follows the direct Z-scheme photocatalytic mechanism.By coupling nickel coating on Mg alloy with TiO2/TpBD photoanode,the coating improves the photoelectrochemical performance for effective anti-corrosion.According to the electrochemical test results in a 3.5 wt.% NaCl solution,theEcorrandIcorrof the Mg alloy are -1.50 V (vs.SCE)and 7.08×10-6A cm-2,respectively.After being covered by the Ni coating,these parameters change to -0.33 V and 3.43 × 10-6A cm-2,respectively,showing that the Ni layer can be used as a physical barrier to protect the Mg alloy.After coupling with the TiO2/TpBD photoanode,theEcorrof the Ni/TiO2/TpBD layer decreases to -1.21 V,while theIcorrincreases to 6.96 × 10-5A cm-2under visible light,indicating the TiO2/TpBD layer can protect the Ni layer through cathodic polarization.Although this case is not a self-repairing coating,but the excellent properties of COFs make them show outstanding application potential in the field of corrosion prevention.Therefore,COFs will be an emerging hot spot in the future research on Mg alloy self-repairing coatings waiting for researchers to explore.

Fig.13.Multiple 2D COFs with layered stacking structures and nanopores [170].

Fig.14.Schematic diagram of TiO2/TpBD composite coating preparation process [53].

Fig.15.Chemical bonding between phytic acid coatings and the Mg surface[101].
4.The main mechanisms of self-repairing and characterization methods
The working mechanism of anti-corrosion self-repairing coatings is to form a flat and protective coating at the cracks.Shape restoring coatings swell,shrink,or reassociate reversible bonds to return to the original state and morphology.For example,because the glass transition temperatures of SMP is about 60 °C and the melting point of ceresine wax is about 80 °C,the composite coating composed of shape memory polymers (SMP) and ceresine wax on AZ31B Mg alloy plates (30 mm × 30 mm × 3 mm) show excellent selfrepairing capability after treated by a two-step heating process(65 and 80 °C,respectively) to trigger the self-repairing process [64].
The difference between function restoring coatings and shape restoring coatings lies in the presence of corrosion inhibitors.The key to the mechanism of function restoring coatings is that the redox reaction or coordination reaction with metal ions occurs to form new coatings at the crack after the inhibitors are released.The composition of the new coatings depends on the type of inhibitors.Inorganic inhibitors will mainly generate precipitation layers via redox reactions,while organic inhibitors will mainly form coordinate covalent bond with metal atoms through polar groups (N,S and O) to stabilize the system and form a protective layer on the Mg substrate.Polymer inhibitors adsorb and form a single or multiple layers of dense protective film on the Mg substrate.For example,the self-repairing capability of PEO coatings loaded with inhibitors (8-hydroxyquinoline) on the MA8 Mg alloy is attributed to the chelation of the Mg2+with 8-HQ to form a passive Mg(8-HQ)2layer [70].

Fig.16.(a–d) Snapshots of a scratched Cr(III) CCC on Mg alloy in 3.5 wt.% NaCl aqueous solution at room temperature for various times and (e and f)schematic diagram of self-repair mechanism [87].

Fig.17.SEM images and EDS spectra of the PMTMS/(AgNPs/PEI)5 coating after 0 h (a,d and e),24 h (b,f and g) and 72 h (c,h and i) of immersion in water [63].
For various application fields including biomedical,portable devices and automotive industries,several characteristics in terms of the bonding strength,layer-to-layer cohesion,self-repairing activity (kinetics and durations) should be considered in depth for better corrosion resistant properties.For instance,chemical conversion coatings adopt chemical bonding to enhance bonding strength between chemical conversion coatings and the Mg surface(Fig.15)and layer-tolayer cohesion among the multiple composite layers of composite coatings.The high bonding energy prevents the coating from peeling off during the application of magnesium alloys and to perform long-lasting protection [101].
For self-repairing coatings,their self-repairing activities are important factors in evaluating coating properties.Now,various methods,including optical photograph,optical microscopy,SEM,electrochemical impedance spectroscopy(EIS)test,and localized electrochemical techniques have been used to evaluate self-repairing activities (kinetics and durations).Optical photograph,optical microscopy,and SEM can more intuitively observe the repair activity of coatings,usually by scratching the coating and immersing it in NaCl aqueous solution (Figs.16 and 17) [63,87].
The efficiency of self-repairing can be used to evaluate the repairing ability of coatings.Normally,it could be calculated by the EIS tests in 3.5 wt.% NaCl solution according to Eq.(2) [64].
Whereηis the self-repairing efficiency.Log(|Z|0.1Hz)Repaired,Log(|Z|0.1Hz)Scratched,and Log(|Z|0.1Hz)Intactrepresent the Log value of low frequency (0.1 Hz) impedance modulus of the self-repaired coating,scratched coating,and intact coating,respectively.
Zhang et al.adopted Eq.(2) to illustrate the efficiency of SMP coated Mg alloy.Accordingly,the efficiency coefficients of SMP-BTA coated Mg alloy,fluoroATP coated Mg alloy,SMP-BTA/fluoroATP coated Mg alloy were calculated to be 41.47%,40%,and 98.18%,respectively,demonstrating the best repair ability of SMP-BTA/fluoroATP coating [64].
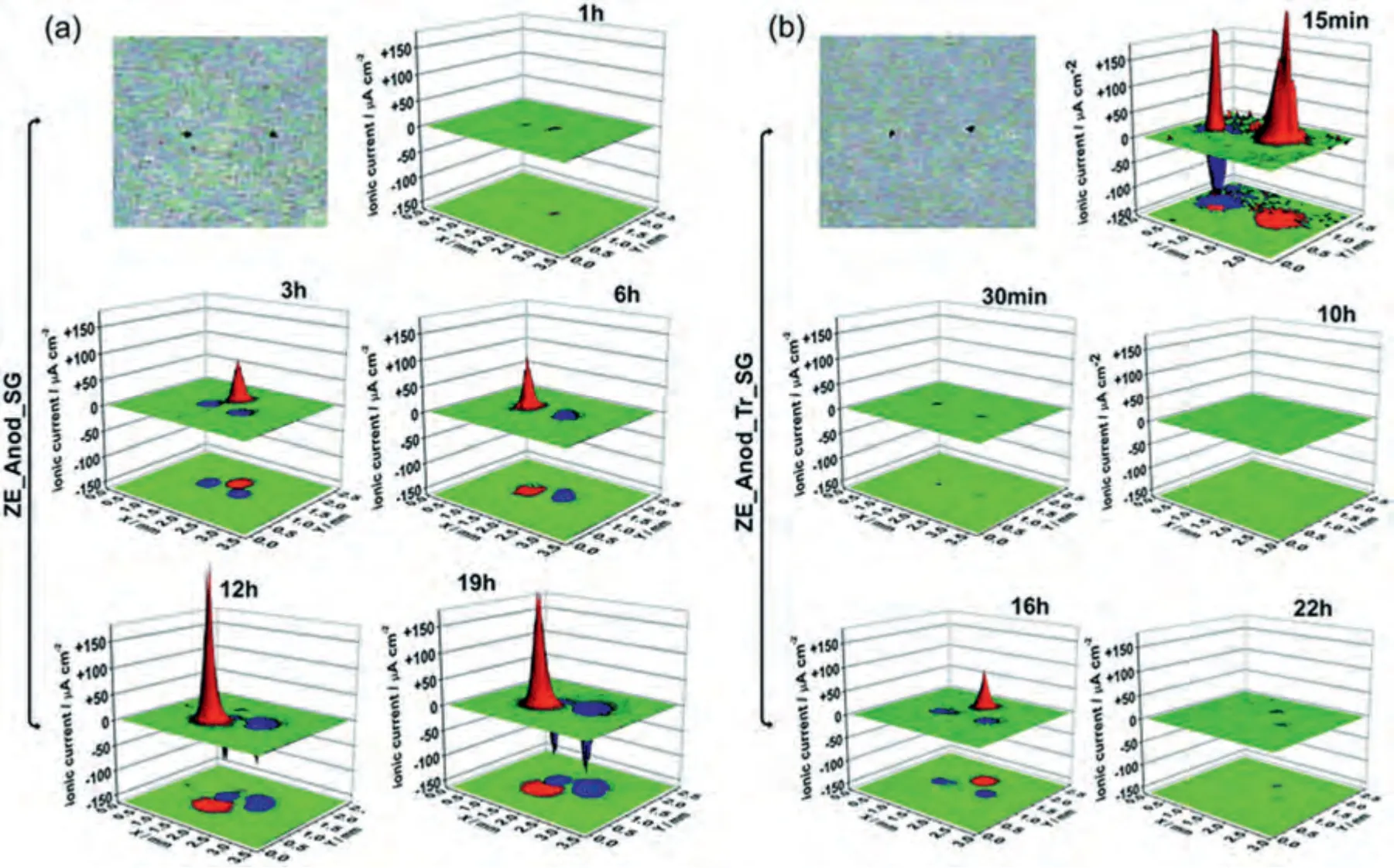
Fig.18.Microphotographs of scratched area and 3D distribution of currents for (a) ZE-Anod-SG and (b) ZE-Anod-Tr-SG immersed in 0.05 M NaCl solution[171].

Table 4 Self-repairing time by SVET of reported coatings in 0.05 M NaCl solution on Mg alloys.
Self-healing kinetics and durations were normally investigated by the scanning vibrating electrode technique (SVET),as a kind of localized electrochemical techniques,it evaluates the self-repairing activity of coatings by real-time andin-situdetection of corrosion current changes in the scratched area of the coating.After the coating is artificially scratched,Mg alloy with coatings is immersed in solutions (such as NaCl solution,simulated body fluid and Hank’s balanced salt solution),and the current changes in the scratched area are detected by the probe for presenting a three-dimensional current map to illustrate the self-healing kinetics and durations of the coatings(Fig.18)[171].As shown in Table 4,CP-SFAC coatings have a strong capability to repair broken spots in about 13 h probed by SVET in 0.05 M NaCl solution on Mg alloys.
In future,multiple techniques can be combined to achieve the goal of multifunctional detections,such as SVET technology was conducted under light irradiation to detect the self-repairing activity of light stimulus-responsive coatings.Thermal radiation is able to be combined with optical photograph to evaluate the variation of morphology of thermal stimulus-responsive coating during self-repairing process.InsituRaman technology could be adopted to probe the realtime detection of composition and structural changes in damaged coating.Local electrochemical impedance spectra technique (LEIS) could be used at different pH values to detect the responsive behaviors of self-repairing coatings under acid or alkaline environments.
5.Summary and prospect
This review illustrates the important progress in the construction of self-repairing anti-corrosion coatings on Mg alloys.We have systematically summarized the representative studies,mainly including shape and function restoring coatings,and described their unique merits in terms of structural design,coating preparation,and potential applications.
Shape restoring coatings can deform to restore the coating morphology under external conditions (light,heat,or pH).Therefore,it is suitable for preparing coatings on lessprecision surfaces,especially those that are prone to or often experience damage.Shape restoring coatings are mainly composed of synthetic polymers,including poly (ethylene imine)(PEI),poly (acrylic acid) (PAA),perfluorodecyl polysiloxane (PFPOS),polymethyltrimethoxysilane (PMTMS) etc.,which are promising candidates for preparing intelligent selfrepairing coatings with stimulus-response ability via shrinkage or expansion.However,there are still some problems that need to be overcome.One is that most of the synthetic polymers are environmentally unfriendly,so the development of coatings composed of green and environmentally friendly polymers is an important research direction in the future.In response to this issue,silane sol-gel coatings with some plastic deformation capability show the unique advantage of being bioenvironmentally friendly.Moreover,the silane sol-gel materials have a large number of pore cavities in the size range of tens of nanometers to several micrometers,which allow them to be composited with other functional materials to exhibit even better corrosion protection.
Function restoring coatings are mainly divided into three categories,namely conversion coatings,coatings directly loaded with inhibitors,and coatings loaded with containers.Conversion coatings combine the advantages of structure and function.Their physical structure effectively prevents the contact between Mg substrate and corrosive media,while their microstructure serves as corrosion inhibitors to repair damaged coatings.Conversion coatings are mainly composed of metal-based coatings,metal hydroxides and hydrotalcite coatings,metal oxide coatings and so on.The preparation process is relatively facile and does not require complex equipment.However,due to the relatively thin thickness,existing conversion coatings are often used for temporary protection or pre-treatment of Mg alloy surface to further prepare composite coatings.In future research work,this class of coatings can be combined with human essential metal elements for the design and assembly of coatings.Based on the thin thickness,it is beneficial to realize the controlled degradation of magnesium alloys inside living organisms.
For coatings directly loaded with inhibitors,their preparation process is relatively simple and thus exhibits high economic value.When the coating breaks,corrosion inhibitors adsorb on the Mg substrate surface through polar groups (N,S and O) to form coordinate bonds with metal atoms and stabilize the system.In the meantime,the coordination complex forms a hydrophobic protective layer on the Mg substrate.At present,PEO,epoxy resin,graphite oxide,etc.,loaded with corrosion inhibitors are mainly used to cover Mg alloy surface.Nevertheless,the existence of micro-and meso-pores in the coating architecture facilitates the release of the corrosion inhibitors before the coating breaks,leading to the failure of corrosion protection.Therefore,the main issue with such coatings is the modification of functional groups inside the architecture to prevent the premature release of inhibitors by increasing their interaction with the coating.
As for coatings loaded with containers,this class of materials relies on the presence and modification of the containers,which in turn enhances its load capacity and binding ability to the corrosion inhibitors.Since containers play a huge role,which are extensively investigated,including halloysite nanotubes,magnesium silicate nanotubes,biological polymers (chitosan and proteins),zeolites,MOFs,etc.Aiming at large loads and rapid release of corrosion inhibitors,the carriers need to have sufficient internal space and suitable pore wall environment and pore channel components for the combination of corrosion inhibitors.Halloysite nanotubes,magnesium silicate nanotubes,and zeolites are composed of pure inorganic components,making them suitable for loading inorganic corrosion inhibitors,but they have a limited pore volume.As an inorganic-organic hybrid material,MOFs can load both inorganic and organic corrosion inhibitors,but their acid/base stability is relatively poor.Biological polymers are constituted by organic building groups,which are suitable for organic corrosion inhibitors,but their acid,alkali and salt stability needs to be improved.
In future development,the self-repairing coatings for Mg alloy will be developed in the direction of intelligent and multi-functional,whose functions and effects mainly depend on the design and modification of carriers.So novel stable carriers should be designed and synthetized.Based on the reasonable design,the inhibitors can be released intelligently through the stimulus-response to the environment so as to exhibit different self-repairing effects for different environments.
A talented carrier should meet the following three basic requirements.First,the carrier is supposed to have enough internal space to contain inhibitors.Secondly,the carrier should have a moderate interaction with the inhibitor to avoid too fast or too slow release.Last but not least,there should be stimulus-responsive active groups in the carrier to realize the intellectualization of the coatings.Based on the above summary and analysis,COFs are a class of promising candidate for loading inhibitors to prepare self-repairing coatings.Because COFs have large Brunauer–Emmett–Teller (BET) surface areas and uniform channels,they can accommodate more corrosion inhibitorswhile modifying various functional groups(–COOH,–NH2,–SH,–SO3H,–CHO,–OH,–C≡N,etc.) to enhance interaction,thus being endowed with multiple functions.On the basis of the state-of-the art molecular design of building blocks,one is able to synthesize diverse COF frameworks with defined but tailored microstructures,endowing the coating with stimulus response function to achieve better corrosion prevention effect.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (Nos.52204389,U19A2084 and 52234009),the National Key Research and Development Program (No.2022YFE0122000)and Program for the Central University Youth Innovation Team.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Improvements in mechanical,corrosion,and biocompatibility properties of Mg–Zr–Sr–Dy alloys via extrusion for biodegradable implant applications
- Ultrasonic solidification mechanism and optimized application performances of ternary Mg71.5Zn26.1Y2.4 alloy
- Superplastic behavior of a fine-grained Mg-Gd-Y-Ag alloy processed by equal channel angular pressing
- GO/MgO/Mg interface mediated strengthening and electromagnetic interference shielding in AZ31 composite
- Experimental and theoretical studies on two-dimensional vanadium carbide hybrid nanomaterials derived from V4AlC3 as excellent catalyst for MgH2
- Electrochemical synthesis of boron-containing coatings on Mg alloy for thermal neutron shielding
