Structure-function integrated magnesium alloys and their composites
2023-12-27JunbinHouDingLiZejiLiuZhikngJiShoufuGunChongchoLiXiogungQioIgorGolovinMingyiZheng
Junbin Hou ,Ding Li ,Zeji Liu ,Zhikng Ji ,Shoufu Gun ,Chongcho Li ,Xiogung Qio,∗,Igor S.Golovin,Mingyi Zheng,∗
a School of Material Science and Engineering, Harbin Institute of Technology, Harbin 150001, China
b Department of Physical Metallurgy of Non-Ferrous Metals, National Research Technological University ‘‘MISiS’’, Leninskiy pr.4, Moscow 119049, Russia
c Moscow Polytechnic University, B.Semenovskay 38, Moscow 107023, Russia
Abstract Magnesium-based materials not only exhibit desirable characteristics such as low density and high specific strength,but also possess exceptional functional properties,including high damping capacity,high thermal conductivity,high electromagnetic interference shielding capacity,flame retardancy,and dissolvability.However,achieving a balance between strength and functional properties remains a significant challenge in Mg alloys community.Typically,strength depends on the pinning effect of defects,such as solute atoms and second phases,which hinder dislocation motion.On the other hand,optimal functional properties usually necessitate relative perfect crystal structures,as the presence of solute atoms and second phases can have adverse effects on damping capacity and thermal conductivity.Balancing these conflicting requirements is difficult.The trade-off between strength and functional properties of the Mg alloys should be broken to meet the urgent need in aerospace,automotive,3C (computers,communications,and consumer electronics) and energy industries for high performance structural-functional integrated Mg-based materials.This review summarizes recent progress in understanding the mechanisms and influencing factors for the functional properties of Mg alloys.The mechanisms underlying the trade-off between strength and functional properties of Mg alloys is discussed.The latest developed structural-functional integrated Mg alloys and their composites are summarized,including high strength Mg-based materials with high damping capacity/ high thermal conductivity/ strong electromagnetic shielding capability/ excellent flame-resistance/ high dissolution rate.The future works of developing structure-function integrated Mg-based materials are proposed.
Keywords: Structure-function integrated Mg alloys;Composites;Strength;Damping capacity;Thermal conductivity;Electromagnetic interference shielding;Ignition resistance;Dissolvability.
1.Introduction
Magnesium has the lowest density among commercially available structural metals,high specific strength and specific stiffness [1].In addition,Mg has some unique functional properties.For example,Mg has very high damping capacity and shock absorption properties[2],high thermal conductivity[3],electromagnetic shielding effectiveness [4],and excellent biocompatibility in physiological conditions [5].The dissolution rate of Mg is high due to its lowest standard electrode potential of all structural metals in an NaCl solution [6] as well as a loose and porous surface layer [7].Therefore,Mgbased materials are one of the most promising lightweight materials with good structural and functional characteristics,which makes them attract considerable attention in the fields of transportation,aerospace,electronics,energy and defense industries.
At present,magnesium-based materials are mainly used as lightweight structural materials,whilst their functional properties have not been fully exploited.The development of high-performance magnesium alloys with integrated structural and functional properties is of great significance for solving the problems of lightweight and functionalization of key structural components and alleviating the increasingly severe global environmental and energy crisis.However,some of the functional properties of Mg-based materials are difficult to achieve simultaneously with their strength since strength and functional properties originate usually from two contradictive mechanisms [8].For example,high strength requires high density of defects,such as solute atoms,second phases etc.,to impede dislocation movement.However,the second phase and solute atoms cause lattice distortion,and thus scatter electrons or phonons,which is undesirable for thermal conductivity and electromagnetic interference shielding effectiveness;the pinning effect of solute atoms on dislocations reduces the dislocation mobility,then decreases damping capacities of Mg alloys[8–10];some strengthening solute atoms and second phases may have significant effect on the surface layers and matrix,influencing the ignition-proof and dissolvable properties of the Mg alloys [11].Hence,there is a trade-off between the functional properties and strength of Mg-based materials.To meet the urgent needs from industries,efforts have been devoted in the recent years to develop high performance structural-functional integrated Mg-based materials.
This review summarizes the recent progress in the mechanisms for some typical functional properties,such as damping capacity,thermal conductivity,electromagnetic interference shielding effectiveness,flame retardancy,and dissolvability.Additionally,the latest developments of high-performance structural-functional integrated Mg-based materials are summarized,and avenues for future research on the structural and functional integrated Mg alloys and their composites are proposed.
2.Mg alloys and their composites with high strength and high damping capacity
Magnesium has excellent damping capacity among the structural metals owing to its easily activated dislocation slip and twinning as well as weak dislocation pinning at defects or impurities,making it an attractive choice for structure components requiring light weight and reduced vibration and noise.However,the tensile strength and damping capacity are often mutually exclusive properties since common strengthening approaches in magnesium invariably lower its damping capacity.This is because the strength and damping capacity originate principally from two contradictive strategies -the respective obstruction and promotion of dislocation motion[2,12–14].The strengthening mechanisms of Mg alloys are mainly solid solution strengthening and precipitation strengthening.On the other hand,these solute atoms and precipitation phases act as pinning points for slip dislocations,resulting in greatly reduced damping performance of the Mg alloys.In the 1960s,the United States developed a high-damping magnesium alloy K1×1 (Mg-0.6Zr) for aerospace products,while the tensile yield strength of the alloy is very low [15].Significant efforts have been devoted in the recent years to develop Mg-based materials with high damping capacity combined with high strength to circumvent the usual damping-strength trade-off.
2.1.Mechanisms for damping capacity of Mg alloys
Damping capacity is a measure of a material’s ability to dissipate elastic strain energy during mechanical vibration or wave propagation [16].Due to the time-dependent thermally activated relaxation effects,there exists a phase lag between the applied load and the resulting deformation in a material.In practical situations,the material’s response to the load includes a time-independent synchronous strain (elastic strainεe) and a time-dependent strain (anelastic strainεa) that lags behind the load.Consequently,the stress-strain relationship can be expressed as [17]:
where,σ0is the stress,ε0is strain amplitude.ϕdenotes the phase difference,andωstands for the angular frequency(ω=2πf).
As a result,a hysteresis loop will be formed in the stressstrain curve when the material is under cyclic loading.The area enclosed by the hysteresis loop represents the energy dissipated inside the material during one cycle.This dynamic hysteresis at low stress levels is defined as anelasticity or damping [16].
Several quantities (specific damping capacity (Ψ),loss angle (ϕ),loss coefficient (tanϕ),logarithmic decrement (δ),inverse quality factor (Q-1)) have been used to characterize damping capacity.Specific damping capacity (Ψ=ΔW/W)represents the ratio of energy absorption during one cycle(ΔW) to the maximum elastic stored energy during the same cycle(W).Loss angle(ϕ)is the angle by which the strain lags behind the stress.Loss factor (η) or loss tangent (tanϕ=E′′/ E′) is the ratio of loss modulus (E′′=(σ0/ε0) sinϕ) to the storage modulus (E′=(σ0/ε0) cosϕ).Logarithmic decrement(δ) represents the rate at which the amplitude decay during the free vibration process,δis given byδ=log (An/An+1),where Anand An+1denote the amplitudes of the nthand(n+1)thvibrations,respectively.The inverse quality factors(Q-1) is commonly used to characterize the damping capacity of materials throughQ-1=(f2-f1)/fr,wherefris the resonant frequencies corresponding to the amplitude,f1andf2are the half-width peak frequencies in the frequency spectrum of the sample.
If the damping capacity is low (tanϕ<0.1),all the damping quantities can be approximately related byΨ/2π=η=tanϕ=ϕ=δ/π=Q-1.Among them,Q-1is the most frequently adopted measure of damping capacity.
There are several different experimental techniques developed to measure damping capacity of Mg alloys.The torsion pendulum is extensively used to evaluate damping capacity by measuring logarithmic decrement (δ) through free decay method.Both free-decay and resonant-vibration techniques using cantilever beams have been used to determine logarithmic decrement (δ) and the inverse quality factor (Q-1),respectively [18].Dynamic mechanical analysis (DMA) [17] is a technique that is widely used to measure a material’s dynamic modulus (E′and E′′) and loss tangent (tanϕ) at various temperatures and frequencies under sinusoidal load.
It is underlined that damping of the same material measured by different techniques,e.g.free-decay,forced vibration and different clamping (free-free or free-clamped vibration reed,single or dual cantilever,three points bending etc.) may have different absolute values.Therefore it is very important to provide along with damping values such information,otherwise the comparison of the damping capacity may be misleading[19].Further details and limitations of internal friction techniques and calculations can be found in a Handbook [19].
High damping metals,often abbreviated as “Hidamets” or HDM,is the biggest and probably most important group of high damping materials widely used in various practical applications (noise and vibration reduction,preventing fatigue problems,increasing the quality of different cutting tools etc.)[20–22].High damping alloys can convert mechanical energy of vibration into heat through different damping mechanisms on atomic level [23].In addition to the obvious demand to have a certain level of strength,the Hidamets must have a stable and powerful source of damping [24].
Mg and its alloys are one of the most typical representatives of high damping metals with dislocation damping mechanism.The theoretical basis of dislocation movement producing damping was introduced by Granato and Lücke (G-L theory) for relatively low temperatures at which the pinning points are not movable [25].The model suggests that dislocations are pinned by relatively weak and unmovable pinning points,causing irreversible reciprocating unpinning-pinning motion between weak pinning points under external forces and consuming energy.
The G–L model was developed for “pure” metals,with a relatively small concentration of impurities.In alloys,the dislocation lines may be occupied with solute atoms diffused to the dislocation cores where they find places with lower position energy and are considered as unmovable pinning points[26].More general the defects in the crystal can be divided into two types with respect to their interaction with dislocation: first type is some really immobile point defects,such as dislocation network nodes and precipitated phases,and this type is called strong pinning points;the other is weak pinning points,such as solid solution atoms,vacancies,and point defects.The dislocation-pinning points interaction (GL model) can be explained by the model shown in Fig.1[25].At low stress levels,dislocations undergo bowing motion between weak pinning points.However,when the stress is sufficiently high to overcome weak pinning points,dislocations begin to make reciprocal motion between strong pinning points,resulting in “depinning-pinning” of dislocation lines and generating significant amplitude-related damping.
It can be inferred that the damping capacity (Q-1) consists of a strain -independent component (Q-10) and a strain-dependent component (Q-1h),
It should be also noticed that the first amplitude dependent internal friction (ADIF) curve is in most cases different to the second and subsequent curves.This difference is caused by the fact that during first ADIF test the dislocation structure is changed and new dislocations are generated due to work of Frank-Read mechanism.Thus,first curve is not representative and it is necessary to run at least three-five subsequent tests after the first one in order to have trustable ADIF curves,and to average these curves to have reliable results.
2.2.Factors influencing damping capacity of Mg alloys
The main factors affecting damping capacity of Mg alloys include solute elements,second phases,mobile dislocation density,etc.[12,27,28].
2.2.1.Solute elements
According to G-L theory,if alloying elements are dissolved in the solid solution of magnesium alloys,they act as weak pinning points for dislocations,affecting the amplitudeindependent damping performance of Mg alloys.Fig.2 shows the effect of alloying elements on damping capacity (logarithmic decrement (δ)) of binary Mg alloys [29].The solubility of alloying elements has crucial effect on damping capacity of binary Mg alloys.The low dislocation breakaway stress in the binary Mg alloys with the addition of alloying elements with very small solid solubility,such as Ni,Ca,Mn,Si,La,etc.,leads to extraordinary high damping capacity.Especially,the Mg alloys containing 0.1–0.2wt% Ni exhibit obvious higher damping capacity then pure Mg.While the addition of alloying elements with high solid solubility in Mg,such as Al,Cd and Nd,etc.decreases sharply the damping capacity of Mg since the mobility of dislocations is decreased to a large extent.With increasing concentration of the alloying elements,the damping capacity of Mg alloys is decreased obviously.
2.2.2.Second phase
The second phases,such as precipitates,act as strong pinning points for dislocations,and hence the damping capacity of the Mg alloys will be reduced[30].With increasing amount of second phases,the damping capacity of Mg alloys is decreased since the mobility distance of dislocations is reduced[31].
2.2.3.Grain size
Grain boundaries act as strong obstacles for dislocations.With decreasing grain size,the distance for dislocation motion becomes short.Consequently,the area that dislocations sweep through becomes small,resulting in low damping of Mg alloys [32].
2.2.4.Texture
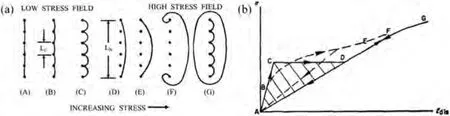
Fig.1.The schematic diagram of G-L model [25].(a) The bowing out of a pinned dislocation line by an increasing applied stress.The length of loop determined by impurity pinning is denoted by Lc,and that determined by the network by LN.As the stress increases,the loops Lc bow out until breakaway occurs.For very large stresses,the dislocations multiply according to the Frank-Read mechanism.(b) The solid line shows the stress strain law that results for the model shown in Fig.1(a).The elastic strain has been subtracted out so that only the dislocation strain is shown.The path ABCDEF is followed for increasing stress,while the path FA is followed for decreasing stress.The dashed line curve is that which would result if not all the loops have the same length,but there is a distribution of lengths Lc.

Fig.2.The effect of alloying elements on damping capacity of binary Mg alloys [29].(a):Mg-Ni;(b):Mg-Zr;(c):Mg-Mn;(d):Mg-Si;(e):Mg-La;(f):Mg-Ca;(g):Mg-Ce;(h):Mg-Al;(i):Mg-Cd;(j):Mg-Nd.
The damping of magnesium alloys is very sensitive to their crystallographic texture since the Schmid′law holds for the crystallographic orientation dependence of the breakaway stress,at which the basal dislocations bow out to move against the binding force of impurity atoms [33].The larger the Schmid factor,the smaller the breakaway stress of dislocations and thus the higher damping capacity of Mg alloys[34].Therefore,the damping capacity of Mg alloys is usually decreased after deformation due to the formation of the basal texture with basal planes parallel to the extrusion or rolling direction [35].
2.2.5.Twinning
Twinning is an important deformation mechanism in Mg alloys,and (102) twins are easily activated in wrought Mg alloys under compression along the extrusion/rolling direction.The introduction of (102) twins can enhance the damping capacity of Mg alloys because of the alternative shrinkage and growth of pre-existing twins during cyclic loading,which is attributed to the high mobility of (102) twin boundaries[36–39].
2.2.6.Dislocation density
The increase in the mobile dislocation density contributes to the enhancement of damping capacity of Mg alloys.However,too high dislocation density may lead to dislocation tangles,resulting in a decrease in the mobile dislocation density and reduced damping capacity of Mg alloys [40].
2.3.Mg alloys with high strength and high damping capacity
Classical approach to increase strength without significant lost in intrinsic damping capacity of Mg is introducing second phases to Mg (e.g.,Mg-Ni,Mg-Si,or Mg-Zr).These second phases act as pinning points to impede long-range dislocation motion,thereby increasing the yield strength,while dislocation loops between precipitates must be relatively free to move [41–44].To overcome the trade-off between damping capacity and strength,researchers have also been exploring novel strengthening mechanisms that are different from the damping mechanism found in Mg-based materials.
Sugimoto et al.[45] proposed guidelines for producing high damping capacity Mg alloys with enhanced strength:
(1) solution atoms can weaken pinning effects on dislocations,so the solubility of alloying elements inα-Mg should be minimized;

Fig.3.The damping capacity at the strain amplitude of 10-3 v.s.ultimate tensile strength of Mg alloys.Damping measurements were carried out using forced vibrations (single cantilever, f=1 Hz,Room temperature) on dynamical mechanical analyzers of different trade marks.
(2) the second phase in the Mg alloys should have stress concentration effects;
(3) the grain size of Mg alloys should not be less than 10μm,otherwise dislocations cannot depin under high strain.
The damping capacity and ultimate tensile strength of Mg alloys reported in the literatures are summarized in Fig.3.It can be seen that the Mg-RE alloys exhibit excellent damping capacity and strength.This chapter summarizes the latest developed Mg alloys with high-damping capacity (Q-1>0.01 at a strain amplitude of 1×10-3) and strength higher than 300 MPa.
2.3.1.Mg-RE alloys
Mg-RE alloys containing RE elements (such as Y,Gd,Er,etc.) with high solid solubility usually have low damping capacity [46–48].However,recent researches indicate that Mg-RE-TM (transition metals) alloys containing long period stacking ordered (LPSO) phase and stacking faults (SF) exhibit both high strength and high damping capacity[2,46–50],making them promising candidates for new high-damping Mg alloys.
As shown in Table 1,Yuan et al.[49] develop a highstrength Mg-7Gd-3Y-1Nd-1Zn-0.5Zr Mg alloy with high damping capacity,which contained 14H LPSO phase,through extrusion and T5 treatment.The alloy achieves a UTS of 390 MPa,TYS of 346 MPa,EL of 9.5%,andQ-1value of 0.035 at a strain amplitude of 1 × 10-3.The 14H-type LPSO phase formed in the alloy not only strengthens the alloy but also enhances its damping capacity.The formation of the 14H-LPSO phase leads to the consumption of RE and Zn elements in theα-Mg matrix,reducing the concentration of solute atoms and contributing to the high damping capacity of the alloy.They consider that the 14H-LPSO phase induces cross-slip of dislocations,resulting in an increase in the density of mobile dislocations and an improvement in damping capacity.
Ma et al.[47] develop an extruded Mg-3.16Y-1.85Zn-0.37Zr (wt.%) alloy containing 14H LPSO phase with UTS of 330MPa,TYS of 280MPa,EL to failure of 21% andQ-1of 0.023 at a strain amplitude of 1×10-3.The strengthening mechanisms of the extruded alloy include solution strengthening,dislocation strengthening,LPSO phase strengthening and grain boundary strengthening.They propose that LPSO phase may be a new damping source that improves the damping capacity of Mg-RE alloys.
Stacking faults formed in Mg-RE alloys contribute to the high strength and damping capacity of the alloys.The extruded Mg-4Er-4Gd-1Zn alloy [46] and Mg-1.5Gd-1Zn alloy[2] containing stacking faults exhibit a good combination of strength,ductility,and damping capacity.The extruded Mg-1.5Gd-1Zn alloy exhibits a TYS of 314 MPa,an EL of 21.0%,and aQ-1value of 0.017 at a strain amplitude of 10-3,as shown in Fig.4.The damping mechanism of the Mg alloys containing stacking faults does not agree with the G-L theory.In addition to dislocation motion,the stacking faults may introduce interfaces and dislocation/cluster interactions,contributing to the high damping capacity of the Mg-RE alloys[46].
2.3.2.RE-free Mg alloys
Mg-Al/Zn alloys are the most commonly used Mg alloys.However,the large solubility of Al and Zn elements inα-Mg leads to a low damping capacity in Mg-Al/Zn alloys [53].Alloying,thermomechanical processing,or heat treatment can be utilized to facilitate the precipitation of second phases and reduce the solute content dissolved inα-Mg,thereby improving both the mechanical properties and damping capacity of Mg-Al/Zn alloys [54–57].As shown in Table 2,the strength and damping capacity of the ZK60 alloy are improved through high strain rate rolling (HRSS) [58] and multi-directional forging (MDF) [55].The ZK60 alloy processed by HRSS and subsequent 1 h annealing at 340◦C exhibits a UTS of 333 MPa,TYS of 268 MPa,EL of 19%,and aQ-1value of 0.018 at a strain amplitude of 10-3[58].The increased strength is mainly attributed to grain refinement and the presence of fine MgZn2precipitates.The increased damping capacity is related to the purifiedα-Mg matrix due to the presence of abundant precipitates and an increase of mobile dislocation density.The ZK60 alloy processed by MDF achieves UTS of 333 MPa,YS of 293 MPa,EL of 19%,and aQ-1value of 0.014 at a strain amplitude of 10-3[55].The remarkably improved strain-dependent damping capacity is ascribed to the high density of mobile dislocations in the MDFed alloy,which effectively increased their sweep areas,as shown in Fig.5.
An ultralight extruded Mg-4Li-3Al-0.3Mn alloy is developed with TYS of 248 MPa,UTS of 332 MPa,EL of 14.3%,andQ-1value of 0.028 at a strain amplitude of 1×10-3.The high strength is attributed to the refined grain and the precipitation of large amount of nanoscale Al-Mn phases.The highdamping capacity is due to the high dislocation density and the high Schmid factor for non-basal slip [59].

Table 1 The mechanical properties and Q-1 of Mg-RE alloy with high damping capacity and high strength.

Fig.4.TEM-BF,HAADF-STEM images,EDS mapping analyses of the as-extruded Mg-1.5Gd–1Zn alloys: (a,b,d–f) extruded at 300 °C,(c,g–i) extruded at 420 °C and (j) damping-strain amplitude curves,(k) tensile stress-strain curves [2].

Table 2 The mechanical properties and Q-1 of high-damping RE-free Mg alloys with high strength.
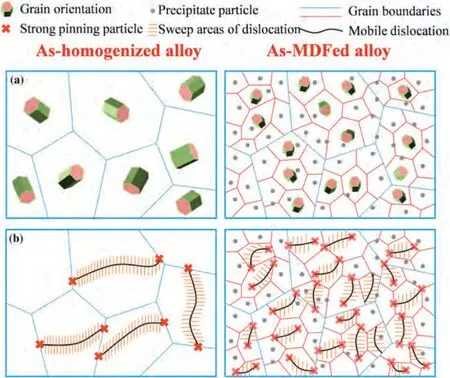
Fig.5.Schematic diagrams of the (a) strengthening (b) damping mechanisms for the ZK60 alloys before and after MDF process [55].
Recent researches indicate that extruded Mg-Mn [60] and Mg-Ca [61] alloys exhibit significant solute segregation at fine-grain boundaries.This segregation may facilitate grain boundary sliding at ambient temperature,making them potential low-cost high-damping Mg alloys with high strength and high ductility.
2.4.Magnesium-matrix composites with high strength and high damping capacity
Alloying often has a negative effect on the damping capacity of Mg alloys.Addition of reinforcements with high damping capacity and high strength into Mg matrix is a promising approach to enhance both mechanical properties and damping capacity of Mg alloys [62,63].The damping mechanisms of Mg-matrix composites include dislocation damping,grain boundary damping,interface damping,interaction damping and the rule of mixtures damping [64].
Due to the mismatch in thermal expansion coefficient between the reinforcement and the matrix,residual stress induced by thermal misfit generates at and near the interface.This stress induces plastic flow in the matrix,and thus high density of dislocations,which may enhance damping capacity.
As shown in Table 3,Wu et al.[65] develop Grp/AZ91 composites with high damping capacity through stir casting and subsequent extrusion.The as-extruded Grp/AZ91 com-posite exhibits a TYS of 252 MPa,UTS of 351 MPa,EL of 5.6%,and aQ-1value of 0.015 at a strain amplitude of 1×10-3.The addition of graphite refines the grain size of the matrix,leading to high strength.TheQ-1value increases significantly with the increasing volume fraction of graphite particles.The damping capacities of the Grp/AZ91 composite satisfy the G-L model within a limited strain amplitude range.In addition to dislocation damping,other mechanisms such as intrinsic damping of graphite particles,particle/matrix interface damping,or grain boundary damping,also contribute to the damping capacity of Grp/AZ91 composites at room temperature.
Table 3 The mechanical properties and Q-1 of Mg-matrix composites with high strength and high damping capacity.
Zhang et al.[63] develop a novel Mg-NiTi composite with a bicontinuous interpenetrating-phase architecture by infiltrating Mg melt into a three-dimensionally printed Nitinol scaffold.The formation and 3D architecture of the Mg-NiTi interpenetrating-phase composite are shown in Fig.6.The bicontinuous interpenetrating-phase architecture ensures a significant strengthening effect through the Nitinol reinforcement while promoting good damping capacity through effective load transfer.The Mg-NiTi interpenetrating-phase composite has an ultimate compressive strength of about 320 MPa,which is well above the estimates from the rule-of-mixtures,and exhibits an exceptional damping capacityQ-1value of 0.08 (at a strain amplitude of 0.1%) which is higher than that of pure magnesium and the Nitinol scaffold.The superior damping capacity (Q-1) of 0.08 combined with an ultimate compressive strength higher than 320 MPa in the Mg-NiTi composite break through the traditional benchmark of properties for Mg-based materials.The damping capacity of the composite was related to the Ti-rich phases and Mg2Ni phases formed at grain boundaries,which serve as prime sites for microyielding and thus promote the damping capacities of the composite.
Magnesium-matrix composites possess excellent damping capacity and high strength/modulus compared to Mg alloys.However,the ductility of these composites is generally much lower than that of the monolithic Mg alloys.The manufacturing processes for Mg-matrix composites are commonly quite complex,and the reinforcement phases are usually expensive.Future work should focus on exploring cost-effective reinforcements and low-cost fabrication methods.
3.High strength Mg alloys and their composites with high thermal conductivity
With the development of new energy vehicles,aerospace,and 3C industry,the power density of devices is increasing.Therefore,it is essential to efficiently dissipate the heat generated during device operation to ensure their reliability and stability.Consequently,there is an urgent demand for lightweight heat dissipation materials.In comparison to traditional high thermal conductivity materials such as Ag and Cu,which have high density and cost,Mg boasts a lower density,lower cost,and a high thermal conductivity of 158 W·m-1·K-1.These characteristics make Mg an excellent choice for lightweight heat dissipation components [66].
3.1.The mechanisms for thermal conductivity of Mg alloys
From a macroscopic view,thermal conduction is the process through which heat spontaneously flows from a hightemperature area to a low-temperature area in the presence of a temperature gradient.The thermal conductivity of a material is a parameter that characterizes its ability to conduct heat.Thermal conductivity is defined as the amount of heat transmitted per unit time and per unit area through per unit temperature gradient,as calculated by Fourier’s law [67].Thermal conductivity is usually represented by the symbolλand is measured in units of W·m-1·K-1as shown in Eq.(4):
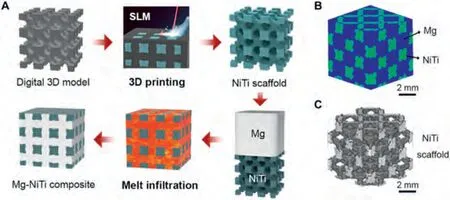
Fig.6.Fabrication and 3D architecture of Mg-NiTi interpenetrating-phase composite [63].(A) Schematic illustration of the fabrication process of the Mg-NiTi interpenetrating-phase composite by 3D printing of a Nitinol scaffold and subsequent pressureless infiltration of the scaffold with magnesium melt.SLM,selective laser melting.X-ray tomography (XRT) volume renderings of (B) the infiltrated composite and (C) Nitinol reinforcement in the form of rhombic dodecahedrons within the composite,obtained by filtering out the signal from the magnesium.
whereQis the heat flux (W·m2) and ∇Tis the temperature gradient (K·m-1),respectively.
From a microscopic view,efficient heat transport requires a sufficient number of collisions among energy carriers to occur.In solid materials,particles can only undergo small vibrations near their equilibrium positions [68].Therefore,heat conduction in solid materials primarily relies on the lattice vibrations (phonons) and the movement of free electrons.Consequently,the thermal conductivity of solid materials is given by [69,70]:
whereλphdenoted as phonon thermal conductivity,λedenoted as electron thermal conductivity.Therefore,thermal conduction depends on the availability and scattering rates of electrons or phonons.For metals,the movement of free electrons can be analogized to the free movement of gas molecules[71].
Where,Cvis the total specific heat capacity andlis the average free path of electrons.
Therefore,the thermal conductivity of metals and alloys is influenced by the mean free path of electrons or phonons,and it tends to be proportional to their mean free path.Interactions between phonons and electrons determine the thermal conductivity in a pure metal.In alloys,the thermal conductivity is significantly reduced due to the presence of lattice vibrations,impurities,defects,and interfaces,leading to a reduction in the mean free path of electrons [68].
In Mg alloys,solute atoms and precipitates act as scattering centers for both free electrons and phonons.The scattering of free electrons by solute atoms is primarily governed by two mechanisms: (1) electron-impurity scattering,where the presence of solute atoms disrupts the regular electron-lattice interaction,leading to increased electron scattering and reduced electron mobility,and (2) electron-phonon scattering,where electrons interact with lattice vibrations (phonons) and exchange energy,thereby reducing their mean free path [72].Solute atoms and precipitates introduce local strain fields and lattice distortions,scattering the propagating phonons and impeding their transport,thereby reducing the thermal conductivity.
The methods to measure the thermal conductivity of alloys can be classified into steady-state methods and transientstate methods [68].Steady-state methods directly measure the thermal conductivity based on Fourier’s law by measuring the temperature difference under a steady-state heat flow through the sample.However,steady-state methods require relatively large samples and longer test times compared to transient methods.The transient-state methods involve subjecting the sample to a sudden change in temperature and measuring the subsequent temperature response over time to determine the thermal diffusivity.Among the transient-state methods,the flash method is the most popular for measuring thermal diffusivity due to its convenience and simplicity [66,71,73].
The thermal conductivity is determined by the product of the thermal diffusivityα(m2·s-1),densityρ(g·cm-3) and special heat capacity Cp(J·g-1·K-1) [74,75].
3.2.Factors influencing thermal conductivity of Mg alloys
The factors influencing the thermal conductivity of Mg alloys are complex.At a microscopic level,factors that influence the mean free path of electrons and phonons will influence the thermal conductivity of Mg alloys.The main factors influencing the thermal conductivity of Mg alloys include solute atoms,second phases,texture,and temperature[9,66,76,77].

Fig.7.Effects of solute atoms on the thermal conductivity of some binary Mg alloys in: (a) RE-free binary Mg alloys [78] and (b) Mg-RE binary alloys [79].
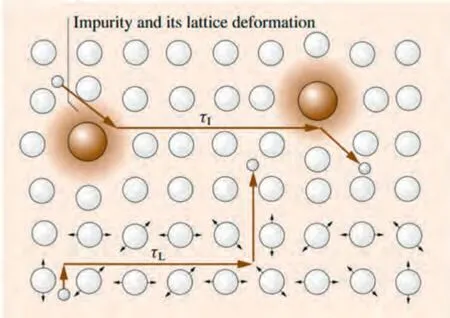
Fig.8.Scattering effects of lattice vibrations and impurities on electrons[80].
3.2.1.Solute atoms
The addition of alloying elements decreases the thermal conductivity of binary Mg alloys.As shown in Fig.7 [78,79],the addition of solute atoms reduces the thermal conductivity of Mg.In binary Mg alloys,different solute elements have varying effects on the thermal conductivity.The thermal conductivity is determined by the difference in atomic volume and valence between the solute atoms and Mg,both of which can cause lattice distortion and deteriorate the thermal conductivity.Fig.8 shows the scattering effects of impurities and lattice vibrations on electrons.Lattice vibrations and the presence of impurities alter the direction of electron motion and shorten the mean free path of electrons and phonons,thereby reducing the thermal conductivity of the alloy.Therefore,alloying elements that have a small difference in atomic volume and valence compared to magnesium show a weak effect on reducing the thermal conductivity of Mg alloys.Non-RE alloying elements decrease the thermal conductivity of Mg in the following sequence: Zn 3.2.2.Second phase Due to the mismatch in lattice constants between the second phases and the Mg-matrix,lattice distortion occurs at the interface,which becomes a scattering center for electrons and phonons.This leads to a decrease in the thermal conductivity of Mg [81].Compared to the incoherent interface,the coherent interface induces more significant lattice distortion due to the large stress field introduced by the occupation of theα-Mg lattice by atoms of the second phases.This distortion leads to a more substantial decrease in the thermal conductivity of Mg alloys [81]. Intermetallic compounds generally have much lower thermal conductivity compared to the Mg-matrix due to a significantly reduced number of free electrons.Increasing content of the intermetallic compounds usually reduces thermal conductivity of Mg alloys.Although the second phases deteriorate the thermal conductivity of Mg alloys,their influence on thermal conductivity is much lower than that of the solute atoms[79,82]. 3.2.3.Texture Magnesium alloys exhibit obvious anisotropy in thermal conductivity [83,84].The thermal conductivity of Mg alloys along the close-packed direction or plane is lower than that along the non-close-packed directions or planes.This is attributed to increased scattering of electrons and phonons by atoms on the close-packed plane,resulting in a decrease in the mean free path and,consequently,a lower thermal conductivity parallel to the basal plane compared to that along other planes.Weakening the basal texture can enhance the thermal conductivity of Mg alloys [84,85]. 3.2.4.Temperature Fig.9.The thermal conductivity at room temperature v.s.ultimate tensile strength of Mg-base materials. Temperature is an important factor that affects thermal conductivity.Different temperature ranges are characterized by distinct main scattering mechanisms for thermal conductivity.In the low-temperature range (0–300 K),pure Mg and dilute Mg alloys exhibit a peak in thermal conductivity between 20 and 40 K.This peak is attributed to the competition between electron-phonon scattering and electron-defect scattering.However,no noticeable peak is observed in the thermal conductivity of Mg alloys with high solute content.In this case,the thermal conductivity monotonically increases with temperature due to strong electron-defect scattering [76,85].In the high-temperature range (>300 K),the thermal conductivity of pure Mg and dilute Mg alloys decreases with increasing temperature due to enhanced electron-phonon and phonon-phonon scattering.On the other hand,the thermal conductivity of Mg alloys with high solute content increases with temperature [83,86,87]. The contradiction between strength and thermal conductivity can be addressed by precipitation of second phases since the adverse effect of precipitates on thermal conductivity is much weaker compared to that of solute atoms [73].Therefore,it is feasible to design Mg alloys with high yield strength and high thermal conductivity by carefully selecting alloying elements with low solid solubility to promote precipitation.Additionally,optimizing heat treatment and deformation processes can also enhance both thermal conductivity and strength by effectively controlling the type,morphology,and quantity of the second phases [88]. The thermal conductivity and tensile yield strength of Mg alloys reported in the literatures are summarized in Fig.9.It can be seen that the thermal conductivities of Mg-Zn and Mg-Mn alloys are usually higher than that of Mg-Al alloys.This chapter summarizes the latest developed high-strength and high-thermal conductivity Mg alloys with a UTS>300 MPa and thermal conductivity>100 W·m-1·K-1,with main focus on the Mg-RE,Mg-Zn,Mg-Al,and Mg-Mn series wrought alloys. 3.3.1.Mg-RE alloys Mg-RE alloys are typical high-strength Mg alloys [89].However,the addition of RE elements,particularly heavy RE elements such as Gd and Y,results in a significant decrease in the thermal conductivity of Mg alloys due to the high solid solubility of heavy RE elements in Mg [90].For instance,the thermal conductivity of the high-strength GW103 (Mg-10Gd-3Y-0.5Zr) alloy and GW123 (Mg-12Gd-3Y-0.5Zr) alloy was only 30.4 W·m-1·K-1and 29.9 W·m-1·K-1,respectively[66].Therefore,efforts have been dedicated to enhancing the thermal conductivity of high strength Mg-RE alloys by incorporating third elements to form intermetallic compounds that contain RE,thereby reducing the concentration of RE dissolved in theα-Mg phase. Another approach involves adding light RE elements with low solid solubility,such as La and Ce.As shown in Table 4,Mg-La binary alloys exhibited high strength and high thermal conductivity [91].With increasing La content,the strength of the Mg-La alloys increased,while the thermal conductivity decreased slightly due to the very low solid solubility of La inα-Mg.The as-extruded Mg-3.1La alloy exhibited a yield strength of 332 MPa and a high thermal conductivity of 136 W·m-1·K-1.As the La content increased,the grain size became refined,and the volume fraction of the Mg12La second phase increased,resulting in an improvement in strength. After extrusion,the second phase was broken into small particles,which reduced the scattering of free electrons and phonons,contributing to an increase in thermal conductivity.To further enhance the strength of the alloy,low-solubility alloying elements such as Zr and Mn were added to the Mg-La alloys.Liu [91] developed a series of extruded Mg-La-Zr(Mn) alloys with high yield strength ranging from 290 to 380 MPa and high thermal conductivity ranging from 120 to 137 W·m-1·K-1. 3.3.2.Mg-Zn alloys The atomic volume and chemical valence of Zn are similar to those of Mg.Therefore,its influence on thermal conductivity is less significant compared to other elements such as Al,Mn,and Ca due to the small lattice distortion caused by alloying with Zn [92].Additionally,Zn exhibits high solid solubility in Mg,and the solubility decreases as temperature decreases.This property allows for significant solid solution strengthening and aging hardening effects while minimizing the impact on thermal conductivity,thus making Mg-Zn alloys potential non-RE Mg alloys with high strength and high thermal conductivity. Mg-Zn alloys with the addition of Zr [93–95],Mn[75,96,97],La [86,98,99],Al [97],Y,Ce,Ga [100,101],Ca [96,102],Cu [103–105] achieve high strength (UTS of 300–380 MPa) as well as high thermal conductivity (100–120 W·m-1·K-1).The precipitation of Zn-containing secondphases,such as Mg-Zn-Mn phase [42,76],MgZn2[106] and LPSO phase [56],in the Mg-Zn alloys can improve the thermal conductivity while enhancing their strength. Table 4 Thermal conductivity and strength of high strength Mg-RE alloys with high thermal conductivity. Yuan et al.[107] developed a Mg-8Zn-1Mn (wt.%) alloy through extrusion and T6 treatment.The alloy exhibited a yield strength of 354 MPa,an ultimate tensile strength of 383 MPa,an elongation to failure of 12%,and a thermal conductivity of 115 W·m-1·K-1.The precipitation of the MgZn2phase during extrusion and T6 treatment,along with the reduction in the dissolved Zn content into the matrix,contributed to the increase in the thermal conductivity of the Mg alloy. 3.3.3.Mg-Al alloys The addition of Al significantly reduces the thermal conductivity of Mg alloys due to the large solid solubility of Al in Mg [108].Ying et al.[83] found that the thermal conductivity of a cast Mg alloy drops sharply to around 100 W·m-1·K-1with the addition of only 2.5 wt.% Al to pure Mg.Therefore,in order to obtain Mg-Al alloys with high strength and high thermal conductivity,other elements need to be added to limit the solubility of Al inα-Mg.As shown in Table 5,Mg-Al alloys with the addition of Ca,La,Mn,Ce,etc.,exhibit excellent strength (UTS>300 MPa) and high thermal conductivity (100–120 W·m-1·K-1).Liu et al.[10] developed Mg-Al-Mn-La alloys through the dynamic precipitation of Al11La3during hot extrusion,resulting in a yield strength of 271 MPa,an ultimate tensile strength of 332 MPa,and a high thermal conductivity of 117 W·m-1·K-1. 3.3.4.Mg-Mn and other alloys Due to the low solid solubility of Mn inα-Mg and the formation of Mn particles,the addition of 0.5% Mn can increase the ultimate tensile strength to 200 MPa for as-extruded Mg alloys,with a decrease in thermal conductivity of around 20 W·m-1·K-1compared to pure Mg [109].However,a further increase in Mn content will decrease the thermal conductivity of Mg-Mn alloys due to the increased precipitation ofα-Mn,resulting in more interfaces betweenα-Mn and the matrix[110].The addition of third alloying elements,such as La and Ce,in Mg-Mn alloys can increase both the thermal conductivity and strength of as-extruded Mg-Mn alloys.During extrusion,these alloying elements tended to exist in the form of precipitates,reducing the lattice distortion in the alloys and improving thermal conductivity (as shown in Fig.10)[109,111].For example,the as-extruded Mg-2.2Mn-0.6La alloy can achieve a UTS of 372 MPa and a thermal conductivity of 137 W·m-1·K-1[111].Similarly,the as-extruded Mg-0.5Mn-0.3Ce alloy exhibited a UTS of 321 MPa and a thermal conductivity of 140 W·m-1·K-1[109],exhibiting excellent comprehensive properties. Recent research indicates that solute segregation at grain boundaries of fine DRXed grains can significantly reduce the solute content in theα-Mg matrix [112],providing a new approach to developing Mg alloys with high strength and high thermal conductivity through enhanced grain boundary strengthening and increased thermal conductivity due to solute depletion in the matrix. Incorporating reinforcements with high thermal conductivity and high strength into Mg alloys is another viable approach to developing Mg matrix composites with high strength as well as excellent thermal conductivity which may be higher than that of pure Mg.As shown in Table 6,the addition of reinforcements such as carbon nanotubes (CNTs)[120,121],graphene flakes (GFs) [122],nano-diamonds (ND)[123],and graphene nanoplatelets (GNPs) [124] significantly increases both the thermal conductivity and strength of Mg alloys.The thermal conductivity of the composites primarily depends on the volume fraction of the reinforcements.However,due to the mismatch between the reinforcements and Mg matrix,the addition of reinforcements may increase the interfacial thermal resistance of the alloys,thereby reducing their thermal conductivity.As shown in Fig.11,Zhang et al.[122] developed an ultra-fine-grained GFs/ZX50 Mg-matrix composite with a thermal conductivity of 174 W·m-1·K-1,UTS of 395 MPa,and TYS of 361 MPa using the semi-solid stir casting technique assisted by ultrasonic vibration.The high thermal conductivity and strength of the GFs/ZX50 composites can be attributed to the clean Mg-graphite interface,controlled orientation of GFs,ultrafine grains,and MgZn2nano-precipitates. Table 5 Thermal conductivity and strength of non–RE Mg alloys. Fig.10.Nano precipitates in the extruded Mg-2Mn-2.5La alloy [111]: (a) TEM bright field image;(b) HAADF image (c–e) Elemental mapping of Mg,La and Mn. Table 6 Thermal conductivity and strength of Mg-matrix composites. With the rapid development of information technology and the widespread usage of electronic devices,unwanted electromagnetic interference (EMI) and radiation are generated,which affect the performance of electronic products and have the potential harm to human health.To ensure the normal operation of electronic equipment and protect human health,the development of materials with high EMI shielding capacity is desirable.Aerospace electronic equipment and portable electronic devices particularly require lightweight EMI shielding materials.Among these materials,lightweight Mg alloys with high electrical conductivity show great potential in reducing EMI,making them promising for electromagnetic shielding applications where weight savings are crucial. Fig.11.SEM/TEM images of extruded GFs/ZX50 composite materials [122]: (a) High magnified SEM image of as-extruded GFs/ZX50 composites;(b) EDS element distribution images of (a);(c) TEM morphology of as-extruded GFs/ ZX50 composites;(d) HRTEM of the Graphite-Mg interface;(e) FFT pattern of figure (d);(f)–(h) Inverse Fourier-filtered (IFFT) images of the green box b-1,b-2 and b-3,respectively;(i) XRD spectrums of as-extruded GFs/ZX50 composites. However,the strategies utilized to enhance the strength of Mg alloys,such as alloying,often lead to a decrease in their EMI shielding effectiveness (SE) due to the presence of various complex influencing factors.There is an urgent demand for the development of lightweight,high-strength Mg-based materials that demonstrate high EMI shielding effectiveness (EMISE) to meet the requirements from industry. Fig.12.Mechanisms of EMI shielding [129]. Electromagnetic interference (EMI) shielding refers to the reflection and/or adsorption of electromagnetic radiation by a material,which thereby acts as a shield against the penetration of the radiation through the shield [127].Shielding effectiveness (SE),measured in dB,is employed to characterize the shielding properties of materials [128]. Fig.12 displays a schematic diagram illustrating the EMI shielding mechanism based on transmission line theory,which treats the electromagnetic wave shield as a transmission line[129].The EMI shielding mechanism of EMI shielding materials comprises three components: surface reflection (SER),absorption (SEA),and multiple reflections inside the materials(SEM) [130].Hence,the total shielding effectiveness (SEtot)can be expressed as follows: where SER,SEAand SEMare the reflection,absorption and multiple refection losses,respectively [131]. When incident electromagnetic waves reach the surface of shielding materials,they undergo significant reflection at the outer surface due to the impedance mismatch between the external free space and the shielding materials.The electric field of the incident wave interacts with the free electrons near the interface,generating induced currents within the material.These induced currents,in turn,generate opposing magnetic fields that hinder the propagation of the EM waves.Consequently,a portion of the electromagnetic wave energy is reflected back at the interface [132].This reflection loss (SER)can be expressed as follows: wherekis the ratio of Zs/Zo,Zoand Zsare the characteristic impedance of free space and shielding material,respectively.Zois usually a constant.For low-Z matched or high-Z mismatched shields,kis minimal,giving rise to a large SER.The magnitude of SERprimarily depends on the relative mismatch between the incoming waves and the surface impedance (Z)of the shield. Residual EM waves that penetrate the surface of shielding materials are continuously attenuated during transmission.Materials with active electric and/or magnetic dipoles are expected to attenuate the EM energy through absorption mechanisms.These dipoles interact with the radiation and consume energy for polarization processes [133,134].The absorption loss (SEA) can be expressed as follows: wheredis the shield thickness andδis the skin depth.μandσrepresent the permeability and electrical conductivity of material,respectively. When an electromagnetic wave propagates through the surface of a magnesium alloy,this part of the EM waves undergo multiple reflection upon encountering various surfaces or interfaces in the shielding material due to the mismatch impedance,leading to the scattering and absorption of electromagnetic waves within the shielding materials,consequently diminishing the propagation capability of the electromagnetic waves.The multiple reflection loss (SEM) can be expressed as: The primary EMI shielding mechanism of most Mg alloys is surface reflection (SER),which typically depends on the electrical conductivity of Mg alloys.Higher electrical conductivity results in a greater amount of electromagnetic waves being reflected from the surface of Mg alloys.Therefore,the key to enhancing the EMI shielding properties of Mg-matrix materials lies in improving their electrical conductivity. Solute elements,second phases,grain size and texture play a significant role in EMI shielding performance of Mg alloys. 4.2.1.Solute elements The addition of solute atoms decreases the EMI shielding effectiveness (SE) of Mg.The presence of solute atoms induces lattice distortion,reduces mean free path of the free electrons,and thus electrical conductivity.The decreased electrical conductivity leads to an increase of impedance (Zs)and reduction of SERof Mg alloys.At the same time,the decreased electrical conductivity leads to the decrease of internal electromagnetic induction eddy current loss,reducing SEAof Mg alloys [135].The addition of solute atoms which induce less lattice distortion will has weak effect on deteriorating conductivity and thus SE of Mg alloys.The solute elements decrease the SE of Mg in the sequence of Zn 4.2.2.Second phases The amount,size and orientation of second phases significantly affect shielding properties of Mg alloys.The precipitation of a second phase not only consumes solute atoms within the matrix,thereby increasing electrical conductivity of the Mg alloys,but also leads to increased multiple reflection losses due to the impedance mismatch between the second phase and the matrix,resulting to the increased SE of the Mg alloys [137].However,large amount of second phases can decrease the electrical conductivity of Mg alloys,thus reducing the SE.Coarse second phases reduce area of the interface between the precipitate and matrix,which leads to a decrease in the multiple reflection loss of EM waves and poor electromagnetic shielding performance of Mg alloys[138].The presence of second phases with good conductivity and permeability improves the EMI shielding performance of Mg alloys [139]. Crystallographic orientation of second phases have a significant influence on the shielding properties of Mg alloys.When the incident direction of electromagnetic waves is perpendicular to the basal plane,the SE of the Mg alloys containing precipitates formed on the basal plane is increased due to the increased SERand SEM. 4.2.3.Grain size Electron-scattering caused by grain boundaries weakens vortex electrical currents and reduces conductivity.However,the electrical conductivity is not sensitive to the grain size at the micron scale.The grain refinement leads to large area of grain boundary,which increases the multiple reflection loss of EM waves.However,the low impedance mismatch between adjacent grains results in weak multiple reflection loss of EM waves.Thus,effect of grain size on the SE of Mg alloys is not obvious.[140]. 4.2.4.Texture Texture is a crucial factor influencing the EMI shielding properties of Mg alloys.Mg alloys exhibits significant anisotropy of EMI shielding properties [141].SE of Mg rolling sheet increases with intensity of basal texture when incident direction of electromagnetic waves is perpendicular to the basal plane.The SE increment is mainly attributed to the improvement in the reflection attenuation of the incident electromagnetic wave,which is caused by the enhanced impedance mismatch between the air and Mg sheet surface due to the reduced surface impedance of the Mg sheet [142].The reduced surface impedance of the Mg sheet is due to the increase of electrical conductivity along the c axis direction with increasing intensity of basal texture,which is attributed to the increase of the mean free path of the electrons along the c axis with less close-packed crystal lattice. The EMI shielding effectiveness of Mg-based materials mainly depends on the electrical conductivity.To increase the electrical conductivity,the solute atoms dissolved in theα-Mg should be reduced to alleviate lattice distortion,which leads to a decrease in strength.Therefore,there is a trade-off between EMI shielding effectiveness and strength in Mg alloys.To overcome this trade-off,high-performance Mg alloys with both high strength and superior shielding properties have been developed on the basis of a deep understanding of the factors influencing the shielding properties of Mg alloys [136]. Fig.13.The EMI shielding effectiveness(f=1500 MHz)v.s.ultimate tensile strength of Mg-base materials. The EMI shielding effectiveness and tensile strength of some Mg alloys reported in the literatures are summarized in Fig.13.It can be seen that Mg-RE and Mg-Zn alloys exhibit excellent electromagnetic shielding performance and high strength.SE (>60 dB) can satisfy most of the requirements for EMI shielding in practical applications [143].Therefore,this chapter summarizes the latest developed highstrength and high-EMISE Mg alloys with UTS>300 MPa and SE>60 dB. 4.3.1.High-strength Mg alloys with high EMISE The addition of RE to Mg alloys will significantly improve the strength and EMI shielding effectiveness due to the formation of RE-containing second phases [4,142,144,145].However,an excessive addition of RE elements can lead to an increase in solution atoms dissolved in theα-Mg,resulting in lattice distortion and a decrease in EMISE.The main high EMISE Mg-RE alloys with high strength are the Mg-Y and Mg-Gd series alloys.Table 7 summarizes the strength and SE of some high-performance Mg-RE alloys. The Mg-13Gd-4Y-2Zn-0.5Zr alloy containing the LPSO phase exhibits high strength and EMI shielding effectiveness after extrusion.When the deformation amount reaches 80%,this alloy shows a SE of 93–107 dB at frequencies of 30–1500 MHz,with a UTS of 380 MPa,YS of 310 MPa,and EL of 10.8% [146].The significant precipitation of the Mg5(Gd,Y) phase during the aging process improves the electrical conductivity of the Mg-RE alloy,thereby increasing reflection and absorption losses.Additionally,the increase in phase interface density leads to an increase in internal multiple reflection losses.The lamellar LPSO phase increases the area of internal interface and promotes the reflection of electromagnetic waves passing through the LPSO phase,resulting in increase of shielding effectiveness.However,the influencing mechanism of LPSO on electromagnetic shielding requires further investigation. Table 7 The strength and SE of Mg-RE alloys. The addition of 1.5% Sn to the Mg-12Gd-3Y alloy promotes the precipitation of Mg24(Gd,Y)5,Mg5(Gd,Y),and Mg2Sn phases.After extrusion and aging treatment,the alloy exhibits excellent strength (UTS of 415 MPa,EL of 5%)and high EMI shielding effectiveness (EMISE) (112–81 dB at 30–1500 MHz) [4].When the incident direction of electromagnetic wave is perpendicular to the basal plane,the Mg2Sn precipitated on the (0001) basal plane can increase the effective reflection area of the electromagnetic waves within the alloy. 4.3.2.Mg-Zn alloys Mg-Zn binary alloys with high electrical conductivity exhibit excellent EMI shielding effectiveness (SE) but poor strength [149].EMI shielding Mg-Zn-X alloys with high strength have been successfully developed by alloying with Zr,Cu,Sn,Mn,and RE elements,as summarized in Table 8.Among these alloys,Mg-Zn-RE alloys exhibit excellent electromagnetic shielding performance and high strength. An extruded Mg-6Zn-3Sn-0.5Cu alloy exhibits an SE of 100–105 dB in the range of 30–1500 MHz and a UTS of 319 MPa [150].The addition of Cu induces precipitation of the MgZnCu phase,resulting in a reduction of the solution content inα-Mg and an improvement in electrical conductivity.The EMI shielding mechanisms of the extruded Mg-6Zn-3Sn-0.5Cu alloy are illustrated in Fig.14.The precipitation of the Mg2Sn,β′1,andβ′2phases significantly increase the phase interface in the alloy,enhancing the multiple reflections of electromagnetic waves and thereby improving the EMI shielding effectiveness of the alloy. Fig.14.Schematic diagram of the electromagnetic shielding mechanism of extruded Mg-6Zn-3Sn-0.5Cu alloy [150]: (A) reflection attenuation,absorption attenuation and multiple reflection attenuation,(B) multiple reflection attenuation of different second phases for electromagnetic waves. A Mg-6Zn-1Y-0.5Nd-0.5Zr alloy processed by extrusion and T5 treatment exhibits excellent strength,with a UTS of 421 MPa,TYS of 386 MPa,EL of 9.8%,and SE of 73–111 dB in the range of 30–1500 MHz[151].With the addition of Y/Nd,a large amount of Mg3Zn3Y2and Mg3Zn3(Y,Nd)2precipitate,resulting in high strength.At the same time,lattice distortion is reduced and phase interfaces are increased,which improve the electrical conductivity thus ensuring excellent electromagnetic shielding performance. Table 8 The strength and SE of Mg-Zn alloys. The shielding Mg-matrix composite can be developed by selecting proper reinforcements and fabrication methods to generate a specific structure that effectively reflects electromagnetic waves. Xu et al.[157] develop a SnO2-coated Graphene oxide(GO) reinforced AZ31 Mg matrix composite.During the smelting process,the MgO layer is generatedin situat the interface between GO and the molten Mg matrix by consuming SnO2,enhancing the interface bonding between GO and Mg,and generating stacking faults.The composite reinforced with trace amounts of modified GO exhibits a high ultimate tensile strength of 308 MPa and elongation of 12.9%.Additionally,as shown in Fig.15,the multi-layer structure of GO and the presence of the MgO mid-layer lead to a multi-level electromagnetic reflection and interface polarization,thereby significantly enhancing the EMI shielding performance. Wang et al.[158] develop a magnesium-based composite reinforced with multi-walled carbon nanotubes (MWCNTs)by using electrophoretic deposition (EPD) and accumulative roll bonding (ARB).The ultralight MWCNTs/Mg-9Li-3Zn-0.5Gd composite processed by 5-cycle ARB exhibits a UTS of 280 MPa,TYS of 264 MPa,and an SE of 89–100 dB in the frequency range of 8.2–12.4 GHz.The strengthening of the composite is mainly attributed to precipitation strengthening,grain refinement strengthening,and dislocation strengthening caused by the presence of MWCNTs.The excellent electromagnetic shielding performance is ascribed to the dynamic precipitation of nano-scale MgZn2and ultrafine nano-scale W phases induced by uniformly distributed MWCNTs,which strongly promotes the transfer of free electrons by consuming solute atoms in the matrix,thereby increasing the reflection loss and absorption loss of the composite sheet. Lightweight magnesium alloys are highly attractive for use in aerospace,automotive,and railway vehicles.However,due to their high reactivity and flammability,magnesium undergoes significant oxidation and even ignition at temperatures above 400–550◦C [159].This poses serious safety risks and thus restricts their applications. Fig.15.Schematic diagram of EMI shielding mechanism of GO-SnO2/AZ31 composites [157]. Fig.16.The diagram of the oxidation and ignition process of Mg alloys at the high temperature [160]. Ignition of Mg alloys is a violent oxidation process,as illustrated in Fig.16 [160].Initially,a thin and dense oxide film forms.As the oxidation reaction progresses,Mg2+diffuses outward,causing the film to thicken and generate gaps between the substrate and the oxide film.This leads to internal stress and higher vapor pressure,resulting in the formation of cracks in the oxide film.These cracks act as pathways for oxygen transport.Mg vapor reacts with oxygen,leading to the formation of oxide ridges within the cracks.As the number of cracks and oxide ridges increases,the oxidation rate accelerates,resulting in the formation of oxide nodules on the surface.These nodules constantly expand,forming a porous oxide film.If the heat input surpasses the heat released during Mg oxidation,the local temperature will experience a significant increase,ultimately leading to the ignition and combustion of the Mg alloys. The ignition of Mg alloys is closely linked to hightemperature oxidation.The Pilling-Bedworth ratio (PBR) is a widely employed model for assessing the effectiveness of oxide film protection.It is defined as follows: Fig.17.(a) ΔGθ of Li,Na,Al,Si,Mn,Co,Ni,Cu,Zn,Sr,Sn,Sb,and Ba at different temperature.(b) ΔGθ of Be,Ca,Sc,Y,La,Ce,Pr,Nd,Sm,Gd,Dy,Ho,and Yb at different temperature [164]. where Voxrepresents the volume of metal oxides produced,while VMrepresents the volume of metal consumed in the formation of metal oxides.When the PBR is less than 1,the oxide film formed on the substrate is porous and loose,making it ineffective in protecting the metal.This is because oxygen from the air can easily penetrate the porous structure and reach the interior of the material.On the other hand,a PBR greater than 2 results in excessively high internal stress within the oxide film,leading to crack formation and subsequent loss of its protective capabilities.In the range of PBR values between 1 and 2,the oxide film is compact enough to prevent oxygen from oxidizing the underlying matrix,providing effective protection.However,it should be noted that there is no meaningful relationship between PBR values and the ignition temperatures of Mg alloys [161].Therefore,PBR is not a useful factor for explaining the high-temperature oxidation resistance of Mg alloys. The thermodynamic factor influencing the oxidation behavior at the surface of Mg at high temperature is the change in Gibbs free energy (ΔG) for oxidation reaction [162]: whereΔGθrefers to the standard Gibbs free energy change;R is gas constant;T is oxidation temperature;αrepresents activity. As shown in Fig.17,the Gibbs free energy change for the oxidation of reactive alloying elements such as Be,Ca,Sc,Y,La,Ce,Pr,Nd,Sm,Gd,Dy,Ho,and Yb is negative at elevated temperatures.Consequently,the addition of these alloying elements results in the formation of new oxides XbOarather than MgO,which becomes unstable above 500◦C.The oxidation process is slowed down if XbOaprovides better protection compared to MgO [163].Mg alloys that contain reactive elements typically exhibit higher ignition temperature.However,there is no strong correlation between the Gibbs free energy change and the ignition point of Mg alloys [160]. Oxidation dynamics depends on the oxidation temperature and time.Fig.18 shows the oxidation kinetics of Mg alloys at different temperature [165].Below a critical temperature,the oxidation rate of Mg alloys is slow,and the growth of the oxide film follows a parabolic law.When the temperature exceeds this critical value,the oxidation process is characterized by two stages: an initial incubation period following the parabolic law and an acceleration stage following the linear law [165].Oxide film is dense and protective in the parabolic oxidation process,while in the linear oxidation stage,voids and cracks are formed in the oxide film,which makes the film lose protection [166].Extending the duration of the incubation (parabolic oxidation) stage can effectively improve ignition resistance of Mg alloys. Fig.18.Oxidation dynamics under different temperature of Mg alloys [165]. The key to enhance the ignition-proof performance of Mg alloys lies in achieving continuous and dense oxide films to prevent further oxidation.The solute elements and second phases play crucial roles in the preservation and effectiveness of the oxide films,thus are key factors influencing the ignition resistance of Mg alloys. 5.2.1.Solute elements In addition to the PBR and the Gibbs free energy change for oxidation,the solubility limit of alloying elements is one of the key factor influencing the ignition resistance of magnesium alloy[161].The addition of elements with low solubility limit,such as La,Pr,Si,Sr and Ce,leads to poor ignition resistance of Mg alloys,although these elements can form protective oxide layer.This is due to fact that solute concentration dissolved in Mg matrix was too small to form a protective oxide film.The addition of elements with solubility limit higher than 2 at.%,such as Y,Gd,Dy and Er increases significantly the ignition temperature of Mg.The higher the concentration of solute elements inα-Mg,the higher the ignition temperature of Mg alloys.The addition of alloying elements with an intermediate solubility limit (0.5–1.0 at.%),such as Ca and Sm,improves the ignition resistance of Mg alloys since the solute concentration inα-Mg increases due to the dissolution of the eutectic phases during heating. The reactive elements,such as Be,Ca and rare earth elements enhance the ignition resistance of Mg alloys by reactive element effect (REE).These reactive elements react with oxygen to form a compact oxide film,retards the diffusion of Mg2+cations and oxygen ions,thus restricting the growth of MgO.In addition,the reactive elements diffuse to the boundaries,voids or cracks of MgO,and exist in the form cations or oxides,inhibiting effectively the outward diffusion of Mg2+[165]. 5.2.2.Second phases The low-melting-point second phases may lower the ignition temperature of Mg alloys.For example,the addition of alloying elements,such as Al,Zn and Cu,deteriorate the ignition resistance of Mg alloys due to the formation of lowmelting second phases[167].These second phases melt before the Mg matrix forms the liquid phase,which accelerates the oxidation and evaporation of Mg[168].In addition,higher vapor pressure from the second phases with low melting points promotes the growth of the porous oxides and cracks,leading to the rupturing of the surface protective film [169]. According to the discussion above,the efficient alloying elements to improve the ignition resistance of Mg alloys should have PBR between 1 and 2,the negative ΔG for the for the oxidation reaction with MgO,large solid solubility limit as well as the ability to form second phases with high melting points [170–172].Active elements such as RE (rare earth),Ca,Be,and Sr have been found to be the most effective alloying elements for retarding ignition in magnesium alloys[170].These elements can significantly improve the ignition temperature of Mg alloys,and can even result in non-combustibility.Ca and Y are effective in strengthening Mg alloys.Furthermore,the incorporation of high-melting-point second phases,such as (Mg,Al)2Ca and LPSO (long-period stacking ordered) structures,not only enhances ignition resistance but also acts as dislocation pinning points,thereby strengthening the alloys [115]. Fig.19.The ignition temperatures v.s.tensile yield strength of Mg base materials (ingot form) [170]. As shown in Fig.19,Tekumalla and Gupta compiled the tensile yield strength and ignition temperatures of various Mg alloys [170].Mg-RE alloys containing LPSO (longperiod stacking ordered) structures,Mg-Al-Ca alloys with C36 phase,and Mg matrix composites demonstrate both high strength and excellent ignition resistance.This chapter provides a summary of the latest advancements in Mg-based materials that exhibit high ultimate tensile strength (UTS>300 MPa) and high ignition temperatures (>700◦C). 5.3.1.Mg-RE alloys Rare earth elements are efficient alloying elements for improving the strength and ignition resistance of Mg alloys.This is attributed to their large solid solubility and strong affinity for oxygen.Table 9 provides a summary of the strength and ignition temperatures of various Mg-RE alloys.It is important to note that there is currently no standardized testing method for ignition temperature,which may result in different testing results due to variations in testing methods and sample dimensions used by different research groups. Magnesium Elektron Company of UK develops a highstrength Elektron 43 wrought Mg-RE alloy with ignition temperature above 690◦C,which have passed flammability testing by the Federal Aviation Administration (FAA),indicating that the use of Elektron 43 in aircraft seat frames does not reduce the level of safety of the aircraft when compared to heavier aluminum seat frames [170,173].Šašek et al.[174] achieved ignition temperature of 1072◦C in a WE43 alloy processed by equal channel angular processing (ECAP),which maybe be the highest ignition temperatures currently achieved in WE43 alloy whist a yield strength of 356 MPa,and tensile strength of 367 MPa.However,the mechanisms for enhanced ignition resistance of the WE43 alloy processed by sever plastic deformation needs further investigation. As shown in Table 9,combined addition of RE and Ca significantly increases the ignition temperature and strength of Mg-RE alloys.Kubasek et al.[11] develop an extruded Mg-4.5Gd-3.4Y-2.6Ca alloy with ignition temperature of 1100◦C,tensile yield strength of 229 MPa,ultimate tensile strength of 302 MPa,and elongation to failure of 10%.As shown in Fig.20,the formation of dense (Y,Gd)2O3and CaO layers hinders further oxidation reactions. Mg-RE alloys strengthened by LPSO phase show excellent ignition resistance [175,176].Kawamura [177] develops an ultrahigh-strength Mg96.75Y2Zn0.75Al0.5alloy containing LPSO phase with tensile yield strength of 359–520 MPa,elongation to failure of 5–15% and ignition temperature of 780–940◦C by rapidly solidified powder metallurgy(RS P/M)processing.The improved ignition temperature in the LPSOcontaining Mg alloys is attributed to the formation of dense oxide layer containing RE elements and excellent thermal stability of the LPSO phase [178]. Table 9 The strength and ignition temperature of several Mg-RE alloys. Fig.20.Distribution of elements (EDX) in oxide layers of Mg-4.5Gd-3.4Y-2.6Ca alloy [11]: (a) sample after heating to 950 ◦C and immediate furnace cooling;(b) after additional thermal exposure at 950 ◦C for 2h. 5.3.2.Ca-containing Mg-Al alloys The addition of Ca increases the ignition temperature of Mg-Al alloy through the formation of CaO surface layer and the formation of a thermally stable Al2Ca phase [115].In addition,the addition of Ca strengthens Mg alloys by grain refinement and formation of high-strength second phases.Asshown in Table 10,the Mg-Al alloys containing minor amount of Ca and Y,and the Mg-Al-Ca alloys with Ca content greater than 2 wt.% exhibit excellent ignition-proof performance and strength. Table 10 The strength and ignition temperature of several Mg-Al-Ca alloys. The addition of Ca to AZ91 alloys results in the formation of a brittle and thick oxide film at high temperature,which makes it susceptible to cracking under applied stress [184].While the combined addition of trace amounts of Ca and Y refines the matrix grain and impedes the formation of brittle phases containing Ca,leading to the significant increase of ignition temperature and strength of the Mg alloy [185–188].A Mg-8Al-0.3Zn-0.1Mn-0.3Ca-0.2Y (AZXW8000) alloy processed by low-temperature and low-speed indirect extrusion [189] exhibits a yield strength of 379 MPa,ultimate tensile strength of 422 MPa,elongation to failure of 11.3%,and ignition temperature of 820◦C.The high strength of the alloy is attributed to the fine recrystallized grains,large amounts of fine Mg17Al12precipitates and nonrecrystallized zone with a strong basal texture.With the addition of a minute quantity of Ca and Y,a protective Y2O3/CaO layer can form,leading to a substantial increase in ignition temperature. Although the PBR of Ca is 0.65,which does not meet the rules for ignition-proof elements,the PBR value of CaO/Mg-10Al-5Ca is 1.17.The anion volume ratio of the CaO/MgO interface is 1.48,and CaO is subjected to compressive stress resulting in a denser film layer [190].Furthermore,the addition of Ca can suppress the formation ofβ-Mg17Al12with low melting point.As a result,the ignition temperature of the Mg-Al-Ca alloys is higher than 1000◦C.In addition,due to the pinning effect of the high-melting-point phases of Mg2Ca,Al2Ca,and (Mg,Al)2Ca,Mg-Al-Ca alloy exhibits high tensile strength of 300–400 MPa whilst low ductility less than 6% [191,192]. Kawamura develops a series of Mg-Al-Ca alloys with high strength and ignition point,called "KUMADAI nonflammable Mg alloy".Their yield strength is as high as 410–460 MPa,and the ignition point is above 1091◦C [115,177].The superior ignition resistance of Mg-10Al-5Ca alloy is attributed to the formation of protective oxide film composed of fine CaO outermost layer,fine MgO intermediate layer,and coarse MgO innermost layer [190].Zhang [193] develops a high-strength non-flammable extruded Mg-17Al-8Ca-0.2Mn alloy with UTS of 362 MPa and the ignition temperature of 1250◦C.The strengthening is ascribed to the pinning effect of a large amount of Laves phases and grain refinement,while the excellent ignition-proof properties are attributed to the dense oxidation film composed of a large amount of MgAl2O4spinel and a small amount of Al2O3. 5.3.3.Magnesium matrix composites with high strength and high ignition resistance Magnesium-matrix composites can be potential candidate materials with high strength and high ignition resistance through selection of suitable reinforcements and matrix.As shown in Fig.21,the incorporation of nanoparticles (SiC,SiO2,B4C,Si3N4,ZnO,CaO,CeO2,etc.) into the magnesium matrix considerably increases the ignition temperature by enhancing its oxidation kinetics [126].The addition of nano particles can promote the formation of a stable and moreprotective oxide layer on the surface of magnesium alloys.Furthermore,the low thermal conductivity of the nanoparticles compared to magnesium reduces the thermal conductivity of the composites and improves thermal stability and ignition resistance [196,197]. Table 11 The strength and ignition temperature of Mg-matrix composites. Fig.21.Ignition temperature of Mg/nanoparticles composites [173]. As shown in Table 11,the addition of 1% CaO in the Mg92Zn2Y5–1CaO composite increases the ignition temperature from 735◦C to 1026◦C by the formation of dense and MgO/CaO oxide layer on the surface of alloys during oxidation [198].At the same time,the Mg92Zn2Y5–1CaO composite exhibits excellent strength with TYS of 310 MPa and UTS of 350 MPa.Further researches are needed to search for novel reinforcements which can satisfy the required mechanical and ignition-proof properties to develop novel magnesium matrix composites with superior strength and ignition-proof properties. The downhole materials used in hydraulic fracturing (HF)technology for oil and gas extraction must possess high strength,light weight,and a controlled dissolution rate.This allows the fracturing tools to effectively dissolve after the fracturing operation,eliminating the need for drill-out.Mg is highly susceptible to corrosion in water,and the resulting corrosion product is loose,which does not impede further corrosion.Thus,dissolvable magnesium alloys,which exhibit high dissolution rates and can dissolve in neutral solutions,are a smart choice for temporary isolation tools in hydraulic fracturing applications.As these tools are subjected to high pressures,there is an urgent need for dissolvable magnesium alloys with high strength.In recent years,extensive researches have been conducted on the development of high-strength dissolvable Mg-based materials. Dissolvable magnesium alloys rely on galvanic corrosion induced by noble impurities in chloride-bearing wellbore fluids [200].The dissolution rate of the dissolvable Mg alloys is dependent on the type and fraction of second phases in the Mg matrix.The second phases are more cathodic than the Mg matrix,causing the formation of hydrogen and magnesium ions according to the reaction in brine solution: Magnesium is the least noble structural metal and can form a galvanic couple with almost any of the other metals.Therefore,it is possible to develop dissolvable Mg alloys for oil and gas industry by the addition of noble metals based on galvanic corrosion. The reaction kinetics of the dissolution of dissolvable Mg alloys is dependent on temperature and salinity[200].Increasing the chloride concentration leads to a nonlinear increase in the dissolution rate.For a given chloride concentration,the dissolution rate of Mg alloys increases almost linearly with increasing temperature. The factors influencing the dissolution rate of dissolvable Mg alloys include second phase,texture,dislocation density and grain size,etc. 6.2.1.Second phase Second phase is the key factors affect the dissolution rate of dissolvable Mg alloys.Due to the potential difference between the second phases and the Mg matrix,the presence of the second phase leads to the formation of micro galvanic couples within the Mg alloy,accelerating the galvanic corrosion process.Addition of Cu,Ni and/or Fe to Mg alloys significantly accelerates dissolution rate due to their low solid solubility and formation of high-electropotential intermetallic compounds along the grain boundaries of the Mg matrix.The dissolution rate of Mg alloys dramatically increases even with minor addition of Cu,Ni and/or Fe [201,202]. The morphology and distribution of second phases have obvious effect on dissolution rate of Mg alloys.The presence of discontinuous block-like second phases leads to enhanced micro galvanic corrosion in the Mg alloy due to their larger anode-to-cathode area ratio.On the other hand,continuous network-like second phases act as barriers,impeding the ingress of corrosive electrolytes and thus reducing the dissolution rate of the Mg alloy [203]. 6.2.2.Texture Dissolution rate of Mg alloys is strongly dependent on the crystal orientation.The basal plane of Mg alloys has the lowest dissolution rate among all the crystallographic planes because of its lowest surface energy and compact corrosion films [204].Dissolvable wrought Mg alloys commonly display strong texture,which has obvious effect on their dissolution rate.If more crystallographic planes with low corrosion resistance are exposed to corrosive medium by modification of alloying composition and thermomechanical processing,the dissolution rate of the Mg alloys will be increased [205]. 6.2.3.Dislocation density Dislocations induce the crystallographic lattice distortion,and atoms in the distorted lattice are much more active than those in a normal lattice.Hence,a dislocation is always a preferentially dissolving site in metal [206].Dissolution rate of the wrought Mg alloys are dramatically reduced after annealing because the density of dislocations introduced by deformation is significant decreased after static recrystallization[207]. Dissolvable Mg alloys used in hydraulic fracturing technology usually contain Ni or Cu with very low solid solubility inα-Mg since the addition of these elements significantly increase dissolution rate of Mg alloys by formation of Nior Cu-containing secondary phases with high potential and deteriorating the corrosion product layers [208,209].At the same time,these Ni-or Cu-containing secondary phases act as strengthening phases.As shown in Fig.22 [210],among the dissolvable Mg alloys developed,the Mg-RE and Mg-Zn alloys achieve both excellent strength and high dissolution rates. Fig.22.Corrosion rate and ultimate compressive strength of partial dissolvable Mg alloys and composites [210].(corrosion rates were measured by weight loss or hydrogen evolution in 3.0–3.5 wt.% NaCl or KCl solution at room temperature). This chapter summarizes the latest developed high-strength dissolvable Mg alloys with a TYS>300 MPa or UCS>400 MPa,and a dissolution rate of 500 mm·y-1at room temperature in a 3.0–3.5 wt.% KCl/NaCl solution. 6.3.1.Mg-RE alloys Mg-RE alloys exhibit high strength as well as excellent corrosion resistance due to their relatively close standard electrode potentials with Mg and the formation of more protective corrosion layers [209,211].Adding Ni or Cu elements to Mg-RE alloys can dramatically increase the dissolution rate while improving their strength,thanks to the formation of Nior Cu-containing phases,especially the LPSO phases.Thus,Mg-RE-Ni/Cu alloys can be considered as ideal dissolvable magnesium alloys with high strength,achieved by regulating the content of Ni/Cu,LPSO phase,and other second phases. Table 12 summarizes the strength and dissolution rates of some high-strength dissolvable Mg-RE alloys.Hou et al.[208] develop high-strength dissolvable extruded Mg-12.9Y-4.6Ni alloy containing 18R LPSO phase,with a tensile strength of 538 MPa,a tensile yield strength of 409 MPa,and a dissolution rate of 1180 mm·y-1at 93◦C.The alloy is strengthened mainly by the high volume fraction of the Nicontaining 18R LPSO phase.Meanwhile,the Ni-containing 18R LPSO phase acts as an anodic phase due to its lower potential than that of Mg,leading to its preferential corrosion and thus the formation of channels beneficial for rapid penetration of corrosive ions into theα-Mg,which contributes to the higher dissolution rate of the Mg-Y-Ni alloys [205]. Liu et al.[209,211] develop extruded Mg-9.5Gd-2.7Y-0.9Zn-0.8Cu-0.4Ni alloys containing 14H-LPSO.The alloy shows a high UCS ranging from 605 to 621 MPa and a high dissolution rate ranging from 786.2 to 2363.8 mm ·y-1.The 14H LPSO phase acts as a cathodic phase,accelerating the dissolution rate of the alloy.The presence ofβ′precipitates contributes to the ultrahigh UCS of 621 MPa.After T5 treat-ment,the dissolution rate of the alloy decreases due to the preferential corrosion of theβ′phase over the matrix,thus protecting the matrix.The corrosion mechanism of the Mg-Gd-Y-Zn-Cu(-Ni) alloys is illustrated in Fig.23. Table 12 The strength and dissolution rate of high-strength dissolvable Mg-RE alloys. 6.3.2.Mg-Zn alloys The dissolution rate of Mg-Zn alloys increases rapidly when the concentration of Zn is greater than 2.5% due to the formation of Mg-Zn second phases.When Ni or Cu is added to the Mg-Zn alloys,the formation of MgZnCu,MgZnNi,Mg2Cu,and Mg2Ni phases with higher potential than the matrix further increases the strength and dissolution rate of the alloys [218]. As shown in Table 13,Mg-Zn-Ni alloys are promising high-strength dissolvable Mg alloys.Both the strength and dissolution rate increase with decreasing extrusion temperature of the Mg-4Zn-2Ni alloy.This is because the increased amount of unDRXed regions containing a high density of dislocations and more dynamic precipitates which act as both a strengthening effect and preferential galvanic corrosion sites[218].The Mg-4Zn-2Ni alloy extruded at 200◦C achieves a UCS of 513 MPa and a high dissolution rate of 619 mm· y–1.With an increase in Ni content,both the strength and dissolution rate of the extruded Mg-4Zn-xNi alloy increase due to the formation of more Mg2Ni phase.The as-extruded Mg-4Zn-4Ni alloy exhibits a high UCS of 585 MPa and a high dissolution rate of 913 mm ·y–1[207].As shown in Fig.24,the extruded Mg-4Zn-2Ni alloy contains the Mg2Ni phase,τphase,and MgZn phase,all of which have higher potentials than the Mg matrix.Thus,the galvanic corrosion between the second phases and the Mg matrix occurs primarily in the initial stage of corrosion.Moreover,galvanic corrosion preferentially occurs in the unDRXed regions due to the high density of dislocations. In addition to the alloying approach,the addition of reinforcements such as hollow glass microspheres (HGM) and fly ash cenospheres (FAC) mainly composed of SiO2,Fe,and SiC,into Mg alloys is an effective method to enhance the strength and dissolution rate of Mg alloys [220–225]. Fig.23.Schematic of the corrosion mechanism for Mg-Gd-Y-Zn-Cu(-Ni) alloy [209].(a–c) As-extruded and (d–f) T5 alloy. Table 13 The strength and dissolution rate of high-strength dissolvable Mg-Al/Mg-Zn alloys. As shown in Table 14,FAC-or HGM-reinforced Mg-15Al-6Zn-2Cu-1.5Ni composites fabricated by stir casting exhibit superior strength and a high dissolution rate.The refinement of the matrix grain and the formation of a large amount of Mg2Si at the interface between FAC or HGM and the matrix contribute to the high compressive strength of the composites.The 3% HGM/Mg-15Al-6Zn-2Cu-1.5Ni composite achieves the highest ultimate compressive strength of 415 MPa,while the UCS gradually decreases with a further increase in HGM content.The dissolution rate of the composites increases significantly with an increasing content of FAC or HGM,which is attributed to the formation of more corrosion micro-galvanic couples and the exfoliation of corrosion products during the dissolution process [220].The 5%HGM/Mg-15Al-6Zn-2Cu-1.5Ni composite exhibits a very high dissolution rate of 1629 mm ·y–1.Furthermore,both the UCS and dissolution rate increase with increasing Ni content in the 4%HGM/Mg-15Al-6Zn-2Cu-xNi composites due to the increasing amount of Al3Ni2phase.This phase disrupts the local continuity of the Mg17Al12phase and weakens its corrosion barrier effect [220].The 4%HGM/Mg-15Al-6Zn-2Cu-3Ni composite achieves a UCS of 404 MPa and a dissolution rate of 604 mm ·y–1.As shown in Fig.25,corrosion occurs primarily in the areas around the HGM.During the degradation process,α-Mg andβphase near the Al3Ni2phase corrode and peel off.With an increase in Ni content,both the Al3Ni2phase and moreβphase are detected in the corrosion products,indicating that the Al3Ni2phase acts as the cathode phase and facilitates the peeling off of theβphase during the dissolution process. In the recent years,most of the researches have been focused on dissolvable Mg alloys with high strength at ambient temperature.However,to meet the increasing demands of downhole tools,it is necessary to explore high temperature dissolvable Mg alloys and high ductility dissolvable Mg alloys with controllable dissolution rates. Magnesium alloys are one of the most promising lightweight structure-function integrated materials.This article provides a review of the mechanisms behind various functional properties such as damping capacity,thermal conductivity,electromagnetic shielding,flame retardancy,and dissolvability in Mg alloys.It also discusses the trade-off between strength and functional properties.In recent years,through advancements in understanding the influence of solute atoms,second phases,grain boundaries,and texture on the mechanical and functional properties of magnesium alloys,new structure-function integrated Mg alloys and their composites with superior comprehensive properties have been developed by optimizing chemical composition and processing technologies.The article summarizes the latest developed structure-function integrated Mg alloys and their composites,including high strength Mg-based materials with high damping capacity/ high thermal conductivity/ strong electromagnetic shielding effectiveness/ excellent flame resistance/ high dissolution rate. Among the alloys developed up to now,Mg-RE-TM (transition metals) alloys containing long period stacking ordered(LPSO) phase and stacking faults (SF) show promise as highstrength Mg alloys with high damping.Mg-Zn-X and Mg-Mn-La/Ce series alloys demonstrate superior thermal conductivity and high strength.Mg-Y/Gd-X and Mg-Zn-RE alloys exhibit excellent electromagnetic shielding performance and high strength due to their high electrical conductivity and the increased interface through precipitation of different kinds of second phases.Mg-Al-Ca alloys containing C36 phase and Mg-RE alloys containing LPSO phase exhibit superior ignition resistance and high strength due to the formation of a dense and protective oxide film and the significant precipitation strengthening.Mg-RE-Ni/Cu alloys are ideal dissolvable magnesium alloys with high strength.Additionally,the addition of reinforcements with specific functional properties can significantly enhance the functional properties and strength of Mg alloys. Fig.25.SEM images of the 4%HGM/Mg-15Al-6Zn-2Cu-xNi composites containing Ni after immersion in 3.5 wt.% KCl solution for (a) 15 min;(b) 30 min;(c,d) 2 h;Schematic diagrams of corrosion morphology during degradation process: (e) and (f) correspond to the composites containing Ni;(g) correspond to the composites without Ni [222]. The key points that are of particular interest for future research on structure-function integrated Mg-based materials are outlined: (1) The mechanisms for mechanical and functional properties of magnesium alloys and their coupling effects need to be further clarified through experimental study and theoretical simulation.Special attention should be paid on the influencing mechanisms of novel phases and defects,such as LPSO,Laves phases,SFs,twins,kink bands,ordered and clustering structures,etc.on the mechanical and functional properties of Mg alloys. (2) Quantitative prediction models of mechanical and functional properties should be established considering the influence of microstructure on mechanical properties and functional properties of Mg alloys.Multi-scale modeling approaches using atomistic,mesoscale,and continuum modeling techniques can offer valuable insights into the underlying mechanisms governing the properties of Mg alloys.Machine learning (ML) paves a new way toward develop of novel Mg alloys with excellent integrated mechanical and functional properties. (3) In the current research on the structure-function integrated Mg alloys,the process performance of the Mg alloys is rarely considered.In addition to improve the structural and functional properties,formability (castability,extrudability,forgeability,etc.) of the Mg alloys should be taken into account in order to promote the commercial applications of the new alloys. (4) The cost of the structure-function integrated Mg-based materials is an important issue to be considered.Therefore,the development of low-cost structure-function integrated Mg-based materials is one of the key research directions in the future.This may be realized by optimization of the microstructure of the materials through reduced addition of expensive alloying elements (REfree or low RE) and adopting low-cost fabrication techniques,such as high-speed extrusion/rolling by designing novel second phases with a high melting point. (5) It is desirable to develop super Mg-based materials exhibiting superior mechanical properties (high strength,high ductility and toughness)in combined with excellent multi-functional properties (high damping capacity,high thermal conductivity,excellent electromagnetic shielding performance,superior corrosion and ignition resistance) simultaneously,which requires the more comprehensive and profound understanding of the mechanisms involved. (6) Mg-matrix composites are promising structure-function integrated Mg-based materials due to their designability and high elastic modulus.Future work will focus on the selection of novel reinforcements which can satisfy both mechanical and functional properties and new processing technologies to develop novel magnesium matrix composites with superior mechanical and functional properties through architectural design of the reinforcements and Mg matrix. Declaration of competing interest None. Acknowledgments This work is partially supported by National Natural Science Foundation of China (No.U21A2047,No.51971076,and No.51771062) and National Key Research and Development Program of China (No.2022YFE0109600).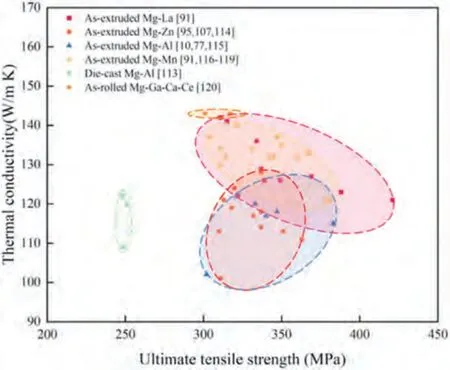
3.3.Mg alloys with both high strength and high thermal conductivity

3.4.Magnesium matrix composites with high strength and high thermal conductivity



4.Mg alloys and their composites with high strength and high electromagnetic interference shielding effectiveness

4.1.Mechanism for EMI shielding of Mg alloys
4.2.Factors influencing EMI shielding effectiveness of Mg alloys
4.3.Mg alloys with high strength and high EMISE




4.4.Magnesium-matrix composites with high strength and high EMISE
5.High-strength ignition-proof Mg alloys and their composites

5.1.Ignition mechanisms of Mg alloys
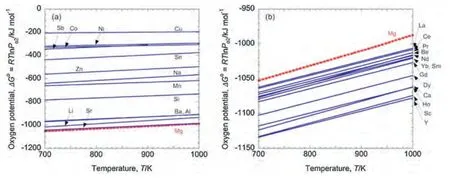
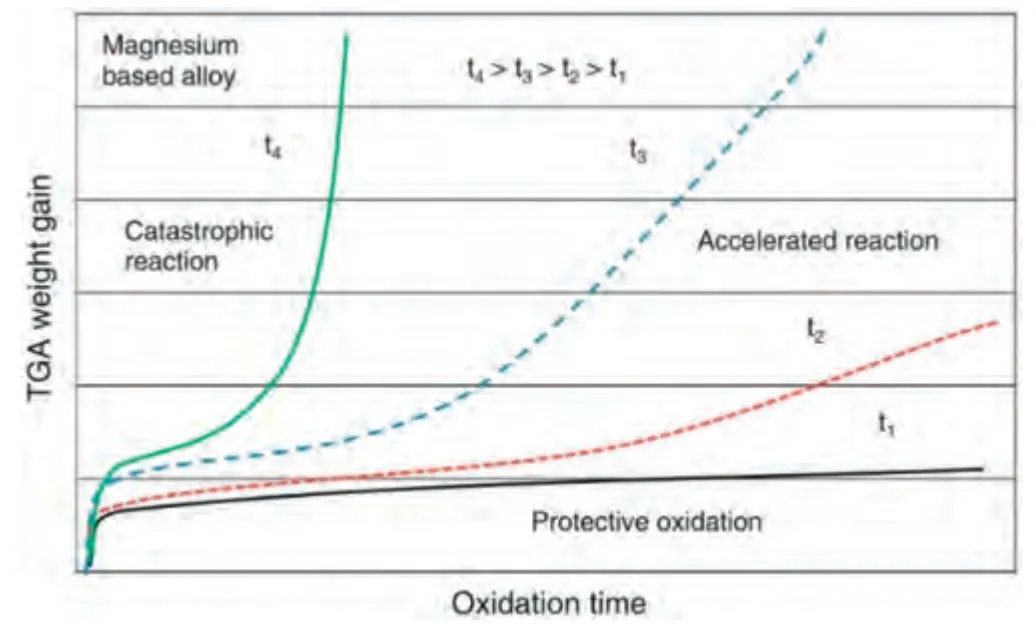
5.2.Factors influencing ignition resistance of Mg alloys
5.3.Mg alloys with high strength and excellent ignition-proof performance
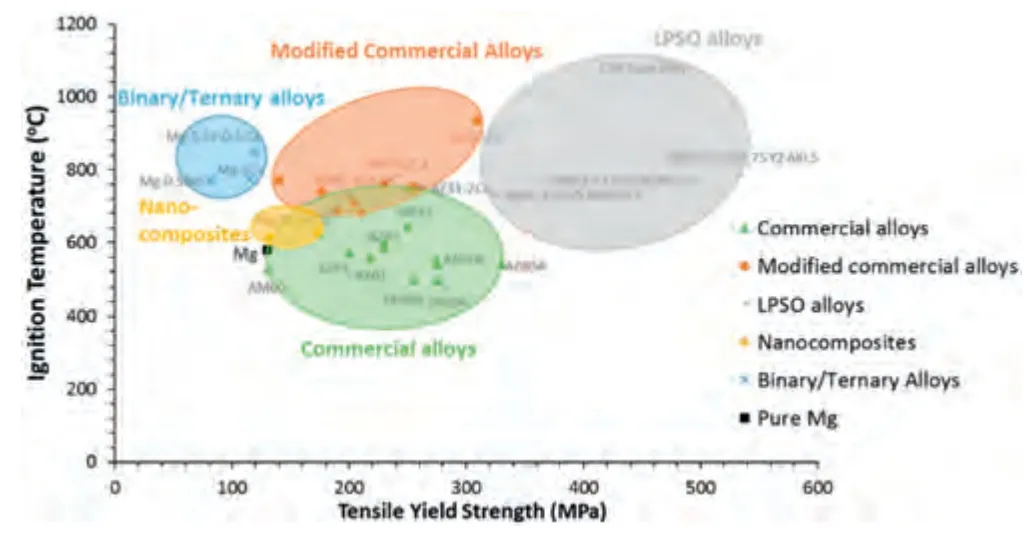


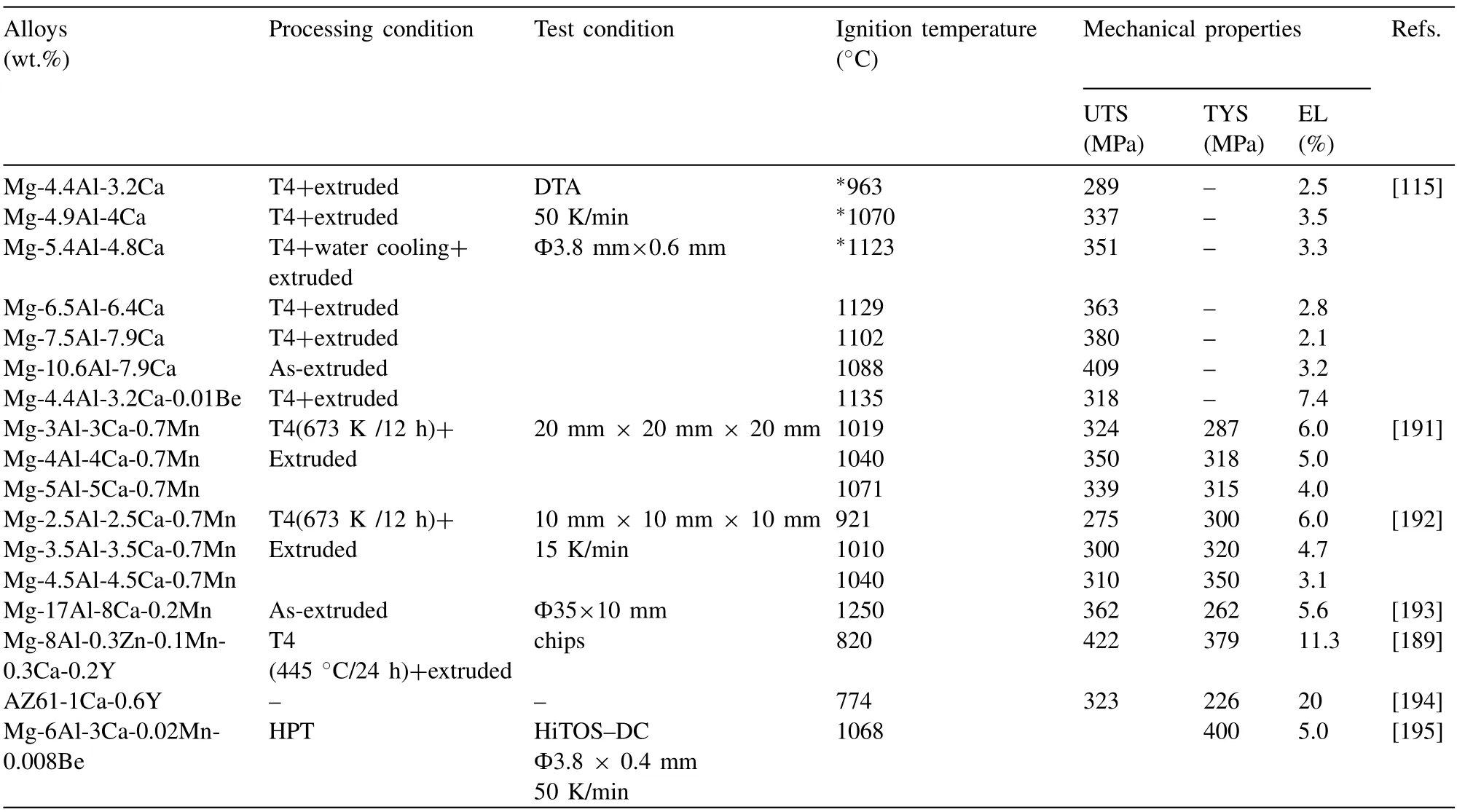


6.High-strength dissolvable Mg alloys and their composites
6.1.Dissolution mechanism of dissolvable Mg alloys
6.2.Factors influencing dissolution rate of dissolvable Mg alloys
6.3.High-strength dissolvable Mg alloys
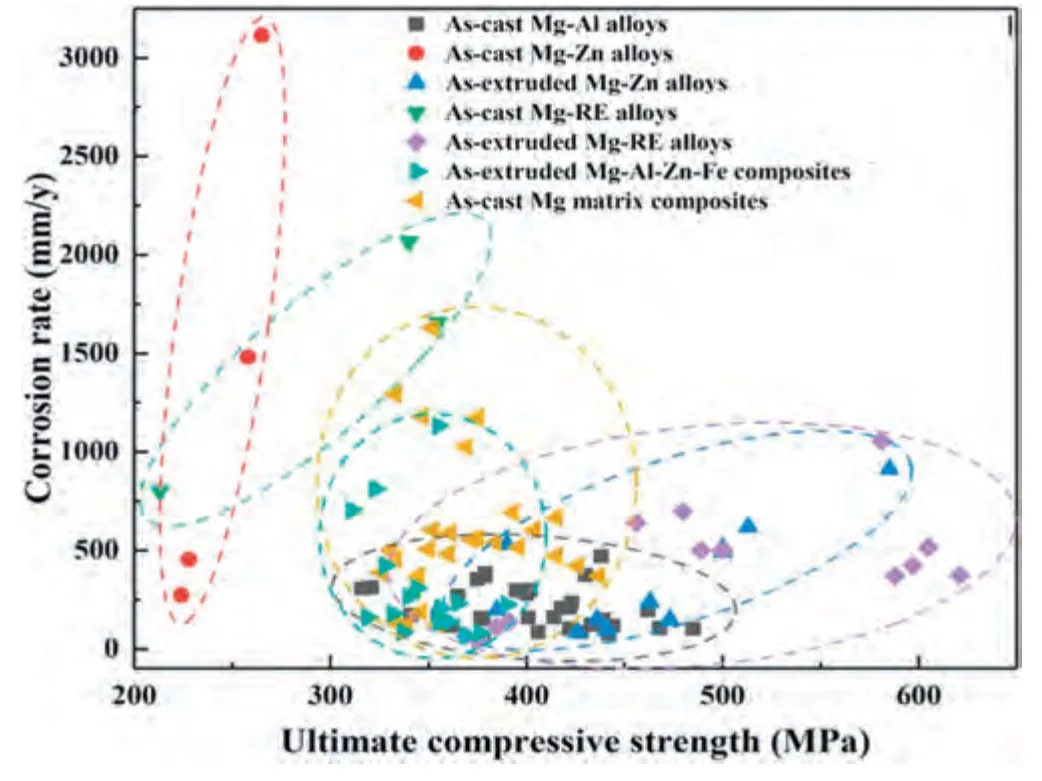
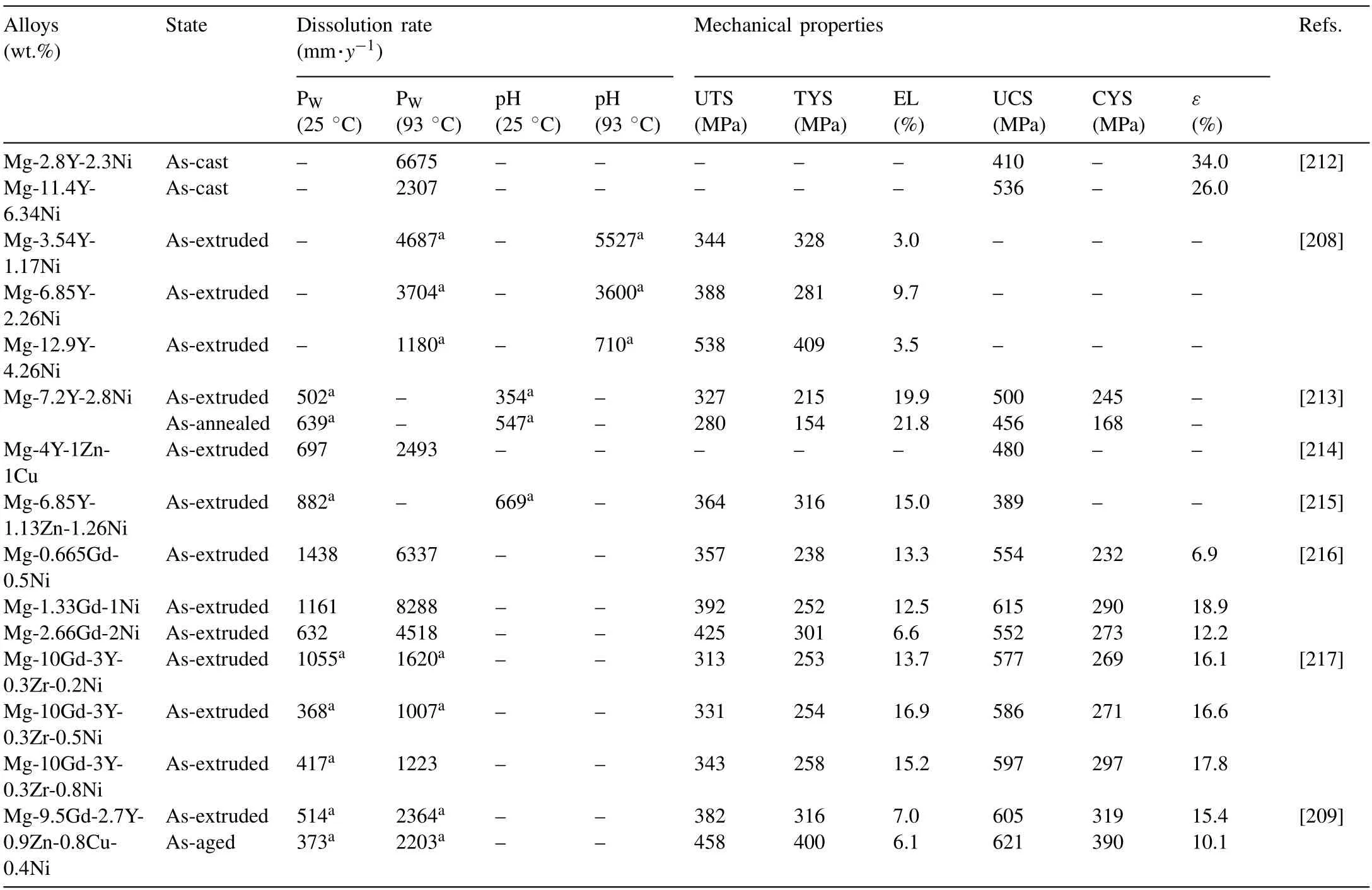
6.4.High-strength dissolvable Mg matrix composites

7.Summary and outlook
杂志排行
Journal of Magnesium and Alloys的其它文章
- Improvements in mechanical,corrosion,and biocompatibility properties of Mg–Zr–Sr–Dy alloys via extrusion for biodegradable implant applications
- Ultrasonic solidification mechanism and optimized application performances of ternary Mg71.5Zn26.1Y2.4 alloy
- Superplastic behavior of a fine-grained Mg-Gd-Y-Ag alloy processed by equal channel angular pressing
- GO/MgO/Mg interface mediated strengthening and electromagnetic interference shielding in AZ31 composite
- Experimental and theoretical studies on two-dimensional vanadium carbide hybrid nanomaterials derived from V4AlC3 as excellent catalyst for MgH2
- Electrochemical synthesis of boron-containing coatings on Mg alloy for thermal neutron shielding
