Effect of metal promoters on catalytic performance of Co/AC for higher alcohols synthesis from syngas
2023-11-21GUOLeiLIUPeigongGONGKunQIXingzhenLINTiejun
GUO Lei,LIU Pei-gong,GONG Kun,QI Xing-zhen,LIN Tie-jun,2,*
(1.School of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China;2.CAS Key Laboratory of Low -Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China;3.University of Chinese Academy of Sciences, Beijing 100049, China)
Abstract: Shifting products of Fischer-Tropsch Synthesis (FTS) from paraffins to value-added higher alcohols receives great attention but remains great challenge.Herein,metal oxides of Mn,Zn,La and Zr are investigated as promoters to tune the activity and product distributions of Co/AC catalyst for syngas conversion.It is found that these promoters show different promotion effect on CO dissociation rate,the formation of Co2C phase and the alcohols selectivity.The formed Co2C/Co0 constitutes the dual active site for higher alcohols synthesis.The strongest CO dissociation rate is observed for Zn-promoted Co/AC catalyst,resulting in the highest activity and space-time yield (STY) of alcohols.The Mn promoter is most conducive to the formation of Co2C,but slightly decreases the activity.The similar CO dissociation rate and CO conversion are obtained over both Zr-and La-promoted Co/AC catalysts,but the Zr-promoted Co/AC catalyst exhibits the highest alcohols selectivity due to the function balance between CO non-dissociative insertion and CO dissociation.
Key words: syngas;higher alcohols;CO hydrogenation;promoter;cobalt carbide
As a prime example of C1 chemistry,Fischer-Tropsch Synthesis (FTS) is a crucial process in both coal liquefaction and gas to liquids technologies for creating hydrocarbons fuels or value-added chemicals such as olefins,aromatics and higher alcohols[1].Feand Co-based are the most investigated catalysts for FTS,and the production of long-chain paraffins over these two types of catalysts also realize industrialization and commercialization[2].The economic value of FTS technology would be greatly improved,provided the product selectivity could be transferred from low-cost paraffins to value-added chemicals[3].However,the production of chemicals via FTS route remains grand challenge due to the lack of solid catalysts that can provide sufficient activity,selectivity and stability[4].Many efforts are made to achieve the selectivity control of FTS,and some labscale significant achievements also has been reported[5-9].
Among these chemicals,higher alcohols (referring to terminal linear α-alcohols and α-aldehydes) with two or more carbon numbers receive great interest due to their wide application as fuel additives,feedstocks in fine chemicals[10].In addition,the higher alcohols synthesis (HAS) also possess high degree of atom utilization efficiency since C and O atoms are both incorporated into the final products.Compared to direct syngas conversion to hydrocarbons products,the HAS is more conducive to carbon emission reduction.Traditionally,biofermentation,methanol carbonization,and homologation are used to produce higher alcohols[10].
Till now,four typical types of catalyst systems are reported for HAS: modified methanol synthesis (MS)catalyst,Rh-based catalyst,Mo-based catalyst,and modified Fischer-Tropsch (FT) catalyst[10,11].CO hydrogenation with MS catalysts usually produce high selectivity towards low-cost methanol[12].Rh-based catalysts show high selectivity to ethanol,but the higher cost of Rh as well as its limited reserves restrain its industrial usage.Mo-based catalysts operated under high temperature and high pressure typically require the addition of trace amount of H2S in the feed gas and lead to the largely production of mixed lower alcohols(C2-5OH).The modified FT catalysts including Cobased and Fe-based have been demonstrated to show most promising for HAS due to the high activity and selectivity to higher alcohols[11].Compared to Co-based catalyst,the easy phase transformation of Fe species and its quickly deactivation is severe issues for Febased case.Co-based catalyst with metallic Co as active site for CO dissociation is an excellent candidate for FTS to produce paraffins.Many studies suggest that the addition of second metal or metal oxides promoter could benefit the construction of another site for CO nondissociative adsorption and insertion,thus effectively shifting the products from paraffins to alcohols[10].One of the representative dual-sites is Co0-Co2C,where the metallic Co is responsible for CO dissociation and carbon chain growth,while Co2C provides a CO insertion function[13-18].It is pointed out the addition of promoters[4]including Na[19],K[20],Mn[17],La[21],Zn[22,23],Ca[24]can promote the formation of Co2C.Especially,the alkali metal is most conducive to construct Co0-Co2C interface sites and has been most extensively investigated[15,25].However,the addition of alkali metal simultaneously leads to the significant decrease of activity.It is desirable to explore another promoter that both improve the activity and alcohols selectivity.Moreover,the different role of metal promoters on Co2C formation and catalytic performance of Co-based catalysts has not been extensively compared.
Herein,we systematically compare the effect of the addition of small amounts of different promoters(Zn,La,Mn,Zr) on the structure and catalytic performance of the activated carbon supported Co(Co/AC) catalyst for CO hydrogenation.Of particular interest is the role of these metal promoter on Co2C formation and CO dissociation rate as well as the production of higher alcohols.
1 Experimental
1.1 Catalyst preparation
Incipient wetness impregnation was used to prepare Co/AC and metal promoted Co/AC catalyst(CoX/AC,X=Zn,La,Mn,Zr).Before impregnation,the activated carbon was pretreated with concentrated nitric acid at a mass concentration of 68% in an oil bath at 120 °C for 4 h.The treated activated carbon was then washed with deionized water for severe times until the pH is close to 7.Subsequently,the treated activated carbon was dried in a vacuum drying oven at 120 °C for 2 h.The mass loading of Co (Co(NO3)2·6H2O as precursor salt,Sinopharm Chemical Reagent Co.,Ltd.)was controlled at 20% with respect to the weight of the activated carbon.The mass loading of metal promoter of Zn,La,Mn,Zr was controlled at 1% with respect to the weight of the activated carbon.The La(NO3)3·6H2O,50% Mn(NO3)2and Zr(NO3)4·5H2O were purchased from Sinopharm Chemical Reagent Co.,Ltd.and were used as precursor salt.
The impregnated samples were dried for 12 h at room temperature and calcined in a tube furnace under Ar flow at 350 °C for 4 h.After calcination,the catalyst was passivated with 1%O2/Ar for 2 h at 30 °C.
1.2 Catalyst characterization
Nitrogen physisorption experiment was conducted on a micromerics 2420 device at -196 °C.The sample was firstly degassed under vacuum at 300 °C for 6 h before test.The specific surface areas were calculated by the Brunauer-Emmett-Teller (BET) equation.The total pore volume and average pore size were determined using the Barrett-Joyner-Halenda (BJH)method.
X-ray diffraction (XRD) patterns of samples were obtained on a Rigaku Ultima IV X-ray powder diffractometer using CuKα radiation with the wavelength of 1.54056 Å at 40 kV and 40 mA.The samples were scanned from 5° to 90° at a rate of 2(°)/min.The crystallite structure phase was identified by JCPDS standard cards.
X-ray photoelectron spectra (XPS) were obtained from a Thermo Fisher Scientific K-Alpha spectrometer equipped with an AlKα (1486.6 eV) radiation source.The binding energy of all spectra was calibrated by setting the C 1speak at 284.8 eV.
The transmission electron microscope (TEM) and high-resolution transmission electron microscope(HRTEM) measurements were performed on a JEOLJEM 2011 electron microscope operating at an accelerating voltage of 200 kV.The samples were prepared by ultrasonic dispersion of the catalysts in ethanol followed by adding the suspension dropwise to a carbon-coated copper microgrid for measurement.
1.3 Catalytic reaction
The HAS reaction was performed in a stainless steel fixed-bed reactor with 10 mm inner diameter.The reaction temperature was measured with a K-type thermocouple buried in the catalyst bed.The fresh catalyst (1 g,40-60 mesh) was diluted with 3 g of silica sand (40-60 mesh) and loaded into the reactor tube.Prior to reaction,each fresh catalyst wasin situreduced in a flow of hydrogen (200 mL/min) at 450 °C for 5 h.After reduction,the reactor was cooled down to 160 °C,and pressurized to reaction pressure (4 MPa)with a syngas flow (H2/CO/N2=64.7∶32.3∶3.0,3.0%N2as the internal standard).And then the reactor was heated to the reaction temperature (220 °C) with heating rate of 1 °C/min.The effluent gas was analyzed by an online Agilent 7890B gas chromatograph equipped with a thermal conductivity detector (TCD)and a flame ionization detector (FID).The aqueous,oil and wax products were collected by a hot trap (120 °C)and a cold trap (0 °C) and analyzed off-line with Shimadzu GC.Detail GC analysis information can be found in our previous work[7].The mass balance and carbon balance were calculated and kept better than 95%.All performance data were obtained at 48 h under the steady state.
For a typical reaction,the CO conversion and product selectivity were calculated by the following equations:
where COinletand COoutletmeans moles of CO at the inlet and the outlet,respectively,simeans the selectivity to production a carbon basis,Niis the molar fraction of producti,andniis the carbon number of producti.
The chain-growth probability (α) was calculated based on the Anderson-Schulz-Flory distribution (3):
The catalytic activity for CO dissociation and disproportionation to form surface carbon and CO2was performed by exposing the reduced catalyst with CO atmosphere at 220 °C in a U-types fixed-bed reactor.The CO2signal in the effluent gas was continuously recorded using a TILON LC-D200 M mass spectrometer.
2 Results and discussion
2.1 Catalysts characterizations
Figure 1 shows the N2adsorption-desorption isotherms and the pore size distributions of various calcined samples.According to the IUPAC classification,these isotherms are the typical adsorption profile of Type-I.The presence of an H4 hysteresis loops indicates that these samples belong to microporous structured material,which could further be clearly observed from the pore size distributions in Figure 1(b).According to Table 1,the specific surface areas of AC is as high as 1396.0 m2/g,while that of Co/AC,CoMn/AC,CoZn/AC,CoLa/AC and CoZr/AC catalyst is 319.1,416.2,368.1,409.5 and 492.6 m2/g,respectively.The catalysts' specific surface areas and pore volume drastically decrease after the addition of Co and/or promoter,demonstrating that the catalysts'active ingredients are thoroughly deposited in the carrier pores.It is noteworthy that the specific surface areas of promoted CoX/AC catalyst show a slightly higher trend than that of Co/AC,which might be due to the collapse of some of the pore channels within the carrier caused by the decomposition of nitrate salts,resulting in the creation of some smaller pore channels.
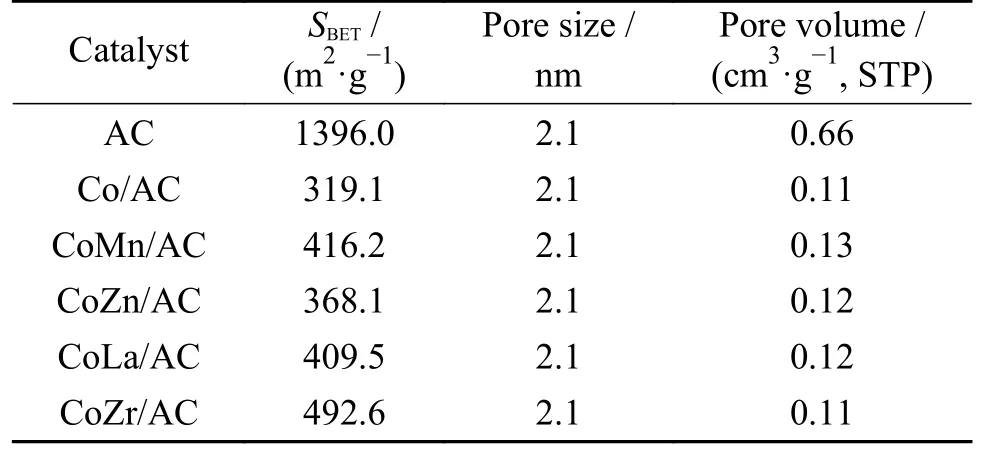
Table 1 Textural properties of the fresh CoX/AC catalysts
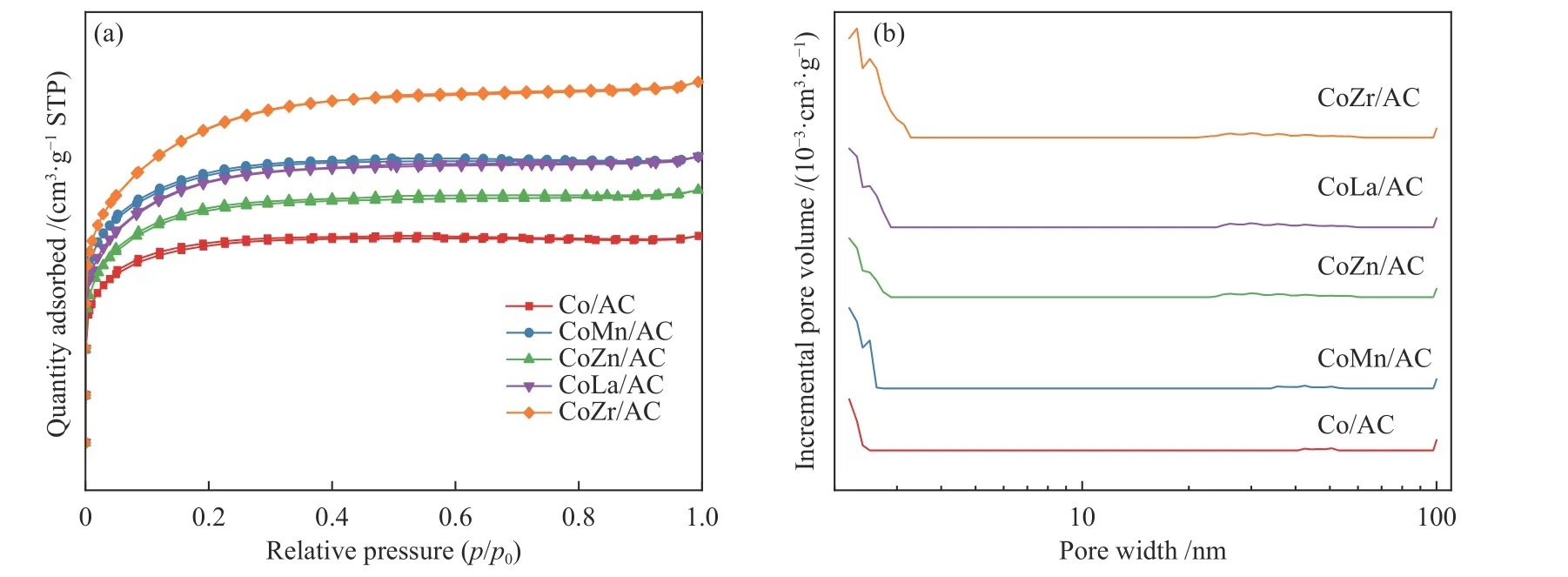
Figure 1 Isotherm curves (a) and pore size distributions (b) for the fresh CoX/AC catalysts
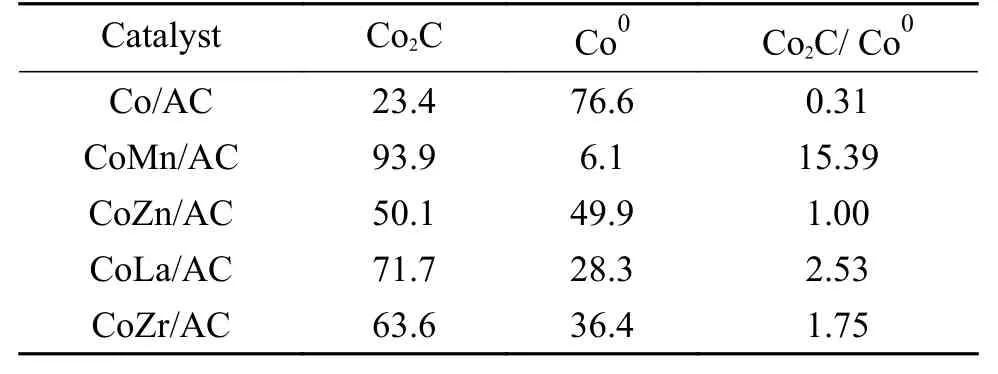
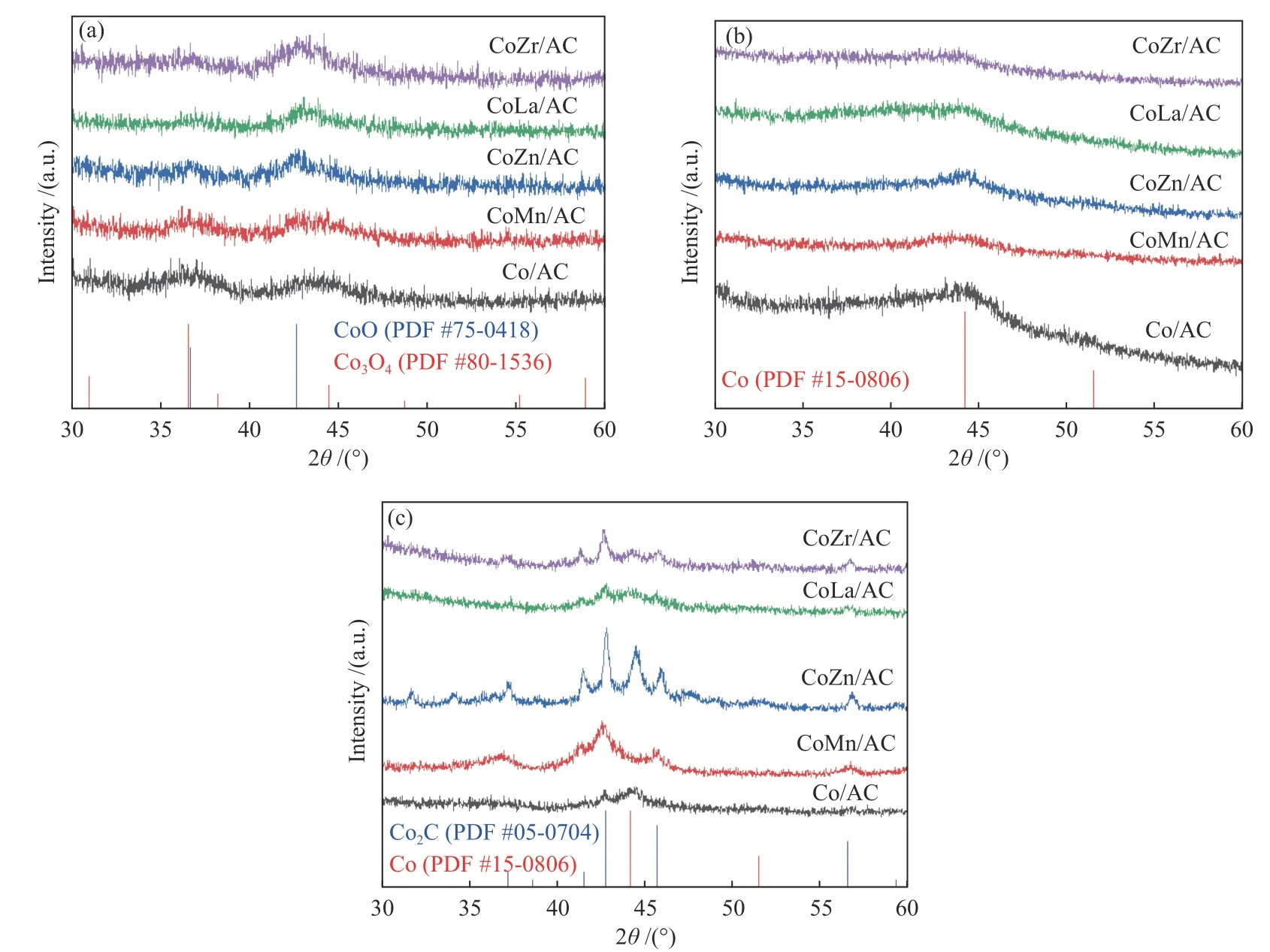
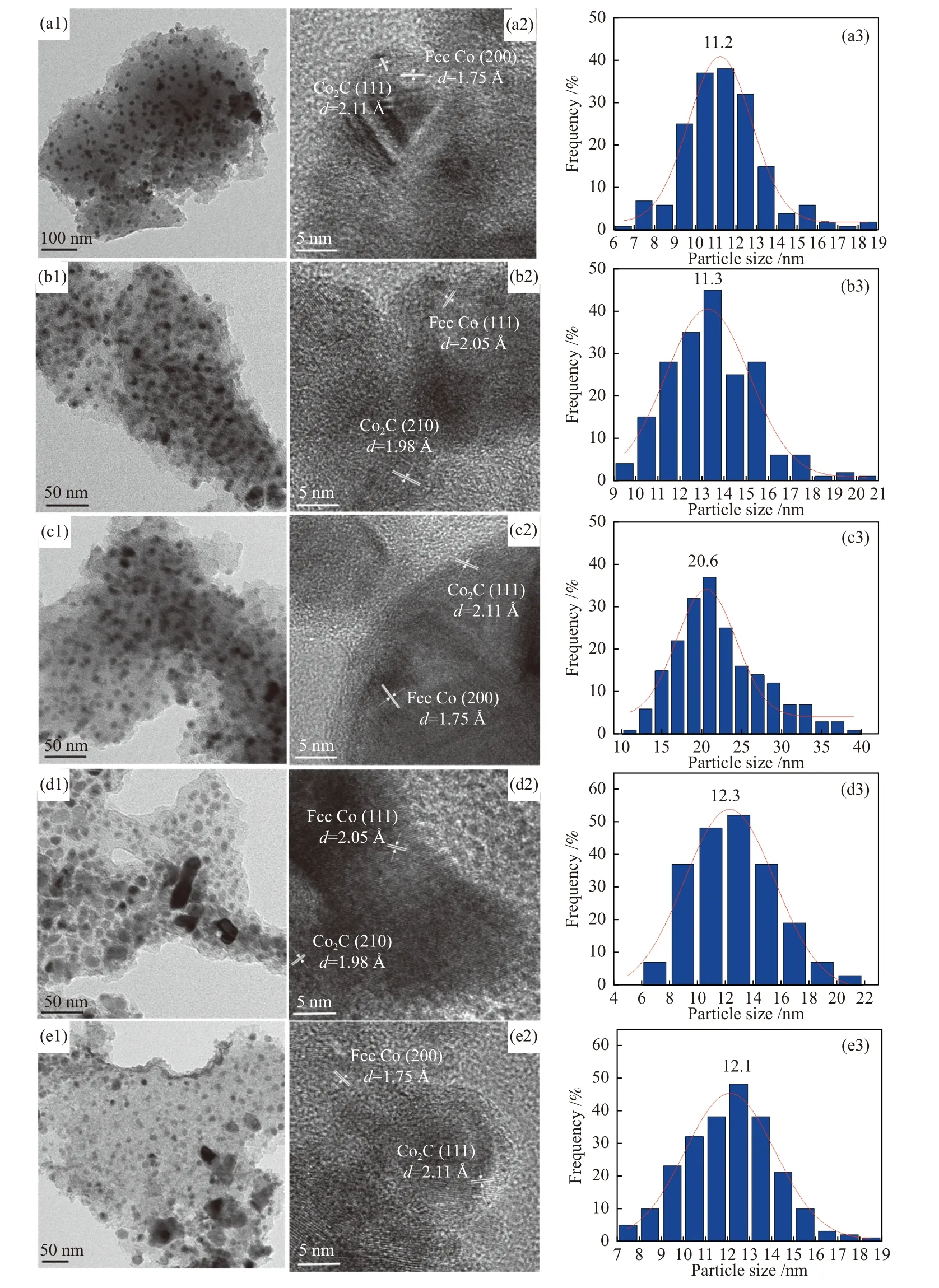
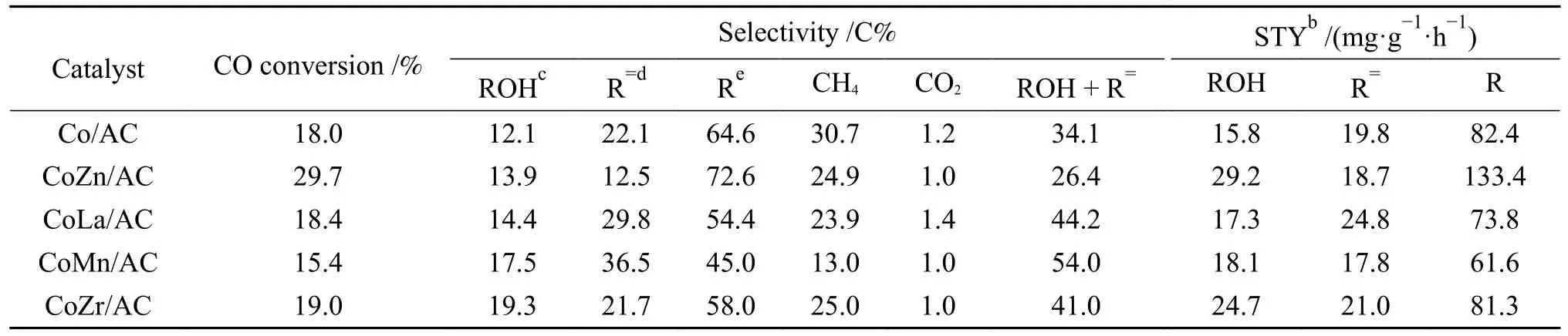
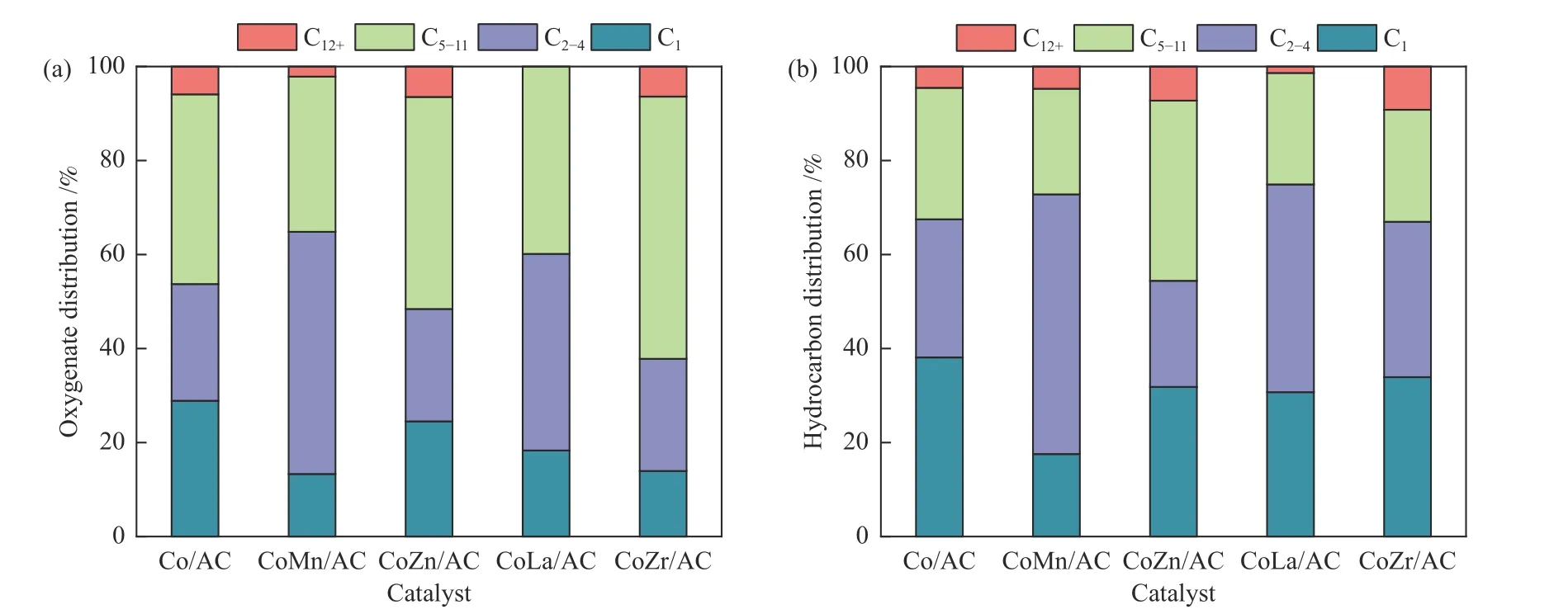
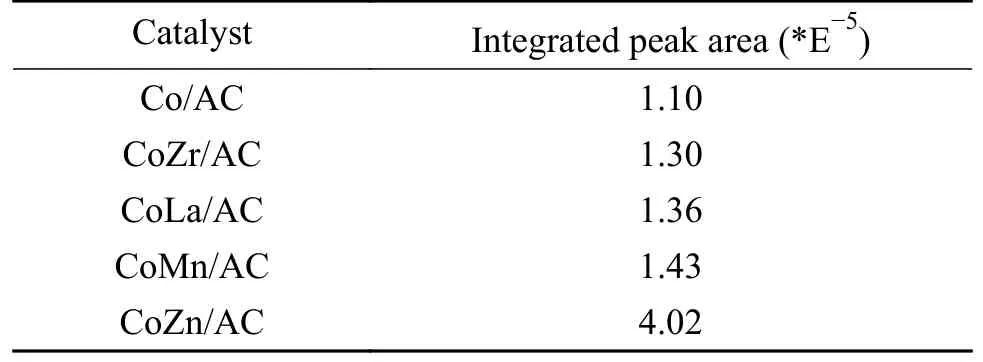
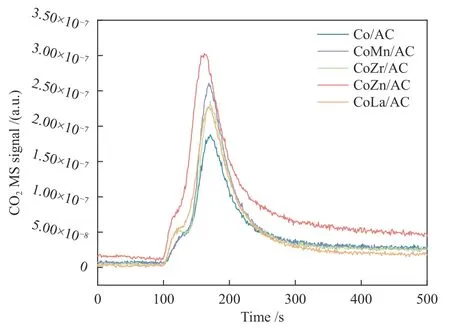
The crystal structures of the catalysts after calcination,reduction and reaction were studied by XRD,as shown in Figure 2.The catalysts after calcination all show characteristic diffraction peaks of CoO (PDF#75-0418) and Co3O4(PDF#80-1536),no other obvious species are observed,indicating the highly dispersed active components on the AC support due to its special high specific surface areas.The addition of the promoters does not affect the crystalline phase structure of the catalysts.The appearance of CoO is caused by the self-reducibility property of AC during high temperature treatment under Ar flow,as similar to that reported in other references[17,25].To clearly reflect the actual crystalline phase,the reduced sample was firstly transferred into liquid paraffins under N2flow to prevent being oxidized by air,and then the sample was submitted to XRD characterization.As shown in Figure 2(b),the metallic Co is detected as the exclusive Co species,suggesting that the cobalt oxides are completely reduced to Co metal under H2treatment.After reaction,the diffraction peaks at 2θof 41.56°,42.76°,45.73° are clearly observed,which is attributed to the (020),(111) and (210) crystal planes of Co2C(PDF#05-0704).In addition,the diffraction peak of 2θof 44.22° is attributed to the (111) crystal plane of Co0(PDF#15-0806).Obviously,the metallic Co is partially carburized into Co2C after exposing reduced sample to syngas atmosphere.The formation of Co0and Co2C as well as the possible Co0/Co2C interface might be responsible for the alcohols synthesis.The crystalline size of metallic Co and Co2C was roughly calculated according to Scherrer Equation.As shown in Table 2,the crystalline size of Co2C for Co/AC is about 23.0 nm,while the metal-promoted samples show a slightly decrease with the following order: CoMn/AC Table 2 Average nanoparticle sizes of Co species over spent CoX/AC catalysts characterized by HRTEM and XRD Table 3 Relative content of cobalt species in the spent CoX/AC catalysts Figure 2 XRD patterns of various catalysts at different stages TEM and HRTEM characterization were performed to investigate the structure information of various spent catalysts,as shown in Figure 3.The crystalline spacing of 1.75 and 2.05 Å corresponds to the (200) and (111) facet of fcc Co phase,respectively,while crystalline spacing of 1.98 and 2.11 Å is attributed to the (210) and (111) facet of Co2C,respectively.The particle size of Cocontaining species was counted according to the TEM images and the average size was listed on Table 2.The CoZn/AC catalyst has the largest particle size of Co at~ 20.6 nm,while the other catalysts show similar particle size. Figure 3 (HR)TEM images and particle size distributions of Co-containing species for various spent catalysts a1-a3 for Co/AC,b1-b3 for CoMn/AC,c1-c3 for CoZn/AC,d1-d3 for CoLa/AC,e1-e3 for CoZr/AC In order to investigate the valence state of Co species for different samples,XPS analysis was performed for the various spent catalysts.The C 1s(a)and Co 2p(b) XPS spectra of spent CoX/AC catalysts are shown in Figure 4.The C 1sin Figure 4(a) suggests that the generation of Co-C species can be ascribed to the cobalt carbide for all spent sample,confirming that the syngas atmosphere induces the structure evolution of Co0to Co2C.For the spent catalyst,the peak at binding energy (BE) of 777.9 eV in the Co 2p3/2represents Co0.The peak centered at around of 780.5 eV in the Co 2p3/2belongs to Coδ+.For CoMn/AC,the peaks corresponding to Co0could be clearly observed since the Mn promoter typically leads to the formation of an interfacial state of Co0adhering to the surface of Co2C[17].For the other samples,the weak Co0might be caused by the oxidation of surface Co0species. The catalytic performances for HAS reaction over various CoX/AC catalysts were evaluated at the conditions of 220 °C,4 MPa,3000 mL/(g∙h) and H2/CO=2.The results are shown in Table 4.The AC is perfect support candidate that benefits the formation of alcohols[13].Therefore,the prepared Co/AC catalyst shows 12.1% of alcohols selectivity at 18.0% CO conversion.The CO2selectivity is as low as 1.2% and the space-time yield (STY) of alcohols is 15.8 mg/(g∙h).After introducing metal promoters into Co/AC,the catalytic performance changes significantly.The addition of metal promoter of Zn,La,Mn and Zr lead to an increase in the selectivity of the alcohols to 13.9%,14.4%,17.5%,and 19.3%,respectively.Except for CoMn/AC,the other promotermodified catalysts show an increased trend for CO conversion.Among them,the CoZn/AC exhibits the highest CO conversion of 29.7%.The STY of alcohols over these catalysts show the following order:CoZn/AC >CoZr/AC >CoMn/AC >CoLa/AC >Co/AC.Notably,the selectivity of methane decreases from 30.7% for Co/AC to 25.0% for CoZr/AC,24.9%for CoZn/AC,23.9% for CoLa/AC and 13.0% for CoMn/AC,respectively. Table 4 Catalytic performance of various catalysts for syngas conversiona Obviously,the addition of Zn,La,Mn,Zr could effectively suppress the production of methane.For the CoMn/AC catalysts,we can see that the selectivity of olefins also reaches to 36.5%,indicating that the addition of Mn promoter effectively prevents the secondary hydrogenation of olefins. Figure 5 shows the distributions of products containing oxygenates and hydrocarbons.It can be seen from oxygenates distributions that the fraction of methanol is as high as 28.9% for Co/AC.The addition of metal promotes effectively decreases the CH3OH fraction to 24.5% for CoZn/AC,18.3% for CoLa/AC,13.9% for CoZr/AC and 13.3% for CoMn/AC,respectively.Therefore,it can be concluded that these promoters benefit the formation of C2+OH.Similarly,the fraction of methane in hydrocarbon distributions(Figure 5(b)) significantly decreases.Especially for CoMn/AC catalyst,the CH4fraction decreased to 17.5% from 38.1% for Co/AC.The findings show that Zn,La,Mn and Zr is effective promoter to suppress CH4formation. Figure 5 Product distributions of CoX/AC catalysts Figure 6 plots the ASF distributions of hydrocarbons and oxygenates over various catalysts.The corresponding chain-growth probability (α) was also calculated.The fitted linear distribution of products suggests the addition of promoter does not change the reaction mechanism of FTS over Co/AC and CoX/AC catalyst.The chain-growth probability (α)for hydrocarbons or oxygenates over Co/AC is higher than these promoted catalysts,which is caused by the transformation of Co species from metallic Co to Coδ+. Based on the above results,we can see that the addition of metal promoters leads to significant changes in both the structure of active site and catalytic performance of Co/AC.Especially,the products distributions can be effectively tuned from paraffins to alcohols without sacrificing the activity.Previous studies have confirmed that the Co0-Co2C interface sites can catalyze alcohols production[13,15].The metallic Co phase mainly appears in Co/AC catalyst,and it is hard to form Co2C without any promoters[13].The lower Co2C/Co0ratio leads to the main production of paraffins.After adding promote of Zn,La,Mn and Zr,the carburization rate of metallic Co is boosted,and the Co0-Co2C interface sites are thus readily constructed.Therefore,the alcohols selectivity is greatly improved.However,the different Co2C/Co0ratios over spent catalysts suggest that different promoters exhibit different promotion effect on carburization rate.Among them,the Mn-promoted Co/AC shows the maximum content of Co2C phase along with the observed highest olefins selectivity and lowest fraction of C1 by products.Many studies have indicated that the Mn is a very effective promoter to suppress reactivity of chemisorbed H species,thus benefiting the C5+production in FTS[26,27]or favoring olefins formation in FTO[28,29]or facilitating CO insertion to give alcohols products[17].Whereas,the Mn-promoted Co/AC shows slight decreased activity,which might be due to the relatively high Co2C/Co0ratio.It is known that the Co2C itself shows very low intrinsic activity than Co0nanoparticles[13].Inversely,the Zn promoter shows special promotional effect on activity,alcohols selectivity and STY of alcohols. To explore the influence of these promoter on CO dissociation rate,we exposed the reduced catalysts to CO atmosphere at 220 °C based on the method reported by Zhao et al.[17].The formation rate of surface carbonaceous species is accessed by comparing the fitted peak areas of CO2signal recorded by MS in the effluent gas.As shown in Figure 7,under the same treatment conditions,the addition of promoters can effectively catalyze disproportionation reaction of CO to form CO2and surface carbonaceous species.The CO2signal intensity of CoX/AC is stronger than that of Co/AC.The fitted peak area of CO2was calculated and shown in Table 5. Table 5 Catalyst surface carbon species concentration Figure 7 MS signal of CO2 for disproportionation reaction of CO to form CO2 and surface carbonaceous species Experiments were conducted at 220 °C,0.1 MPa,and 12000 mL/(g·h) The CO2concentration of these catalysts follows the order: CoZn/AC >CoMn/AC >CoLa/AC >CoZr/AC >Co/AC.The high CO2concentration typically indicates high CO dissociation rate and high initial surface carbonaceous species coverage.The concentration of surface carbonaceous species commonly determines the carburization rate and Co2C formation.Therefore,it is easily to understand that the CoX/AC catalysts show much larger amount of Co2C phase and high Co2C/Co0ratio.Meanwhile,the amount of carbon species on the surface of the CoZn/AC is much higher than the other samples,i.e.,four times higher than that of Co/AC,indicating that the Znpromoted Co/AC shows strong CO dissociation rate.This phenomenon also explains the observed highest activity of CoZn/AC catalyst.The existence of surface carbonaceous species and its coverage also can decrease the surface H* coverage,thus creating a COrich and H-lean local microenvironment that benefits CO nondissocitive adsorption and insertion[30].It should be noted that different properties of promoter including electronegativity,acidity would largely affect the reactivity of surface carbonaceous species and surface adsorbed intermediates,leading to different surface active sites structure and catalytic performance of CoX/AC catalysts.For CoZr/AC catalyst,the appropriate ratio of Co2C/Co0ratio and appropriate surface carbonaceous species coverage could benefit the function balance of CO non-dissociative insertion and CO dissociation,which gives the highest total alcohols selectivity. In conclusion,the promotion of Co/AC catalysts with metal promoters including Mn,Zn,La and Zr leads to a modification in the structure and catalytic performance for CO hydrogenation.For all modified catalysts,the addition of promoter could accelerate CO dissociation rate and enhance surface carbonaceous species that promotes the carburization rate of metallic Co and the formation of Co2C phase.The as-formed Co2C/Co0interface sites can effectively shift the products selectivity from paraffins to alcohols without sacrificing activity.Among them,CoZn/AC shows strongest CO dissociation rate and highest activity and space-time yield (STY) of alcohols.The Mn-promoted Co/AC produce highest ratio of Co2C/Co0and lowest C1 by products with slight decrease of activity.The activity of CoZr/AC and CoLa/AC is similar but the former exhibits the highest alcohols selectivity of 19.3% due to the appropriate Co2C/Co0ratio that could achieve function balance between CO dissociation and CO non-dissociative adsorption,thus promoting the production of alcohols products.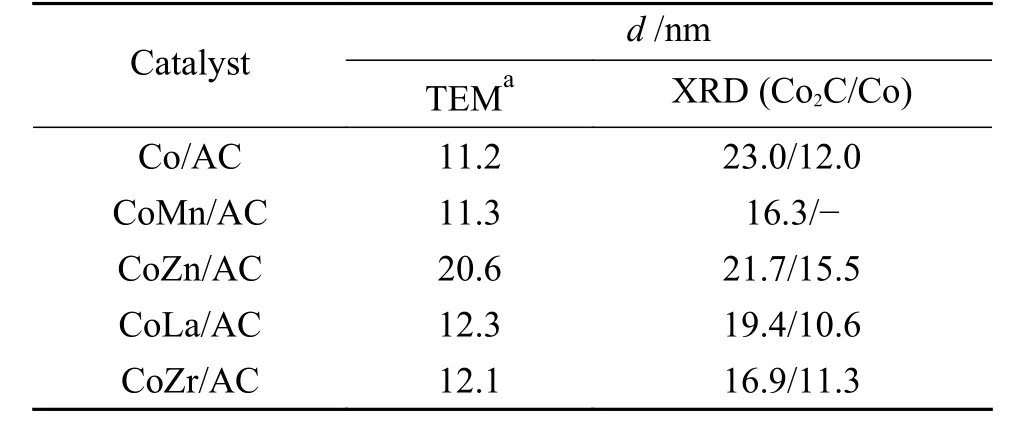
2.2 Catalytic performance
2.3 Effect of promoter on structure-performance relationship
3 Conclusions
