影响神经危重症患者预后不良的危险因素分析及预测模型构建
2023-11-20顾沈燕王玉琴柯开富成亚琴卞艳红钱海兰张元媛
顾沈燕 王玉琴 柯开富 成亚琴 卞艳红 钱海兰 张元媛
[摘 要] 目的:探討影响神经危重症患者短期预后不良的危险因素,构建预后列线图预测模型。方法:回顾性分析首次在南通大学附属医院神经内科重症监护病房(neurological intensive care unit,NICU)住院的神经内科危重症患者310例临床资料,根据出院时APACHE Ⅱ评分作为短期预后指标分为预后良好组161例和预后不良组149例。将患者入院时的指标作为自变量,采用Lasso回归及交叉验证的方法筛选变量,通过二元Logistic回归筛选影响患者短期预后的独立危险因素。搭建预测模型并绘制列线图,采用Bootstrap检验进行内部验证,以受试者工作特征曲线(receiver operator characteristic curve,ROC)、校准曲线、决策曲线分析(decision curve analysis,DCA)及临床影响曲线(clinical impact curve,CIC)验证模型的区分度、校准度及临床适用性。结果:机械通气、房颤、入院时血钙水平以及GCS评分是影响NICU患者短期预后的独立危险因素。入院时GCS评分、机械通气、入院时血钙水平、房颤的ROC曲线下面积(area under curve,AUC)分别为0.787、0.655、0.629及0.598,入院时GCS评分的AUC大于其他独立危险因素(P<0.001)。联合预测因子模型的AUC为0.861,校准后为0.854,显著大于其他独立危险因素(P<0.001)。ROC曲线及C统计量提示本模型区分度良好,校准曲线提示本模型校准度良好,而 DCA曲线及CIC曲线则显示本模型具有良好的临床适用性。结论:机械通气、房颤、入院时血钙水平以及GCS评分是引起神经危重症患者预后不良的危险因素,构建的联合预测因子模型具有良好的预测能力,能定量而简明评价不同危险因素的风险,为神经危重症患者的精准医疗提供依据。
[关键词] 神经危重症;预后;危险因素;列线图;预测模型
[中图分类号] R745.7 [文献标志码] A [DOI] 10.19767/j.cnki.32-1412.2023.03.004
Analysis of risk factors affecting the poor prognosis of neurocritical patients and construction of prediction model
GU Shenyan1,2, WANG Yuqin3, KE Kaifu3, CHENG Yaqin3, BIAN Yanhong3, QIAN Hailan3, ZHANG Yuanyuan3
(1Medical School, Nantong University, Jiangsu 226001; 2Department of Neurology, Peoples Hospital of Tongzhou;
3Department of Neurology, Affiliated Hospital of Nantong University)
[Abstract] Objective: To explore the risk factors affecting short-term poor prognosis in neurocritical patients, and to establish a nomogram prediction model for predicting these risks. Methods: The clinical data of 310 neurocritical patients who were first admitted to the Neurological Intensive Care Unit (NICU) of Affliated Hospital of Nantong University were retrospectively analyzed, and they were divided into 161 cases in the good prognosis group and 149 cases in the poor prognosis group based on the APACHEⅡ score at discharge as a short-term prognostic indicator. The admission indicators of patients with different prognosis were selected as independent variables by Lasso regression and cross-validation, and independent risk factors affecting short-term prognosis were selected by binary Logistic regression. The prediction model was constructed and the nomogram was drawn, the Bootstrap method was used for internal verification, and the differentiation, calibration and clinical applicability were verified by ROC curve, calibration curve, DCA and CIC curve. Results: Ventilator, atrial fibrillation, blood calcium level at admission, and GCS score were independent risk factors affecting the short-term prognosis of NICU patients. The area under ROC curve (AUC) for GCS score at admission, ventilator, blood calcium level at admission, and atrial fibrillation were 0.787, 0.655, 0.629, and 0.598, and the AUC for GCS score at admission was greater than other independent risk factors (P<0.001). The joint prediction model had an AUC of 0.861 and a calibrated AUC of 0.854, which was significantly greater than other independent risk factors (P<0.001). The ROC curve and C-statistic suggested that the model was well differentiated, the calibration curve suggested that the model was well calibrated, and the DCA curve and CIC curve showed that the model had good clinical applicability. Conclusion: Ventilator, atrial fibrillation, blood calcium level at admission and GCS scores are risk factors for poor prognosis in neurocritical patients, and the nomogram prediction model has good predictive ability to quantitatively and concisely assess the risk of different risk factors, which provides a basis for precise medical treatment of neurocritical patients.
[Key words] neurocritical; prognosis; risk factor; nomogram; prediction model
神经内科危重症包括大面积脑梗死、脑出血、蛛网膜下腔出血、吉兰-巴雷综合征、重症肌无力危象、重症颅内感染、癫痫持续状态等[1]。目前临床有多项危重症量化评分来判断疾病严重程度,其中急性生理学及慢性健康状况评分系统(acute physiology and chronic health evaluation scoring system,APACHE Ⅱ)[2-3]最具权威性、应用最广泛,但缺少针对神经危重症的评估系统。本研究试图构建列线图预测模型,更直观分析影响神经危重症患者预后的危险因素,为临床精准化治疗和判断预后提供依据。
1 资料与方法
1.1 一般资料 回顾性分析2019年1月—2021年10月首次入住南通大学附属医院神经内科重症监护病房(neurological intensive care unit,NICU)310例危重症患者的临床资料,其中男性212例,女性98例,年龄18~93岁,平均67.44±13.17岁。纳入标准:(1)符合《中国急性缺血性脑卒中诊治指南2018》[4]、《中国脑出血诊治指南2019》[5]、《中国吉兰-巴雷综合征诊治指南2019》[6]及《中国重症肌无力诊断和治疗指南2020》[7]中关于脑梗死、脑出血、吉兰-巴雷综合征或重症肌无力诊断标准;(2)年龄≥18岁,发病7天内入院;(3)临床基线资料完整。排除入院时合并其他重要脏器损害或恶性肿瘤等预期寿命小于1年的患者。以出院时APACHE Ⅱ评分作为短期预后评价指标,310例患者中161例(51.94%)>15分为预后良好组,149例(48.06%)≤15分为预后不良组。本研究通过南通大学附属医院伦理委员会审批,所有患者签署知情同意书。
1.2 统计学处理 按照临床预测模型研究报告准则TRIPOD指南[8]进行模型构建。应用SPSS 26.0及R4.1.1统计学软件对数据进行分析处理。符合正态分布的计量资料以■±s表示,组间比较采用t检验,不符合正态分布的计量资料以M(P25、P75)表示,组间比较采用非参数检验;计数资料以频数和率表示,组间比较采用χ2检验;采用Lasso回归及二元Logistic回归筛选变量。所有检验均使用双侧检验,P<0.05为差异有统计学意义。采用R软件中glmnet包进行Lasso回归,rms包进行列线图构建及Bootstrap检验,rmda包绘制决策曲线分析(decision curve analysis,DCA)及临床影响曲线(clinical impact curve,CIC)。使用校准曲线、Hosmer-Lemeshow检验评估模型的校准度。采用Graphpad Prism 9.0软件绘制森林图及受试者工作特征曲线(receiver operator characteristic curve,ROC),根据ROC曲线下面积(area under curve,AUC)判断模型的预测价值(0.5~0.7为一般,~0.9为较好,>0.9为极好)。
2 结 果
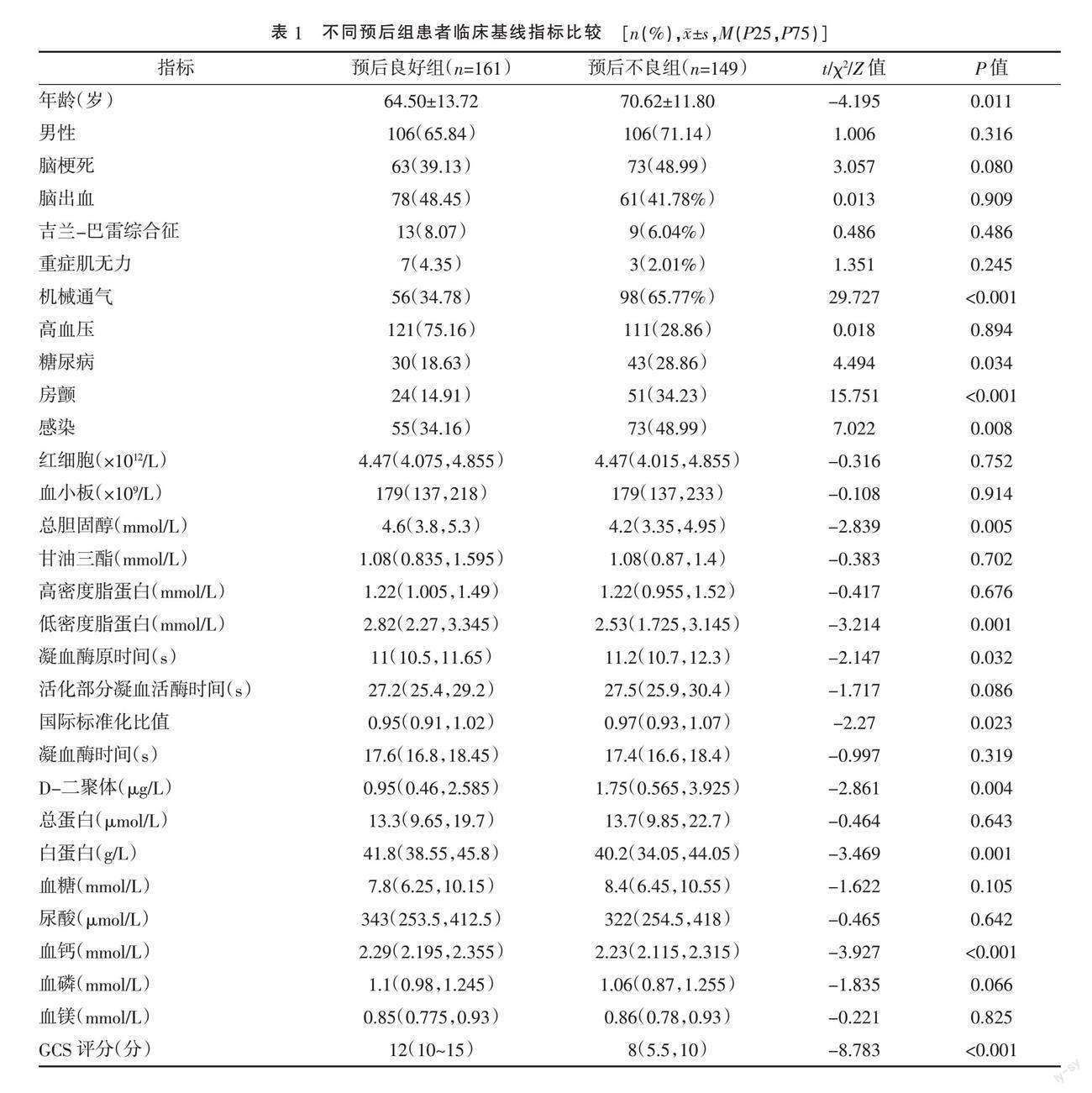
2.1 不同预后组患者临床基线指标比较 单因素分析显示,预后不良组患者机械通气、房颤、糖尿病、感染率、入院24小时内凝血酶原时间、国际标准化比值、D-二聚体均显著高于预后良好组,入院24小时内总胆固醇、低密度脂蛋白、白蛋白、血钙水平以及格拉斯哥昏迷评分(Glasgow coma scale,GCS)均显著低于预后良好组,差异均有统计学意义(P<0.05)。见表1。
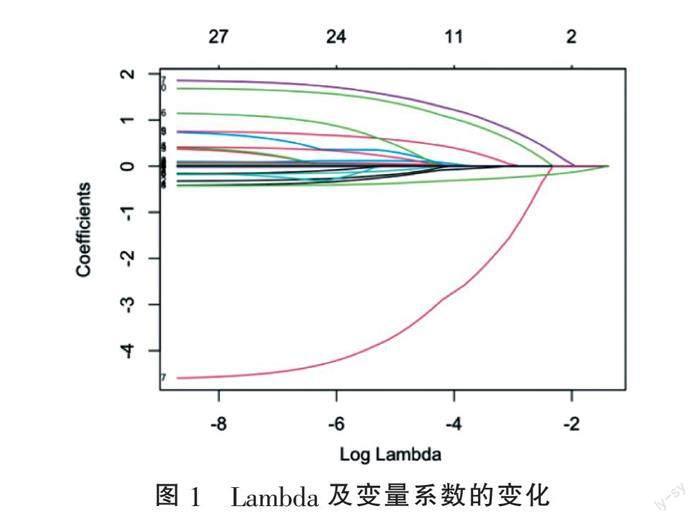
2.2 影响患者不良预后危险因素初筛 为避免变量之间多重共线性,将纳入的临床基线指标为自变量,患者出院时APACHE Ⅱ评分为因变量,利用Lasso回归筛选变量(图1)。通过十倍交叉验证选取最优λ,如图2中第一条虚线所示,即误差最小的λ为0.02640866,Log λ为-3.634063。此时入选的变量为机械通气、房颤、糖尿病、总胆固醇、低密度脂蛋白、血钙及GCS评分,经计算方差膨胀因子均小于2,各变量之间无交叉共线性。将入选的变量纳入二元Logistic回归分析,结果显示总胆固醇及低密度脂蛋白的P值>0.05,予以剔除。绘制森林图(图3),结果显示机械通气、房颤、合并糖尿病、入院时血钙水平以及GCS评分为影响神经危重症患者短期预后的独立危险因素。
2.3 独立危险因素ROC曲线分析 基于以上变量筛选结果,采用ROC曲线比较各独立危险因素的预测效能,见圖4。合并糖尿病的AUC为0.551,不具有预测意义(P=0.120);入院时GCS评分、机械通气、入院时血钙水平、房颤的AUC分别为0.787、0.655、0.629和0.598,入院时GCS评分的AUC明显大于机械通气、房颤、糖尿病、入院时血钙水平,差异均有统计学意义(P<0.001),其余独立危险因素之间差异均无统计学意义(P>0.05),表明入院时GCS评分的预测价值更优。
2.4 联合预测因子模型构建 将机械通气、房颤、入院时血钙水平及GCS评分4个自变量纳入二元Logistic回归(表3),依据回归系数进行赋分,建立联合预测因子,logit(P)=11.976+1.566×机械通气+1.452×房颤-4.186×血钙-0.369×GCS评分。可按以下公式计算每例患者预后不良的概率:P=e^logit(P)/1+e^logit(P)。将联合预测因子评分(PRE)作为自变量,纳入上述ROC曲线分析(图3),AUC为0.861(95%CI=0.820~0.901),显著高于其他独立危险因素(P<0.001),提示该联合预测因子较独立危险因素对模型有更好的区分能力。见表2。
2.5 列线图预测模型建立 为了更直观显示各独立危险因素对于NICU患者预后不良的风险评分,使用R软件中的rms包构建列线图(图5),并制作相应变量的赋分表(表4)。该模型预测总分为240分,机械通气为29分,房颤为27分,同理可根据入院时血钙水平及GCS评分得出相应分数,当总分为72~153分时,患者预后不良的概率为10%~90%。
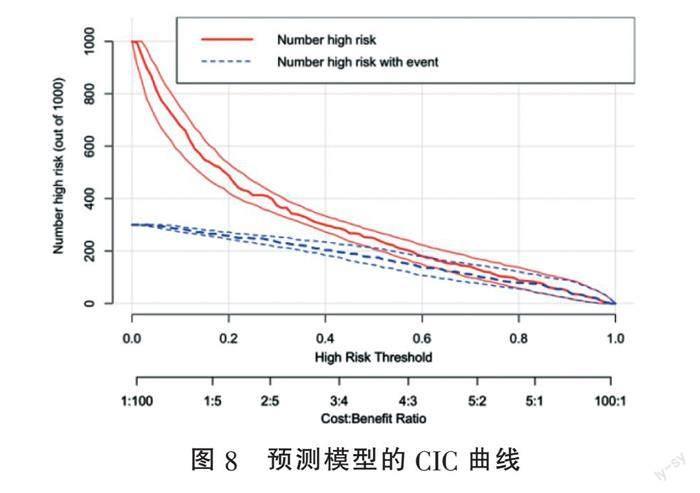
2.6 模型验证 为防止模型过度拟合,采用加强Bootstrap法重抽样1 000次的方式进行内部验证,C统计量(C-statistic)为0.8608,调整高估值,最终得出校准C统计量为0.8540,对于二元Logistic回归,C统计量等价于AUC,即校准C统计量=校准AUC=0.854,提示本模型区分度较好。采用校准曲线评估列线图校准度(图6),Hosmer-Lemeshow拟合优度检验差异无统计学意义(χ2=6.095,df=8,P=0.641),提示本模型的校准度较好。采用DCA曲线及CIC曲线评估模型的临床适用性,图7提示当阈概率(threshold probability)大于1%时,预测模型的净收益(net benefit)显著高于完全干预(All)及完全不干预(None)两种极端情况;图8直观显示各个阈概率下预测模型所划分的高风险人数与真实情况之间的比较,提示使用本模型有良好的临床适用性。
3 讨 论
神经危重症患者有严重神经功能障碍,多合并心、肺、肾等多器官功能障碍,年龄总体偏高,治疗周期长,并发症多、致残致死率高,往往预后不佳。卒中相关性肺炎是卒中后常见的严重并发症,部分患者需要使用呼吸支持设备。高龄、人工气道建立、APACHE Ⅱ高评分、合并慢性阻塞性肺疾病均可导致机械通气患者呼吸机相关肺炎(ventilator associated pneumonia,VAP)发生几率增高,易出现撤机困难,死亡率高[9-11]。房颤是缺血性脑卒中病因之一,新发的阵发性房颤较永久性房颤引起栓塞事件发生的风险更高。WETTERSLEV等[12]研究显示,连续心电图监测可以捕捉到许多ICU患者住院期间的新发房颤,新发房颤与不良预后密切相关。ZHANG等[13]对MIMIC II数据库分析显示,血钙水平与危重患者临床结局间呈U型曲线关系,轻中度血钙增高具有一定保护作用,而轻中度血钙降低与死亡风险的增加密切相关。GCS量表是床边评估昏迷患者脑损伤程度的常用方法,GCS评分与疾病严重程度及预后密切相关[14-17]。
本研究单因素分析和Logistic回归分析显示,机械通气、房颤、入院时血钙水平及GCS评分为影响患者短期预后的独立危险因素。ROC曲线分析显示,入院时GCS评分的AUC为0.787,大于机械通气、入院时血钙水平、合并房颤的AUC,差异均有统计学意义(P<0.001),表明入院时GCS评分预后预测价值更优。神经危重症患者除了出现意识和神经功能障碍,还合并多系统功能障碍,仅用GCS评分或其他单独因素不能全面评估患者的病情及器官受损情况。列线图(nomogram)是一种图形统计工具[18],通过Logistic回归或Cox回归明确独立危险因素,并将危险因素转化为连续的评分系统,从而计算出特定患者发生特定事件的精确风险概率,具有直观、便捷等特点,已广泛应用于癌症预后[18-19]、术后并发症[20-21]等领域。本研究将机械通气、房颤、入院时血钙水平以及GCS评分构建联合多个危险因素的列线图预测模型,联合预测因子模型的AUC为0.861,校准后为0.854,显著高于其他独立危险因素,差异均有统计学意义(P<0.001),表明该预测模型的预测效能显著优于各独立危险因素。ROC曲线、C统计量、DCA曲线及CIC曲线检验结果显示本模型具有良好的校准度、区分度及临床适用性,能定量而简明评价不同危险因素的相应风险,为神经危重症患者的预后判断和精准医疗提供依据。
本研究是单中心回顾性研究,可能受到研究者的主观影响,今后需要开展多中心前瞻性研究,并进行外部验证以优化列线图。
[参考文献]
[1] HUANG M,WANG J,NI X,et al. Neurocritical care in China:past,present,and future[J]. World Neurosurg,2016,95:502-506.
[2] CHHANGANI N P,AMANDEEP M,CHOUDHARY S,et al. Role of acute physiology and chronic health evaluation II scoring system in determining the severity and prognosis of critically ill patients in pediatric intensive care unit[J]. Indian J Crit Care Med,2015,19(8):462-465.
[3] KNAUS W A,DRAPER E A,WAGNER D P,et al. APACHE II: a severity of disease classification system[J]. Crit Care Med,1985,13(10):818-829.
[4] 中華医学会神经病学分会,中华医学会神经病学分会脑血管病学组. 中国急性缺血性脑卒中诊治指南2018[J]. 中华神经科杂志,2018,51(9):666-682.
[5] 中华医学会神经病学分会,中华医学会神经病学分会脑血管病学组. 中国脑出血诊治指南(2019)[J]. 中华神经科杂志,2019,52(12):994-1005.
[6] 中华医学会神经病学分会神经肌肉病学组,中华医学会神经病学分会肌电图及临床神经电生理学组,中华医学会神经病学分会神经免疫学组. 中国吉兰-巴雷综合征诊治指南[J]. 中华神经科杂志,2010,43(8):583-586.
[7] 中国免疫学会神经免疫分会,常婷,李柱一,等. 中国重症肌无力诊断和治疗指南(2020版)[J]. 中国神经免疫学和神经病学杂志,2021,28(1):1-12.
[8] COLLINS G S,REITSMA J B,ALTMAN D G,et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD):the TRIPOD Statement[J]. Br J Surg,2015,102(3):148-158.
[9] KUMAR S T,YASSIN A,BHOWMICK T,et al. Recommendations from the 2016 guidelines for the management of adults with hospital-acquired or ventilator-associated pneumonia[J]. P and T,2017,42(12):767-772.
[10] MIROLIAEE A E,SALAMZADEH J,SHOKOUHI S,et al. Effect of vitamin D supplementation on procalcitonin as prognostic biomarker in patients with ventilator associated pneumonia complicated with vitamin D deficiency[J]. Iran J Pharm Res,2017,16(3):1254-1263.
[11] KAO C C,CHIANG H T,CHEN C Y,et al. National bundle care program implementation to reduce ventilator-associated pneumonia in intensive care units in Taiwan[J]. J Microbiol Immunol Infect,2019,52(4):592-597.
[12] WETTERSLEV M,HAASE N,HASSAGER C,et al. New-onset atrial fibrillation in adult critically ill patients: a scoping review[J]. Intensive Care Med,2019,45(7):928-938.
[13] ZHANG Z,XU X,NI H,et al. Predictive value of ionized calcium in critically ill patients: an analysis of a large clinical database MIMIC II[J]. PLoS One,2014,9(4):e95204.
[14] TEASDALE G,JENNETT B. Assessment of Coma and impaired consciousness. A practical scale[J]. Lancet,1974,2(7872):81-84.
[15] TEASDALE G,MAAS A,LECKY F,et al. The Glasgow Coma Scale at 40 years: standing the test of time[J]. Lancet Neurol,2014,13(8):844-854.
[16] LI A,ATEM F D,VENKATACHALAM A M,et al. Admission Glasgow coma scale score as a predictor of outcome in patients without traumatic brain injury[J]. Am J Crit Care,2021,30(5):350-355.
[17] WIJDICKS E F,RABINSTEIN A A,BAMLET W R,et al. FOUR score and Glasgow Coma Scale in predicting outcome of comatose patients: a pooled analysis[J]. Neurology,2011,77(1):84-85.
[18] ZELIC R,GARMO H,ZUGNA D,et al. Predicting prostate cancer death with different pretreatment risk stratification tools: a head-to-head comparison in a nationwide cohort study[J]. Eur Urol,2020,77(2):180-188.
[19] BALACHANDRAN V P,GONEN M,SMITH J J,et al. Nomograms in oncology:more than meets the eye[J]. Lancet Oncol,2015,16(4):e173-e180.
[20] GROOT KOERKAMP B,WIGGERS J K,GONEN M,et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram[J]. Ann Oncol,2015,26(9):1930-1935.
[21] TANVETYANON T,FINLEY D J,FABIAN T,et al. Prognostic nomogram to predict survival after surgery for synchronous multiple lung cancers in multiple lobes[J]. J Thorac Oncol,2015,10(2):338-345.
[收稿日期] 2022-09-26
(本文編辑 赵喜)
