Simultaneous Determination of Six Components in Wanjinxiang Shushuang Ointment by QAMS
2023-10-31YanniMAOLinYANGXiaoyaoXIEJinyanJIAQingWUCunWU
Yanni MAO, Lin YANG, Xiaoyao XIE, Jinyan JIA, Qing WU, Cun WU
1. Key Laboratory of Mountain Environment Information System and Environment Protection, Guizhou Normal University, Guiyang 550001, China; 2. Guizhou Hongyu Pharmaceutical Co., Ltd., Guiyang 550001, China; 3. The Second Affiliated Hospital of Guizhou College of Traditional Chinese Medicine, Guiyang 550001, China
Abstract [Objectives] To regulate the quality of Wanjinxiang Shushuang Ointment through simultaneously quantifying menthol, camphor, 1, 8-cineole, linalool, borneol and caryophyllene oxide by QAMS (quantitative analysis of multi-components by single-marker). [Methods] The method was performed using an Agilent DB-WAX (30 m×0.32 mm, 0.25 μm) polyethylene glycol chromatographic column; with nitrogen employed as a carrier gas. The constant pressure was 4.73 psi; and the injection temperature was 240 ℃, with a shunt ratio:of 10:1; The hydrogen flame ion detector with a detector temperature of 240 ℃; the injection volume was 0.3 μL. To verify the accuracy and applicability of QAMS, the results were compared with those obtained using the internal standard method (naphthalene). [Results] In Wanjinxiang Shushuang Ointment, menthol, camphor, 1, 8-cineole, linalool, borneol, caryophyllene oxide and naphthalene were well separated by the same chromatography with good linearity in their respective ranges (R≥0.999 2). The average recoveries were 99.66%, 101.03%, 98.07%, 98.24%, 101.39%, and 103.39% with RSDs of 0.69%, 1.52%, 1.25%, 1.94%, 1.44%, and 2.74%, respectively. The QAMS is similar to the internal standard method. [Conclusions] This simple, accurate method with high precision, separation and reproducibility can serve as a reference for the quality control of Wanjinxiang Shushuang Ointment.
Key words Wanjinxiang Shushuang Ointment, Blumea balsamifera (L.) DC., Chemical component, Quantitative analysis of mult-components by single-marker(QAMS), Quality control
1 Introduction
The Wanjinxiang Shushuang Ointment containsBlumeabalsamifera(L.) DC., 1, 8-cineole, and menthol as its main components. This ointment has disinfectant and anti-inflammatory properties, which can help reduce swelling, pain, and prevent mosquito bites.B.balsamifera(L.) DC., also called Niuer wormwood and Dafeng wormwood, is a herbaceous perennial plant from the asteraceae family’s genus Blumea. It is a popular medicinal plant in ethnic minority areas of China, like Li, Miao, and Zhuang, as well as in Southeast Asian countries due to its effects of clearing heat, reducing itching, inhibiting inflammation, and providing antioxidant properties[4-6]. Several studies have demonstrated thatB.balsamifera(L.) DC. contains several active components like borneol, camphor, linalool, among others. These components promote osmosis, antimicrobial, and other essential pharmacological activities[7-12]. Additionally, research has indicated that the ointment’s menthol is present in topical pain-relieving, itch-reducing, antiseptic and cooling products[13-15], 1, 8-cineole exhibits insect repellent properties[16-17]. It is consistent with the primary therapeutic function of this formula and can serve as an indicator for quality control purposes.
Currently, there is a lack of comprehensive quality control standards for Wanjinxiang Shushuang Ointment, and only a limited number of studies have addressed its quality standards. Chinese medicine has a complex component, an unclear target point, and mechanism of action, making it challenging to fully evaluate its quality based solely on the analysis of a single component’s content and it is only through the joint control of multiple index components to ensure the quality control of the required herbs that the quality of the medicines can be more comprehensively and fully controlled[18-19]. In this study, we simultaneously determined the content of six active components using QAMS, a simple, reliable, and accurate method that provides scientific reference for the quality control of Wanjinxiang Shushuang Ointment.
2 Instruments and materials
Agilent 7890, Agilent 6890 Gas Chromatographs, DB-WAX, HP-INNOWax, DB-1701 polyethylene glycol chromatographic column, produced by Agilent USA;XS105DU electronic analytical balance produced by Mettler Toledo, Switzerland.
1, 8-cineole, caryophyllene oxide, camphor, linalool reference substance(purity: ≥98%; batch No.: wkq22011706, wkq21092408, wkq21112403, wkq21052711), purchased from Sichuan Weikeqi Biological Technology CO., LTD; menthol, borneol (purity: ≥98%; batch No.: 110728-201707, 110881-200706), purchased from Sinopharm Chemical Reagent Co.Ltd.; naphthalene (purity: ≥99.7%; batch No.: 20080515), purchased from Tianjin Zhiyuan Chemical Reagent Co.; methanol(analytically pure, batch No.: 20210906)purchased from Tianjin Fuyu Fine Chemical Co., Ltd.; Wanjinxiang Shushuang Ointment (batch No.: 20210219, 20210415, 20210620) from Guizhou Hongyu Pharmaceutical Co., Ltd.
3 Methods
3.1 Gas chromatographic conditionsColumn: Agilent DB-WAX (30 m×0.32 mm, 0.25 μm); carrier gas: high-purity nitrogen (purity≥99.999%); Injection temperature: 240 ℃; shunt ratio: 10:1; hydrogen flame ion detector: 240 ℃; the injection volume: 0.3 μL. Initial temperature: 40 ℃ (retention for 2 min), rising to 230 ℃ at 10 ℃/min (retention for 10 min).
3.2 Solution preparation
3.2.1Preparation of internal standard solution. Precise quantities of naphthalene were placed in a 25 mL volumetric flask, which was then diluted and adjusted with methanol to be scaled up and prepared into an internal standard solution with a concentration of 0.446 0 mg/mL.
3.2.2Preparation of tested sample solution. A weight of 2.0 g of Wanjinxiang Shushuang Ointment was carefully measured and placed into a 10 mL volumetric flask. Then, 0.5 mL of internal standard solution was added, the mixture was diluted, and fixed with methanol to the necessary scale.
3.2.3Preparation of deficiency sample solution. According to the prescription and production process of Wanjinxiang Shushuang Ointment make 1, 8-cineole deficiency sample, menthol deficiency sample andBlumeabalsamifera(L.) DC. deficiency sample.
3.2.4Preparation of reference solution. Precise amounts of menthol, camphor, 1, 8-cineole, linalool, borneol and caryophyllene oxide were accurately measured in methanol to create a mixture of reference solution containing 4.650 mg/mL menthol, 0.220 mg/mL camphor, 1.690 mg/mL cineole, 0.270 mg/mL linalool, 0.800 mg/mL borneol, and 0.690 mg/mL caryophyllene oxide.
3.3 Methodology examination
3.3.1Exclusivity Test. In accordance with Section2.2, separately tested the tested sample solution, deficiency sample solution and reference solution. The peaks of menthol, camphor, 1, 8-cineole, linalool, borneol and caryophyllene oxide and naphthalene were separated well, and the peak positions of the tested sample substances and the reference substance have the same retention time, and the peak positions of the components to be tested show no corresponding peaks in the deficiency sample solution. The results are illustrated in Fig.1.
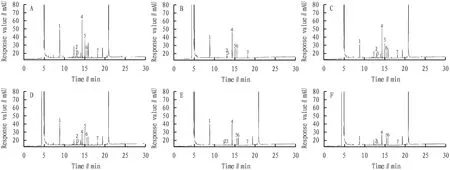
Note: A. sample solution; B. mixed standard solution; C.1, 8-cineole deficiency sample; D. menthol deficiency sample; E. Blumea balsamifera (L.) DC. deficiency sample; F. cineole, menthol, Blumea balsamifera (L.) DC. deficiency sample; 1. 1, 8-cineole; 2. camphor; 3. linalool; 4. menthol; 5. borneol; 6. naphthalene; 7. caryophyllene oxide.
3.3.2Linear relationship examination. Precisely measured 0.30, 0.40, 0.50, 1.00, and 2.00 mL of mixed reference solution in a 5 mL volumetric flask. Then, added 0.5 mL of the internal standard solution of naphthalene and dilute with a solution of methanol to reach the mark. Five different concentrations of the standard mixed solution were derived in this manner. A standard curve was plotted with calculated by the concentration of the reference substance and the ratio of the peak area of each component to the peak area of the internal standard as the abscissa and ordinate coordinates, the results showed that there was a strong linear correlation among the contents of 6 components. The results are shown in Table 1.
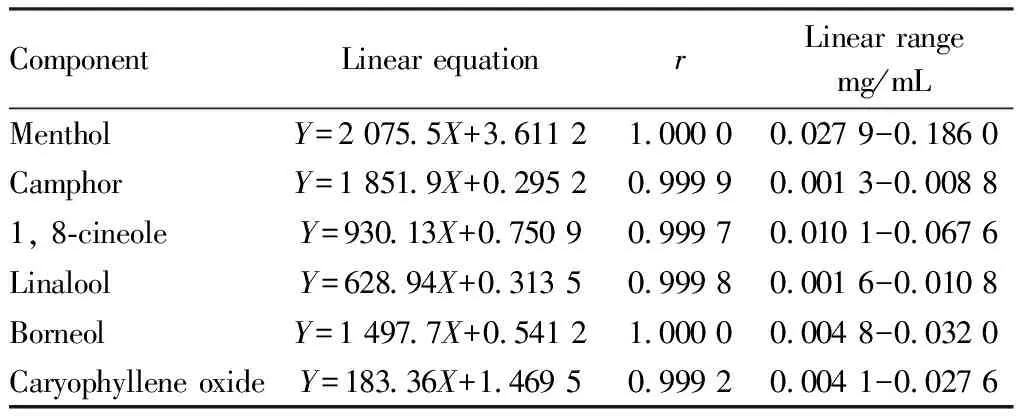
Table 1 Linear relationship of various constituents
3.3.3Precision test. The working mixed reference solution injected for six consecutive times under chromatographic conditions, the peak area of each component was recorded and theirRSDs were calculated. TheRSDof menthol, camphor, 1, 8-cineole, linalool, borneol and caryophyllene oxide was 0.94%, 1.75%, 2.25%, 1.65%, 1.93% and 1.76%, respectively, which indicated that the developed method had a good precision.
3.3.4Repeatability test. Stability was investigated by analyzing the tested sample solution at 0, 3, 6, 12, and 24 h at room temperature and recording peak area of each component. The results in the showed that theRSDvalue of peak area of menthol, camphor, 1, 8-cineole, linalool, borneol and caryophyllene oxide was 1.69%, 2.87%, 2.51%, 1.87%, 2.39% and 1.06%, respectiviesly, indicating that the tested sample solutions were stable within 24 h.
3.3.5Sample recovery test. Accurately weighed 2.0 g of the Wanjinxiang Shushuang Ointment(batch No.: 20210219), 9 portions, added the mixed reference solution according to 80%, 100%, 120% of each component, diluted with methanol to the scale line, and inject the sample for detection. The average recoveries of 6 samples are shown in Table 2. The results show that the method is accurate.
4 Results and analysis
4.2 Robustness of RCFsIn order to investigate the robustness of RCFs in different chromatographic conditions, Agilent 7890, Agilent 6890 of gas chromatographs and DB-WAX (30 m×0.32 mm, 0.25 μm), HP-INNOWax (30 m×0.32 mm, 0.25 μm), DB-1701 column (30 m×0.25 mm, 0.25 μm) three different models of chromatographic columns were examined, the mixed standard solution was injected and analyzed. The results showed that the RCFs of each component were not significantly different under different types of instruments and columns. The results are shown in Table 4.
4.3 Relative retention timeCalculation of the content of each component through the relative correction factor needs to confirm the location of the peaks of menthol, camphor, linalool, borneol and caryophyllene oxide to achieve the purpose of one measurement and multiple evaluations. In this study, the relative retention time method was used to calculate the relative retention time of menthol, camphor, linalool, borneol and caryophyllene oxide through the retention time of 1, 8-cineole, the mixed standard solution was injected and analyzed. (ΔtRs/R1, 8-cineole=tRs-tR1, 8-cineole,tRsis the retention time of the sample component,tR1, 8-cineoleisthe retention time of internal reference standard. )The results are shown in Table 5 and none of them exhibit a significant difference.
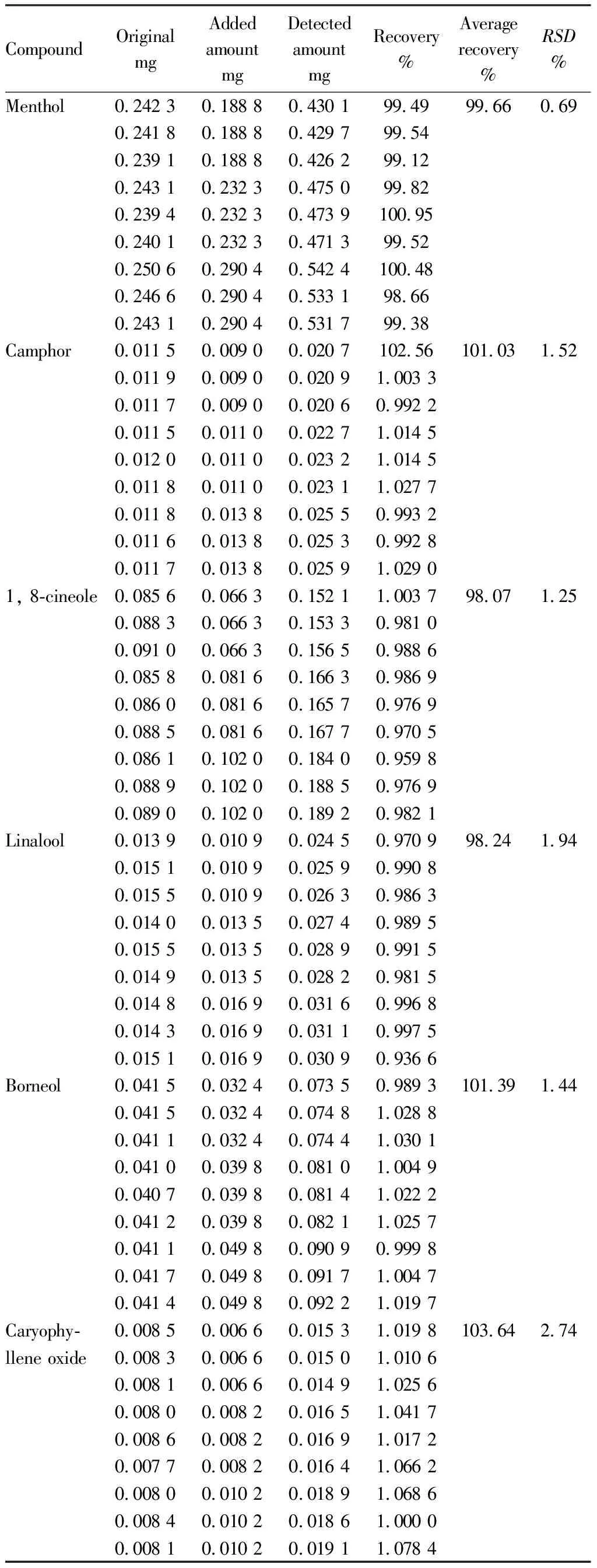
Table 2 Determination results of recovery test
4.4 Determination of sample contentPrecise weighing of a sample of Wanjinxiang Shushuang Ointment (batch No.: 20210219, 20210415, 20210620). Three batches of samples were prepared as tested sample solution, and three portions of each batch were prepared in parallel. To evaluate the feasibility of the QAMS method, the contents of menthol, camphor, 1, 8-cineole, linalool, borneol and caryophyllene oxide were determined and calculated by QAMS method and internal standard method respectively, and the results of the above two kinds of results were analyzed byt-test[15]. The results demonstrate that there is no significant statistical difference between the internal standard method and the QAMS method. Hence, it proves that QAMS is a feasible option for quality controlling the active components in Wanjinxiang Shushuang Ointment. The results are shown in Table 6.
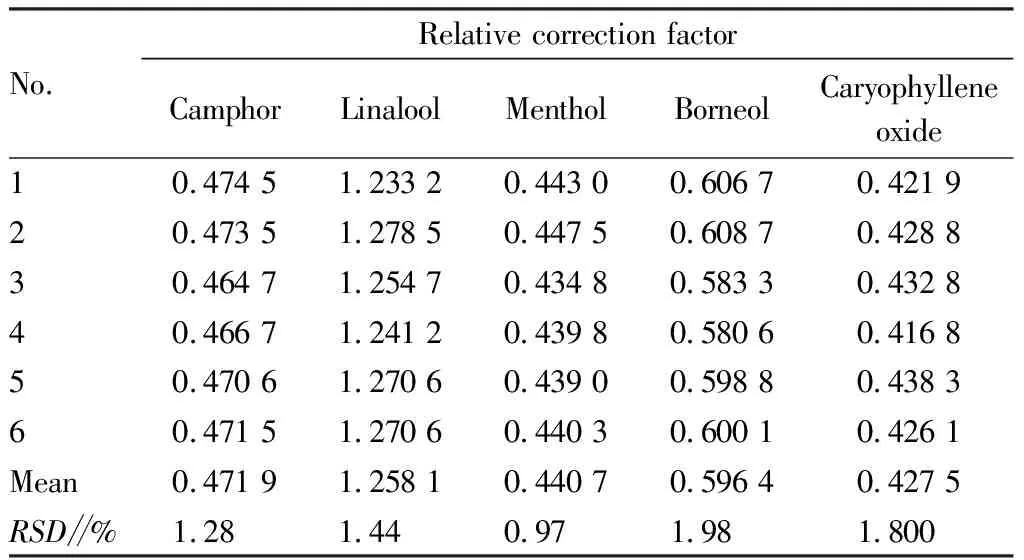
Table 3 Relative correction factors for various constituents

Table 6 Sample content determination results (n=3)
5 Discussion
5.1 Selection of internal standardsChinese medicines have complex components. In this study, the internal standard method can be utilized to eliminate errors due to changes in operating conditions. This makes the measurement and calculation of the relative peak areas of the internal standard and the components to be measured more precise. To reduce experimental error, it is advisable to choose an internal standard with a peak position that is either close to the measured component’s peak position or falls between the multiple chromatographic peaks of the measured component. This experiment evaluated diethyl malonate, m-xylene, n-propanol, n-butanol, and naphthalene as internal standards. The use of naphthalene as an internal standard revealed that it was positioned between borneol and caryophyllene oxide peaks, the duration of its peak was more optimal. Subsequently, the components intended to be measured could be accurately differentiated. This approach could effectively prevent the occurrence of systematic errors.
5.2 Chromatographic conditionsIt was found that under constant temperature, the separation effect was poor due to the long interval between the peaks of the active components and the inability of the separation degree to meet the standard, and the separation effect was poor. Compared with the constant temperature determination, the temperature programmed can accelerate the peak time of high boiling point substances, reduce the diffusion and shorten the time, so this study adopts the temperature programmed method. The effect on the chromatographic peak separation was also investigated from several aspects, such as: injection volume, shunt ratio, initial temperature, temperature increase rate,etc.After experimental verification, the initial temperature was determined to be 40 ℃, the shunt ratio was 10:1, and the injection volume was 0.3 μL. Examination of three different models of columns, including DB-WAX, HP-INNOWax and DB-1701, showed that the DB-WAX column performed better than the other models in terms of the response value, the peak shape and the degree of separation. The DB-WAX column was found to be better than the other columns in terms of response value, peak shape and separation, which was more suitable for the analysis of the active components of Wanjinxiang Shushuang Ointment.
5.3 Selection internal reference standardThe initial study reveals that the 1, 8-cineole extract content in the samples was high and low-cost. It also had a short peak time, and there were fewer interfering peaks in the vicinity of 1, 8-cineole. As an internal reference standard adhering to the QAMS choice principle, it is stable, inexpensive and readily available.
6 Conclusions
Focusing on Wanjinxiang Shushuang Ointment, we established a reliable and efficient QAMS method to simultaneously determine the concentration levels of menthol, camphor, cineole, linalool, borneol, and caryophyllene oxide. Through a comparison of the QAMS and internal standard methods, the results indicated no significant differences, which is consistent with the modernized quality control of traditional Chinese medicine. This suggests that the QAMS method is a feasible option for the quality control of Wanjinxiang Shushuang Ointment and provides insight into the comprehensive quality control of this preparation.
杂志排行
Medicinal Plant的其它文章
- Observation on Therapeutic Effect of Erxian Decoction on Relieving Low Back Pain after PVP of PMOP-derived Vertebral Fracture
- Experimental Study on Anatomic Reduction of Lateral Pterygoid Muscle (Simulated Manipulation Fracture Reduction) and Condylar Free Reduction for Condylar Fracture
- Protective Effect and Molecular Mechanism of Shui People’s Classic Prescription Jipei Dilong Ointment on Osteoarthritis in Rats
- Effects of Cigu Xiaozhi Formula on miR-378a-3p Expression and Hh Signaling Pathway in TGF-β1 Induced LX2 Cells
- Chlorophyll Fluorescence Response of Persimmon Plants under Salt Stress
- Optimization of Solid-state Fermentation Conditions of Sophora japonica cv. jinhuai by Response Surface Methodology
