Effects of Cigu Xiaozhi Formula on miR-378a-3p Expression and Hh Signaling Pathway in TGF-β1 Induced LX2 Cells
2023-10-31AidiWANGYanhuaMALiWANGXiupingZHAO
Aidi WANG, Yanhua MA, Li WANG, Xiuping ZHAO
The First Clinical Medical College, Gansu University of Chinese Medicine, Lanzhou 730000, China
Abstract [Objectives] To observe the effects of Cigu Xiaozhi Formula on miR-378a-3p expression and Hh signaling pathway in TGF-β1 induced and activated LX2 cells. [Methods] Cells were divided into control group, induction group, drug-containing serum group, miR-378a-3p inhibitor group, and miR inhibitor NC group. CCK-8 method was used to detect the cell viability of each group, and flow cytometry was used to detect the apoptosis rate of each group. RT-qPCR was used to detect the expression of miR-378a-3p in each group’s cells, and RT-qPCR and Western blot were used to detect mRNA and protein expression of Shh, Gli1, Gli2, Col-I, and α-SMA in each group’s cells. [Results] Compared with the control group, the cell viability and expression of Shh, Gli1, Gli2, Col-I, and α-SMA mRNA and protein in induction group increased (P<0.01), while the expression of miR-378a-3p decreased (P<0.01). Compared with the induction group, the cell viability and expression of Shh, Gli1, Gli2, Col-I, α-SMA mRNA and α-SMA and Gli2 protein decreased in drug-containing serum group (P<0.05), while cell apoptosis rate and miR-378a-3p expression increased (P<0.01). In miR-378a-3p inhibitor group, cell viability and the expression of Shh, Gli1, Gli2, Col-I, α-SMA mRNA and Gli1, Gli2, α-SMA protein increased (P<0.05, P<0.01), while the apoptosis rate and miR-378a-3p expression decreased (P<0.05, P<0.01). [Conclusions] Cigu Xiaozhi Formula containing serum can upregulate miR-378a-3p expression and downregulate the expression of Gli2 and α-SMA in TGF-β1 induced LX2 cells, thereby inhibiting the activation of LX2 cells and exerting the effects of anti liver fibrosis.
Key words Cigu Xiaozhi Formula, LX2 cells, TGF-β1, miR-378a-3p, Hh signaling pathway
1 Introduction
The mechanism of liver fibrosis is relatively complex, with the core link being abnormal activation of hepatic stellate cell (HSC). When the liver is damaged, the liver tissue undergoes excessive repair or loss of control. Under the action of inflammation and cytokines, HSC in a stationary state is activated and transformed into myofibroblast expressing α-smooth muscle actin (α-SMA) label, which synthesizes and secretes various extracellular matrix (ECM). It leads to abnormal proliferation and excessive deposition of extracellular matrix in liver tissue, thereby resulting in liver fibrosis[1-2]. Among them, collagen type I (Col-I) is the earliest ECM component formed during the fibrosis process and one of the main components. Many studies have shown that microRNA (miRNA) participates in the occurrence of liver fibrosis and the activation of HSC by targeting multiple signaling pathways[3-7]. In the early stage, the research team analyzed and screened out the differential expressionmiR-378a-3prelated to the Hh signal pathway through microRNAs second generation sequencing, bioinformatics and computer simulation technology, and showed that the serum containing Cigu Xiaozhi Formula could upregulate the expression ofmiR-378a-3pin transforming growth factor β1 (TGF-β1) induced and activated human hepatic stellate cells (LX2). Based on the above research, by adopting TGF-β1 to stimulate and activate LX2 cells, a hepatocellular fibrosis model was established[8-12], and the Cigu Xiaozhi Formula containing serum was used to intervene. At the same time, amiR-378a-3pinhibitor group was established as a control to observe the effect of Cigu Xiaozhi Formula on the expression ofmiR-378a-3pand Hh signaling pathway related protein in TGF-β induced and activated LX2 cells, in order to further explore the molecular mechanism of Cigu Xiaozhi Formula in anti liver fibrosis and provide a theoretical basis for the prevention and treatment of liver fibrosis.
2 Materials and methods
2.1 Materials
2.1.1Cells and animals. Human liver stellate LX2 cells (337957) was provided by Beijing Beina Chuanglian Biotechnology Research Institute. 80 healthy SPF-level KM mice, male, with a body weight of about 25 g, were purchased from Chongqing Ensiweier Biotechnology Co., Ltd. Production license number of experimental animals: SCXK(Xiang)2019-0004. Mice were raised in an environment with a temperature of 23-25 ℃ and alternating day and night for 12 h/12 h, and were free to drink and eat. The experiment began after 2 weeks of adaptive feeding. The animal experiment was approved by the Experimental Animal Ethics Committee of Gansu University of Chinese Medicine (ethics number: 2018-030).
2.1.2Drugs. Cigu Xiaozhi Formula consisted of 10 g of Cremastrae Pseudobulbus, 15 g of Rhizoma Pinelliae, 20 g of Poria, 12 g of Radix Bupleuri, 20 g of Salviae Miltiorrhizae, 20 g of Eupolyphaga, 30 g of Coicis Semen, 10 g of Scutellariae Radix, 15 g of Oriental Waterplantain Rhizome, 20 g of Lycii Fructus, 20 g of raw Crataegi Fructus, 30 g of raw Polygoni Multiflori Radix, 20 g of Cassia Seed, and 10 g of Radix Glycyrrhizae Preparata. The above medicinal materials were provided by the Preparation Center of the Affiliated Hospital of Gansu University of Chinese Medicine, and were identified as authentic by Chief Pharmacist Wang Xiaoli from the Preparation Center of the Affiliated Hospital of Gansu University of Traditional Chinese Medicine to authenticate. The medicinal solution was prepared by decoction method with a mass concentration of 0.225 g/mL.
2.1.3Reagents. RNA extraction kit (lot No.:AK70880A, Japan TaKaRa Company); CCK-8 test kit, HRP labeled goat anti rabbit IgG, HRP labeled goat anti mouse IgG (lot No.:C0093, A0201, A0216, Shanghai Biyuntian Biotechnology Co., Ltd.); TGF-β1 (lot No.:1218209, America PeproTech Company); apoptosis kit FITC-PI (lot No.:3KJEJNLKAY, Wuhan Elabscience Biotechnology Co., Ltd.); Universal Blue Qpcr SYBR Green Master Mix (lot No.:H1125090, Yeasen Biotechnology (Shanghai) Co., Ltd.); Lipofectamine2000 (lot No.:2125329, America Invitrogen Company); Shh, Gli2, Col-I primary antibody (lot No.:79d6337, 47z1318, 26u3150, America Affinity Company); Gli1, α-SMA, β-actin primary antibody (lot No.:HN0921, HN0810, R1207-1, Hangzhou Hua’an Biotechnology Co., Ltd.)
2.1.4Instrument. ECLIPSE Ti type of invert microscope (Japan Nikon Company); real-time fluorescence quantitative PCR instrument (America ABI Company); gel imager stabilized voltage DNA electrophoresis apparatus, Tanon-1600 gel imaging system (Shanghai Tanon Technology Co., Ltd.); Varioskan LUX type of multifunctional fluorescent enzyme labeling instrument (America Thermo Fisher Company); vertical plate electrophoresis transfer device, Trans-Blot membrane transfer device, and electrophoresis apparatus (America Bio-Rad Company).
2.2 Methods
2.2.1Cell culture. LX2 cells were cultured in a DMEM medium containing 10% high-quality fetal bovine serum and 1% penicillin-streptomycin double antibody at 37 ℃, 5% CO2, saturated humidity incubator, and the liquid was changed once for 2 to 3 d. When the culture bottle was adhered to the wall and fused to 70% to 80%, it was digested and centrifuged. In a ratio of 1:3, it was passed and cultured.
2.2.2Preparation of Cigu Xiaozhi Formula containing serum. The mice were randomly divided into control group and Cigu Xiaozhi Formula group. The Cigu Xiaozhi Formula group was given 20 mg/kg of medication by gavage, while the control group was given an equal amount of physiological saline by gavage, administered once a day in the morning and evening, for a continuous week. One hour after the last gavage administration, blood was collected from the eyeball and left to stand at 4 ℃ for 2 h. The serum was separated by centrifugation at a low temperature of 3 500 r/min for 15 min. The same group of serum was mixed and inactivated at a constant temperature of 56 ℃ for 30 min. After filtered and sterilized through 0.22 μm of microporous filter membrane, it was stored in a refrigerator at -20 ℃ for later use.
2.2.3Chemical synthesis and cell transfection ofmiR-378a-3pinhibitor. Cells were inoculated onto a 6-hole plate and transfected when the fusion degree reached 50%-60%. The prepared Lipofectamine 2000 diluent and siRNA diluent were mixed gently and incubated at room temperature for 20 min. Then the above transfection complex was added into a 6-hole plate. After incubated in an incubator at 37 ℃ for 6 h, the fresh and complete culture medium was used to change.
2.2.4Grouping and administration. LX2 cells in logarithmic growth phase were taken. After adjusting the density to 1×105cells/mL, it was inoculated in a 6-hole plate. After cultured until the cells adhered to the wall, they were divided into control group, induction group, drug-containing serum group, miR-378a-3p inhibitor group, and miR inhibitor NC group, with 3 holes in each group. Except for the control group, all other groups discarded the complete culture medium, and 5 ng/mL of TGF-β1 was added to treat the cells, and it continued to culture in the incubator for 24 h. The complete culture medium was discarded from the drug-containing serum group, and the serum culture medium containing 6% Cigu Xiaozhi Formula (choosing the intervention dose based on the pre experimental results) was added. After transfected cells for 6 h, the complete culture medium was replaced in the miR-378a-3p inhibitor group and miR inhibitor NC group, and it continued to be cultured in the incubator for 72 h.
2.2.5RT-qPCR method for detecting the expression ofmiR-378a-3pin each group of cells. Each group of cells were collected, and the total RNA of each group of LX2 cells was extracted according to the steps described in the total RNA extraction kit, and the quality and concentration of RNA were determined by an ultramicro spectrophotometer. Referring to the instructions of the reverse transcription kit, the total RNA was reversely transcribed into cDNA, and then fluorescence quantitative PCR detection was conducted. The reaction conditions were preheating at 95 ℃ for 2 min; 95 ℃, 10 s, 60 ℃, 30 s, and 40 cycles. Forward sequence ofmiR-378a-3p: GCGGCACTGGACTTGGAGTCA, reverse sequence: CCTGTTGTCTCCAGCCACAAAAGAGGCACAATATTTC AGGAGACAACAGGGCCTTC; forward sequence of U6: CTCG CTTCGGCAGCACA, reverse sequence: AACGCTTCACGAATTTGCGT. TakingU6 as internal reference, 2-ΔΔCTmethod was used to calculate relative expression amount ofmiR-378a-3p.
2.2.6CCK-8 method for detecting cell viability in each group. After the intervention treatment of each group of cells, 10 μL of CCK-8 solution was added in each hole to incubate for 3 h in an incubator. The absorbance value at 450 nm was measured using a microplate reader, and cell viability was calculated.
2.2.7AnexinV/PI staining for detecting the apoptosis rate of cells in each group. Cells were collected from each group, resuspended with PBS and counted. Taking approximately 1×105resuspended cells in a 1.5 mL of EP tube. After centrifuged at 1 000 r/min for 5 m, the supernatant was discarded, and 195 μL of Annexin V-FITC binding solution was added to gently resuspend the cells. Then 5 μL of Annexin V-FITC was added to gently mix, and it was incubated at room temperature in dark for 10 min. After centrifuged at 1 000 r/min for 5 min, the supernatant was discarded, and 190 μL of Annexin V-FITC binding solution was added to gently resuspend cells. Then 10 μL of propidium iodide staining solution was added for ice bath away from light. Immediately, flow cytometry was performed, with Annexin V-FITC showing green fluorescence and PI showing red fluorescence.
2.2.8RT-qPCR method for detecting the expression ofShh,Gli1,Gli2,Col-I, andα-SMAmRNA in cells. Using TRIzol method to extract total RNA from cells, amplification reaction was carried out using the method of Section2.2.5. UsingGAPDHas the internal reference, relative expression level ofShh,Gli1,Gli2,Col-I, andα-SMAmRNA was calculated. The primer sequence was shown in Table 1.
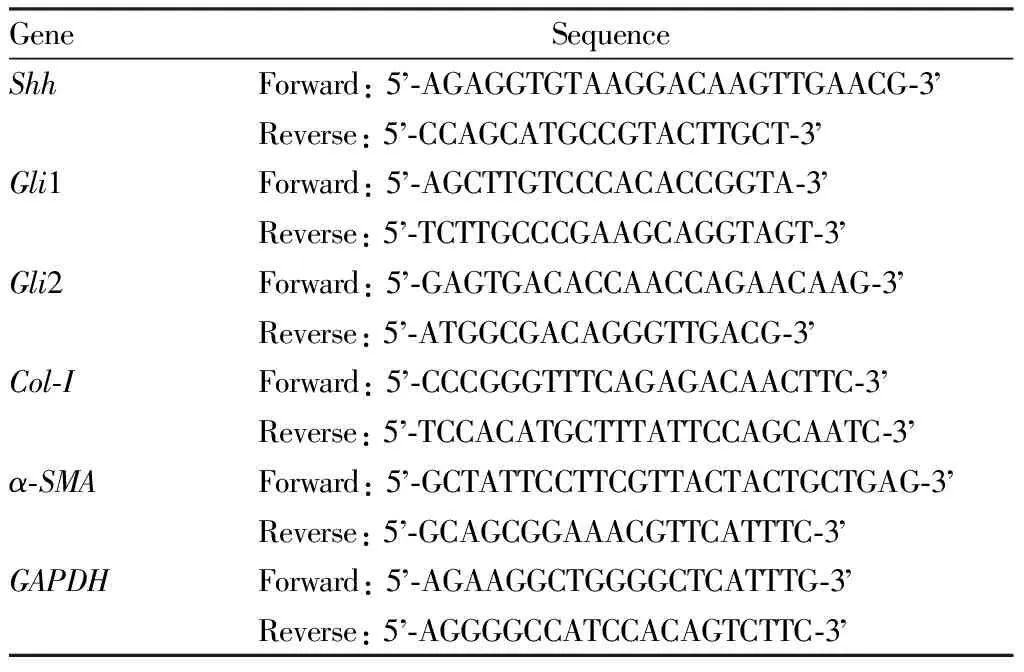
Table 1 Primer sequence
2.2.9Western blot method for detecting the expression of Shh, Gli1, Gli2, Col-I, and α-SMA protein in cells. Each group of cells were collected, and 100 μL of protein lysis solution was added to lyse cells. After centrifuged at 4 ℃ and 12 000 r/min for 15 min, the supernatant was taken. The protein concentration was measured by BCA method. The protein loading buffer was added, and it was boiled in boiling water for 10 min after mixing evenly to denature the protein. Each group took the same amount of protein to load sample, and gel electrophoresis was used to separate. It was transferred to PVDF membrane and sealed with 5% skim milk sealing solution at room temperature for 1 h. It was incubated at 4 ℃ with primary antibody (1:1 000) overnight, and TBST was used to wash the membrane. It was incubated at room temperature with secondary antibody (1:1 000) for 1 h, and TBST was used to wash the membrane. After added ECL luminescent solution, it was exposed to the exposure meter, and image analysis software was used to measure the band gray value. Taking β-actin as the internal parameter, the relative expression level of the target protein was calculated.
3 Results and analysis
3.1 MorphologyLX2 cells were cultivated normally and liked fibroblasts observed under the microscope at low density. At high density, the cells were tightly arranged and were epithelioid cells (Fig.1). After cell transfection, fluorescence reactions can be observed under a fluorescence microscope, indicating successful cell transfection (Fig.2).
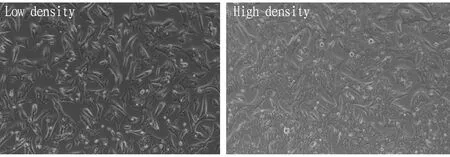
Fig.1 Normal LX2 cells (×100)
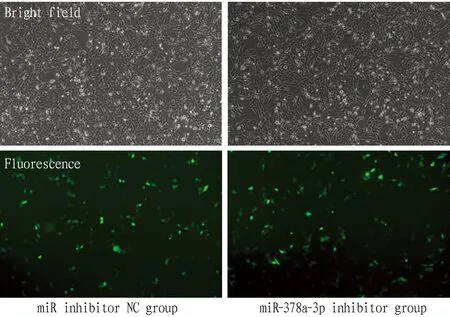
Fig.2 LX2 cells after transfection (×100)
3.2 Effect of Cigu Xiaozhi Formula containing serum on the expression ofmiR-378a-3pin LX2 cellsCompared with control group, the expression ofmiR-378a-3pin induction group reduced (P<0.01). Compared with induction group, the expression ofmiR-378a-3pin drug-containing serum group increased (P<0.01), while the expression ofmiR-378a-3pin miR-378a-3p inhibitor group reduced (P<0.01), and the expression ofmiR-378a-3pin miR inhibitor NC group changed slightly (P>0.05, Table 2). The above experimental results not only confirmed the successful transfection of cell plasmids, but also confirmed that 6% Cigu Xiaozhi Formula containing serum can upregulate the expression ofmiR-378a-3p.
3.4 Effect of Cigu Xiaozhi Formula containing serum on LX2 cell apoptosisCompared with control group, there was no significant change in apoptosis rate in the induction group (P>0.05). Compared with induction group, cell apoptosis rate in the drug-containing serum group increased (P<0.01), while cell apoptosis rate in miR-378a-3p inhibitor group declined (P<0.05), and cell apoptosis rate in miR inhibitor NC group changed slightly (P>0. 05, Table 2, Fig.3).

Fig.3 Flow cytometry of cell apoptosis in each group
3.3 Effect of Cigu Xiaozhi Formula containing serum on the viability of LX2 cellsCompared with the control group, the induction group showed an increase in cell viability (P<0.01). Compared with induction group, cell viability in the drug-containing serum group decreased (P<0.05), while cell viability in miR-378a-3p inhibitor group rose (P<0.01), and cell viability in miR inhibitor NC group changed slightly (P>0.05, Table 2).
3.5 Effect of Cigu Xiaozhi Formula containing serum on the expression ofShh,Gli1,Gli2,Col-I, andα-SMAmRNA in LX2 cellsCompared with control group, the expression ofShh,Gli1,Gli2,Col-I, andα-SMAmRNA in induction group rose (P<0.01). Compared with induction group, the expression ofShh,Gli1,Gli2,Col-I, andα-SMAmRNA in drug-containing serum group declined (P<0.05), while the expression ofShh,Gli1,Gli2,Col-I, andα-SMAmRNA in miR-378a-3p inhibitor group rose (P<0.05), and the expression ofShh,Gli1,Gli2,Col-I, andα-SMAmRNA in miR inhibitor NC changed slightly (P>0.05, Table 3).
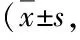
Table 2 miR-378a-3p expression, vitality, and apoptosis rate of cells in each group n=3)
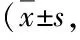
Table 3 Expression of Shh, Gli1, Gli2, Col-I, and α-SMA mRNA in cells of each group n=3)
3.6 Effect of Cigu Xiaozhi Formula containing serum on the expression of Shh, Gli1, Gli2, Col-I, and α-SMA protein in LX2 cellsCompared with control group, the expression of Shh, Gli1, Gli2, Col-I, and α-SMA protein in induction group rose (P<0.01). Compared with induction group, the expression of α-SMA and Gli2 protein in drug-containing serum group declined (P<0.05), and the expression of Shh, Col-I, and Gli1 protein had no obvious change (P>0.05), and the expression of Gli1, Gli2, and α-SMA protein in miR-378a-3p inhibitor group rose (P<0.05). In miR inhibitor NC group, the expression of Shh, Gli1, Gli2, Col-I, and α-SMA protein had no obvious change (P>0.05, Table 4, Fig.4).
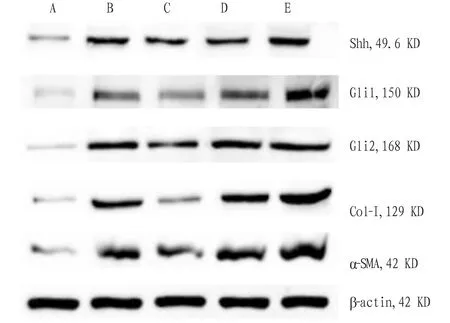
Note: A. Control group; B. Induction group; C. Drug-containing serum group; D. miR inhibitor NC group; E. miR-378a-3p inhibitor group.
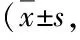
Table 4 Expression of Shh, Gli1, Gli2, Col-I, and α-SMA protein in cells of each group n=3)
4 Discussion
Hh signaling pathway plays a crucial role in regulating liver growth and repair responses. Numerous studies have shown that the Hh signaling pathway is closely related to HSC activation, and its expression is generally upregulated in liver fibrosis, which is one of the key determinants of liver fibrosis development[13]. It is worth noting that the instantaneous activation of the Hh signaling pathway is necessary for normal liver regeneration, but if liver cell damage persists, the Hh signaling pathway will be excessively activated, thereby promoting HSC activation and liver fibrosis[14]. The research results show that the excessive activation of the Hh signaling pathway is closely related to various diseases, including liver fibrosis[15-17].
miRNA typically drives liver fibrosis by targeting various substrates involved in molecular processes related to activation of hepatic stellate cells (HSCs), immune cell sensitization, and liver cell apoptosis[7]. Hyunetal.[18]found that the expression ofmiR-378 family, includingmiR-378a-3p,miR-378b, andmiR-378d, were downregulated in liver tissue of CCl4induced liver fibrosis rats, while the expression of Gli2 and Gli3 was upregulated.Invitroexperiments have found thatmiR-378a-3pled to the inactivation of HSCs by reducing the expression of Gli2 and Gli3 in HSCs. In this experiment, the miR-378a-3p inhibitor group was designed as a control, and plasmid transfection led to low expression ofmiR-378a-3pin TGF-β1 induced and activated LX2 cells. The results showed that LX2 cells had increased vitality and significantly reduced apoptosis rate. The marker protein for LX2 cell activation, α-SMA, its expression was upregulated, and the expression of key proteins Gli1 and Gli2 in the Hh signaling pathway was upregulated, suggesting that down regulation ofmiR-378a-3pexpression promoted the activation of LX2 cells induced by TGF-β1, and it was mainly achieved by up regulating the expression of Gli1 and Gli2.
Cigu Xiaozhi Formula is a self-designed prescription by the research group, and it has the effects of detoxifying, resolving phlegm, and reducing turbid fat. This prescription has been clinically applied for many years and has achieved good therapeutic effects in treating fatty liver disease, liver fibrosis, hyperlipidemia, and obese patients. Previous research of the research team showed that this prescription has anti-inflammatory, anti fibrosis, regulation of ceramide and other effects, and can reduce the degree of steatosis in the liver tissue of rats with nonalcoholic fatty liver disease[19-21]. Based on the previous research results, the Cigu Xiaozhi Formula containing serum was used to intervene in TGF-β1 induced and activated LX2 cells in this study. By combining "dry experiment" with "wet experiment", the research results showed that Cigu Xiaozhi Formula containing serum can upregulate the expression ofmiR-378a-3pin TGF-β1 induced and activated LX2 cells, reduce cell viability, and promote cell apoptosis. Meanwhile, Cigu Xiaozhi Formula containing serum can downregulate the expression of Gli2 and α-SMA protein in cells, suggesting that it may inhibit the Hh signaling pathway by up regulatingmiR-378a-3pexpression, thereby reducing the activity of LX2 cells and exerting an anti liver fibrosis effect.
In summary, Cigu Xiaozhi Formula containing serum can upregulate the expression ofmiR-378a-3pin TGF-β1 induced and activated LX2 cells and downregulate the expression of Gli2 and α-SMA, thereby inhibiting the activation of LX2 cells and exerting anti liver fibrosis effects. The study results could provide a theoretical basis for the prevention and treatment of liver fibrosis with traditional Chinese medicine. It also provides new ideas and directions for the treatment of liver fibrosis by studying the mechanism of its occurrence from the perspective of miRNA.
杂志排行
Medicinal Plant的其它文章
- Quality Control of Zhuang Medicine Xiaoyan Zhiyang Lotion
- Research Progress and Ideas on the Anti-liver Fibrosis Effect of Ethnic Medicine Plumbagin Based on microRNAs/TLR4/NF-κB and NLRP3 Inflammasome Activation
- Gastroprotective Effect of Alpinia zerumbet (Pers.) Burttet Smith on Ethanol-induced Gastric Ulcers in vivo and vitro
- Exploring the Mechanism of Blumea balsamifera (L.) DC in Preventing and Treating Alzheimer’s Disease Based on HPLC-ESI-HRMS and Network Pharmacology
- Observation on Therapeutic Effect of Erxian Decoction on Relieving Low Back Pain after PVP of PMOP-derived Vertebral Fracture
- Effects of Early-stage Phased Rehabilitation Training on Acute Respiratory Distress Syndrome: A Systematic Review and Meta-analysis
