Chlorophyll Fluorescence Response of Persimmon Plants under Salt Stress
2023-10-31XiningGENGLihuaXIEJingwenXURuiyuanWANG
Xining GENG, Lihua XIE, Jingwen XU, Ruiyuan WANG
Pingdingshan University, Henan Province Key Laboratory of Germplasm Innovation and Utilization of Eco-economic Wood Plant, Pingdingshang 467000, China
Abstract [Objectives] To study the photosynthetic response mechanism of persimmon seedlings to salt stress. [Methods] The chlorophyll fluorescence parameters of Diospyros virginiana and Diospyros lotus seedlings under 4% salt stress were studied by pot culture salt control method, including the minimal fluorescence (F0), maximum fluorescence (Fm), potential activity of PS II (Fv/F0), maximum photochemical efficiency of PS II (Fv/Fm), electron transport rate (ETR), actual photochemical efficiency of PS II (YII), and photochemical quenching coefficient (qp). [Results] Under 4% salt stress, the maximum fluorescence (Fm), maximum photochemical efficiency of PS II (Fv/Fm), and photochemical quenching coefficient (qp) of two persimmon plants decreased with time. The potential activity of PS II (Fv/F0), actual photochemical efficiency of PS II (YII), and electron transport rate (ETR) decreased under salt stress. [Conclusions] This study indicates that the PS II reaction center in the persimmon leaves was damaged and the electron transport at the acceptor side was damaged under salt stress. It is expected to lay a foundation for the analysis of salt-tolerance mechanism of persimmon plants.
Key words Chlorophyll fluorescence, Salt stress, Diospyros virginiana, Diospyros lotus
1 Introduction
Diospyrosis the largest genus in Ebenaceae that has more than 500 species with remarkable economical values, especiallyDiospyroskakiThunb which has traditionally been used as an important food resource in China, Korea, and Japan[1].Diospyrosplants are pantropical and thrive in warm regions of the world such as China, Korea, Japan and the United States. In 2007, the global production of persimmon reached over 3.3 million t, with 70% coming from China[2].Diospyrosspecies have been ubiquitous to ethnic medication throughout the tropical regions. Leaves, barks, fruits, hard wood, and roots have been used as tonic, powder, and poultice to heal a wide range of illnesses such as asthma, dermatitis, hypertension, atherosclerosis, lumbago, hemorrhage, insomnia, biliousness, among others. Common usage includes febrifuge, carminative, astringent, sedative, anti-hypertentsive, vermifuge, constipation, and antidiuretic[3].
Some natural factors such as climate and unscientific agricultural operation will cause soil salinization. At present, the degree of soil salinization is becoming more and more serious, and the area is also expanding, which has become an ecological problem plaguing many countries[4]. With soil salinization becoming more and more serious, salt stress has gradually evolved into abiotic stress factors that seriously hinder plant growth and development. Salt stress is one of the main environmental factors that inhibit plant photosynthesis, which can lead to the damage of photosynthetic organs and inhibit plant photosynthesis. Chlorophyll fluorescence kinetics has been widely used in the research of photosynthetic physiology of plant stress resistance, which can quickly determine photosynthetic function without causing damage to plant leaves and reflect the changes of plant photosynthetic capacity under stress conditions[5]. Previous studies have been conducted on the growth, enzyme activity, photosynthesis and transcriptional response of persimmon plants under salt stress[6-9], but few studies have reported the chlorophyll fluorescence response of persimmon plants to salt stress. In this study, we explored the effects of salt stress at different time points on chlorophyll fluorescence parameters of persimmon leaves.
2 Materials and methods
2.1 Experimental materialsThe seeds ofDiospyrosvirginianaandD.lotuswere collected and sown for seedling cultivation. When the tested plants grew to 3-4 leaves, the seedlings were planted in medium-size thickened plastic POTS with 3 plants per pot. The soil was mixed with matrix, sand and vermiculite (2:1:1). In May 2022, NaCl salt control test was carried out in the intelligent greenhouse of Pingdingshan University, Henan Province.
2.2 Salt treatment of persimmon plantsThe method of multiple salt application was adopted, and 1.00% NaCl solution was prepared for each concentration under salt treatment for the first time, and the concentration was increased by 1.00% step by step every 24 h. When the salt concentration reaches the set concentration, water is then watered according to the evaporation situation to balance the evaporation amount, and at the same time, the plastic tray under the plastic pot is thickened, so that the solution flowing out of the water can be returned to the basin in time to prevent the loss of salt. The salt concentration was set at 4.00% and 6 strains were repeated. After 8 d of salt solution treatment, the basic chlorophyll fluorescence parameters of the leaves of American persimmon seedlings were determined by portable chlorophyll fluorescence analyzer PAM-2500 (WALZ, Germany), and the leaves at the same position of each plant were selected for continuous measurement for 4 days.
2.3 Determination of chlorophyll fluorescence parametersAfter dark adaptation treatment for 15 min, the minimal fluorescence (F0) and maximum fluorescence (Fm) were determined, and the variable fluorescenceFv(Fv=Fm-F0) and the maximum photochemical efficiency of PS II (Fv/Fm) were calculated. After light adaptation, the minimum fluorescence (F0), maximum fluorescence (Fm), electron transport rate (ETR), actual photochemical efficiency of PS II (YII), photochemical quenching coefficient (qp) are automatically given.
3 Results and analysis
3.1 Effects of salt stress on chlorophyll a fluorescence parameters of persimmon leavesOn the third day after chlorophyll fluorescence parameters measurement, the leaves ofD.virginianain the treatment group were normal, while the leaves ofD.lotusin the treatment group were shed. The results (Table 1) showed that with the increase in time after 4% salt solution treatment, the minimal fluorescence (F0) of the leaves ofD.virginianaandD.lotusfirst increased and then gradually decreased, indicating that the PS II reaction center was damaged or reversibly deactivated.Fmis the maximum fluorescence in the dark, which refers to the fluorescence intensity when all PS II reaction centers are closed after complete dark adaptation, and all non-photochemical processes are minimized, which can reflect the electron transfer situation after PS II.
With the increase in time after treatment with 4% salt solution, theFmof persimmon leaves first increased and then gradually decreased, theFmof persimmon leaves gradually decreased by 32.5% compared with the first day on the 4thday, theFmofD.virginianaleaves gradually decreased, and theFmofD.lotusleaves on the 3rdday decreased by 22.1% compared with the first day, indicating that the electron transfer ability of plant PS II reaction center was weakened.
Fv/F0is often used to measure the potential activity of PS II,Fv/Fmrefers to the maximum photochemical efficiency of PS II. They are two important parameters to indicate the photochemical reaction status. With the increase in time after treatment with 4% salt solution,Fv/F0andFv/Fmof the two persimmon genera showed a slow increase at first and then a sharp decrease (Table 1). The decrease inD.virginianaon the 4thday was 53.3% compared with the 1stday, and the decrease ofD.lotuson the 3rdday was 24.0% compared with the 1stday, indicating that the salt stress caused damage to PS II. The potential activity and primary light energy conversion efficiency of PS II were weakened, and photoinhibition of photosynthesis occurred in plants.
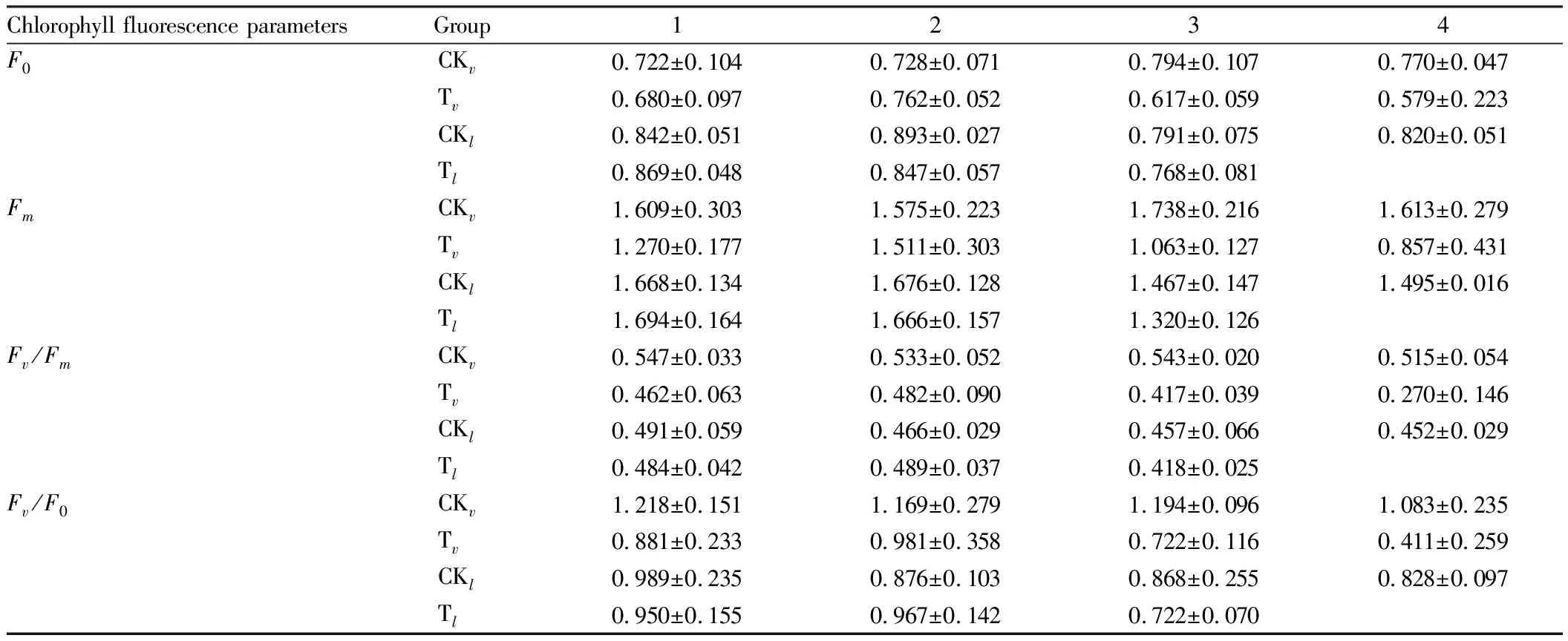
Table 1 Effects of salt stress on F0, Fm, Fv/Fm, Fv/F0
3.2 Effects of salt stress on kinetic parameters of chlorophyll a fluorescence induction in persimmon leavesYIIis the actual photochemical quantum yield of PS II, which reflects the proportion of excitation energy used in photochemical pathways to excitation energy entering PS II, and is an important index of plant photosynthetic capacity. Y(II) of persimmon leaf decreased under salt stress, which decreased by 55.1% and 23.9%, respectively, on the 4thand 3rdday of treatment (Table 2). The photochemical quenching coefficient (qp) is related to the photochemical reaction of PS II, reflecting the share of light energy absorbed by the pigment of PS II antenna for the electron transfer of photochemical reaction, and reflecting the openness of the PS II reaction center to a certain extent.
With the increase in time after 4% salt solution treatment, the photochemical quenching coefficientqpshowed a slow increase at first and then a sharp decline, and the two species of persimmon decreased by 37.1% and 10.1% on the 4thand 3rdday of treatment, respectively (Table 2), indicating that the reoxidation capacity ofQAwas weakened and the electron transfer on the PS II accepter side was damaged by salt stress.ETRis the apparent photosynthetic electron transport rate.
With the increase in time after salt solution treatment,ETRshowed a downward trend in general, and theETRdecreased by 55.1% and 23.9% for two persimmon species, respectively (Table 2).
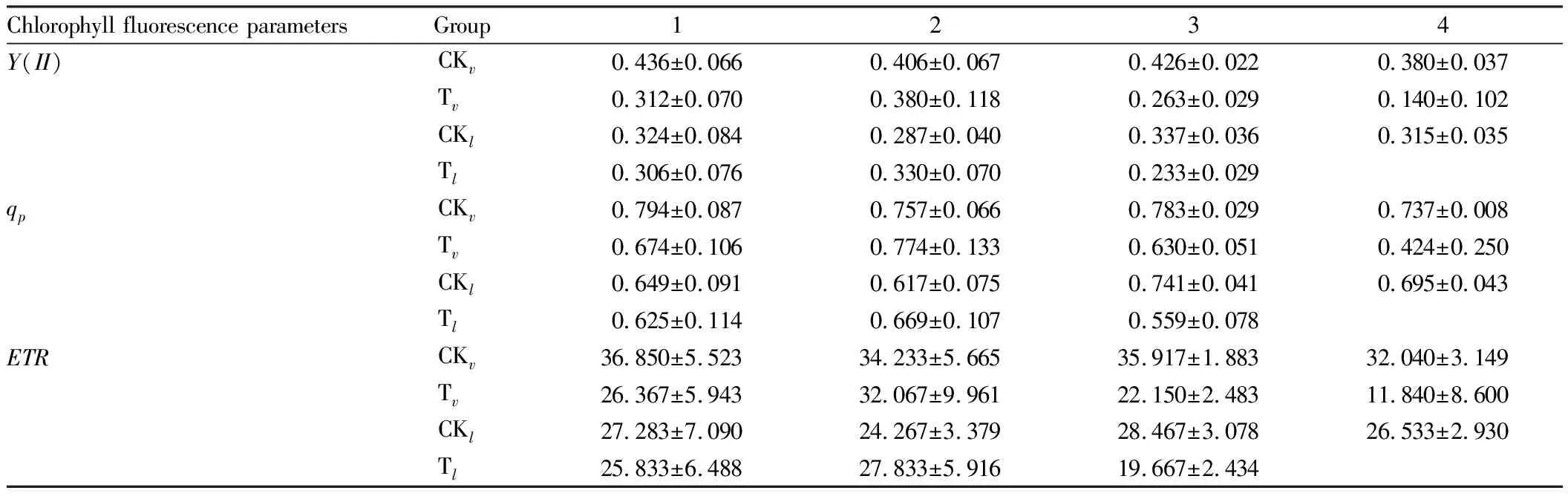
Table 2 Effects of salt stress on Y(II), qp, ETR
4 Discussion
Under normal circumstances, light energy absorbed by chlorophyll is mainly consumed through three ways: photosynthetic electron transfer, chlorophyll fluorescence emission and heat dissipation[10]. There is a relationship between these three pathways, and the change in photosynthesis and heat dissipation will cause the corresponding change of fluorescence emission. Therefore, photosynthesis and heat dissipation can be explored through the observation of fluorescence. The results of this study showed that the maximum fluorescence (Fm), the maximum photochemical efficiency of PS II (Fv/Fm) and photochemical quenching coefficient (qp) of the leaves of two persimmon plants decreased with the increase of time after salt stress, and the potential activity (Fv/F0), the actual photochemical efficiency (YII) and the electron transport rate (ETR) of the leaves decreased under salt stress. The results indicated that salt stress had caused photoinhibition, resulting in damage to the potential active center of PS II, inhibition of photochemical activity of PS II, and affected the transfer of photosynthetic electrons from PS II reaction center toQA,QBandPQlibrary, which was not conducive to the transfer of excitation energy from light-trapping pigment protein complex (LHC) to PS II. These results were similar to those of rice[11], tomato[12], lettuce[13], and Amur grape[14]when exposed to salt stress.
杂志排行
Medicinal Plant的其它文章
- Quality Control of Zhuang Medicine Xiaoyan Zhiyang Lotion
- Research Progress and Ideas on the Anti-liver Fibrosis Effect of Ethnic Medicine Plumbagin Based on microRNAs/TLR4/NF-κB and NLRP3 Inflammasome Activation
- Gastroprotective Effect of Alpinia zerumbet (Pers.) Burttet Smith on Ethanol-induced Gastric Ulcers in vivo and vitro
- Exploring the Mechanism of Blumea balsamifera (L.) DC in Preventing and Treating Alzheimer’s Disease Based on HPLC-ESI-HRMS and Network Pharmacology
- Observation on Therapeutic Effect of Erxian Decoction on Relieving Low Back Pain after PVP of PMOP-derived Vertebral Fracture
- Effects of Early-stage Phased Rehabilitation Training on Acute Respiratory Distress Syndrome: A Systematic Review and Meta-analysis
