Seasonal variation in the biochemical composition, condition index, and meat yield of the non-indigenous pearl oyster Pinctada imbricata radiata(Leach, 1814) from the West of the Aegean Sea, Greece
2023-10-19JohnTheodorouMriMkriXnthiDouviAlexisRmfosEfthimiosSpinos
John A.Theodorou, Mri Mkri, Xnthi Douvi, Alexis Rmfos, Efthimios Spinos
a Department of Animal Production, Fisheries & Aquaculture, University of Patras, Mesolongi, 30200, Greece
b Fisheries Department of Directorate of Agricultural Economy, Region of Western Greece, Patras, 26443, Greece
Keywords:Pearl oyster Condition index Meat yield Biochemical composition Aegean
ABSTRACT The average values of the seasonal flesh biochemical composition (%) of the pearl oyster Pinctada imbricata radiata originated form 2 sampling sites, the gulfs of Evoikos (E) and Saronikos (S) in the Western Aegean Sea,showed that is rich in proteins (64.00 ± 1.86 -(E), 64.67 ± 2.95-(S)) with low fat content (10.96 ± 1.04-(E),11.86 ± 1.13-(S)) and carbohydrates (13.29 ± 2.48- (E), 9.94 ± 4.32 (S)).The condition index ranged from 26.16% ±5.04 in the autumn in (E), to 44.73% ±7.50 in the summer in (S).The meat yield varied from 20.49%± 3.20% in the summer in (E) to 30.73% ± 3.47% in the summer in (S).Both results demonstrate the high nutritional profile of the pearl oyster, supporting its suitability as a potential new Mediterranean seafood source for human consumption.
1.Introduction
Pinctada imbricata radiata(Leach, 1814) (pearl oyster) is a benthic species that lives on sandy or hard substrate bottoms and coral reefs(Strack, 2008; Zenetos et al., 2005).It originates from the Indo-Pacific Ocean region and has been recorded in the Mediterranean as a non-endemic species since the 19th century (1874), immediately after the opening of the Suez Canal (Zenetos et al., 2005; Barbieri et al., 2016;Antit et al., 2011).Since then, the pearl oyster has spread and settled in areas of the Central-Eastern (CE) Mediterranean with a significant presence in Aegean Sea (Theodorou, Perdikaris, et al., 2019; Theodorou,Leech, et al., 2019; Yigitkurt et al., 2017).
A recent wild stock evaluation in the Aegean Sea indicates the resource availability at adequate quantities for potential fisheries exploitation (Yigitkurt et al., 2020; Moutopoulos et al., 2021) for other uses than cosmetics, such as that of seafood for human consumption.In order to enhance this effort, the pearl oyster promotion as a “prestigious” edible bivalve is recommended.There is a great interest in the health benefits of shellfish by the consumers, and the nutritional profile of the flesh is requested to support the image of the “new” coming product into the market (Harris & Von Schacky 2004; Zhukova 2019).The moisture content of the edible part indicates the quality and the freshness of the shellfish since it affects the physical and chemical processes of the flesh.Water is a fundamental medium for chemical reactions involved in many physiological processes, such as nutrient transport, removal of excess and minerals, nerve transmission and muscle contractions (Aberoumad et al., 2010).
Edible bivalves are a source of high quality proteins, vitamins,essential amino acids, minerals and low lipids in addition to beneficial ones such as polyunsaturated fatty acids (PUFAs), which offer such benefits to the human body (Biandolino et al., 2020; Rittenschober et al.,2013; Anacleto et al., 2014; Prato et al.2018, 2019 Tan et al., 2021).
Research to this direction for the Pteriidae is limited, since pearl oyster species have been widely studied over the past years and are considered to be the most promising pearly production (Andr´efou¨et et al., 2012; Kishore & Southgate 2015; Kishore et al., 2018).For this purpose, emphasis is given on the gonad cycle development and the relevant changes on the condition index (CI) as well as on the biochemical variations among seasons (Behzadi et al., 1997; Choi &Chang 2003; Derbali et, al., 2009).
Studies on the winged oysterPteria colympus(Y´a˜nez et al., 2010;Pe˜nuela-Jim´enez et al., 2020) showed the dependence of the proximate composition and the fatty acid profile from the local environmental conditions in Caribbean Sea (Punda Arena, Estado Sucre, Venezouela).
In the eastern Mediteranean Sea (Gulf of Antalya, Turkey), early observations by Gokoglu et al., (2006) for the pearl oyster (Pinctadaradiata, Leach, 1814), showed that the proteins ranged between 65.9 and 160.4 g/kg-1the fats between 4.3 and 10.9 g/kg-1and the ash 4.6–27.0 g/kg-1with maximum protein and fat values during summer months.Since then, there is not any other similar report in the region as the pearl oyster is treated as a Non-Indigenous Species (NIS) and the research interest up to now is rather focused on the species distribution,the life cycle and the ecological interactions (Yassien et al., 2000;Tlig-Zouari et al., 2010;, 2009; Yigitkurt, 2021) than its nutritional value for human consumption.The present effort aims to cover this gap by determining the seasonal variation of the pearl oyster condition index(CI), meat yield (MY) and biochemical composition fromspecimenscollected in western Aegean Sea.
2.Materials &methods
2.1. Sampling location
Pearl oyster samples were collected from a high (Saronikos Gulf, 37 59 ‘113’ ’/23 26′059′′) and a low (Evoikos Gulf, 38 30′783’’/23 32′254′′) productivity region, according to satellite data (http://data.eu ropa.eu/88u/dataset/10161412-a76c-42b0-b4e1-5fcccdc412b2) in Western (W) Aegean Sea, central east coastline of the continental Greece(Fig.1).
During the period February 2019–July 2020, a total of 30 individuals per season per site were collected by hand, through 30 min autonomous diving at a depth of 1–4 m.The summer 2019 sampling was lost, so it was repeated in the next year (summer 2020) following the same procedure to cover the missing values.In order to ensure that potential size dependent analytical differences are minimized (Biandolino et al.,2020), commercial size individuals with approximately similar length range (>50 mm) were chosen to be collected (Table 1).
Samples were stored in ice in 10 kg insulated polystyrene boxes (6–9◦C) in order to be kept alive during their transportation to the laboratory.
2.2. Morphometric characteristics
Shells of live bivalves from each seasonal batch were externally cleaned by brushing and rinsing with distilled water, in order to remove derbis and epibiots.The external morphometrics shell height (SH), shell length (SL), shell hinge (Sh) and shell width (SW) were measured (mm)with a vernier caliper.
Prior to weighing, each animal was left on an absorbent tissue surface for 15 min to remove the extraneous water.Afterwards, the total wet weight (Twe) of each individual was measured (precision 0.01g).The Condition Index (CI) and the Meat Yield (MY) were estimated in each individual as it represents the percentage edibility of the species.Each individual was sucked to remove the fresh flesh (Fwe) from the shell (Swe).Each separate component was weighted following the previous procedure.
The selected formulas to evaluate the flesh quantity of the pearl oyster expressed on wet weight, as it is commonly used in the shellfish market (Davenport & Chen, 1987; Theodorou, Leech, et al., 2019) and consequently best fits with the aim of the present effort.
The Condition Index (%) and Meat Yield (%) calculated as follows:
CI =[Fwe (g)/Swe (g)] ×100 (Davenport & Chen, 1987; Okumus &Stirling, 1988).
MY = [Fwe (g)/Twe (g)] × 100 (Mohite et al., 2009).
2.3. Proximate composition analyses
After the removal and the weighing of the soft tissues of oysters, the tissues were freeze-dried for 24 h.The dry weight (g) of each tissue was measured using an analytical balance for water content determinations.Since the freeze-dried weight of a single tissue was not enough for performing all assays of interest, five pooled samples were prepared by homogenizing together six dried tissues per site and season.These freeze-dried pooled samples (named from now on “sample”) were used for ash, crude protein and lipid determinations.The use of pooled samples assumes that the values of assays obtained from the pooled samples are the weighted average values of the same assay obtained from analyses of each individual tissue that consists the pooled sample(Linehan et al., 1999).
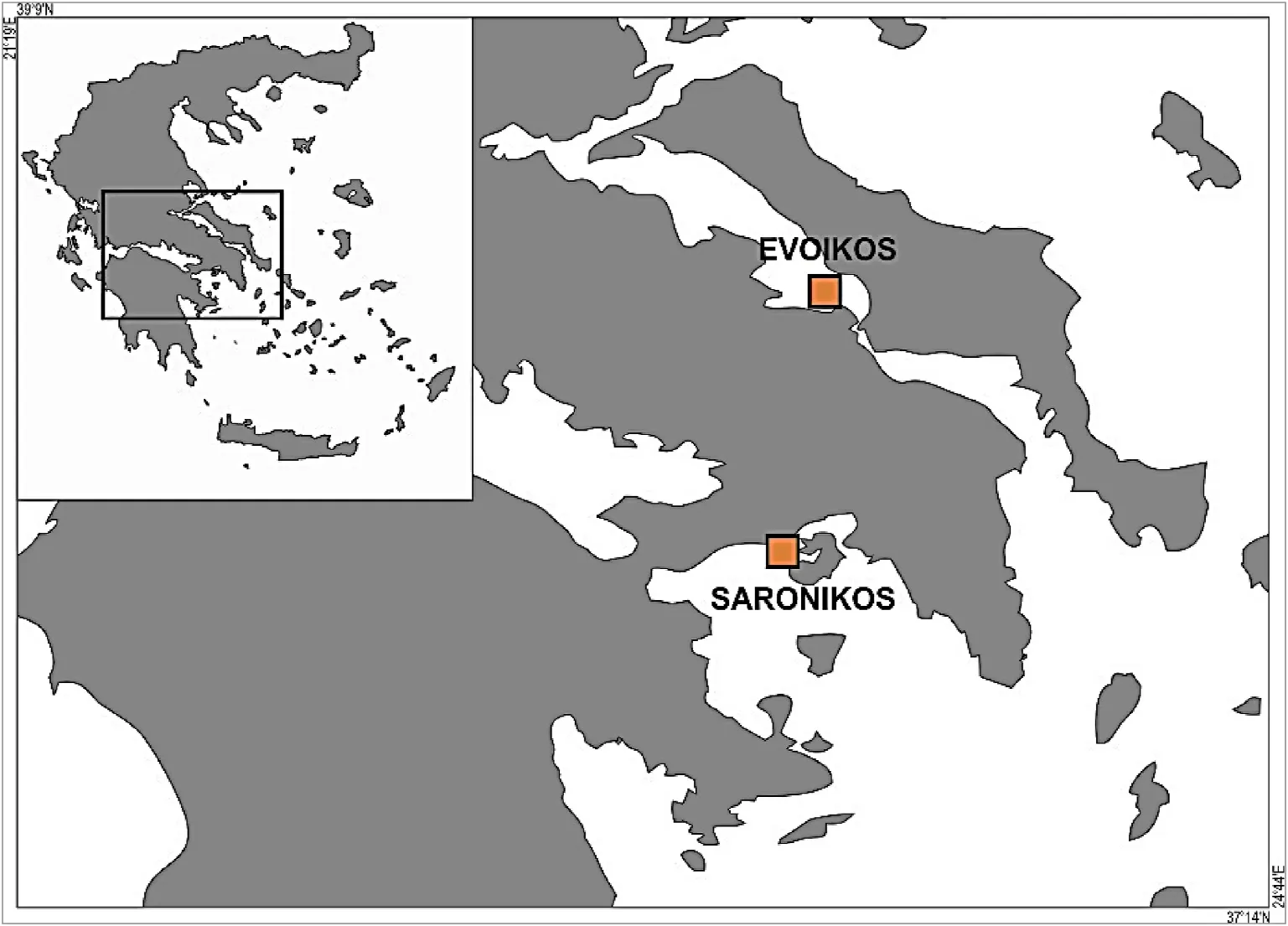
Fig.1.Pearl oyster sampling positions in the gulfs of Saronikos (37 59 ‘113’ ’/23 26′ 059 ′′) and Evoikos (38 30 ‘783’ ’/23 32′ 254 ′′), W.Aegean Sea, Greece.
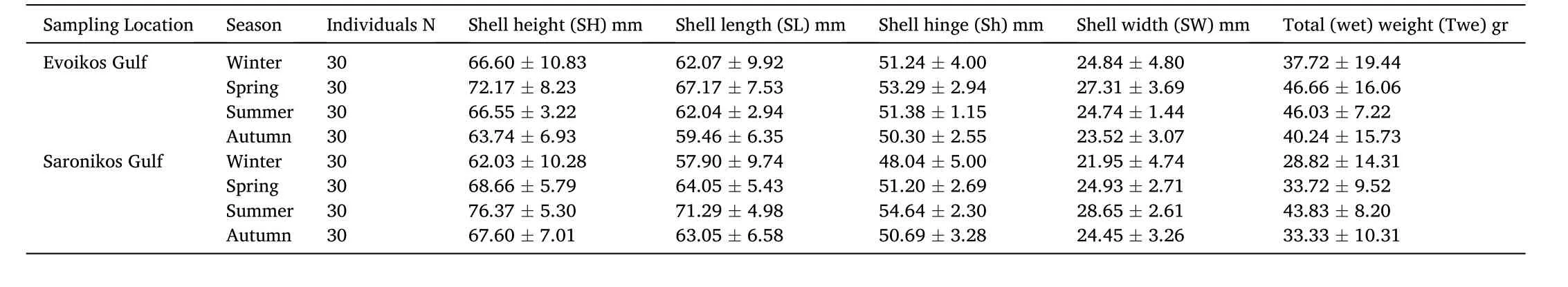
Table 1 Pearl oyster morphometric characteristics sampled in the gulfs of Evoikos and Saronikos (W.Aegean Sea).
Water content (%, w/w) was determined from the weight of each tissue before and after freeze-drying.The ash content was obtained by heating a portion (0.1 g) of sample in a furnace at 550◦C for 24 h, while crude protein content was measured by the Kjeldahl method using 2g sample (AOAC,1997).Crude lipid was extracted from 1 g of sample using 20 ml of an extracting solution consisting of chloroform and methanol (2:1, v/v) following the method Folch et al.(1957) as modified by Vareltzis et al.(1997).Triplicate measurements were performed on each sample for ash, crude protein and lipid determinations and the results were expressed as mean±S.D.from five samples per site and season on dry base.
Carbohydrate content was calculated based on difference calculation following the type: Carbohydrate (%) =100 – [moisture (%) +ash (%)+ crude protein (%) +fat (%)] (Nurnadia et al., 2011).
In the mentioned type, the values of ash, crude protein and fat were expressed on wet bases.
2.4. Statistical analysis
Statistical analyses of data from proximate analyses were performed with Minitab14 for Windows (Minitab Inc., 2002).The assumption of normality of distributions was tested with the Anderson-Darling test and that of homogeneity of variances with the Bartlett’s test.Data were log –transformed when necessary.Two-way analyses of variance (site and season) were performed to test for site and season effects on parameters measured.
Primary data sets of morphometrics and biochemical composition saved in Excel sheets (Microsoft Corp., Redmond, WA) and are further processed with Statistical Package for Social Science version 11.01 software (SPSS Inc., Chicago, Illinois, USA).
Since values of all parameters were estimated as percentages and in many cases the sample size was small (N = 5), the statistical comparisons between the two areas and among the four seasons was performed with the non-parametric Kruskal-Wallis test at 95% significance level(Zar, 1999).
3.Results
3.1. Morphometric characteristics
The morphometric characteristics (SH, SL,Sh, SW) of pearl oyster samples for each area and season are shown in Table 1.SL ranged from 52.05 ±16.52 cm (winter) to 66.18 ±5.21 cm (summer) in Evoikos gulf and from 51.25 ± 13.63 cm (winter) to 68.00 ± 6.08 cm (summer) in Saronikos gulf.Fig.2 shows the seasonal variation of the MY and CI in both sampling regions.CI ranged from 26.16% ±5.04% in the autumn in Evoikos, to 44.73% ± 7.50% in the summer in Saronikos.The MY ranged from 20.49% ± 3.20% in the summer in Evoikos to 30.73% ±3.47% in the summer in Saronikos.
Highest MY values in Evoikos were observed in winter and spring,while in Saronikos highest values were observed a few months later(spring and summer).The CI in Saronikos has similar values all year around, whereas in Evoikos higher CI values were observed in winter and spring (Fig.2).
3.2. Biochemical composition
3.2.1.Seasonal profile
Fig.3 shows the seasonal variation of the pearl oyster flesh biochemical composition, in both sampling sites.The peak of the protein content (%) reported in Evoikos in winter while in Saronikos in autumn.In Evoikos samples, the fat content (%) was higher during summer than the rest of the year.Ash (%) content was differentiated in Saronikos during winter and autumn.In Evoikos, carbohydrates (%) and moisture(%) content remained stable all year around.In contrast, the carbohydrates (%) content of Saronikos samples, was higher during spring and summer, while moisture (%) remained at a minimum level at the same time.
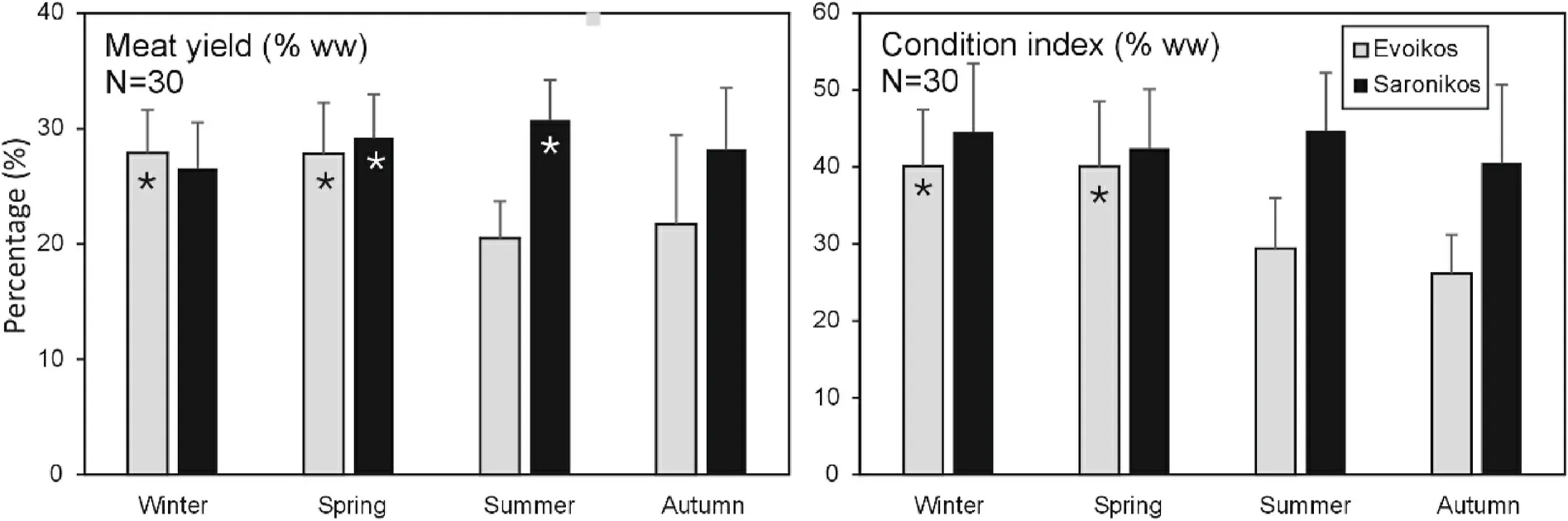
Fig.2.Seasonal variation of condition index (CI) and meat yield (MY) of pearl oyster in Evoikos and Saronikos gulfs (mean values and SD).Where shown, asterisks denote statistically higher values among seasons in each region (Kruskal-Wallis, P < 0.05).
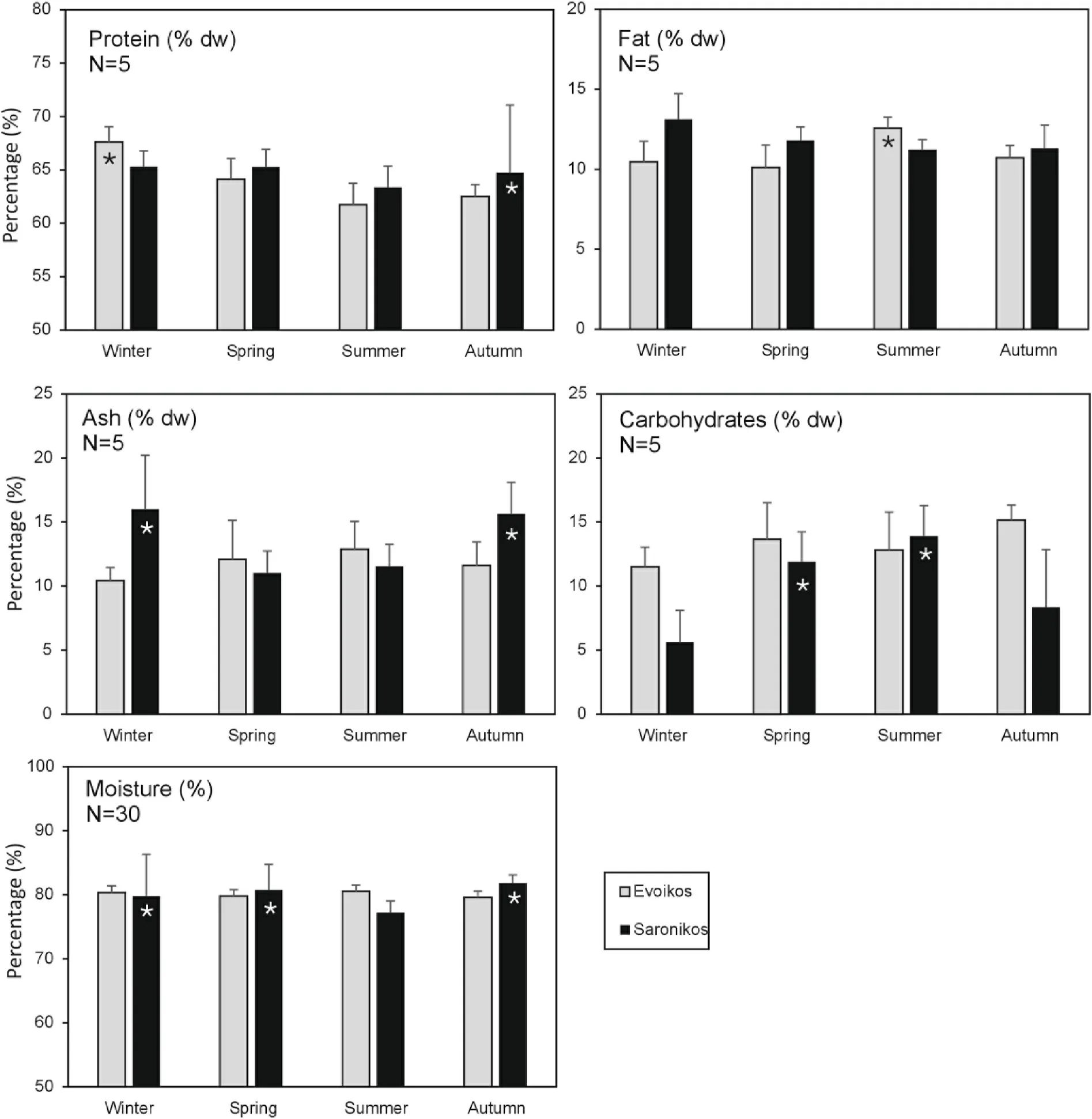
Fig.3.Seasonal variation of pearl oyster biochemical composition for the gulfs of Evoikos and Saronikos in W.Aegean Sea (mean values and SD).Where shown,asterisks denote statistically higher values among seasons in each region (Kruskal-Wallis, P < 0.05).
The statistical analysis indicated that moisture content was higher in samples originating from the Evoikos compared to the Saronikos.These differences were statistically significant during the autumn and summer seasons (P< 0.05).Protein content did not exhibit statistically significant differences between the two sampling sites, ranging between 61%and 67% for the different seasons and geographic locations (P>0.05).Lipid content was lower in samples harvested in Evoikos compared to the respective samples from the Saronikos, showing statistically significant differences during winter and summer periods (P<0.05).
3.2.2.Nutritional comparison/evaluation
The biochemical composition of the pearl oyster in Evoikos (Table 2)defined as protein (64.00% ± 1.86%), fat (10.96% ± 1.04%), carbohydrates (13.29% ± 2.48%), ash (11.76% ± 2.01%) and moisture(80.07% ±2.38%), while in Saronikos as protein (64.67% ±2.95%), fat(11.86% ± 1.13%), carbohydrates (9.94% ± 4.32%), ash (13.54% ±2.54%) and moisture (79.88% ±3.49%).
This nutritional profile can easily compete with that of the native oystersOstrea edulisobserved in CE Mediterranean (Table 2).Furthermore, the protein content (%) of thePinctada imbricata radiataflesh is much higher than the maximum observed in other edible commercial species of the region, such as the nativesRuditapes decussatus(56.27 ±1.98) and the exotics clamsRuditapes philippinarium(55.88 ±2.04), the musselsMytilus gallopronviciallis(54.03 ± 2.82), the pectenidsFlexopecten glaber(8.84 ±0.32) andClamys varia(9.31 ± 1.18).
4.Discussion
ΤheP.imbricata radiatais a commercial fishing edible species in the Persian Gulf, Red Sea and few Mediterranean regions.It represents about the 95% of the total oyster catches in Qatar (Mohammed & Yassien, 2003), while is a significant fishing resource for the artisanal fisheries in Saudi Arabia in Red Sea (Gladstone, 2002).Along the Lebanese coastline is exploited as a high value commercial edible species(Nader & Talhouk, 2002) while in Tunise the evaluation of the wildstocks are examined for potential for commercial catches (Derbali et al.,2019).In Greece despite that pearl oyster was a species of minor commercial interest in the past decades (Koutsoubas et al., 2007; Katsanevakis 2008; Τheodorou et al., 2011), recently is a significant exploitable resource for human consumption, especially in regions with high and medium productivity such as Saronikos and Evoikos gulf where the native edible commercial bivalves such as native oysters, mussels, warty venus and smooth clams are under “suppression” or “overexploitation”.

Table 2 Biochemical composition of edible bivalves originated from various regions of the Central and Eastern Mediterranean Sea.
Moutopoulos et al., (2021) suggested that the morphometric characteristics ofP.imbricata radiatacannot be attributed to specific environmental factors despite that Evoikos gulf is more productive than Saronikos.
The high meat yield (over 20%) of the pearl oyster, remains juicy(80% approx) all year around and is rich in proteins (60% approx).However, the CI has lower values in autumn and winter and higher in spring and summer.This might be explained by the reproduction biology of the species as a similar pattern of CI is reported by Yigitkurt(2021), in the Eastern Aegean (coast of Izmir in Turkey).The pearl oyster biochemical composition is very attractive for human consumption and is competitive with the other regional edible species such as native oysters, mussels, clams (native, exotics) and pectenids in CE Mediterranean Sea.However, the total lipids has to be further analyzed to identify the polyunsaturated fatty acids profile as it could be further support the nutritional profile of the species as a healthy diet or a “super seafood”(Theodorou 2017; Prato et al., 2018, 2019; Biandolino et al.,2020).In addition, heavy metals and biotoxins accumulation has to be constantly checked prior harvesting, according to the edible bivalves health and safety monitoring standards of the exploitation areas(Gokoglu et al., 2006; Rodrigues et al., 2015; Theodorou et al., 2020;Zgouridou et al., 2021).
5.Conclusion
• Τhe biochemical composition of the pearl oyster edible part demonstrates its high nutritional value and supports its promotion as a“new” seafood source for human consumption.
• The high meat yield content of the bivalve, almost all year around indicates its suitability for further processing to develop new added value seafood products.
CRediT authorship contribution statement
John A.Theodorou:Conceptualization, Methodology, Formal analysis, Writing – original draft, Supervision, Fund Raising.Maria Makri:Methodology, Data curation, Formal analysis.Xanthi Douvi:Laboratory assistance, Data gathering.Alexis Ramfos:Field sampling,Statistics, and graph presentation.Efthimios Spinos:Writing – original draft, Formal analysis.
Declaration of competing interest
The authors delare that there is no conflict of interest.
Acknowledgements
The present work is a part of the project “Commercial exploitation of the pearl oysterPinctada imbricata radiataby adding value through the development of processed products (Code MIS: 5010850) funded by the“Innovation in Fisheries” EU-Greece Operational Program of Fisheries,EPAL 2014–2020.
杂志排行
Aquaculture and Fisheries的其它文章
- A framework for risk analysis of the shellfish aquaculture: The case of the Mediterranean mussel farming in Greece
- Slurry ice as an alternative cooling medium for fish harvesting and transportation: Study of the effect on seabass flesh quality and shelf life
- Application of hurdle technology for the shelf life extension of European eel(Anguilla anguilla) fillets
- Physicochemical properties of silver carp (Hypophthalmichthys molitrix)mince sausages as influenced by washing and frozen storage
- Bacterial community in response to packaging conditions in farmed gilthead seabream
- Effective algorithmic operational framework for fish texture evaluation in industry: Achieving maturity
