Application of hurdle technology for the shelf life extension of European eel(Anguilla anguilla) fillets
2023-10-19MriGinnkourouNtliStvropoulouTheofniTsironiVldimirosLougovoisVssilikiKyrnSpyrosKontelesVssiliSinnoglou
Mri C.Ginnkourou, Ntli Stvropoulou, Theofni Tsironi, Vldimiros Lougovois,Vssiliki Kyrn, Spyros J.Konteles, Vssili J.Sinnoglou
a Analysis & Design of Food Processes, Laboratory of Chemistry, School of Food Sciences, Department of Food Science and Technology, University of West Attica, Agiou Spyridonos, 12243, Aigaleo, Athens, Greece
b Laboratory of Food Process Engineering, Department of Food Science and Human Nutrition, Agricultural University of Athens, Iera Odos 75, Athens, 11855, Greece
Keywords:Hurdle Eel fillets Osmotic treatment Rosemary extract Shelf life
ABSTRACT Fresh fish, and especially fatty species, are highly perishable due to oxidative deterioration of fish flesh and the elevated microbial load on fish surface.The implementation of a variety of “mild hurdles” may significantly decrease the rates of fish chemical degradation and microbiological spoilage, by better retaining the initial quality, compared to more intense preservation techniques.The aim of this work is the comparative study of different, single or combined, treatments at 15 ◦C on the quality and shelf life of chilled eel fillets.Fish fillets were treated using osmotic solutions consisting of glycerol (30%–40%–45%) and 5% NaCl with and without former antioxidant impregnation by using Rosemary Serum.In all cases, water activity decreased to approximately 0.90 after 420 min of osmotic treatment.Untreated and osmotically treated fish fillets were subsequently stored at 4 ◦C and their stability under chill conditions was assessed based on microbial growth and oxidative deterioration.Microbial growth of treated samples was significantly delayed, especially due to the osmotic step(OS) and the derived water activity decrease.Lipid oxidation, a major cause of rejection for fatty fish such as eel,was greatly inhibited in treated fillets, owing to both ‘hurdles’, aw lowering (OS) and antioxidant impregnation with rosemary serum (RS/OS), showing the synergistic effect of these successive procedures.Shelf life of treated eel fillets exhibited a more than 10-fold increase, as compared to the untreated samples, based on chemical composition and a 2 to 3-fold shelf life improvement, in terms of microbial growth.
1.Introduction
Fish and other aquatic products play an essential role in a healthy and balanced diet, as they are sources of valuable animal protein, vitamins and minerals (Hassoun & Karoui, 2017).Their consumption has long been associated with several health benefits, including decreased cardiac and stroke risk and decreasing systemic inflammation, neurological diseases, cancer and the progression of coronary atherosclerosis,associated to a number of nutrients, including protein, long-chain omega-3 polyunsaturated fatty acids such as eicosapentaenoic and docosahexaenoic acids, and a number of vitamins and minerals (Giannakourou et al., 2019; Hassoun & Karoui, 2017; Parian & Mullin, 2016).However, finfish and other seafoods are highly perishable food products(Tsironi & Taoukis, 2012), due to their high-water activity (aw), neutral pH, low content of connective tissue and the presence of autolytic enzymes which cause rapid development of undesirable odors and flavors(Socaciu, Semeniuc, & Vodnar, 2018).On the other hand, problematic post-harvest handling, processing, and storage of aquatic products may lead to food losses and waste, related to important physical, economical,or nutritional aspects.As far as quality degradation is concerned,spoilage is the process in which aquatic products deteriorates to the point that becomes unacceptable for human consumption, based either on microbiological or other quality criteria.
Fish quality is considered as the most important quality parameter since it is directly related to the sensory attributes perceived by consumers such as appearance, texture, odour and taste (Hassoun & Karoui,2017; ¨Ozogul, ¨Ozyurt, ¨Ozogul, Kuley, & Polat, 2005).Quality depends on both internal and external parameters, such as the species, the initial microbial load, the handling, processing conditions, microbial spoilage,all of which affect the shelf life of these products (Baklori, Tsironi, &Taoukis, 2012; Lambrianidi, Savvaidis, Tsiraki, & El-Obeid, 2019).The limited and variable shelf life of chilled fish products is a major problem for their quality assurance and commercial viability (Tsironi & Taoukis,2010).
European eel (Anguilla anguilla) is a commercially important species because of its superior quality attributes, such as its white flesh, flavor and high fat content, owing to the abundant ω3 lipids (El-Obeid et al.,2018; Lambrianidi et al., 2019).Its production has steadily increased over the last 30 years, mainly due to the expansion of farming, which accounts for more than 80% of the world’s consumption of the species.Greece possesses an important eel farming activity, following the Netherlands, Italy and Denmark (Choulitoudi et al., 2017).However,owing to their high polyunsaturated lipid content, eels are susceptible to a rapid loss of nutritional quality, mainly due to lipid oxidation,causing a significant decrease of the expected shelf life (Choulitoudi et al., 2017;Küçükgülmez, Yanar, Gerçek, Gülnaz, & Celik, 2013; Yanar, Küçükgülmez, G¨okçin, Gelibolu, & Dikel, 2013).
Eels are presently stored on ice or under refrigeration during their distribution and marketing (Socaciu et al., 2018).In these conditions,the shelf-life of iced eel and refrigerated eel without ice was 12–14 days and 5–7 days, respectively (¨Ozogul et al., 2005).The shelf-life of raw eel samples at 0◦C in atmospheric air, vacuum and MAP conditions was 11,11 and 18 days, respectively.Using the microbial quality indicators, the shelf life of eel packaged in air, vacuum and MAP was estimated to be more than 18, 28 and 34 days, respectively (Arkoudelos, Stamatis, &Samaras, 2007).
Several traditional food preservation methods (e.g.freezing, marinating, canning, salting etc.) are used to effectively control the growth of microorganisms and delay spoilage of fish products, causing however severe damages to food quality.As a method to alleviate quality decrease issues, nonthermal processing has been introduced as an alternative to more intense techniques.Osmotic dehydration (OD) is a technique used to reduce water activity (aw) to improve nutritional,sensorial and functional properties of food.This technology promotes partial removal of water from food by immersion in a concentrated hypertonic solution (Fito et al., 2001) of salts and or low molecular weight carbohydrates.Difference in osmotic pressure between the food and its surrounding solution acts as the driving force for water removal,while the complex cellular structure of food acts as a semipermeable membrane.The preservative effect of osmotic treatment is greater as the awof the final product decreases (Tsironi & Taoukis, 2014).By reducing the awof a food matrix, microbial growth is reduced or inhibited, while the rates of other deteriorative processes in the fish tissues are decreased, leading to prolonged shelf life and improved retention of flavor and nutritional parameters of the product (Tsironi & Taoukis,2012).The careful choice of the osmotic agents is based on issues, such as the awlowering effect, impact on sensory attributes, safety and economical aspects.Considering those requirements, a combined application of sodium chloride and glycerol, which is an effective humectant and food additive to improve texture (plasticizer) (Moreira,Chenlo, Torres, & V´asquez, 2007), has been used to generate intermediate moisture food products (Khubber et al., 2020; Moreira et al., 2007;Sanchez S´anchez Pascua, Casales, & Yeannes, 1994; Semenoglou,Dimopoulos, Tsironi, & Taoukis, 2020).Reviewing current literature, a number of studies have investigated the extension of the shelf life for different aquatic species, obtained by the application of osmotic dehydration, as a sole treatment technique before subsequent chill storage(Arvanitoyannis, Veikou, & Panagiotaki, 2012; Checmarev, Casales,Yeannes, & Bevilacqua, 2014; Semenoglou et al., 20202020; Tsironi,Salapa, & Taoukis, 2009; Tsironi & Taoukis, 2014, 2017).
Although the removal of moisture to a low level delays microbial spoilage and quality deterioration, lipid oxidation, the main deterioration path for aquatic products that are rich in fat content and unsaturated fatty acids (such as eel), still occurs.Therefore, it is essential to seek for methods to minimize or even overcome this detrimental procedure.One effective way could be based on the use of natural preservatives such as essential oils, which do not have any side effects, are found to improve the odour and taste of the food and extend the shelf life of the food products (Rezaeifar, Mehdizadeh, Mojaddar Langroodi, &Rezaei, 2020).In the case of eel, chitosan was found to be effective in delaying lipid oxidation in fish fillets under refrigerated storage(Küçükgülmez et al., 2013; Yanar et al., 2013).Chitosan alone or in combination with oregano were investigated in (Lambrianidi et al.,2019), as a means to extend shelf-life with chitosan-treated eel fillets being organoleptically preferred over oregano-treated fillets (El-Obeid et al., 2018).examined the effect of chitosan, thyme oil and their combination, on the shelf-life of smoked eel fillets stored under vacuum packaging (VP) at 4◦C, and found their advantageous impact on the shelf life of smoked eel.In another study, extracts of myrtle and laurel were found to inhibit lipid oxidation and growth of bacteria in European eel (¨Ozogul et al., 2014).The antioxidant and antimicrobial properties of rosemary essential oil and extracts, entrapped in carboxyl methyl cellulose (CMC) edible coating for smoked eel were studied in (Choulitoudi et al., 2017), and results showed that their effect were rather moderate compared to the inherent microbial stability induced by the smoking process.Rosemary (Rosmarinus officinalis) is one of the most common aromatic herbs belonging to the family Lamiaceae and originated from the Mediterranean region.Some of the effects demonstrated by this plant include the ability to attenuate asthma, atherosclerosis,hepatotoxicity, ischemic heart disease, antioxidant and anti-inflammatory actions, control of hypercholesterolemia and oxidative stress, lipid peroxidation reduction and ability to treat depressive behavior (de Oliveira, Camargo, & de Oliveira, 2019).The main substances responsible for the antioxidant effect of rosemary are phenolic diterpenes, including carnosic acid, 12-methoxy carnosic acid, rosmarinic acid and carnosol (Borr´as-Linares et al., 2014; Linhartov´a et al.,2019; Xie, Van Alstyne, Uhlir, & Yang, 2017) and their effectiveness is similar to that of synthetic phenolic antioxidants (Cuvelier, Richard, &Berset, 1996).
Nonetheless, nowadays, the consumer demands fresh or minimally processed foods of high quality, more “natural”, produced with mild techniques, using the minimum amount of additives, microbiologically safe, nutritious and healthy (Erkmen & Bozoglu, 2016).In this context,hurdle technology promotes the careful combination of traditional and innovative preservation methods in order to establish a series of preservative parameters, called ‘hurdles’, that microorganisms are unable to overcome (Leistner, 2000; Leistner & Gorris, 1995).The individual hurdles may be implemented simultaneously or sequentially, depending on the overall processing procedure (Leistner & Gorris, 1995).The application of this concept is a valuable tool in order to obtain the required microbial stability and safety as well as a minimum quality degradation of the product in question.Taking these advantages into account, it is a challenge to apply complimentary techniques to assure aquatic products stability and superior quality (Tsironi, Houhoula, &Taoukis, 2020).There are limited studies in current literature that have investigated the application of hurdle technology in aquatic products(Semenoglou et al., 2020; Wang, Liu, Cao, Li, & Duan, 2019), and only a few of them using an antioxidant impregnation step complimentary to the osmotic procedure (Giannakourou et al., 2019; Sofra, Tsironi, &Taoukis, 2018; Tsironi & Taoukis, 2010, 2012).
This study is an implementation of the hurdle technology principles,by combining two mild preservation techniques, namely tissue impregnation with bioactive compounds, followed by a water activity reduction step (through OD in hypertonic solutions).By measuring the microbiological, chemical, and sensory changes of air-packaged,refrigerated European eel fillets, pre-treated in different osmotic solutions with and without enrichment in rosemary serum, the ultimate goal was to determine the shelf life extension accomplished during the subsequent chill storage.
2.Materials and methods
2.1. Sample preparation
Eels (A.anguilla) were harvested by V.Geitonas (Arta, Epirus,Greece).Fish was ice shocked, headed, eviscerated and filleted within 2 h post mortem and transported to the Department of Food Science,Technology of the University of West Attica in polystyrene boxes with adequate quantity of flaked ice (0◦C) within 24 h.The average weight of eel fillet was 500 g and the fillets were cut into (3 ×3 ×1) cm3slices in a laminar flow hood, each weighing 13±1 g.For each experimental series(i.e.kinetics of osmotic process, followed by a shelf life study for each one of the tested osmotic solutions and for control samples, including replications), approximately 120 eel fillet slices were used.Osmotic solutions were prepared by dissolving glycerol, food grade (Honeywell,Riedel-de-Haen, USA) and distilled water at concentrations of 30%,40%, and 45%, adding also 5% NaCl.Glycerol is a low molecular weight sugar alcohol with low sweetness which is recognized as a non-toxic and safe additive (Regulation EC No.1333/2008).NaCl was added to strengthen the driving force of the process and counterbalance the slight sweetness obtained during the immersion into glycerol hypertonic solutions.For the antioxidant impregnation procedure, rosemary extract serum was provided by Natural Food Additives (NFA) S.A and is rich in water soluble phenols, including rosmarinic acid (RA) (2.5%–5.3%) and flavonoids (1%).
2.2. Antioxidant impregnation and osmotic treatment
Samples were divided into two groups; half of the eel fillets were directly submitted to the osmotic dehydration procedure and the rest of them were initially immersed into the hypotonic rosemary serum for 60 min, in a ratio of food/serum 1:2, before adding the pre-weighed osmotic compounds (e.g., glycerol and NaCl) (RS/OS), so as to obtain the desired concentration of the hypertonic solutions.This latter, two-step procedure was selected instead of a simultaneous process, as the onestep procedure did not lead to a significant antioxidant enrichment of fish, as it was detailed in Giannakourou et al., 2019 and (Bellary &Rastogi, 2012).
For all sample categories, the osmotic procedure step was performed at 15◦C, for a duration up to 420 min With a food/solution ration of 1:4.Therefore, the overall experimental design consisted of osmotically treating eel fillets in concentrated solutions of glycerol (30%, 40%, and 45%) and 5% NaCl at 15◦C for up to 420 min, either as a unique process(solely osmotically treated, OS) or with the previous immersion into the rosemary serum (RS/OS).To obtain this study, coded beakers filled with pre-weighed osmotic solutions were placed in a controlled incubator(POL-EKO-APARATURA SP.J.type ST 1 B SMART) at 15 ± 1◦C.Preweighed eel samples were immersed in the osmotic solution by means of a grid.At predetermined intervals, one beaker of each osmotic solution was removed and samples were blotted gently with a tissue paper to remove the excess coating solution, and then weighed.
2.2.1.Physicochemical measurements during osmotic treatment
The water activity of fish samples was determined by an aw-meter(AquaLab Dew Point Water Activity Meter 4 TE, METERGroup, Inc.,Pullman, WA, USA), and the◦Brix of the osmotic solution was measured by a hand-held refractometer (Atago, Master refractometer, Yorii,Japan).Salt content was determined using silver nitrate solution by the Mohr method (AOAC,1990).The color of fish samples was also instrumentally determined with a tristimulus chromatometer (model CR-400,Minolta, Tokyo, Japan) calibrated with a white standard plate (L*:97.83, a*: - 0.45, b*: +1.88).The CIELAB color scales were used, with coordinates (L*, a*, b*) being directly read from the chromameter.Lightness (described by the L* parameter) and the hue angle (h*, tan-1(b*/a*)) were chosen as the most representative color attributes.Hue angle is related to the consumer qualitative perception of color since it describes the nuance (e.g.reddish, greenish, etc.), and it is applied to define the difference of a certain color when compared to grey color with the same lightness (Pathare, Opara, & Al-Said, 2013).
Texture measurements were conducted by means of a texture analyzer (TA-XT2i of Stable Micro Systems, Godalming, England), and a TPA (Texture Profile Analysis) test was carried out using eel flesh samples of cylindrical shape.The test was performed on a nonlubricated flat platform using a 60-mm cylindrical compression probe and a 25 kg load cell.Samples were twice compressed using a fixed rate(1 mm/s), at 50% deformation.Texture characteristics such as firmness,elasticity, cohesiveness and chewiness were calculated (Nishinari,Kohyama, Kumagai, Funami, & Bourne, 2013).Amongst the different parameters calculated from texture measurements, elasticity was found to be the more representative.All measurements were performed in triplicate.
Moisture content was calculated after vacuum drying at 70◦C(Heraeus Instruments Vacutherm, ThermoScientific, Waltham, Massachusetts, USA) for 24 h.Each measurement was carried out in three replicates in order to take the average values and standard deviations.
Mass transfer parameters were calculated in terms of water loss (WL)and solid gain (SG) according to the following equations (Eqs.(1) and(2)):
whereM0is the initial mass of fresh material before the osmotic treatment,Mis the mass of fish samples after timetof osmotic treatment,mis the dry mass of fish after timetof osmotic treatment andm0is the dry mass of fresh material.
2.2.2.Total phenolic content determination during immersion within the rosemary serum
The total phenolic content of the rosemary serum used (TPC) and that of the eel fillets during the impregnation and the subsequent osmotic procedure were determined.In the case of fish samples, this was obtained, indirectly, by measuring the TPC of the hypotonic/hypertonic solutions.All measurements were performed in triplicate, according to the modified micromethod of Folin–Ciocalteu’s assay, as described in Andreou et al., 2018.The TPC was expressed as mg of gallic acid equivalents (GAE) per g of fish.The FRAP assay of the rosemary extract was carried out according to the method of Benzie and Strain (1996), as modified by Lantzouraki, Sinanoglou, Zoumpoulakis, and Proestos(2016).The antioxidant activity was expressed as mg FeSO4⋅7H2O per 1 L of extract.
2.3. Comparative stability study under chill storage
The main purpose is to design and implement a shelf life study of the treated samples (both OS and RS/OS), in order to compare their quality degradation versus the corresponding changes of untreated samples.The goal is to assess their shelf life, and evaluate the effect of the treatments imposed.Based on the mass transfer results, the maximum antioxidant impregnation and the minimum sensory alteration of fresh eel fillets,samples osmotically treated in the osmotic solution of 45% glycerol were selected and studied during their subsequent chill storage at controlled isothermal conditions of 4◦C, after being air-packaged and thermally sealed in a laminate PET/Al/LDPE film.
2.3.1.Microbiological analysis
For microbiological determinations, 10 g of representative fish sample were homogenized with 90 mL of sterilized Ringer solution(Merck, Darmstadt, Germany) in a sterile stomacher bag for 60 s using a Stomacher (BagMixer®, interscience, St Nom la Bret`eche, France).Then,0.1 mL of 10-fold serial dilutions of fish homogenates were transferred and spread on the surface of appropriate culture media in Petri dishes for spoilage bacteria enumeration.Plate count agar (PCA, Merck, Darmstadt, Germany) was used for the enumeration of both total viable count(incubation at 30◦C for 72 h) and psychrophiles (incubation at 8◦C for 120 h).Yeasts and molds were enumerated on Rose Bengal Chloramphenicol Agar (RBC, Merck, Darmstadt, Germany) incubated for 168 h at 25◦C.Lactic acid bacteria (LAB) were enumerated on De Man-Rogosa-Sharpe Agar (MRS, Merck, Darmstadt, Germany) followed by incubation at 25◦C for 96 h.For the H2S-producing bacteria Iron Agar was composed as described by Gram, Trolle, and Huss (1987) and incubated at 25◦C for 96 h.Two replicates of at least three appropriate dilutions were enumerated.Microbial growth modeling was carried out using the Baranyi growth model (Baranyi & Roberts, 1995), by fitting curves using the DMFit program (http://www.combase.cc/index.php/en/).Different kinetic parameters, namely the rate (k) of the microbial growth, the lag phase (λ), and the final microbial population predicted (Nmax) were estimated at the processing conditions investigated.
2.3.2.Total lipid extraction and evaluation of lipid oxidation
Total lipids were extracted according to the Bligh and Dyer method(Bligh & Dyer, 1959), and their content was calculated gravimetrically.Oxidation reactions in fish flesh have been evaluated using the TBARs method, which is a standard evaluation method for foods of animal origin, showing high correlation with sensory evaluation data (Fern´andez, P´erez-Alvarez, & Fern´andez-L´opez 1997).More specifically, for fish products, where highly unsaturated fatty acids are found, the later stages of oxidation are characterized by the secondary oxidation products, which are indicative of a history of autoxidation.For the evaluation of secondary oxidation products, the measure of thiobarbituric acid-reactive substances (TBARs) is suggested (FAO, 1995).Lipid oxidation level was estimated by the thiobarbituric acid assay according to the extraction method described in Witte, Krause, & Bailey, 1970.The absorbance was measured at 530 nm using a digital spectrophotometer(Hitachi U-3210; Hitachi, Ltd., Tokyo, Japan).Concentrations of thiobarbituric acid reactive substances (TBARS) were calculated from a standard curve prepared by 1,1,3,3-tetraethoxypropane and expressed as mg malondialdehyde per kg of fish flesh.Each measurement was carried out in three replicates in order to take the average values and standard deviations.
2.3.3.Sensory analysis
The sensory attributes of raw fish were evaluated by a trained sensory panel of 8.Appearance and odour of raw fish slices were evaluated and sensory scores were recorded in appropriate forms, reflecting the organoleptic evolution of quality deterioration.Additionally, panelists were asked to score the overall impression and acceptability.Rating was assigned separately for each parameter on a 1 to 9 hedonic scale (9 =like extremely and 1 =dislike extremely).A sensory score of 5 was taken as the average score for minimum acceptability.
2.3.4.Statistical analysis
The analysis of the rates of quality deterioration for the untreated,OS, and RS/OS treated eel fillets was performed by analysis of variance(ANOVA) at a 95% significance level using STATISTICA® 7.0 (StatSoft Inc., Tulsa, OK, USA).Duncan’s multiple range test was used for the evaluation of significant differences (a =0.05).
3.Results and discussion
3.1. Antioxidant impregnation and osmotic treatment
3.1.1.Antioxidant impregnation using rosemary serum,followed by osmotic treatment(RS/OS)
Rosemary extract solution was measured to have 2007.65 ±112.89 mg GAE/L and its antioxidant activity was found 20166.95 ± 2.33 mg FeSO4⋅7H2O per 1 L (FRAP assay).The impregnation of phenolics in the eel fillets during immersion in Rosemary Serum solution, followed by the osmotic step (RS/OS procedure) is shown in Fig.1, presenting the significant enrichment of fish flesh with the valuable bioactive compounds.Rate of compounds penetration is rapid during the first period where samples are immersed within the hypotonic solution and shows an abrupt increase at the point where the solution becomes hypertonic with the addition of the osmotic solutes.During the second stage of osmotic treatment, phenolics content seems to be stable, without observing any leakage or back diffusion of bioactive compounds to the osmotic solution, a finding that agrees with Giannakourou et al., 2019.A possible explanation could be that phenolic compounds of low molecular weight gradually diffuse towards the centre of the fish fillets due to the increase of osmotic pressure gradient among the outer and inner fish cells, as water is lost faster from the outer cellular structures.Moreover,according to R´ozek, Achaerandio, Güell, L´opez, and Ferrando (2007),during osmotic process, the osmotic solute, e.g.glycerol, concentrates on the fish surface, hindering further transfer of phenolic compounds.On the other hand, glycerol concentration of the osmotic solution was found to significantly (P<0.05) affect the recorded impregnation, with 45% glycerol solution leading to a final content of approximately 3.2 mg GAE/g (3200 ppm), which is considered as a satisfactory antioxidant level for the fish flesh.
3.1.2.Mass transfer during osmotic treatment(both RS/OS and OS procedures)
As far as mass transfer phenomena are concerned, the osmotic treatment (OS) resulted in mild moisture loss and solid uptake and a significant water activity reduction of fish muscle.The mass exchange indices, namely, water loss, solid gain and awchanges of eel samples during osmotic dehydration are illustrated in Fig.2a–d.Regarding the RS/OS process, the first stage of antioxidant impregnation within the hypotonic liquid (60 min) did not seem to modify the trend of mass transfer phenomena, and figures for the combined process (RS/OS) are very similar to the ones depicted in Fig.2 (OS process).In both procedures, when reaching the equilibrium, parameters obtain almost identical values (e.g., awreaching a minimum value of 0.899 ±0.008),slightly affected by glycerol concentration, which seems to positively enhance mass transfer.As far as salt content is concerned (Fig.2d), it increases with processing time and decreased with solute concentration.This may be attributed to the gradual development of a film on the surface of the eel samples which becomes more viscous as the concentration of glycerol increases, leading to a slower salt diffusion through the fish flesh in the osmotic solution and as a result it reduces transfer of salt into fish flesh ((Collignan & Raoult-Wack, 1994); Tsironi et al.,2009).
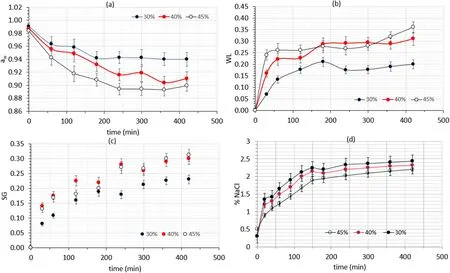
Fig.2.(a) Water activity decrease, (b) Water Loss, (c) Solid Gain and (d) NaCl percentage of eel samples during the (OS) process at different concentrations of the osmotic solution.Error bars represent the ±standard deviation of measurements.
3.1.3.Quality changes-lightness and elasticity retention
Color, expressed as the loss of the initial lightness (L*), showed no significant (p <0.05) change during the osmotic procedure (OS process)(Fig.3a), and the different glycerol concentrations did not seem to have a significant effect on lightness retention.Osmo-treated eel fillets showed significantly (p < 0.05) lower L* (lightness) values and higher b* (yellowness/blueness) and a* (redness/greenness) values than the control samples.After 3 h of osmotic treatment, L* (lightness) value decreased to about 50, a* to a value of - 1 and b* reached a value of 4,with the control samples assuming values of approximately 60, 0 and 0.5, respectively.This increased yellowness is also demonstrated by the hue angle values, that were found to be rather stable, in the range between 70 and 100◦during the OD process, for all glycerol concentrations tested.As far as the combined process (RS/OS) is concerned, when samples were initially immersed into the Rosemary Serum solution up to 60 min, they obtained a more yellowish color (b* value reached a value of 10), and their lightness decreased (L* reached a value of 48), mainly attributed to the slightly colored serum used.In this case, color changes are probably attributed to the colored phenolic compounds transfer from the osmotic solution to the fish tissue.After adding the osmotic compounds and proceeding with the OS process (RS/OS), lightness retained its initial value (L*≈60.00) following a similar pattern as the one shown in Fig.3a for the OS process.
The same good retention was observed in elasticity measurements,both in OS and RS/OS samples, which preserved their initial cohesive texture (Fig.3b).In Fig.4a and b, representative photos of eel samples during both OS and RS/OS, respectively are presented, in comparison to the appearance of the initial, untreated samples.
3.2. Stability study during chill storage
3.2.1.Growth of spoilage bacteria

Fig.3.(a) Lightness decrease and (b) Elasticity change during the (OS) process at different concentrations of the osmotic solution.Error bars represent the ±standard deviation of measurements.

Fig.4.Osmotically treated eel samples with 45% glycerol and 5% salt (a) without and (b) with the addition of rosemary extract in the osmotic solution.
Based on the results of the impregnation procedure, the dehydration performance of the OS process, as well as the quality changes of fresh eel fillets, samples osmotically treated in the osmotic solution of 45%glycerol, for both OS and RS/OS, were selected for the stability study.As far as microbial measurements are concerned, the antimicrobial activity of the extract was assessed, via the total viable count, which was found below the detection limit.The growth curves of the tested spoilage bacteria (total viable count and psychrophiles) in fish samples (untreated, OS, and RS/OS) stored at 4◦C were fitted to the Baranyi growth model, and the growth kinetic parameters at each processing condition were calculated (R2=0.901–0.999).A small lag phase was observed in the growth of total viable count (Table 1).The microbial growth rate (k,days-1), i.e., the rates at the linear phase of the measured microorganisms, the initial (Noin log cfu/g) and final population (Nmaxin log CFU/g), and the estimated lag phase (lag in days) are presented in Table 1.The initial total viable count and psychrophiles count of untreated fish samples averaged 3.0 log CFU/g and 3.2 log CFU/g, respectively, which is slightly below the mean values reported in the literature, indicating good hygiene practices in the fish processing plant (Tsironi et al., 2009;Tsironi & Taoukis, 2012).From the above results (Fig.5 and Table 1),the protective role of both the OD solutes introduced (OS process) into the eel fillets and, the additive effect of the bioactive compounds’impregnation (RS/OS process) is assessed.Lowering the water activity to a level of <0.95 may have a pronounced effect on psychrotropic species growth (Neumeyer et al., 1997a, 1997b).As a consequence,osmotic pre-treatment can extend the shelf life of eel, reducing the initial load and delaying microorganisms’ growth.The beneficial effect is also evident for the growth of total viable count, where the growth rate of the untreated samples was more than six-fold that of the treated fish.This observation is in agreement with previous studies, where an inhibitory effect on microbial activity was recorded as a result of using phenolic-rich extracts on aquatic tissues (de Camargo et al., 2017;Miranda, Carrera, Barros-Vel´azquez, & Aubourg, 2018; Ortiz-Viedma et al., 2017; Oucif et al., 2018).According to Mahmoud et al., 2004, the total viable count in treated carp fillets using carvacrol and thymol (1%solutions) was up to 4 log CFU/g lower than the one measured for the untreated samples after 12 days at 5◦C.In any case, from the results obtained in this study, the water activity decrease (through the OS procedure) was found to be the main inhibitory factor in microbial growth, with rosemary extract having a mild antimicrobial effectiveness.
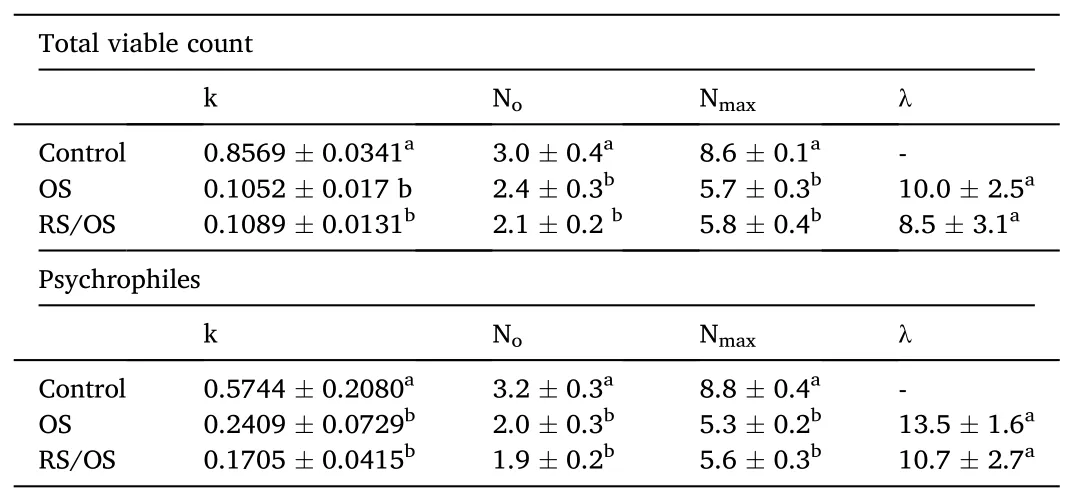
Table 1 Growth rates (k in days-1), initial (No in log CFU/g) and final population (Nmax in log CFU/g), and lag phase (λ in days) of total viable count and psychrophiles of eel slices stored at 4 ◦C.
Slow growth of lactic acid bacteria (LAB) was observed in untreated samples, with microbial load starting from 2 logFU/g at day 0 and reaching final loads of 4.6 logCFU/g after 8 days of refrigerated storage.Osmotic treatment resulted in inhibition of LAB growth, with the final load measured never exceeding 3 logCFU/g during the shelf life study.The addition of rosemary extract in the osmotic solution did not affect significantly the growth of LAB in eel flesh (P>0,05).Yeasts and molds were also monitored during refrigerated storage of eel slices and remained below the detection limit (i.e.<2 logCFU/g) during the storage period for untreated and the osmotically treated eel slices using the alternative osmotic solutions.
Enterobacteriaceae and H2S producing bacteria were only enumerated at the starting and ending points at each processing tested.The observed final populations were well below the TVC and psychrophiles counts (mainlyPseudomonas), indicating that the growth of Enterobacteriaceae and H2S producing bacteria did not play a significant role in eel fillet spoilage This is in agreement with relevant studies in the literature about the spoilage mechanism of aerobically packed from fresh waters (Gram & Huss, 1996).
3.2.2.Lipid oxidation
Content of malondialdehyde in foods is generally associated with oxidative rancidity, which can be measured by the number of.Thiobarbituric acid (TBA) (Tas¸kaya & Yas¸ar, 2018), and then TBA values are used to evaluate the levels of secondary oxidation products.Initial malondialdehyde (MDA) content was in the range between 2.2 and 3.6 mg MDA/kg of fish flesh.As expected, TBA values increased with storage in all samples (Fig.6), and TBA values of all treated samples (OS and RS/OS) were statistically different compared to the untreated samples.The synergistic effect of antioxidant impregnation with the dehydration obtained by the osmotic procedure is particularly evident on the rate of lipid oxidation, which constitutes an important cause of spoilage and rejection of fat fish.

Fig.5.Microbial growth of (a) total viable count and (b) psychrophiles for all samples stored at 4 ◦C (data points indicate the results of representative fish sample).Error bars represent the ±standard deviation of measurements.
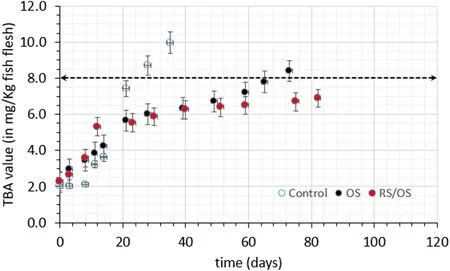
Fig.6.Lipid oxidation of eel fillets (expressed as concentration of malondialdehyde, μmol MDA/g of fat) during storage at 4 ◦C (the limit of acceptability of 8.0 mg/kg fish flesh is also illustrated within the figure).Error bars represent the ±standard deviation of measurements.
In recent literature, it has been reported that such extracts, rich in phenolic compounds can be effectively used to extend aquatic products’shelf life (Ali et al., 2019; Qiu, Jacobsen, & Sørensen, 2018; Raeisi,Quek, Ojagh, & Alishahi, 2016; Tayel, Almabady, Sorour, & Diab, 2018;Viji et al., 2017; Yu et al., 2017), possibly due to the interaction of the phenolic substances with the free lipid-peroxy or lipid-oxy free radicals,preventing their further decomposition.In Yavuzer, ¨Ozogul, & ¨Ozogul,2020 the potential use of vegetable peels as source of antioxidants was investigated during the icing of rainbow trout fillets, while in Giannakourou et al., 2019 the application of by-products of the distillation industry of aromatic herbs was tested in order to extend sea bass fillets shelf life.In Yang et al., 2019 the application of Tartary Buckwheat Extract in a coating, combined with chitosan was tested in tilapia preservation.As far as rosemary extract is concerned, although having been extensively used for the preservation of meat and oil, there are limited surveys on its application for aquatic products (Xie, VanAlstyne,Uhlir, & Yang, 2017).provided a detailed review on the antioxidant capacity of rosemary extract, and its potential use in food industry.In this study, it was shown that rosemary extract has also a positive impact on alleviating the rancid sensation in foods and protecting them from color loss.In Fellenberg, Carlos, Pe˜na, Ib´a˜nez, & Vargas-Bello-P´erez,2020, the effect of using natural antioxidants, such as rosemary, applied in the marinating process on the oxidative and sensory quality in rainbow trout fillets was investigated (Linhartov´a et al., 2019).showed the delay of the oxidation process obtained in rainbow trout fillets when rosemary extracts were used.Martínez, Castillo, Ros, & Nieto, 2019 tested the effect of the application of natural extracts from pomegranate,rosemary, and hydroxytyrosol on both the lipid oxidation and the microbiological spoilage in fish patties, and concluded that a significant extension of their shelf life under retail display conditions was accomplished.The effect of rosemary extract combined with nisin on the quality of pompano (Trachinotus ovatus) was evaluated during cold storage in (Gao et al., 2014) and shelf life was found to be significantly extended (Li et al., 2012).showed that a dipping treatment with 0.2%rosemary extract could effectively delay microbial growth and chemical deterioration, improve sensory attributes and extend the shelf-life of crucian carp.
3.2.3.Color and texture changes
Storage at 4◦C affected color parameters of untreated and treated eel fillets.L* value of all categories of samples remained almost constant during chill storage, with lightness of osmosed samples (both OS and RS/OS samples) being significantly lower than that of the control samples (Fig.7a).Hue angle parameter significantly (P< 0.05) increased throughout the preservation period for the untreated samples (Fig.7b),reaching -after microbial spoilage-values from 160◦to 220◦, close to the green-turquoise region.On the other hand, osmo-treated, previously impregnated with phenolic compounds (RS/OS procedure) eel samples showed a good retention of their yellowish color (values up to 120◦).As far as samples being solely osmo-dehydrated (OS procedure), they also exhibited a gradual increase of their hue angle, without departing from the yellowish region (final values around 140◦).
Greening discoloration in fresh meat has been previously reported(Chen & Hu, 1989; Faustman & Cassens, 1990; Jiang & Lee, 2004) and may be associated with an alteration in heme structure, with two possible myoglobin derivatives being mainly responsible for a green appearance, namely choleglobin and sulfmyoglobin.(Faustman & Cassens, 1990).In our study of white eel flesh, color changes during storage showed that the phenolics, impregnated into the fish tissue, protected more effectively against color alterations, probably attributed to the formation of colored myoglobin derivatives.This observation clearly pointed to the fact that consumer-desirable pale color (white flesh) of eel could be better retained by applying the proposed combined process.
Regarding texture alterations, osmotic treatment, with or without an initial impregnation step, did not seem to significantly affect any of the TPA parameters calculated (data not shown) and texture of all samples was well retained throughout the chill storage.
3.2.4.Sensory evaluation
The sensory characteristics (appearance, odour) of untreated and osmotically pre-treated eel slices, with and without rosemary extract,were evaluated and the results are given representatively in Fig.8.Osmotic pre-treatment maintained the sensory attributes of fish samples,indicating freshness for longer times than the untreated fillets.
3.2.5.Shelf life determination
Regarding lipid oxidation, a limit of acceptability often used in fatty fish is related to the sensory perception of rancid odour and taste, and thus TBA value should be at least 3 in a very good material, close to 5 in a fairly good food and the limit value for rejection is often set at a value of 8 mg MA/kg fish flesh (Alparslan, Baygar, Yapici, & Metin, 2013;Tas¸kaya & Yas¸ar, 2018).In our study, one could roughly estimate eel fillets shelf life based on Fig.5, with control samples reaching the limit of 8 mg MDA/kg of fish flesh after approximately 25 days, OS after 65 days, and RS/OS remaining at lower levels, even after 82 days of storage at 4◦C (approximate values deduced from the experimental curves obtained).Similarly, if the acceptability limit was set based on microbial spoilage and assumed to be equal to a level of 107CFU/g for total viable count (estimated when eel fillets were organoleptically rejected by a preliminary sensory test and also reported in (Arkoudelos et al., 2007)for eel), then, the fish fillet shelf life could be estimated using the following equation (Equation (3), according to (Sofra et al., 2018;Tsironi & Taoukis, 2012):
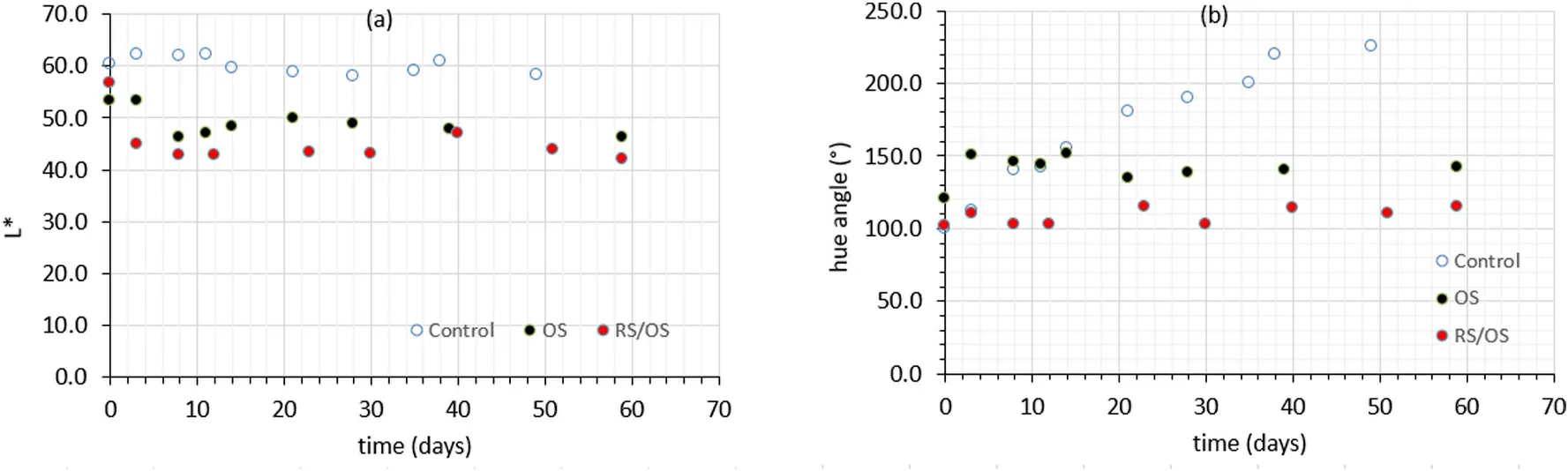
Fig.7.(a) Lightness (L*) decrease and (b) change of hue angle during storage of eel fillets at 4 ◦C.

Fig.8.Sensory scoring for (a) appearance and (b) odour of eel slices (Control: untreated, OS: osmotically treated with 45% glycerol and 5% NaCl, RS/OS: rosemary extract and 45% glycerol and 5% NaCl) during refrigerated storage for 0.3, 8 and 14 days for Control samples and 0.3, 8, 14 and 28 days for OS and RS/OS samples.
where Ntand Noare the final (limit of acceptability) and the initial microbial population, tlagis the lag phase, and k is the relative growth rate (Table 1).Therefore, the shelf life of the eel fillets, treated or not,was found to be 5 days for the untreated samples (in agreement with(¨Ozogul et al., 2005)), 54 days for the OS treated samples, and 52 days for the RS/OS-treated ones.This findings are in agreement with(Choulitoudi et al., 2017) where the incorporation of rosemary essential oil (EO) and extracts in a carboxyl methyl cellulose-based edible coating was found to have an antioxidant and antimicrobial effect when applied to smoked eel.Authors showed that the addition of the extract at 200–800 ppm within the coating offered significant antioxidant protection, whereas the antimicrobial activity of EO and the extracts was moderate, a conclusion that coincides with our findings.Similar observations have been reported in (Choulitoudi et al., 2016), where authors studied the antimicrobial and antioxidant activity of several extracts for gilthead sea bream fillets, showing that each extract has a different level of effectiveness towards microbial inhibition and/or oxidative deterioration.
In any case, it should be stressed out that the level of the shelf life extension accomplished by the application of the specific hurdles implementation is strongly dependent on many factors, such as the initial microbial load, the initial quality status of the raw material, etc.Bearing this in mind, days of perishability provided in this section can only serve as an indicative example for the beneficial role of the processing steps applied.
4.Conclusions
The objective of the present study was to assess the effect of osmotic dehydration with and without a prior bioactive compound impregnation on the stability of eel fillets.Osmotic treatment by its self provided improved quality stability during refrigerated storage, in terms of microbial spoilage and oxidative deterioration.After the application of the selected, optimized osmotic dehydration conditions, the awof eel fillets decreased to a level less than 0.90, which inhibited significantly microbial growth.On the other hand, the immersion of eel samples within the rosemary extract, leading to the incorporation of specific bioactive compounds had a more pronounced effect on lipid oxidation delay (a major cause of degradation for fatty fish), compared to microbial growth spoilage.The indices that could be used to assess eel fillets shelf life during chilled storage were total microflora growth and TBA values, the latter being easily correlated to sensory evaluation.Osmotic treatment,following the impregnation within phenolic-rich rosemary extract was the most effective combined technique, according to hurdle technology principles, for the preservation of eel fillets.
CRediT authorship contribution statement
Maria C.Giannakourou:Conceptualization, Methodology, Supervision, Writing - original draft, Writing - review & editing.Natalia Stavropoulou:Investigation, Writing - original draft.Theofania Tsironi:Methodology, Supervision, Writing - review & editing.Vladimiros Lougovois:Supervision.Vassiliki Kyrana:Investigation.Spyros J.Konteles:Investigation.Vassilia J.Sinanoglou:Conceptualization,Methodology, Supervision, Writing - review & editing.
Acknowledgements
Authors would like to thank Dr Eleni Gogou and Dr Dimitris Tsimogiannis from Natural Food Additives (NFA) S.A for kindly providing the rosemary serum.
杂志排行
Aquaculture and Fisheries的其它文章
- A framework for risk analysis of the shellfish aquaculture: The case of the Mediterranean mussel farming in Greece
- Slurry ice as an alternative cooling medium for fish harvesting and transportation: Study of the effect on seabass flesh quality and shelf life
- Physicochemical properties of silver carp (Hypophthalmichthys molitrix)mince sausages as influenced by washing and frozen storage
- Bacterial community in response to packaging conditions in farmed gilthead seabream
- Effective algorithmic operational framework for fish texture evaluation in industry: Achieving maturity
- Seasonal variation in the biochemical composition, condition index, and meat yield of the non-indigenous pearl oyster Pinctada imbricata radiata(Leach, 1814) from the West of the Aegean Sea, Greece
