Influence of pretreatment conditions on the structure and catalytic performance of supported cobalt catalysts derived from metal-organic frameworks
2023-10-14SUNJiaqiangZHENGShenkeCHENJiangang
SUN Jia-qiang,ZHENG Shen-ke,CHEN Jian-gang
(1.State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2.University of Chinese Academy of Sciences, Beijing 100049, China;3.Hubei Key Laboratory for Processing and Application of Catalytic Materials, College of Chemistry and Chemical Engineering, Huanggang Normal University, Huanggang 438000, China)
Abstract: Supported cobalt catalysts (Co@C-ZnZrO2 and Co/ZnZrO2) were prepared through a metal-organic frameworks(MOFs)-mediated synthesis strategy.The influence of MOFs pyrolysis on the structure and Fischer-Tropsch synthesis performance of supported cobalt catalysts was investigated.The crystalline phase and microstructure of supported cobalt catalysts were characterized by powder X-ray diffraction (XRD),transmission electron microscopy (TEM),high-resolution TEM (HRTEM),N2 adsorption-desorption and X-ray photoelectron spectroscopy (XPS).The Co/ZnZrO2 showed the CO conversion of 18.1% and the C5+ selectivity of 77.4%,whereas the Co@C-ZnZrO2 exhibited the CO conversion of 8.5% and the C5+ selectivity of 35.2%.The excellent CO conversion for Co/ZnZrO2 was attributed to the more exposure of active Co sites.Meanwhile,the activity of Co sites on Co@C-ZnZrO2 catalyst was restricted by the carbon layer,suppressing the adsorption and activation of syngas on Co sites.
Key words: pyrolysis;metal-organic frameworks;supported cobalt catalysts;Fischer-Tropsch synthesis
Supported metal catalysts are objects of great interest in heterogeneous catalysis due to their unique chemical and physical properties[1].They can catalyze the synthesis of bulk chemicals and transportation fuels.Especially,supported cobalt catalysts are widely used for Fischer-Tropsch synthesis (FTS)[2-5].For supported cobalt catalysts,their performance depends strongly on the nature of the active metal sites on the catalyst surface and support[6-9].The nature of active site is closely correlated to the size of Co nanoparticles(NPs),size distribution and crystal phase[10,11].The supports with high specific surface area are expected to disperse the active phase giving a high metal specific surface area and stabilize the active phase against loss of specific surface area during the reaction[12,13].Thus,an ideal supported cobalt catalyst would exhibit not only uniform Co distribution but also highly reduced and dispersed Co sites[14].These factors strongly depend on the preparation method of the catalysts.In this respect,it is not surprising that the development of synthetic methods for the supported cobalt catalysts has received tremendous attentions.Impregnation[15,16],deposition-precipitation[17],and sol-gel methods[18]are among the most commonly used methods to control and tune these factors.By using such methods,the structure performance relationships are also established,aiming to develop better catalysts.However,it is not easy to achieve high Co dispersion by using traditional preparation methods,because it usually means smaller Co particles.The derived stronger interaction between metal and support often leads to the decreased reduction of Co,which lowers the catalytic activity for FTS[19].
Metal-organic frameworks (MOFs),a new class of porous coordination polymer,which are self-assembled by metal nodes and organic ligands through chemical coordination bonds,have been extensively investigated in a number of fields including catalysis,gas adsorption,gas separation,sensors,and drug delivery[20].In particular,MOFs have attracted significant attentions for their application in heterogeneous catalysts design due to the high dispersion of active sites,highly uniform porous structure,large specific surface area and tunable porosity[21-23].In addition to direct applications,MOFs have been widely developed as promising sacrificial templates/precursors to fabricate supported cobalt catalysts with high porosity,high specific surface area and distinctive morphology by applying different thermal and/or chemical treatments.Typically,Co@C catalysts can be prepared by high-temperature pyrolysis of MOFs in inert atmosphere[24].The Co@C catalysts could present a small crystalline size even at high metal loading.Unfortunately,the Co NPs are usually covered by the graphitic carbon shells,which makes the surface of metal NPs difficult to access.Thus,their application in FTS is restricted since the activity and selectivity are unsatisfying[25-28].In order to improve the FTS performance of MOF-derived Co@C catalysts,the Co NPs supported by porous carbon or silica as the FTS catalysts have also been prepared from Co MOF-74 and ZIF-67 by direct pyrolysis or multi-step approaches (pyrolysis,calcination and reduction),and show very competitive activity and selectivity[29,30].In addition,due to the flexibility of MOFs in structure and chemical composition,supported cobalt catalysts derived from MOFs can also be designed rationally by selecting versatile metal centers and ligands.The interesting results demonstrate that the MOF-mediated synthesis strategy is a promising route for the preparation of supported cobalt catalysts with outstanding FTS performance.
Herein,we investigated the MOFs-derived Co@C-ZnZrO2and Co/ZnZrO2catalysts to determine the influence of the thermal treatment methods of the MOFs precursors on the structure and catalytic performance of the supported cobalt catalysts.Detailed characterizations were used to establish the relation between the catalytic performance and structure.By using multi-step approach,Co/ZnZrO2catalysts with highly exposed active sites were synthesized,and exhibited high activity and selectivity.
1 Experimental
1.1 Catalysts preparation
ZCZ-MOFs were prepared by a one-pot solvothermal method.Firstly,certain amounts of Co(NO3)2·6H2O,Zr(NO3)4·5H2O,Zn(NO3)2·6H2O with molar ratios of Co∶Zr∶Zn=1∶1∶2 were dissolved with 200 mL water.Then,40 mmol 1,4-benzenedicarboxylic acid were dissolved in 200 mL DMF to form a clear solution.Afterwards,the asprepared two solutions were mixed under a magnetic stirring.The reaction mixture prepared above was transferred directly into a Teflon-lined stainless steel autoclave and heated at 120 °C for 12 h.The asprepared samples were filtered out and washed with DMF and water and finally dried at 80 °C for 12 h.
The as-prepared ZCZ-MOFs were carbonized at 800 °C for 2 h under Ar.The obtained samples were denoted as Co@C-ZnZrO2(39% Co,0.5% Zn,42%Zr).
The as-prepared ZCZ-MOFs were calcinated at 400 °C for 4 h under air to form CoZnZrO2.The CoZnZrO2was further reduced to Co/ZnZrO2in H2at 400 °C for 4 h.When the temperature decreased to room temperature,the samples were passivated with 1% O2/N2for 1 h,forming CoOx/ZnZrO2(20% Co,31%Zn,31% Zr).
1.2 Characterizations
The transmission electron microscopy (TEM)images and high-resolution TEM (HRTEM) images were obtained on a JEM 2100F HRTEM.The X-ray diffraction (XRD) experiments were performed on a Bruker D8 Advance provided CuKα radiation(λ=1.5418 Å).The N2adsorption-desorption measurement was carried out on a Micromeritics Tristar II 3020 gas adsorption analyzer.The X-ray photoelectron spectroscopy (XPS) studies were performed on the Kratos Axis Ultra DLD.The elemental composition of the sample was collected on the inductively coupled plasma atomic emission spectroscopy (ICP-AES,Thermo iCAP6300).Hydrogen temperature-programmed desorption (H2-TPD) tests were performed on the Quantachrome Chembet TPR/TPD.
1.3 Catalytic performance evaluation
The catalytic behavior was investigated in a fixed bed reactor.The catalysts diluted with quartz powders(60-80 mesh) were reduced at 400 °C for 4 h by H2with a gas hourly space velocity (GHSV) of 2 L/(g·h).After reduction,the reactor was cooled to 100 °C,the syngas (H2/CO=2,v/v) with a GHSV of 1 L/(g·h) was fed into the catalyst bed and the temperature was increased to 200 °C at 4 °C/min.The composition of the reactants and tail gas were analyzed online by gas chromatography (GC).The H2,CO,CO2,CH4and N2were analyzed by using a TDX column and thermal conductivity detector (TCD).The light hydrocarbons were analyzed using a Al2O3capillary column with a flame ionization detector (FID).The oil and wax were analyzed offline using GC with an OV-101 capillary column and an FID.
2 Results and discussion
2.1 Catalyst characterization
We used ZCZ-MOFs as the precursors to synthesize the Co@C-ZnZrO2and Co/ZnZrO2catalysts for FTS.Firstly,the well-defined ZCZ-MOFs were synthesized via a one-pot solvothermal method according to a literature procedure with a few modification[31].As shown by TEM image in Figure 1,the obtained ZCZ-MOFs show a nanosheet morphology.The crystalline phase of the ZCZ-MOFs was demonstrated by XRD (Figure 2).All the peaks of the ZCZ-MOFs can be indexed to MOF-5∙5H2O[31].Secondly,the MOFs were calcined under different atmospheres,during which the solvent molecules were decomposed and discharged.The ZCZ-MOFs were transformed into Co@C-ZnZrO2by the direct hightemperature pyrolysis in inert atmosphere (Ar,800 °C).Meanwhile,the ZCZ-MOFs were transformed into mixed metal oxides (CoZnZrO2) by calcination in air,and were further reduced in H2,forming the Co/ZnZrO2catalysts.Noteworthily,the obtained reduced samples were then passivated with 1% O2/N2to form the CoOx/ZnZrO2samples,for the convenience of structural characterization.Their crystalline nature was also confirmed by XRD.As shown in Figure 3,Co@CZnZrO2displays characteristic peaks of monoclinic ZrO2(JCPDS No.65-1023),cubic ZrO2(JCPDS No.49-1642) and face-centered cubic (fcc) Co (JCPDS No.15-0806).CoOx/ZnZrO2displays characteristic peaks of hexagonal phase ZnO (JCPDS No.36-1451),cubic ZrO2(JCPDS No.49-1642) and fcc Co3O4(JCPDS No.43-1003).In addition,we can find the fcc Co peaks(JCPDS No.15-0806) for Co/ZnZrO2(Figure 4).

Figure 1 TEM image of ZCZ-MOFs
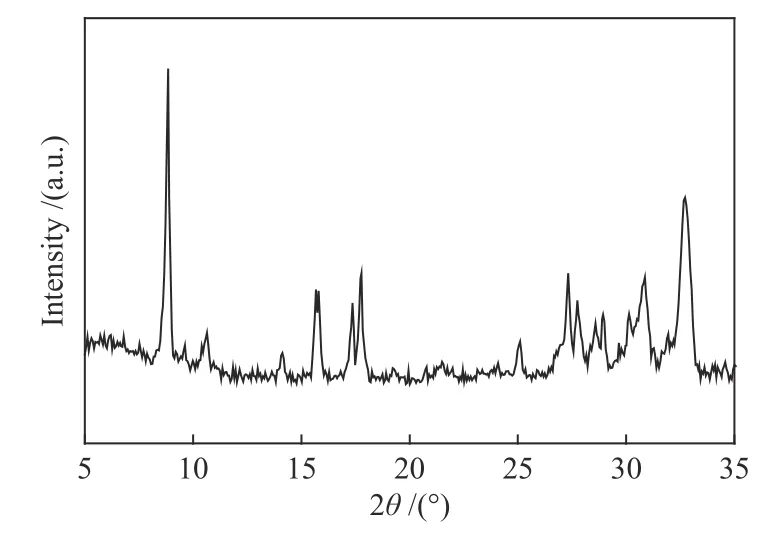
Figure 2 XRD pattern of ZCZ-MOFs

Figure 3 XRD pattern of Co@C-ZnZrO2

Figure 4 XRD patterns of CoOx/ZnZrO2 and Co/ZnZrO2
The morphology and crystal structure of Co@CZnZrO2and CoOx/ZnZrO2were evaluated by TEM and HRTEM.As shown in the TEM image of Co@CZnZrO2catalysts (Figure 5),the sizes of the metallic Co NPs are in the range from 5 to 80 nm.Clearly,metallic Co nanoparticle with the lattice spacing of 0.205 nm is covered by carbon (Figure 5(c)).The CoOx/ZnZrO2catalysts possess a highly porous texture(Figure 6(a)) and some CoOxNPs disperse on the surface of the catalysts (Figure 6(b)).The sizes of the CoOxNPs in CoOx/ZnZrO2are in the range from 2 to 28 nm.HRTEM images further confirm the crystalline feature.The lattice spacing of 0.202 nm corresponds to(400) planes of fcc Co (Figure 6(c)).

Figure 5 TEM ((a),(b)) and HRTEM (c) images of Co@C-ZnZrO2
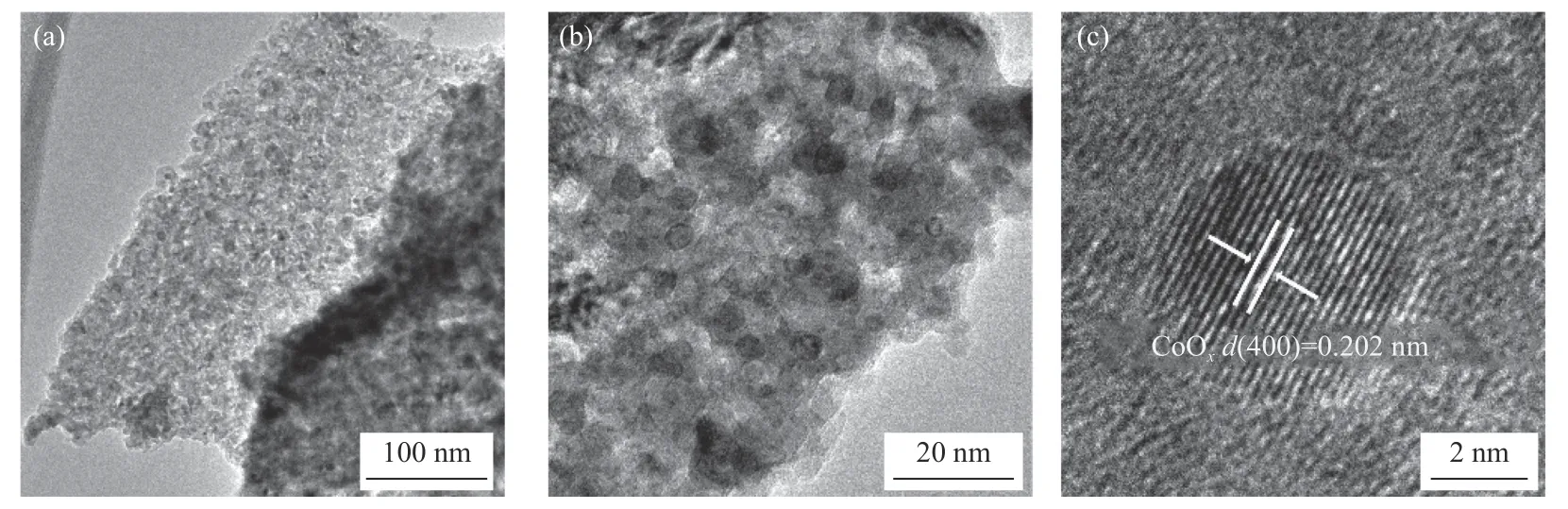
Figure 6 TEM ((a),(b)) and HRTEM (c) images of CoOx/ZnZrO2
The textural properties of the MOFs and the derived catalysts were investigated by N2adsorptiondesorption measurement (Table 1).The MOFs precursors show the Brunauer-Emmett-Teller (BET)specific surface area of 67.2 m2/g and an average pore size of 8.7 nm.Notably,the Co@C-ZnZrO2catalysts obtained by thermal decomposition of the MOFs show the BET specific surface area of 132.6 m2/g and an average pore size of 10.4 nm.Compared with the Co@C-ZnZrO2catalysts,CoOx/ZnZrO2catalysts show smaller BET specific surface area of 28.3 m2/g and an average pore size of 17.5 nm.

Table 1 N2 adsorption-desorption measurement results of the catalysts
XPS was performed to investigate the surface elemental composition as well as chemical properties of those samples.From the full spectra of Co@CZnZrO2,it is observed that the samples show the existence of Co,Zr,O and C species (Figure 7).It is clear that the Co@C-ZnZrO2catalysts show a large amount of C species compared with Co/ZnZrO2catalysts.Meanwhile,CoOx/ZnZrO2and Co/ZnZrO2show the existence of Co,Zr,Zn and O species.As for Co 2pXPS spectra of Co/ZnZrO2and Co@C-ZnZrO2,apart from the existence of Co2+characteristic peaks,the peaks at 778.5 and 792.7 eV corresponding to metallic Co0can also be observed (Figure 8).The Co 2p3/2peak of Co0for Co/ZnZrO2shows lower binding energies compared with that of Co@C-ZnZrO2.In addition,the Zr 3dXPS peaks of Co/ZnZrO2exhibit higher binding energies compared with that of Co@CZnZrO2,indicating the stronger electronic interaction between Zr and Co NPs in Co/ZnZrO2(Figure 9).The XPS peaks of Zn exhibit the same binding energies in all the catalysts (Figure 10).
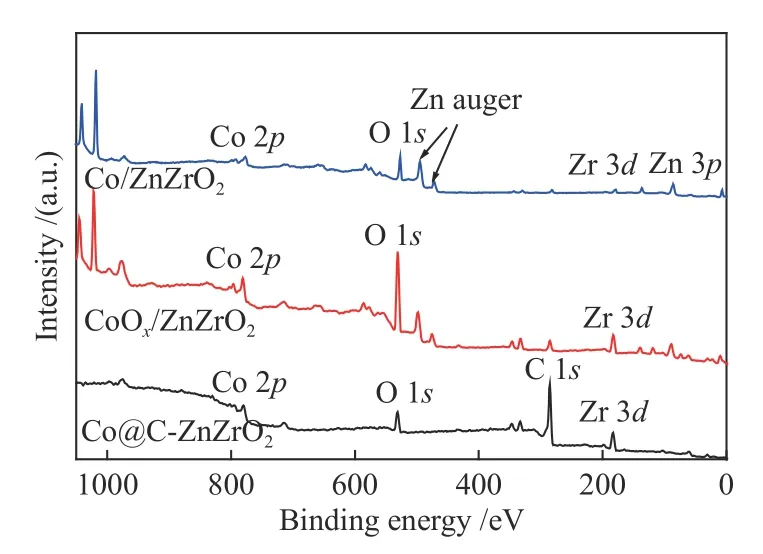
Figure 7 XPS full spectra of the catalysts

Figure 8 Co 2p XPS spectra of the catalysts

Figure 9 Zr 3d XPS spectra of the catalysts
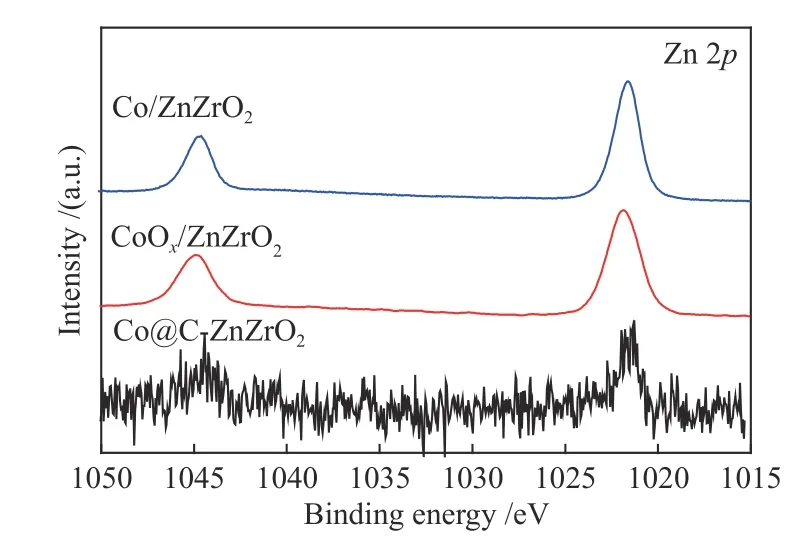
Figure 10 Zn 2p XPS spectra of the catalysts
2.2 Catalytic performance
The catalytic activity of the prepared catalysts with time on stream is shown in Figure 11.The CO conversion of the Co/ZnZrO2catalyst reaches to 18.1%after 24 h at 200 °C.The catalytic activity of Co/ZnZrO2is over 2 times than that of Co@C-ZnZrO2.The turnover frequency (TOF) value of Co/ZnZrO2is 1.0×10-2s-1,while the TOF value of Co@C-ZnZrO2is 0.8×10-2s-1.The reason of the high activity for Co/ZnZrO2may be explained by the fact that Co/ZnZrO2have more active sites than Co@C-ZnZrO2.Furthermore,the activity of the Co/ZnZrO2catalyst did not decrease after 120 h,showing better stability of the catalysts.The selectivity of hydrocarbon products is shown in Figure 12.The CH4selectivity of the Co/ZnZrO2catalyst is about 13%,which is lower than that of the Co@C-ZnZrO2catalyst.The Co/ZnZrO2catalyst exhibits the highest C5+selectivity of 77.4%.On the other hand,the Co@C-ZnZrO2catalyst only exhibits C5+selectivity of 35.2%.The product distributions results show the chain growth probability(α) values of 0.74 for Co/ZnZrO2and 0.45 for Co@CZnZrO2,respectively (Figure 13).

Figure 11 Catalytic activity of the catalysts with time on stream Reaction conditions: v(H2)/v(CO)=2,GHSV=1 L/(g·h),2 MPa,200 °C
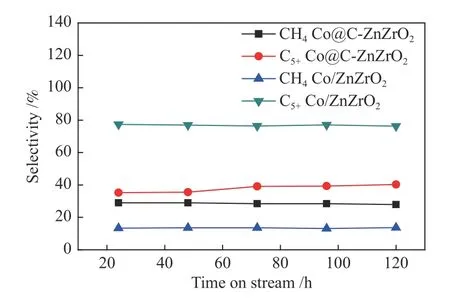
Figure 12 Hydrocarbon product selectivity of the catalysts with time on stream Reaction conditions: v(H2)/v(CO)=2,GHSV=1 L/(g·h),2 MPa,200 °C
To get a thorough understanding of the enhanced activity of Co/ZnZrO2,H2-TPD tests were used for estimating the number of surface active sites of the catalysts.The Co/ZnZrO2shows the hydrogen adsorption amount of 38 μmol/g,while the Co@CZnZrO2shows that of 22 μmol/g,indicating that the Co/ZnZrO2catalyst has more active sites than Co@CZnZrO2catalyst.Despite Co/ZnZrO2exhibits the lower BET specific surface area,the more active sites for Co/ZnZrO2catalyst are responsible for the higher activity.The H2desorption temperature of Co/ZnZrO2is higher than that of Co@C-ZnZrO2,implying that the H2chemisorption on the surface of Co NPs is enhanced for Co/ZnZrO2catalyst,resulting in the higher activity(Figure 14).In addition,it is claimed that zirconium could enhance the activity and heavy hydrocarbon selectivity of Co-based catalysts[32].As clarified by the XPS results,Zr and Co NPs in Co/ZnZrO2have the stronger electronic interaction,which promotes CO adsorption,weakens C-O bond and promotes the chain growth processes,resulting in the higher heavy hydrocarbon selectivity.Zr and Co NPs in Co@CZnZrO2present the weaker interaction.This is because the active Co sites are surrounded by the carbon layer,which suppresses the adsorption of syngas,resulting in the lower activity and heavy hydrocarbon selectivity.Furthermore,Co/ZnZrO2catalyst has the higher chain growth probability than Co@C-ZnZrO2,which reveals that the chain growth on Co/ZnZrO2catalyst is favored,leading to the higher heavy hydrocarbon selectivity.
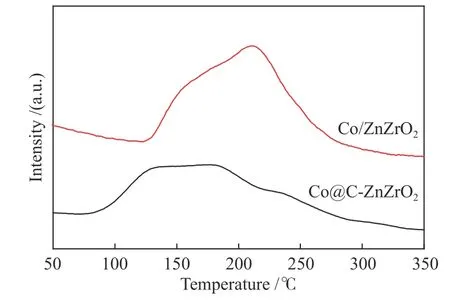
Figure 14 H2-TPD profiles of the catalysts
3 Conclusions
In summary,Co/ZnZrO2catalysts were synthesized by a MOFs-mediated synthesis strategy,which exhibited an excellent catalytic performance for FTS.Co/ZnZrO2showed high CO conversion (18.1%)and high selectivity of C5+products (77.4%).In comparison,Co@C-ZnZrO2exhibited low CO conversion (8.5%) and low selectivity of C5+products(35.2%),indicating that the as-prepared supported Co catalysts exhibited significant differences based on the thermal treatments.The multi-step approach (air calcination following H2reduction) for the preparation of Co/ZnZrO2catalysts circumvents the carbon layer covering the cobalt surface.These results offer an effective strategy for the preparation of the supported metal-based catalysts for diverse reactions.
