Effect of operating conditions on release and transformation of sodium during CFB gasification of Zhundong coal
2023-10-14YANGZijianGUOShuaiWANGXiaofang
YANG Zi-jian,GUO Shuai,WANG Xiao-fang
(1.Institute of Engineering Thermophysics, Chinese Academy of Sciences, Beijing 100190, China;2.China University of Petroleum (Beijing), Beijing 102249, China))
Abstract: To provide some useful suggestions to the operation of circulating fluidized bed (CFB) gasifier,the effect of gasification temperature,residence time and agent on the release and transformation of sodium was studied by using a fixed bed reactor combined with Factsage software.The results indicated that gasification temperature was the significant factor to the release and transformation of sodium.For the promoting effect of sodium release,it was ascribed to the intense of sodium volatilization and competitive reaction between lime and meta-kaolin.Meanwhile,the high temperature promoted the formation of nepheline and slag.The threshold temperature of latter was near 950 °C.It was interesting to find that the release of sodium could be divided into two stages: coal pyrolysis and char gasification.In coal pyrolysis,part of organic and watersoluble sodium was released.The remainder either combined with char structure,or reacted with minerals.In char gasification,Sodium,combined with char structure,was released along with char gasification.Due to the decrease of melting temperature and the formation of NaOH,steam showed a promoting effect on the sodium release.Oppositely,oxygen and nitrogen presented an inhibiting effect.The former was ascribed to the formation of Na2SO4,while the latter was caused by the chemical binding and physical wrapping effect of char.
Key words: Zhundong coal;gasification;sodium;release and transformation
Zhundong (ZD,for short) coalfield is the largest integrated coalfield in China[1-3].It is of great significant significance to ensure national energy security;therefore,the efficient utilization of ZD coal is crucial.Circulating fluidized bed (CFB,for short)gasification is one of the promising ways for clean and efficient conversion of ZD coal,which is featured by wide fuel adaptability,flexible agent,excellent environmental indicators and variable load adjustment range.It has been widely used in industrial gas fields such as alumina roasting,ceramic firing and coal coking.The fields of synthesis gas such as synthetic ammonia and petroleum refining are also extended.So,three CFB gasifiers scaled by 40000 m3/h have been put into the commercial operation in Xinjiang province.However,some sodium-related problems,such as ash sintering and slagging,often encountered.Those problems leaded the shutdown of gasifier as a result of bed defluidization and non-returnable material.Thus,the corresponding reasons behind should be further investigated[4,5].
For CFB gasification,the key to the stable operation is whether the stable fluidization and normal circulation of solid particles can be ensured.However,due to the limitation of available coal resources,the companies in Xinjiang and surrounding areas often purchase high-sodium coal as the feeding coal.The formed low-temperature eutectic could easily cause serious slagging of the bed.Meanwhile,those sodium species volatilized into gas phase could easily lead to serious corrosion of the refractory material of the gasifier,blockage of the syngas outlet,and fouling of the heat exchange surface.Those sodium-related problems will lead to the abnormal shutdown of the gasification system,resulting in huge economic losses and safety hazards.Thus,it is of great significance to deeply understand the effect of gasification conditions on the transformation behaviors of sodium.Wang et al.[6]and Song et al.[7]studied the transformation of sodium species during pyrolysis of ZD coal.Yang et al.[8]compared the sodium transformation and ash deposition characteristics during combustion and gasification of ZD coal in CFB reactor.
At present,there have been considerable reports on the release and transformation of sodium.However,the systematic report is insufficient for CFB gasification.Moreover,the release and transformation behaviors of sodium during different stage,such as coal devolatilization and char gasification,have not been explored.In view of this,on the basis of previous research results,this experimental study combined Factsage simulation calculation had been conducted,the effects of gasification temperature,agent type and residence time on the sodium transformation behaviors was systematically investigated.As a summary,the possible transformation behaviors of sodium species in the CFB gasification process is proposed.The results could provide some theoretical guidance for the reasonable CFB gasification utilization of ZD coal.
1 Experimental
1.1 Test sample
In this experiment,ZD coal,from Xinjiang province,was selected as the test sample.After drying,the sample was ground to a particle size of less than 200 mesh.The corresponding proximate and ultimate analysis are shown in Table 1,and the ash component analysis is shown in Table 2.From Table 1,Table 2,it is known that the coal belongs to medium-high volatile matter,low-ash,low-sulfur,and high sodium coal.Before starting the experiment,the coal samples were dried again at 105 °C for 2 h to eliminate the influence of moisture.
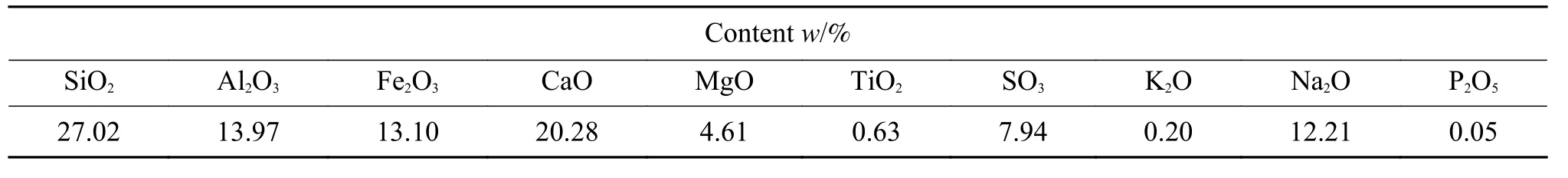
Table 2 Ash chemical compositions
1.2 Experimental process
The fixed-bed gasification experiments were carried out on the apparatus shown in Figure 1.The device mainly includes a horizontal fixed bed reaction system,a gas supply system,a temperature control system,a steam vapor generator and a magnetic sampling system.The specific operations are as follows: the temperature of tube furnace is raised to the predetermined temperature,and a 1.00 g coal sample is weighed and placed in a corundum porcelain boat.Before experiment,the porcelain boat was put into the cold end of equipment,gasification agent (500 mL/min)was pass to remove gas in the tube.After 10 min,the porcelain boat was quickly push to the constant temperature zone.The boat was pulled back to the cold end,and the vaporized residue was ground again and placed in a drying dish to be stored for testing.When examining the effect of temperature,the selected gasification conditions are as follows: gasification agent is 60%H2O+40%N2;temperature is 850,900,950,1000 °C,residence time is 30 min.When examining the effect of residence time,the selected gasification conditions are as follows: gasification agent is 60%H2O+40%N2;temperature is 950 °C;residence time is 5,10,15,20 and 30 min.When investigating the effect of gasification agent,the selected gasification conditions are as follows:gasification agent 60%H2O+40%N2,7.5%O2+92.5%N2,100%CO2,and 100%N2is as reference;temperature is 950 °C;the residence time is 30 min.
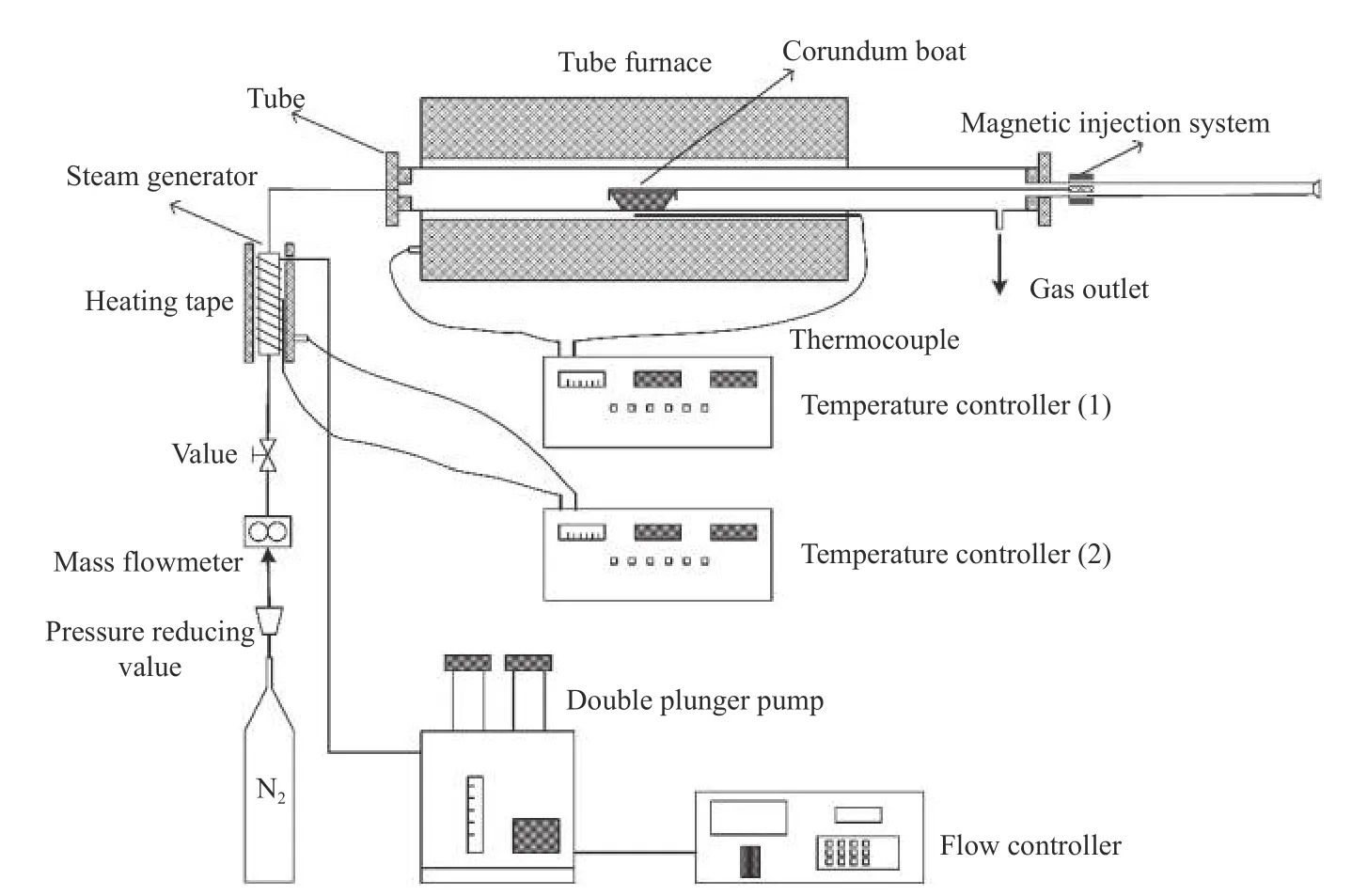
Figure 1 Schematic diagram of fixed bed reactor for coal gasification
1.3 Calculation method
The residue was first ashed in a muffle furnace at 575 °C for several hours to burn off the carbon residue,and then digested according to MT/T 1014—2006.The sodium concentration in solution was accurately and quantitatively determined by ICP-AES.The calculation method of sodium content in the residue is as follows:
mresidue-Na: The content of sodium in the gasification residue,mg;
C: Determination of sodium content in acid solution by ICP-AES,mg/L;
C0: Sodium content in blank test,mg/L;
V: Volume of acid solution after constant volume,L.
The calculation method of volatile amount of sodium in the gasification is as follows:
mcoal-Na: content of sodium in the coal sample,mg;
mresidue-Na: sodium content in the gasification residue,mg;
SR: the percentage of sodium release,%.
The concentration of sodium in the solvent extract and acid solution was quantitatively determined by an inductively coupled plasma chromatograph (ICP-OES,iCAP 6300) produced by Thermo Fisher Scientific in the United States.The mineral components were qualitatively analyzed using an X-ray powder diffraction analyzer (D2 Phaser desktop) produced by Bruker,Germany.The thermodynamic calculation was performed using Equilib module by Factsage software.
2 Results and discussion
2.1 Occurrence form of sodium in coal
Occurrence form and content distribution of sodium in coal are shown in Figure 2.It can be seen that the sodium mainly exists in two forms: Na-W and Na-A/W,and the content of the two accounts for 65.0% and 29.2% of the total sodium,respectively;the Na-I content is very low,only accounting for 5.86% of total sodium.Figure 3 is the XRD pattern of low temperature ash.The main minerals in coal are calcite(CaCO3),quartz (SiO2),nitratine (NaNO3),kaolin[Al2Si2O5(OH)4] and siderite (FeCO3).This result shows that Na-W in coal mainly exists in the form of nitratine,while Na-A/W exists in the form of sodium carboxylate(-COONa),and Na-I may exist in the form of albite(NaAlSi3O8).
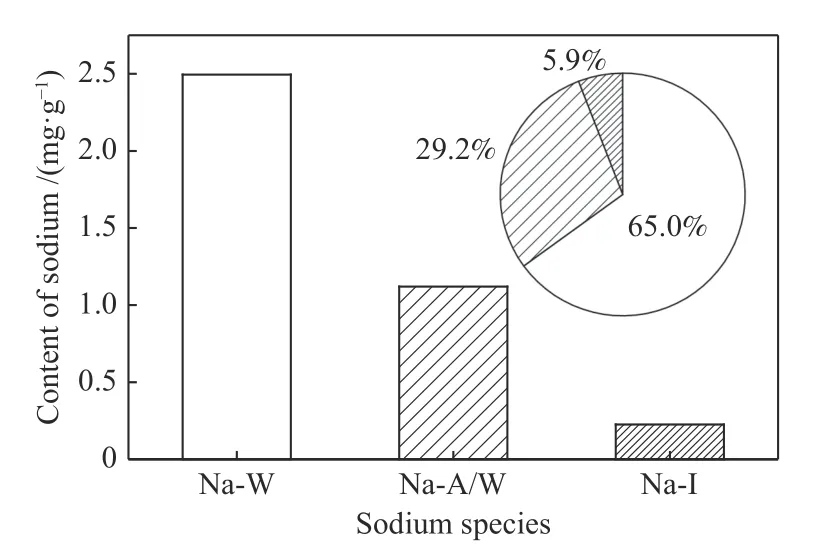
Figure 2 Distribution of sodium species in coal Na-W: water-soluble sodium;Na-A/W: ammonium-acetatesoluble but water-insoluble sodium;Na-I: ammonium-acetateinsoluble sodium

Figure 3 XRD pattern of low-temperature ash C: Calcite (CaCO3);K: Kaolinite (Al2Si2O5(OH)4);N: Nitratine(NaNO3);Q: Quartz (SiO2);S: Siderite (FeCO3)
2.2 Effect of operating conditions on the transformation behaviors of sodium
2.2.1 Effect of temperature
Effect of temperature on the release of sodium is shown in Figure 4.With the increase of temperature from 850 to 1000 °C,the value ofSRgradually increased from 11.5% to 19.2%.Similar phenomena were also found in pyrolysis of Victoria lignite and the combustion of Yanzhou bituminous coal[9,10],indicating that the increase in temperature could significantly promote the release of sodium during coal thermal conversion.However,for coal gasification,some scholars had drawn opposite conclusions.For example,in the fluidized bed gasification device,Zhang et al.[11]found that with the increase of gasification temperature,the value ofSRshowed the trend of increasing first and then decreasing.The researchers believed that the reason for this phenomenon was that high temperature promoted the reaction of sodium species with silico-alumina minerals.Then,the part of sodium fixed in residue increased,resulting in a corresponding decrease inSR.It was concluded that the temperature was a significant factor affecting the release of sodium during CFB gasification of coal.
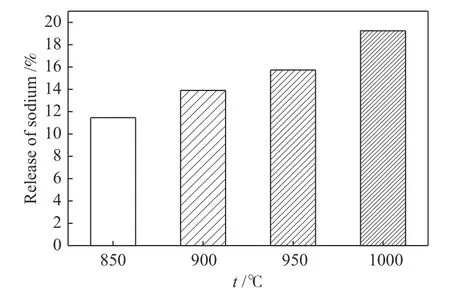
Figure 4 Effect of temperature on the release of sodium during coal gasification
Figure 5 shows XRD patterns of residues at different temperatures.It can be seen that the sodium in residues mainly existed in form of nepheline.During CFB gasification of ZD coal,Song et al.[12]also found the presence of nepheline in the bottom ash.In view of the types of minerals in the low-temperature ash,the possible reactions of sodium species and silico-alumina minerals are suggested as follows:

Figure 5 XRD patterns of gasification residues 1: hematite (Fe2O3);2: lime (CaO);3: larnite (Ca2SiO4);4: nepheline (NaAlSiO4);5: srebrodolskite (Ca2Fe2O5);6: magnetite (Fe3O4);7: calcite (CaCO3);8: gehlenite(Ca2Al2SiO7)
Nitratine was firstly decomposed and sodium oxide (Na2O) formed.Subsequently,it reacted with meta-Kaolin to form stable nepheline.In addition,with the temperature increasing,the bulging phenomenon of XRD patterns between 25° and 40° became more obvious.It meant that the percentage of amorphous matters in residue increased accordingly.The slagging tendency could be more and more serious[13].The reaction (5) between SiO2and Na2O,whose amount are high in coal ash,also promote the formation of slaglike matters.The disappearance of the diffraction peaks of quartz in residue further supported the existence of reaction (5).
The promotion of sodium release was mainly ascribed to two reasons: some low-melting sodium species underwent phase transformation;the increase of temperature also promoted the reaction between lime and those silico-alumina minerals,leading to a decrease silico-alumina minerals and corresponding fixed sodium.As shown in Figure 5,with the increase of temperature,the intensity of CaO diffraction peaks gradually was weakened,while that of Ca2Al2SiO7diffraction peaks continued to increase.
Except experimental study,thermodynamic calculations by equilibrium module of Factsage software were also employed to further investigate the transformation behaviors of sodium during coal gasification.The results are shown in Figure 6 and Figure 7.It can be seen that sodium in gas phase existed in the form of NaCl,Na,(NaCl)2and NaCN.Among them,NaCl is the main volatile form of sodium in the gasification process.Molecular beam mass spectrometry online analysis also confirmed that sodium is mainly volatilized in the form of NaCl[14].With the increase of temperature,the value ofSRcontinued to increase,which was consistent with experimental results.As shown in Figure 7,the sodium in residue was mainly in form of nepheline.With the increase of temperature,the content of nepheline showed a trend of rapid increase at first and then slow decrease.The content of slag phase increased rapidly after 950 °C,indicating that high temperature was beneficial to the formation of slag phase in residue,and the threshold value was near 950 °C.Thus,the safe operating temperature of coal CFB gasification should be controlled below 950 °C.

Figure 6 Effect of temperature on the content of sodium species in gas phase
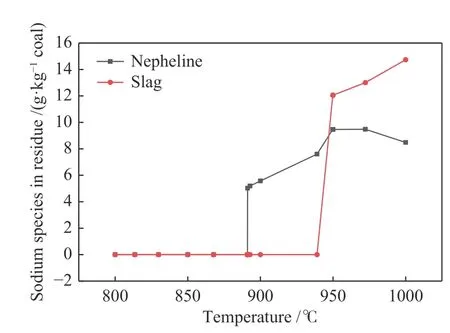
Figure 7 Effect of gasification temperature on the content of nepheline and slag
2.2.2 Effect of residence time
Figure 8 shows the effect of residence time on the carbon conversion and sodium release.It can be seen that within the first 5 min,the raw coal underwent devolatilization process,and a large amount of volatile matter was volatilized,resulting in a rapid increase in carbon conversion.Gasification reaction of semi-coke occurred in the next stage,and the carbon conversion rate increased continuously and approached 100% after 20 min.

Figure 8 Effect of residence time on the carbon conversion and sodium release (gasification agent is 60%H2O+40%N2;gasification temperature is 950 ℃)
The process of sodium release could be divided into two stages: coal pyrolysis and char gasification.The release of sodium mainly occurred in coal pyrolysis,and about 12.1% of sodium was volatilized into gas phase;in semi-coke gasification stage,only 3.6% of sodium was volatilized.Using atomic beam mass spectrometry to study gasification characteristics of Rhine brown coal online,Bläsing et al.[15]also observed that the release of NaCl was divided into two stages: the devolatilization stage and semi-coke gasification stage.Among them,the strong NaCl release was also the same occurred during devolatilization stage.
In the fast devolatilization stage,the release of sodium could be attributed to the release of part of Na-W and Na-A/W in coal[16].Na2O,generated by decomposition of abundant nitratine,was partially converted into NaOH and then transferred to gas phase under water vapor.In addition,part of Na-A/W was volatilized into gas phase as small organic molecules.The possible chemical reactions are as follows:
In the semi-coke gasification stage,with the extending residence time,carbon conversion and sodium release increased synchronously.Moreover,the trend of those two was very similar.This result indicated that the release of sodium in this stage mainly originated in CM-Na.During char gasification,carbon structure was destroyed continuously,and then Na in the form of CM-Na was released.The possible reaction is as follows:
It should be explained that CM-Na was originated in two ways.The first was that the weak carboxyl group in Na-A/W decomposed,and then formed a more thermally stable CM-Na structure.The second way was that some active sites were generated by rapid coal pyrolysis,and Na-W could be bound to active sites to form CM-Na[10,17].
2.2.3 Effect of gasification agent
Effect of gasification agents on the release of sodium is shown in Figure 9.The corresponding XRD patterns of residues are listed in Figure 10.It can be seen that there is a certain difference in the release amount of sodium among different gasification agents.
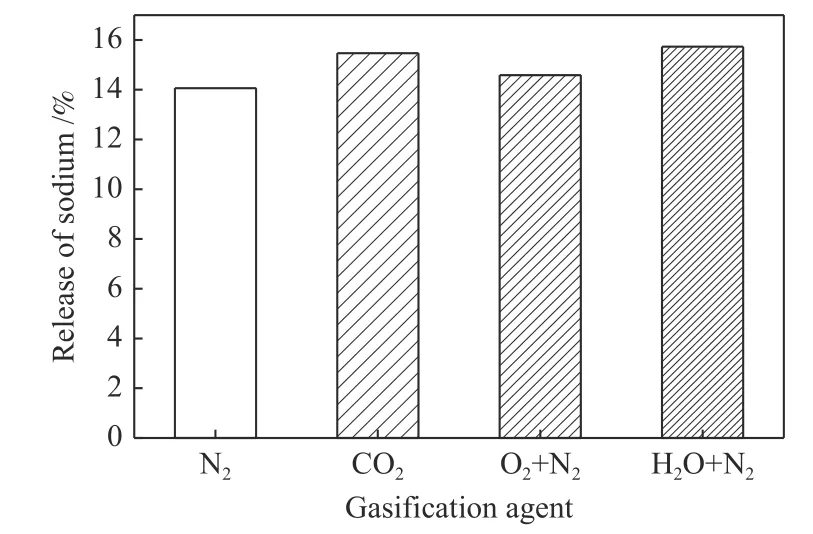
Figure 9 Effect of gasification agent on the release of sodium

Figure 10 XRD patterns of residues under different gasification agent
Compared with carbon dioxide,the release of sodium was more intense during steam gasification.This is because steam could promote the release of sodium[18].During combustion of Shenmu and Yanzhou bituminous coals,researchers found that the injection of water vapor significantly promoted the release of sodium as well[9,19].On the one hand,steam could reduce the melting temperature of sodium salts[20];on the other hand,it is because water vapor promotes the conversion of high melting sodium salts to low melting NaOH (melting point 322 °C)[21,22].
Compared with that in carbon dioxide,the release of sodium was weakened during oxygen gasification.This could be ascribed to the formation of Na2SO4in residue.The possible reactions (12) and (13) were occurred.Sulfur in coal was oxidized to SO2by oxygen,and then reacted with Na2O to form Na2SO4.The existence of Na2SO4diffraction peaks was found in residue (Figure 10).Thermogravimetric temperatureprogrammed experiments confirmed that the release of Na2SO4was negligible within the experimental temperature range[23].
Under nitrogen atmosphere,the amount of sodium release was the smallest.This was because the presence of considerable amount of char inhibited the release of sodium.On the one hand,it was due to the physical wrapping effect of char structure;on the other hand,it was due to the chemical binding effect of char,where CM-Na had a nice thermal stability.
In addition,it can be seen from Figure 10 that the bulging phenomenon of residue XRD pattern between 25° and 40° was the most obvious during gasification by oxygen.This meant that more amorphous minerals(slag phase) were generated.This was because the reaction of carbon and oxygen was an exothermic reaction.It easily caused the local temperature to be higher than the set value.According to the data in Figure 7,the increase in temperature promoted the formation of slag in residue.
2.3 Transformation mechanism of sodium during coal gasification
Figure 11 is a schematic diagram,revealing the transformation behaviors of sodium during steam gasification.NaNO3in raw coal firstly decomposes to form Na2O,or combine with char active sites to form CM-Na;the part of Na2O could react with internal minerals to form nepheline or slag.A large part of CMCOONa is decomposed to form CM-Na,and the rest will be released into the gas phase in the form of small molecular organic sodium.With char gasificationprocess,char structure is gradually destroyed,and Na in CM-Na volatilizes into gas phase.Most of them directly transform to NaOH under steam atmosphere.Small of them transform to Na under the reductive atmosphere.Due to the excessed steam,Na transforms to NaOH in the large probability.Some NaOH may react with Si-Al minerals during the diffusion process,and generate nepheline or slag.Part of NaOH reacts with HCl in the gas phase to generate NaCl.Finally,sodium species in raw coal volatilize into gas phase in the form of gaseous NaOH,NaCl,CM'-COONa and Na,and exist in residue as solid nepheline or liquid slag[24].
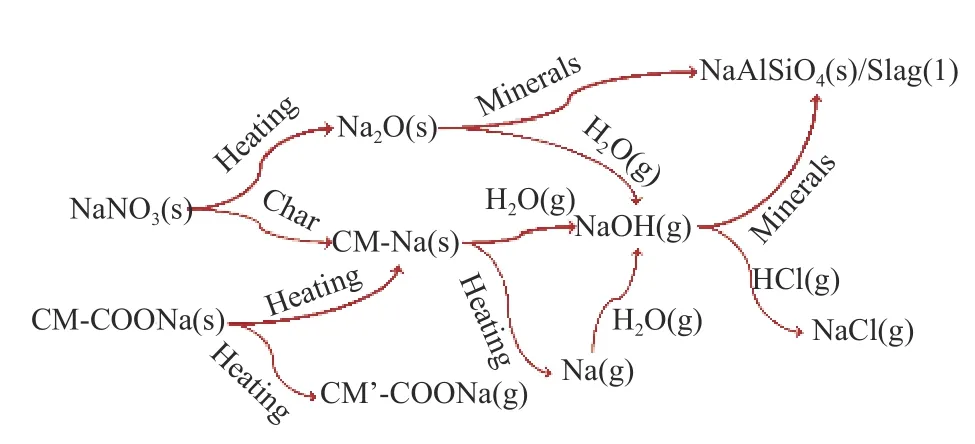
Figure 11 Schematic diagram of transformation behaviors of sodium species during coal steam gasification
3 Conclusions
Gasification temperature is the significant factor to the release and transformation of sodium.For the release of sodium,the high temperature promoted sodium release,ascribing to the intense of sodium volatilization and competitive reaction between lime and meta-kaolin.For the transformation of sodium,the high temperature promoted the formation of nepheline and slag.The threshold temperature of latter was near 950 °C.
The release of sodium in coal during gasification could be divided into two stages: coal pyrolysis and char gasification.In coal pyrolysis,part of organic and water-soluble sodium was released,and others were combined with char structure or reacted with minerals.In char gasification,CM-Na was released along with char gasification.
Due to the decrease of melting temperature and the formation of NaOH,steam showed a promoting effect on sodium release.Oppositely,oxygen and nitrogen presented an inhibiting effect.The former was ascribed to the formation of Na2SO4,while the latter was caused by the chemical binding and physical wrapping effect of char.
