Combustion behavior and mechanism of molecular perovskite energetic material DAP-4-based composites with metal fuel Al
2023-10-09PengDengXueyongGuoHuaFangRuiLiuPengwanChen
Peng Deng,Xue-yong Guo,Hua Fang,Rui Liu,Peng-wan Chen
State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing,100081,PR China
Keywords:DAP-4 Metal fuel al Oxidant Combustion behavior Reaction mechanism
ABSTRACT Combined with the oxidizer anions and fuel cations,molecular perovskite energetic materials show a good potential.In this work,the combustion behavior and mechanism of metal fuel aluminium(Al)with molecular perovskite energetic material (H2dabco)[NH4(ClO4)3] (DAP-4) as a high-energy oxidant was investigated.The DAP-4 based composites with metal fuel Al were designed and fabricated by the different mass ratios.Results showed that DAP-4 exhibits a good oxygen-supplied capacity for enhancing the combustion performance of Al.The maximum combustion heat of DAP-4/Al-3 at the Al/O mass ratio of 38:62 is up to 10,412 J/g in the inert gas,which is higher than those of other ratios and the mixtures of other energetic materials and Al.The evolution of pressure output,pressurization rate,and flame temperature was monitored for DAP-4/Al with different mass ratios.Composites DAP-4/Al/F were characterized by burning rates.The combustion reaction mechanism of metal fuel Al with DAP-4 as a highenergy oxidant was provided.DAP-4 was ignited firstly and released acid and oxidizing gases,which corroded Al2O3 shells on Al particle surfaces and accelerated the combustion reaction with Al to release a lot of energy.This work offered a new idea that molecular perovskite energetic materials have great potential in the high-energy Al-based solid rocket propellants.
1.Introduction
Molecular perovskite energetic materials,as a novel class of energetic materials,have received much attention due to good detonation properties,high thermostability,and cost advantages[1-3].In the perovskite structure ABX3[4,5],organic fuel cations and inorganic oxidizer anions were considered as the sites of ABX3to assemble into the cell units,where the intermolecular interreaction and coulombic force of the different sites form a highsymmetry and stable ternary hybrid structure [6,7].As a result,their high detonation properties and high thermal stability are attributed to the unique hybrid perovskite structures [8-11].Furthermore,perchlorate anions are always acted as main X-bridge candidate in this unit.High oxidation capacities are together in the possession of molecular perovskite energetic materials.
DAP-4,as a promising molecular perovskite energetic material,is composed of perchlorate anions,ammonium cations,and protonated triethylenediamine cations (H2dabco2+).It exhibited the excellent theoretical detonation velocity of 8806 m/s and measured detonation velocity of>8500 m/s,theoretical detonation pressure of 35.2 GPa,and measured detonation heat of 5.69 MJ/kg[2].Considering the combination of simple one-pot assembly synthesis method and excellent energy and safety performance,DAP-4 is considered as a new hot topic in the field of propellants[12-14].Although it has only been four years since its birth,subsequent literature has also been reported to demonstrate the fascinating properties of the molecular perovskite energetic materials [15-20].For example,Cao [21] reported that ball milling technology was used to prepare the micro/nano DAP-4 particles and its impact sensitivity had been reduced obviously,compared from the pure cubic materials.Zhu [22] demonstrated that modified DAP-4 with lower thermal decomposition temperature and higher heat release was obtained by the Fe-doped g-C3N4nanosheet catalysts added.Zhao[23]showed that 1,3,5-triamino-2,4,6-trinitrobenzene (TATB) was introduced into DAP-4 based composites to prepare the petroleum perforating cartridge,which has better detonation penetrability and thermal stability than traditional formula.What’s more,our research group [7,24],in the previous study,also explored the thermal decomposition,combustion,and the energy-releasing mechanism of DAP-4,and revealed that DAP-4 has unique physical and chemical properties,such as high detonation performance and strong oxidation properties.
Recently,with the proposal of the goal of high-energy solid propellant,various kinds of high-energy-density materials,such as hexaazaisowurtzitane (CL-20) [25,26],cyclotetramethylenetetranitramine (HMX) [27-29],cyclotrimethylenetrinitramine (RDX) [30-32] and so on [33,34],were considered to prepare high-energy solid rocket motor successively.Compared from conventional oxidant ammonium perchlorate(AP),on the one hand,these oxidants can be used for the reaction of metal fuel Al by sufficient oxygen provided.On the other hand,they also can provide the unexpected energy by self-reaction [35-43].To the best of our knowledge,DAP-4 has not only high energy from the violent redox reaction of fuel-oxidizer hybrid structure,but also showed strong oxidation capacity from rich perchlorate anions in the cell units.Therefore,it’s necessarily possible to utilize DAP-4 with high energy and strong oxidation in solid rocket propellants.
Inspired by the potential application of DAP-4 in the solid rocket propellants,herein,the combustion behavior and mechanism of DAP-4 based composites with metal fuel Al was investigated.The mixed powders DAP-4/Al were fabricated by physically mixing.Composites DAP-4/Al/F were fabricated by casting method with polymer binder F2602.Other oxidants (CL-20,HMX,RDX,and AP)also were used for comparison.The mixed powders and composites were characterized.The combustion heat,combustion pressure output,ignition and combustion process,and thermal decomposition of samples were studied.The combustion reaction mechanism of metal fuel Al with DAP-4 as a high-energy oxidant were discussed.This offered a proof-of-concept work that DAP-4 has good potential as a high-energy oxidant in the high-energy Albased solid rocket propellants.
2.Experimental sections
2.1.Materials
AP and HClO4solution (concentration,70%) were purchased from Shanxi Jinxi Jiangyang Chem.Eng.Co.,Ltd.Triethylenediamine (dabco) was purchased from Shanghai Aladdin Bio-Chem Technology Co.,Ltd.Ethyl acetate was provided by Tianjin Guoyao Chem Technology Co.,Ltd.Metal powders Al were purchased from Flance Nano Technology Co.,Ltd.Raw materials CL-20,HMX,and RDX were obtained from Gansu Yinguang Chem.Industry Group Co.,Ltd for comparison.
2.2.Preparation of DAP-4
Molecular perovskite energetic materials were synthesized by molecular assembly strategy[2].0.1 mmol AP and 0.1 mmol dabco were added into 20 mL deionized water together and dissolved completely in the mixed solution.0.163 mL HClO4solution(0.2 mmol)added into mixed solution and stirred for 1 h.The white particle samples (DAP-4) were obtained by re-crystallization from the matrix.
2.3.Preparation of DAP-4/Al
DAP-4/Al mixed powders were prepared by simple physically mixing.In order to obtain the highest combustion heat of samples,the mass ratios of the Al and oxidant were designed by weight ratio,as shown in Eq.(1) and Eq.(2).
The Al/O mass ratios of metal fuel Al and oxidants DAP-4 were 33:67 b y calculation based on the stoichiometry ratio.The mixed samples DAP-4/Al with more Al/O mass ratios were designed and fabricated for combustion heat tests.The samples with different Al/O mass ratios of 82:18,72:28,62:38,and 52:48 were selected and named by DAP-4/Al-1,DAP-4/Al-2,DAP-4/Al-3,and DAP-4/Al-4,respectively.CL-20/Al,HMX/Al,RDX/Al,and AP/Al were also prepared.The detailed experiments were shown in SupportingMaterials.
2.4.Preparation of DAP-4/Al/F composites
DAP-4/Al/F were fabricated by casting strategy with fluoropolymer F2602as binder.Al/O mass ratios of mixed powders was used,and polymer binder F2602was added to prepare DAP-4/Al/F.0.1 g F2602was dissolved in 3 mL ethyl acetate for 12 h.0.9 g mixed powders were added into the ethyl acetate solution.The mixture was stirred with ultrasonic treatment for 5 h.The energetic slurry was transferred into a 55×3.5×2.5 mm3mould.They were dried by oven at 60 ℃ for 5 h.The composites was named by DAP-4/Al/F depended on the Al/O mass ratios.
2.5.Sample characterization
XRD patterns of raw materials,mixed powders,and composites were recorded by a D8 advance X-ray diffractometer.SEM and element mapping images was recorded by a S4800 SEM apparatus.The thermal pyrolysis properties were carried out by a thermogravimetric/differential scanning calorimeter (TG-DSC).Coupling TG-FT-IR spectra of the gaseous products of as-prepared samples were recorded by a coupling Nicolet iS 50 spectrophotometer.PYGCMS spectra were collected by EGA/PY-3030D and GCMS apparatus.
2.6.Performance test
Ignition and combustion experiments were planned as followed.Combustion heat values were recorded autocratically by a TRHW-7000C calorimeter.0.2 g raw materials were used to test combustion heat in 30-mL confined space with the oxygen pressure of 3 MPa.0.4 g mixed powders were used to test combustion heat in the atmospheric-pressure closed environment in an inert gas.Pressure-time (p-t) curves were evaluated by combustion cell.Samples were used to observe in time electrically.The ignition and combustion processes of the tested samples were recorded by a high-speed photography device.The mixed powder (~50 mg) and composites(length:30 mm)were used to be ignited by Ni-Cr wire heated and external fire source,respectively.The samples were put into test platform(Fig.S1).The dispersed mixed powders were put in the middle of Ni-Cr wire and the composites were placed and fixed vertically.The flame temperatures of combustion processes were observed by an infrared radiometer.The accuracy of maximum flame temperatures of ignited samples at the condensation and boundary layer was checked depended on the ambient temperature 25 ℃.
3.Results and discussion
The micro morphology of molecular perovskite energetic material DAP-4 was shown in Fig.1(a).Cubic particles with sizes of 10-20 μm can be seen.The A,B,and X sites in the cell units were shown in Fig.1(b),which indicated that unique cubic micro morphology of DAP-4 is originated from the crystal growth of the body-centered cubic cell.Raw Al micro particles were shown in Fig.1(c).Spherical samples with the size of 5-50 μm were observed.Nanoparticles are agglomerated on the surface of large particles.

Fig.1.SEM images of(a)DAP-4 and(c)Al,(b)molecular structure of DAP-4 with ABX3 perovskite structure,and the combustion heat of(c)energetic materials in the rich oxygen environment,and (d) mixed powders with Al in the inert gas.
The combustion heat of energetic materials was characterized in Fig.1(d).The combustion heat of CL-20 under the oxygen enriched environment is 12,319 J/g,which is higher than HMX (12,061 J/g)and RDX(12,096 J/g).But,the tested combustion heat of DAP-4 is up to 15,477 J/g,which is better than highest energy explosive CL-20.However,AP is only used as a simple oxidant and cannot release more energy through self-combustion.
Fig.1(e) showed the combustion heat of the mixed powders with metal fuel Al in the inert gas.Ideally,the maximum combustion heat was obtained at the stoichiometry Al/O ratio of fuel and oxidant.But,for traditional high-energy oxidants CL-20,HMX,and RDX,the maximum combustion heat values of CL-20/Al,HMX/Al,and RDX/Al are 9794 J/g,9017 J/g,and 7945 J/g,respectively.The Al/O mass ratios of obtained maximum combustion heat are 28:72,18:82,and 18:82,respectively,which are lower than their stoichiometry Al/O ratio of 33:67.This indicated that the violent selfreaction consumed lot of oxygen,leading to a more additives of traditional high-energy oxidants.And for conventional oxidant,the combustion heat of AP/Al mixed powder with the stoichiometry ratio is as low as 7943 J/g.However,the tested combustion heat values of DAP-4/Al is above 9850 J/g at the Al/O mass ratios ranged from 23:77 to 38:62,which are higher than 9794 J/g of CL-20/Al.The maximum combustion heat of 10,412 J/g was obtained at the Al/O mass ratio of 38:62,which is higher than the stoichiometry Al/O ratio of DAP-4/Al.The reason could be explained that,on the one hand,the surface oxide layers of Al reduced the active content of metal fuel,High Al contents at the mass ratio of 38:62 is necessary for meeting the redox reaction with actual stoichiometry Al/O ratio.On the other hand,more oxidizing atoms,such as N and Cl,in DAP-4 participated in the redox reaction with Al to release more heat.With the Al contents further increased,the tested combustion heat value of DAP-4/Al also exhibited a sharp downward trend.When Al/O mass ratio became 53:47,the combustion heat is as low as 4366 J/g,indicated that the lack of oxidants in DAP-4/Al will reduce the energy release effect.
DAP-4/Al-1,DAP-4/Al-2,DAP-4/Al-3,and DAP-4/Al-4 with different Al/O ratios were selected through a gradient change(±10 wt% of Al contents) to study the more obvious combustion law,depended on their combustion heat results,as shown in Fig.2(a).The DAP-4 micro particles with cubic morphology and spherical Al are dispersed randomly to generate the mixed powders after mechanical mixing.The structures of samples were characterized by XRD in Fig.3(a).XRD pattern of raw DAP-4 showed main strong diffraction peaks at 21.1°,24.4°,36.6°and 38.2°,which are reflect from crystal planes (222),(400),(531),and (600) of DAP-4 with perovskite structure (CCDC Number: 1,528,108).The peaks of Al in XRD pattern at 38.5°,44.7°,65.1°,and 78.2°showed the main crystal planes (110),(200),(220),and (311) of Al with facecenter cubic structure.With the contents of Al increased,the diffraction intensity of crystal planes(110)of Al had been increased in sample DAP-4/Al-4.The XRD results demonstrated that the mixed powders include the components DAP-4 and Al.

Fig.2.SEM images of (a) DAP-4/Al and (b) composites DAP-4/Al/F;(c),(d) Its magnified local cross-sectional view and corresponding element mappings.
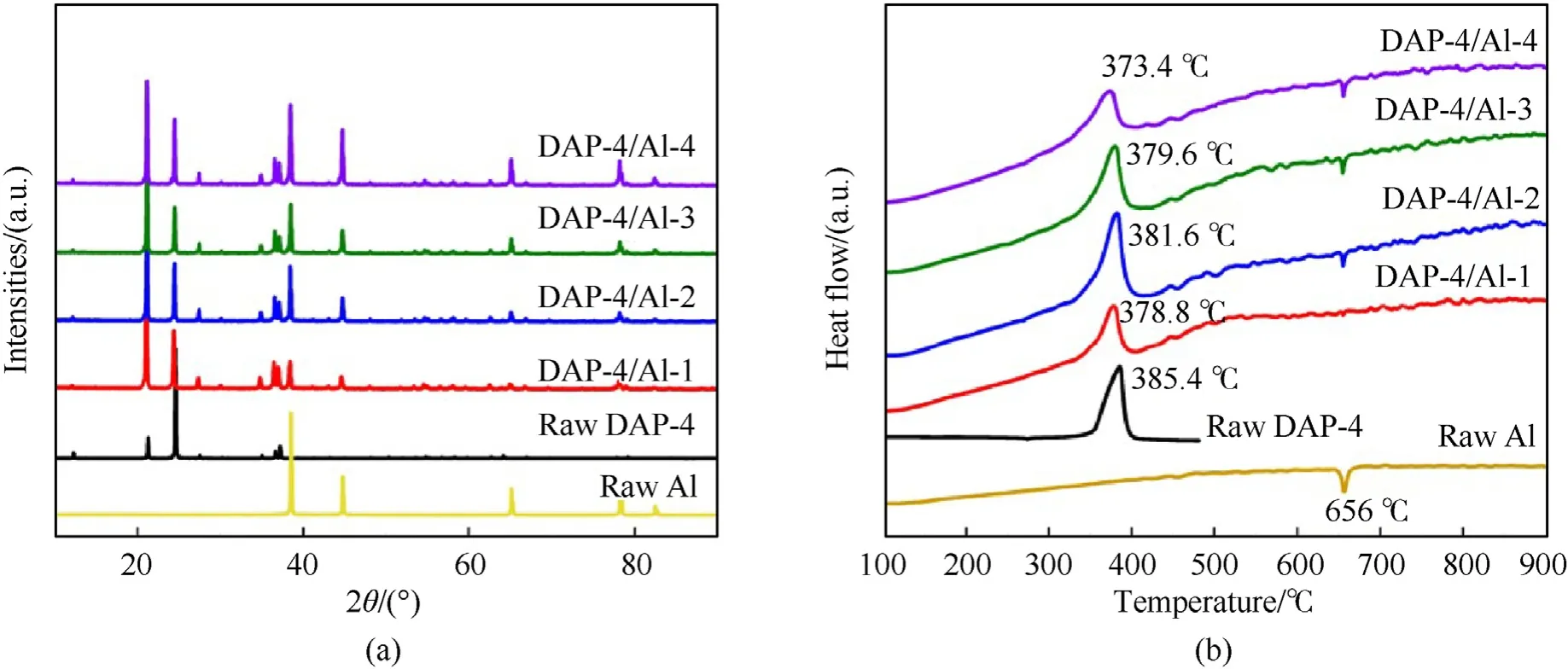
Fig.3.(a) XRD patterns and (b) DSC curves in the N2 atmosphere with the heating rate of 10 ℃/min of DAP-4/Al.
Fluoropolymer binder F2602was used as binder to prepare DAP-4/Al/F in Fig.S2(a).The cross sectional images of DAP-4/Al/F showed stable composite micro structure in Fig.2(b).Fig.2(c)showed the cross-section magnification images of DAP-4/Al/F.The oxidant DAP-4 and metal fuel Al can be observed.F2602appeared in the particle gap and played the role of adhesion of different particle components.The element mappings of samples in Fig.2(d)demonstrated the characteristic element distributions of different components.Al,Cl,and F element are corresponding to Al,DAP-4,and F2602,respectively.The composite DAP/F without Al was prepared in Fig.S3.The SEM images showed micro structure of DAP-4/F,where DAP-4 particles were enclosed by F2602,and adhered closely.The XRD patterns of DAP-4/Al/F was shown in Fig.S4(a).XRD peaks were reflected from DAP-4 and Al.Although 10 wt% F2602was added into DAP-4/Al/F,amorphous polymer showed no obvious diffraction peak.Major changes are caused by different contents of Al.Metal fuel Al and other oxidants also were used to prepare mixed powders and composites for the comparative study in Fig.S5.CL-20/Al,HMX/Al,RDX/Al,and AP/Al and corresponding composites were showed.The structures of samples were also characterized in Fig.S4(b) and Fig.S4(c).
Fig.3(b) showed the DSC curves of thermal decomposition processes of samples with the heating rate of 10 ℃/min.The DSC curve of raw DAP-4 showed a strong exothermic peak at 385.4 ℃,which corresponds to previous report [7].A melting endothermic peak at 656 ℃ of raw Al was seen in the inert gas environment[44,45].The DSC curves of mixed powders DAP-4/Al with different Al/O mass ratios showed that thermal decomposition peak temperatures of the component DAP-4 presented a trend of decline with Al contents increased.But,the melting endothermic peak of the component Al still stayed.In Fig.S6(a)-Fig.S6(e),the DSC curves of DAP-4,other oxidants (CL-20,HMX,RDX,and AP),their mixed powders,and composites were also showed.With metal fuel Al added,the thermal decomposition peaks of both the mixed powders and composites had reduced slightly,as shown in Fig.S6(f).
Furthermore,the combustion performance of DAP-4-based samples had been investigated.The combustion processes of raw DAP-4 and other energetic materials were studied firstly.In Fig.4,CL-20,HMX,RDX,and DAP-4 ignition and combustion processes were showed.CL-20 exhibited the faster combustion process and higher intensity than HMX and RDX.The main combustion reaction process of CL-20 lasted for~539 m s,and the maximum flame temperature is 756 ℃ in Fig.5(a).DAP-4 showed the main combustion process occurred within~290 m s,which is lower than that of CL-20(~539 m s).But the maximum flame temperature of DAP-4 is up to 1065 ℃,which is higher than CL-20 (756 ℃).Higher luminous intensity and quicker flame propagation acceleration of DAP-4 were showed.Different from others,combustion reaction of DAP-4 is originated from the fierce redox reaction between oxidizeranions and fuelH2dabco2+cations.Good combustion characteristics indicated that DAP-4 can be used as high-energy oxidant in solid propellant.

Fig.4.The ignition and combustion processes of high-energy oxidants.

Fig.5.(A),(b) Maximum flame temperatures of raw and mixed powders;(c) The peak pressures of DAP-4/Al and (d) their corresponding pressurization rates.
DAP-4/Al ignition and combustion processes were shown in Fig.6.Metal fuel Al was introduced into DAP-4 to enhance combustion performance of the mixed powders.When the metal fuel Al was added,the luminous intensity,flame propagation acceleration,and maximum flame temperature were improved.When the Al/O mass ratios is 38:62,the combustion reaction process of DAP-4/Al-3 occurred with~200 m s.The maximum combustion flame temperature of DAP-4/Al-3 is up to 1390 ℃ in Fig.5(b),and the luminous intensity and flame propagation acceleration have also been enhanced.This suggested that the maximized combustion properties can be achieved by Al/O mass ratios optimized.With the further increase of Al contents,the maximum combustion flame temperature of DAP-4/Al-4 had is a significant reduction by from 1390 ℃(DAP-4/Al-3)to 902 ℃,which is also lower than 1065 ℃ of raw DAP-4.

Fig.6.The ignition and combustion processes of DAP-4/Al: (a) DAP-4/Al-1;(b) DAP-4/Al-2;(c) DAP-4/Al-3;(d) DAP-4/Al-4.
The mixed powders CL-20/Al,HMX/Al,RDX/Al,and AP/Al were used as comparison in Fig.S7.AP/Al and CL-20/Al showed the fast and violent combustion processes after ignition.The maximum flame temperature of AP/Al and CL-20/Al are 2085℃ and 1602℃ in Fig.5(a),which is higher than that of DAP-4/Al-3.Due to the lower ignition barrier,CL-20 can quickly provide a large amount of oxygen in a shorter time to support the reaction with Al particles.AP,as a conventional oxygen-supply source,can be completely converted to support the reaction of Al with the large amount of heat release.HMX/Al and RDX/Al showed the slow combustion processes in Fig.S7(b)and Fig.S7(c),although the combustion intensity of them has been improved.The maximum flame temperature is so far lower than those of DAP-4/Al-3.This is because more energy is required to make HMX and RDX particles melt in starting combustion process,resulting in a slow combustion reaction rate.
The combustion pressure properties of samples were showed in Fig.5(c) and Fig.5(d) and Fig.S8.The peak pressure and pressurization rate of pure DAP-4 is 268 kPa and 12.4 MPa/s during combustion process.With metal fuel Al added,the peak pressure of DAP-4/Al showed a slow downward trend.When Al/O mass ratios is 38:62,the peak pressure of DAP-4/Al-3 is down to 152 kPa.With the Al content further increased,the peak pressure of DAP-4/Al-4 showed a sharp decline to 83 kPa,resulted from the lack of oxygen supply by the high Al/O ratio.The corresponding pressurization rate of DAP-4/Al showed a trend of increasing first and then decreasing.The maximum pressurization rate is 16.450 MPa/s of DAP-4/Al-1,and the minimum pressurization rate is down to 4.615 MPa/s of DAP-4/Al-4.The combustion pressure properties of CL-20/Al,HMX/Al,RDX/Al,and AP/Al were showed in Fig.S8.AP/Al and CL-20/Al in Fig.S7 revealed a drastic combustion reaction process,and peak pressure and pressurization rate of AP/Al and CL-20/Al are higher than DAP-4/Al-3.But HMX/Al and RDX/Al had a low peak pressure and pressurization rate,which are consistent with their combustion processes.
The burning rates of the composites with F2602as binder were studied.The stable combustion processes of samples were collected randomly within 0.8 s(interval time:0.2 s)in Fig.7 and Fig.S9.The DAP-4/F without Al had a slow combustion process with the burning rate of 9.42 mm/s in Fig.7(a).With the Al added,the violent combustion processes of DAP-4/Al/F were shown in Fig.7(b)-Fig.7(e).The burning rates of DAP-4/Al/F had increased,with Al contents increased.A growing trend of the burning rates had been found from 15.50 mm/s(DAP-4/Al/F-1)to 27.00 mm/s(DAP-4/Al/F-4) in Fig.7(f).And the combustion progresses of other composites were showed in Fig.S9 and Table 1,the burning rate of CL-20/Al/F is 33.50 mm/s,which is higher than that of others.But HMX/Al/F(6.42 mm/s),RDX/Al/F (15.03 mm/s) and AP/Al/F (13.34 mm/s)showed a mid-burning rate.The above results demonstrated that DAP-4 showed good potential as oxidant in the combustion application.
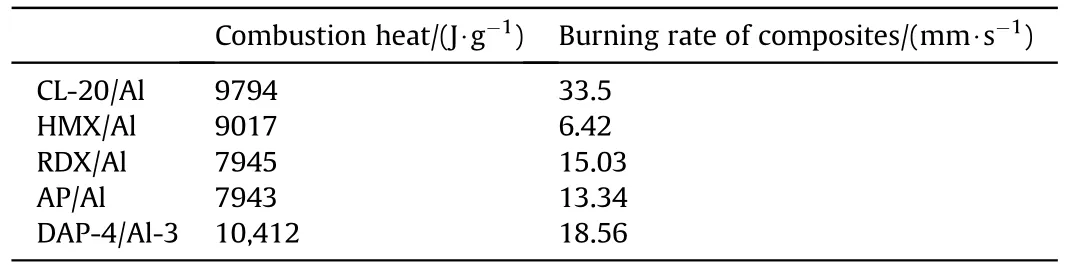
Table 1 Energetic properties of samples.
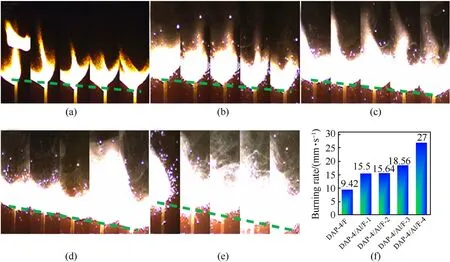
Fig.7.Combustion processes of the samples within 0.8s(interval time:0.2 s):(a)DAP-4/F;(b)DAP-4/Al/F-1;(c)DAP-4/Al/F-2;(d)DAP-4/Al/F-3;(e)DAP-4/Al/F-4;(f)Their burning rates.
The ignition and combustion reaction mechanism of DAP-4 based composites with metal fuel Al were studied in Fig.8-Fig.10.The thermal decomposition behavior and mechanism of DAP-4 was discussed in our previous work [18-22].For DAP-4,the thermal decomposition of DAP-4 occurred first before combustion,which appeared from 360 to 450 ℃ during the thermal decomposition process,as shown in Fig.8(a) and Fig.8(b).Disordered A-site H2dabco2+was activated at~260 ℃ in Fig.3(b)due to the high-temperature phase transition [46].When the heated energy is greater than the coulombic force between X-bridge ClO4-and B-sitethe anionic cage-like skeleton of DAP-4 perovskite structure will collapse.H transfer was accelerated between activated H2dabco2+andwhich facilitated the fast decomposition of HClO4to produce many superoxide radical anionand highly acid gas HCl.Superoxide radical anioncan be used to the reaction of other fuel small molecules and generate many gas products including H2O,CO2,CO,NO2,N2O,HCN,and so on,as PY-GCMS spectrum of DAP-4 at the pyrolysis temperature of 400 ℃ shown in Fig.9(a).This revealed that DAP-4 has strong oxidation and high corrosion capacity.
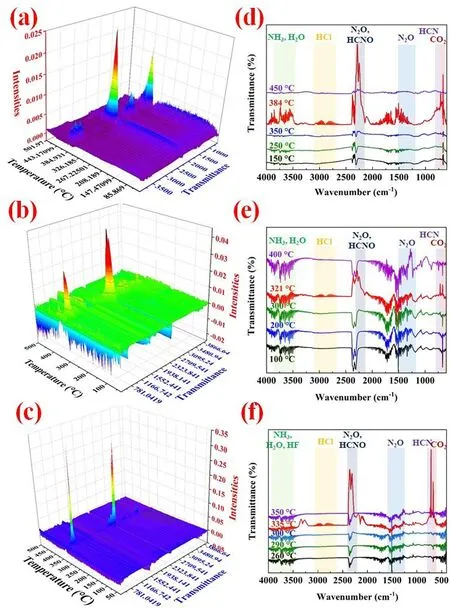
Fig.8.Three-dimensional IR and corresponding in-time FTIR spectra of gas products: (a) Raw DAP-4,(b) DAP-4/Al-3 and (c) DAP-4/Al/F-3.
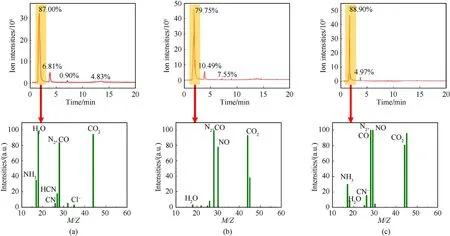
Fig.9.PY-GCMS spectra at 400 ℃: (a) DAP-4;(b) DAP-4/Al-3;(c) DAP-4/Al/F-3.
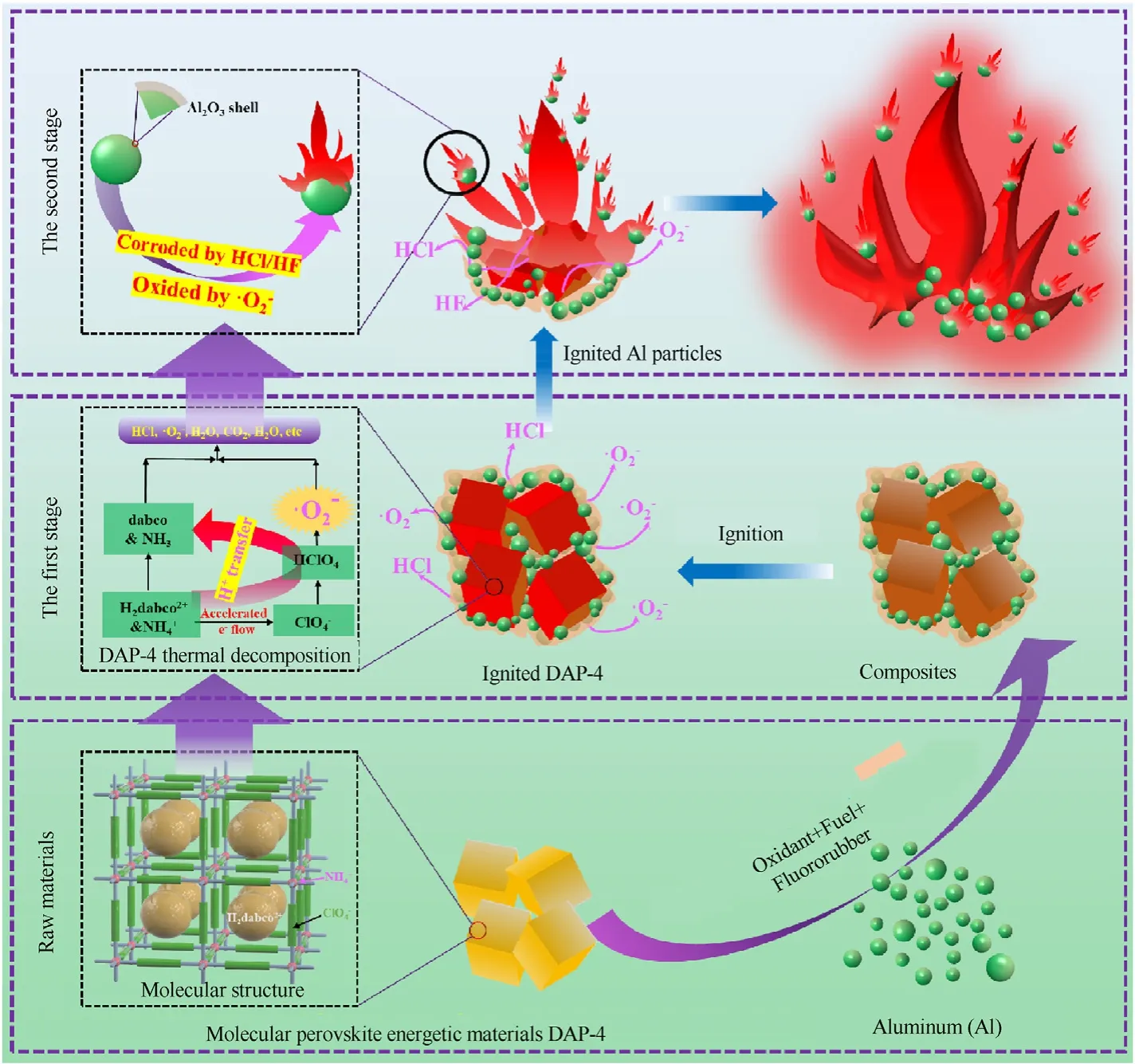
Fig.10.Scheme of ignition and combustion reaction mechanism of metal fuel Al with DAP-4.
Based on the above results and experimental progress,the reaction mechanism of metal fuel Al with DAP-4 was proposed,and two ignition and combustion stages were proposed,as shown in Fig.10.The metal fuel Al particles started the ignition and combustion reaction mainly depended on more energy released by DAP-4 thermal decomposition and combustion reaction at the last stage [47-50],which can accelerate the phase transformation of Al2O3shells at the surfaces of the Al particles and promote melting.
As shown in Fig.3(b),the melting endothermic peak of raw Al was located at 656 ℃.For DAP-4/Al or DAP-4/Al/F,DAP-4 molecules with richanion was easy to be ignited at the first stage[51],to release strong acid and oxidizing gas as well as a lot of energy.As shown in Fig.5(a),the maximum flame temperature of raw DAP-4 was up to 1065 ℃.Acid gases,such as HCl,can directly corrode Al2O3shells on Al particle surfaces under a lower high-temperature environment,which improved ignition performance of Al at the ensuing second stage,and oxidizing gasalso can directly act on Al with melting state through the Al2O3shell.As a result,the contents of HCl and Cl-anion had been reduced during the pyrolysis process at 400 ℃ in Fig.9(b) and Fig.9(c).This will accelerate the combustion reaction of DAP-4/Al.A lot of energy from the self-combustion of DAP-4 can enhanced overall combustion performances of DAP-4/Al.With the thermal decomposition of DAP-4,F2602also begin to be decomposed and generate HF [24],as PYGCMS spectra of DAP-4/F in Fig.S10.More HF was introduced to corrode Al2O3shell on Al particle surfaces and enhance the preignition reaction of Al particle [52,53].
4.Conclusions
In this work,combustion behavior and mechanism of DAP-4 based composites with metal fuel Al were investigated.DAP-4/Al with good dispersion and DAP-4/Al/F were prepared by the physical mixing and casting strategy with F2602.We found that with the metal fuel Al added,the combustion heat,luminous intensity and flame propagation acceleration of DAP-4/Al have also been enhanced.The tested combustion heat value of DAP-4/Al is above 9850 J/g ranged from the Al/O mass ratios of 23:77 to 38:62,which is higher than the maximum combustion heat of other oxidants and Al.The maximum combustion heat of 10,412 J/g at the Al/O mass ratio of 38:62 was obtained.The maximum combustion flame temperature of DAP-4/Al with the maximum flame temperature of 1390 ℃ (DAP-4/Al-3) showed a trend of increasing first and then decreasing.The peak pressure of DAP-4/Al showed a slow downward trend from 200 kPa(DAP-4/Al-1)to 83 kPa(DAP-4/Al-4).But the pressurization rate of DAP-4/Al had a trend of increasing from 12.4 MPa/s of raw DAP-4 to 16.45 MPa/s of DAP-4/Al-1 first and then decreasing to 4.615 MPa/s of DAP-4/Al-4.The two-stage combustion reaction mechanism of metal fuel Al with DAP-4 as a high-energy oxidant was provided: DAP-4 was ignited firstly and released acid and oxidizing gases,and then,they corroded Al2O3shells on Al particle surfaces and accelerated the combustion reaction with Al to release a lot of energy.This work offered a proof of concept that molecular perovskite energetic materials have great potential as an excellent and promising oxidant for the application in the high-energy solid rocket propellants.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank National Natural Science Foundation of China (Grant No.22175026,21975227,11902300),the Foundation of National Key Laboratory of Defense Science and Technology (Grant No.6142602210306),and State Key Laboratory of Explosion Science and Technology (No.QNKT20-07) for the support.Authors also thank Dr.Shaoli Chen(Xi'an Modern Chemistry Research Institute,Xian,China) and Prof.Xiong Cao (North university of China,Taiyuan,China).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2022.10.012.
杂志排行
Defence Technology的其它文章
- An energetic nano-fiber composite based on polystyrene and 1,3,5-trinitro-1,3,5-triazinane fabricated via electrospinning technique
- Dynamic response of UHMWPE plates under combined shock and fragment loading
- Aerial multi-spectral AI-based detection system for unexploded ordnance
- Model-based deep learning for fiber bundle infrared image restoration
- Robust design and analysis for opto-mechanical two array laser warning system
- Perforation studies of concrete panel under high velocity projectile impact based on an improved dynamic constitutive model
