A novel energetic framework with the combination of 5,6-fused triazolo-triazine and nitropyrazole-tetrazole for energy-stability balanced explosive
2023-10-09ChengchuangLiHaoGuJieTangGuojieZhangGuangbinChengHongweiYang
Cheng-chuang Li,Hao Gu,Jie Tang,Guo-jie Zhang,Guang-bin Cheng ,Hong-wei Yang
School of Chemistry and Chemical Engineering,Nanjing University of Science and Technology,Nanjing,210094,China
Keywords:Fused and bridged rings Energetic skeleton Balanced energy-stability Energetic materials
ABSTRACT In recent years,the introduction of fused rings own high density and low sensitivity has promoted the development of energetic materials.However,the development of energetic compounds containing fused and bridged rings by introducing multiple nitrogen heterocycles at different sites of fused rings is still difficult to progress,which seriously limits the emergence of advanced energetic compounds.In this study,a series of energetic materials choosing different nitrogen rich heterocycles at the vacancies of the fused ring,i.e.,neutral compound 5,6 and their ionic derivatives(compounds 7-12)were designed and synthesized.Compounds 5 and 6 were further confirmed by single crystal X-ray diffraction,while the crystal analysis and theoretical calculations were carried out to explore the relationship between crystal structure and physicochemical properties.All of the newly synthesized compounds (5-12) are insensitive to mechanical stimulation (IS >40 J;FS ≥342 N) and they own the high detonation velocity (D:8322-9075 m/s).Notably,hydrazine salt 11 own the higher detonation velocity (9075 m/s) and powder density(1.83 g/cm3),but exhibits lower sensitivity(IS>40 J)than the classical energetic compound RDX(8795 m/s,1.80 g/cm3,7.5 J).It is obvious that the combination of 5,6-fused triazolo-triazine and nitropyrazole-tetrazole may be a new energetic skeleton for synthesising the heterocyclic compounds with balanced energy-stability.
1.Introduction
As a unique branch of chemical materials,the strategies to design High Energy Density Materials(HEDMs)in civil and military has been widely concerned in recent decades[1].The design core of novel HEDMs is to balance high energy and low sensitivity at the same time [2].The existing nitrogen rich heterocyclic compounds like HMX and CL-20 have high heat of formation and the enhanced detonation performance,but their application is limited owing to the high impact and friction sensitivity [3-5].Therefore,it is necessary to give consideration to both the safety and the energy performance when exploring the synthesis strategy of new energetic frameworks.The following effective strategies for designing these kinds of ideal energetic compounds may be helpful for reference: (1) introducing amino groups into energetic frameworks to enhance intramolecular or intermolecular hydrogen bond interactions;(2) constructing energetic metal-organic frameworks;(3) constructing the fused ring skeleton with larger πconjugate system[6-8].
In exploring the synthesis of modern energetic compounds,the construction of fused ring skeleton (3) is an efficient way to improve the performance of new high energetic compounds.Owing to the unique cage structure or polycyclic coplanar structure and the conjugated system,the fused ring compounds show positive heat of formation,high density and good stability [9,10].Various of fused energetic materials containing 1,2,4-triazine have been synthesized with good energetic performance and stability over recent years[11-14].In particular,the fused compounds based on 5-amine-1,2,4-triazine and tetrazole energetic framework have attracted the extensive attention because of their good properties.For instance (Scheme 1),7,8-dinitro-pyrazolium [5,1-C] [1,2,4]triazine-3-(1H-tetrazole-5-yl)-4-amine (I) and 7-nitro-[1,2,4]triazolo [5,1-c] [1,2,4]triazin-3-(1H-tetrazol-5-yl)-4-amine (II)display high detonation velocities(8755 m/s and 8651 m/s)and low sensitivities over 35 J[11,12].The ortho amino group and tetrazole ring on the fused ring stabilize the molecular structure of these energetic compounds through intramolecular and intermolecular hydrogen bond interactions [15].At the same time,incorporating the tetrazole group into the fused framework could improve the stability and density through hydrogen bonding interactions [16].
However,the reported energetic compounds based on 5-amine-1,2,4-triazine and tetrazole energetic framework exhibit undesirably defects(Scheme 1).For instance,compoundIand compoundIIhave high density and detonation properties,but they own the poor thermal stability (Td(onset): 174 ℃ (I),181 ℃ (II)) [11,12].Moreover,3-nitro-6-(1H-tetrazol-5-yl)-[1-3]triazolo [5,1-c] [1,2,4]triazin-7-amine (III) have high sensitivity (IS: 5 J),while compounds
IIIand 6-(1H-tetrazol-5-yl)tetrazolo [5,1-c] [1,2,4]triazin-7-amine(IV) own the low energy density (ρ: 1.77 g/cm3,1.795 g/cm3)[12,13].Herein,in order to design energetic compounds with high explosive properties and good molecular stability,we tried to develop the derivatives of 5,6-fused triazolo-triazine ring which contain 5-amine-1,2,4-triazine and tetrazole [17].There are some significant advantages of the 5,6-fused triazolo-triazine: (a)numerous energetic C-N,N-N,C=N and N=N bonds provide a large energy storage capacity;(b) low mechanical sensitivity;(c)enough vacancies for detonation groups.After that,the most urgent problem to be solved is to construct nitrogen rich heterocycle at the vacancies of the 5,6-fused triazolo-triazine ring.The nitropyrazole rings own high heat of formation and good stability,which can be effective moieties for improving comprehensive properties of energetic compounds [18-21].There are few reports that two vacancies of the fused ring backbone were explosive modified with different nitrogen rich heterocycles through the C-C bond.It is proved that the combination of 5,6-fused triazolo-triazine and nitropyrazole-tetrazole may be a new energetic skeleton for synthesising the heterocyclic compounds with balanced energystability.
Based on the above analysis,two neutral energetic compounds5and6and their divalent cationic salts (compounds7-12) were synthesized (Scheme 1).The prepared compounds were characterized by multinuclear nuclear magnetic resonance (NMR)spectroscopy,elemental analysis (EA) and differential scanning calorimetry(DSC).In addition,based on the obtained single-crystal parameters,quantum chemistry calculations of compounds5and6are performed to investigate the structure-property relationships.
2.Results and discussion
2.1.Synthesis
In this study,5-amine-3-(5-nitro-1H-pyrazol)-1H-1,2,4-triazol(1) can be easily synthesized according to the reported procedure[17].In the first step,compound1was used as the starting material to synthesize compound3through diazotization reaction and condensation reaction with a yield of 89.0%.(Scheme 2).According to the method in literature[11],a closed-loop reaction will occur in compound3when the temperature is heated to 85 ℃.However,only a small amount of flocs separated out after diluting the solution with water in the reaction system.We tried to increase the reaction temperature in a gradient of 10 ℃.To our delight,when the temperature rises to 105 ℃,compound5can be easily collected by filtering with a yield of 92.5%.Considering the energy limitation of compound5,nitro group was attempted to introduce into the vacancy of pyrazole ring by one-step nitration.However,compound6was not successfully obtained(Scheme 2)by using nitrate sulfur mixed acid.Therefore,5-amine-3-(4,5-dinitro-1H-pyrazol)-1H-1,2,4-triazol (2)was selected as substrate to synthesize compound6.Firstly,compound4(yield: 77.8%) were obtained following the similar procedure [22].After that,compound4was further converted to compound6with energy stability equilibrium by reacting with sodium azide at 105 ℃ (yield: 90.6%).
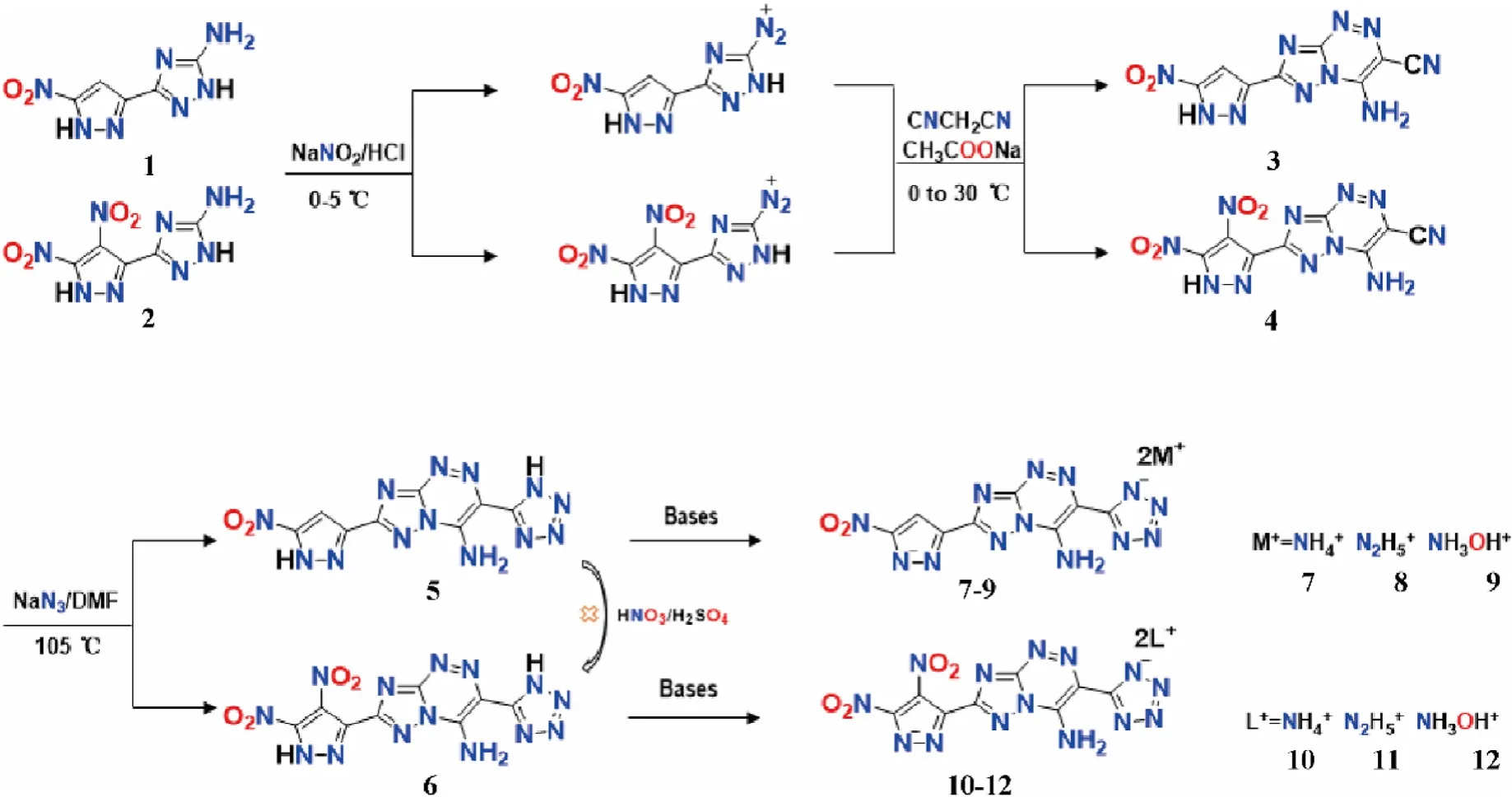
Scheme 2.Synthetic routes of 5-6 and their energetic salts 7-12.
In order to further improve the energy properties of neutral compounds,compounds5and6reacted with monovalent organic bases in acetonitrile at a stoichiometric ratio of 1:2 to produce the corresponding energetic salts7-12 with the yields ranging from 88.5% to 92.4% (Scheme 2).
2.2.Single crystal X-ray structure analysis
In order to determine the structure of the compounds to obtain more structural information,compounds5and6were characterized by single crystal X-ray structure determination.The crystals of both compounds5and6were recrystallized from dimethyl sulfoxide (DMSO),while their molecular structures are depicted in Fig.1(a) and Fig.2(a).Compound5•3DMSO crystallizes in the monoclinic space group P21(Z=2),which has a calculated density of 1.494 g/cm3at 173 K.Compound 6•3DMSO crystallizes in the monoclinic space group P21/n.And there be four molecules per unit cell (Z=4) with the calculated density of 1.526 g/cm3at 173 K.
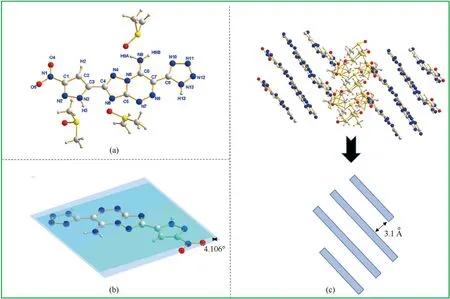
Fig.1.(a) The molecular structure of 5•3DMSO;(b) The planarity of 5•3DMSO;(c) The packing diagram and stacking diagram of 5• 3DMSO.
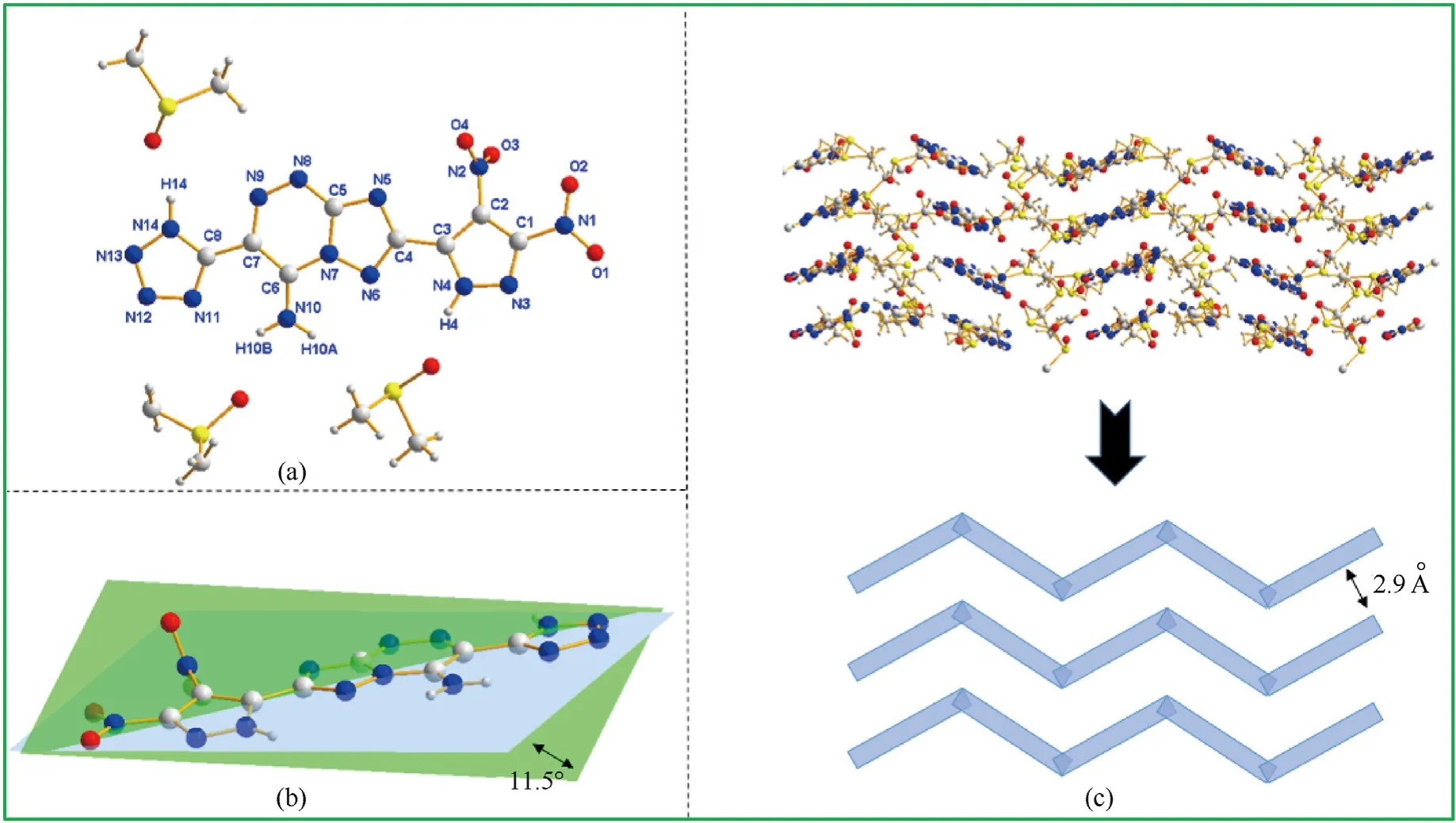
Fig.2.(a) The molecular structure of 6•3DMSO;(b) The planarity of 6•3DMSO;(c) The packing diagram and stacking diagram of 6• 3DMSO.
As shown in Fig.1(b),the dihedral angle(4.106°)confirmed that the triazolo-triazine ring and nitropyrazole ring of compound5•3DMSO are nearly in the same plane.At the same time,the torsion angles display that the nitro and pyrazole are almost coplanar(O4-N1-C1-N2 179.3 (13)°,O5-N1-C1-N2 -3.1 (18)°).On the other side,the amino group and the triazolotriazine bicyclic ring are almost coplanar(N9-C6-C7-N8 180.0(7)°,N9-C6-C7-C8 0.7(12)°).There is an intramolecular hydrogen bond existing in the crystal structure of compound 5•3DMSO(N9-H9A…N12)with the length of 3.012 Å.The crystals of compound 5 exhibit the classic face-to-face stacking,and the nearest distance between the two layers is 3.1 Å(Fig.1(c)).It is worth mentioning that the classic faceto-face stacking mode can effectively explain the low mechanical sensitivity of compound 5 [23].
In the structure of compound6•3DMSO,the fused ring are not coplanar with pyrazole ring,which can be determined by C2-C3-C4-N5(-10.3(8)°)and C2-C3-C4-N6(170.0(5)°)as the dihedral angle of the fused-tetrazolo ring and triazole ring(Fig.2(b)).Furthermore,the two nitro groups in the ortho position on the pyrazole are slightly twisted out of plane.As observed,the torsion angles of one nitro and pyrazole are O1-N1-C1-N3 (10.8(7)°),O2-N1-C1-N3 -168.4 (5)°) while the other nitro group are O3-N2-C2-C1 (74.3 (6)°),O4-N2-C2-C1 (-106.4 (7)°).In the conjoined structure of fused and tetrazole rings,there is a stable system formed by intramolecular hydrogen bond between tetrazole ring and amino group(N10-H10B…N11 2.879 Å),which helps compound 6 to be more stable.As shown in Fig.2(c),a wave-like stacked crystal structure emerges during the stacking process with the interlayer distance of 2.9 Å (Fig.2(c)).
2.3.Physic and energetic properties
All powdered compounds were dried sufficiently at 25 ℃ to remove the solvent before testing,which was judged by differential scanning calorimetry (DSC).The energetic properties of compounds5-12are shown in Table 1.
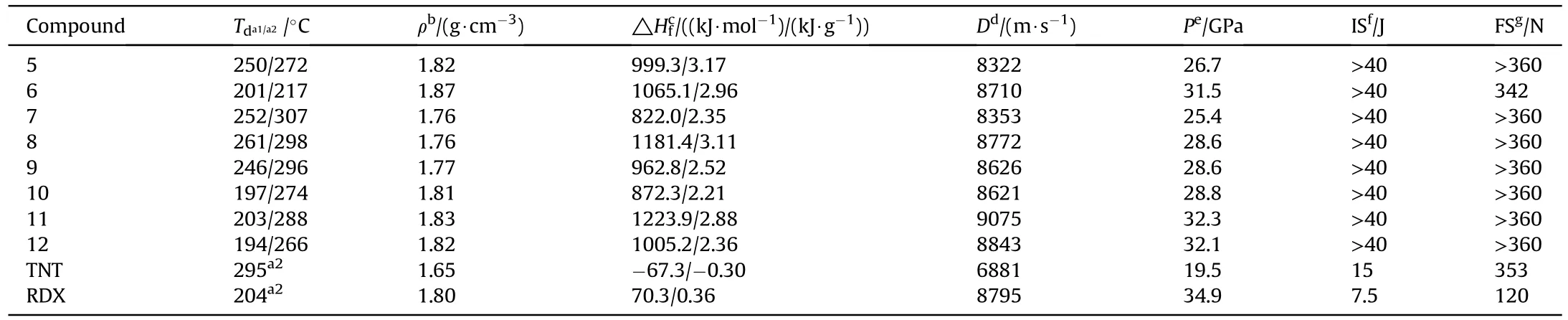
Table 1 The energetic properties of compounds 5-12.
The densities of compounds5-12were measured by using argon gas pycnometer at room temperature [24].Among all these compounds,the compounds5and6(1.82 g/cm3,ρ=1.87 g/cm3)own the higher density than that of traditional energetic compound RDX(ρ=1.80 g/cm3,while the density of anionic salts7-12is in the range of 1.76-1.87 g/cm3.The heats of formation(△Hfc)of compounds5-12was calculated by using Gaussian 09,which exhibits the positive high numerical HOF values due to the high enthalpy of the fused rings.Generally speaking,high nitrogen organic salts have higher nitrogen content,formation enthalpy and detonation performance,but lower density than neutral compounds.Comparing the ionic compounds7-12,it is easy to find that the heat of formation of ammonium salt,hydroxylamine salt and hydrazine salt increase in turn.Although hydrazine salts8and11have a lower density than neutral compounds,their heat of formation is higher,which may be related to the high nitrogen content and rich N=N bonds of hydrazine salts.Using the experimental density at room temperature,the detonation characteristics were calculated by EXPLO5(version 6.01)program.The detonation velocity and detonation pressure of5-12lie in the range from 8322 m/s to 9075 m/s and 25.4-32.3 GPa,respectively.Among them,the detonation velocity of cationic salts7-12is higher than that of their neutral compounds.Notably,the detonation velocity performance of hydrazine salt11(D=9075 m/s) and hydroxylamine salt12(D=8843 m/s) is higher than that of RDX(D=8795 m/s).Hydrazine salts8and11have higher detonation properties than other cationic salts,which may be related to the higher nitrogen content,higher heat of formation and abundant N=N bonds of hydrazine salts.
The sensitivity was tested with standard BAM fall hammer and BAM friction testing machine to evaluate the safety of compounds against external stimuli [25].All of the newly synthesized compounds5-12(IS>40 J,FS ≥342 N)own the lower sensitivity than the traditional explosives TNT (IS=15 J,FS=353 N).Herein,in order to better explain the relationship between sensitivity and structure of compounds5and6,the weak interactions were analyzed by employing two-dimensional fingerprint and Hirshfeld surface [26].Sensitivity can be demonstrated by the shape of the Hirshfeld surface.On the surface of Hirshfeld,red represents the area with high degree of close contact,and blue represents the area with low degree of close contact[27].The molecules of compounds5and6are approximately coplanar,and the red region (intermolecular interaction force) is large and evenly distributed (Fig.3(a) and Fig.3(b)).According to the two-dimensional fingerprints of compounds5and6in Figs.3(b)and 3(d),it can be easily found that the proportions of N-H,H-N,O-H and H-O in the total weak interaction are 61.8% and 59.0% of5and6,respectively.Compound5has a higher hydrogen bond ratio,which can partly explain its lower sensitivity than compound6.In addition,the π-π interaction(C-N and N-C interaction) (9.90%) of compound5is higher than that of compound6(9.30%).Compound5has the stronger π-π stacking interactions,making it more stable.Thus,the above results indicate that the energy of the compound might increase with the increasing of the nitro group number on the pyrazole ring,while the weak interaction will reduce in the meantime.In addition,the strong O-O interaction increases the possibility of accidental explosion,which also leads to higher sensitivity of compounds.As shown in Fig.3(e),the low percentages of O-O interactions for compounds5and6(1.00% for5and 1.90% for6) indicates that compound6has lower sensitivity.
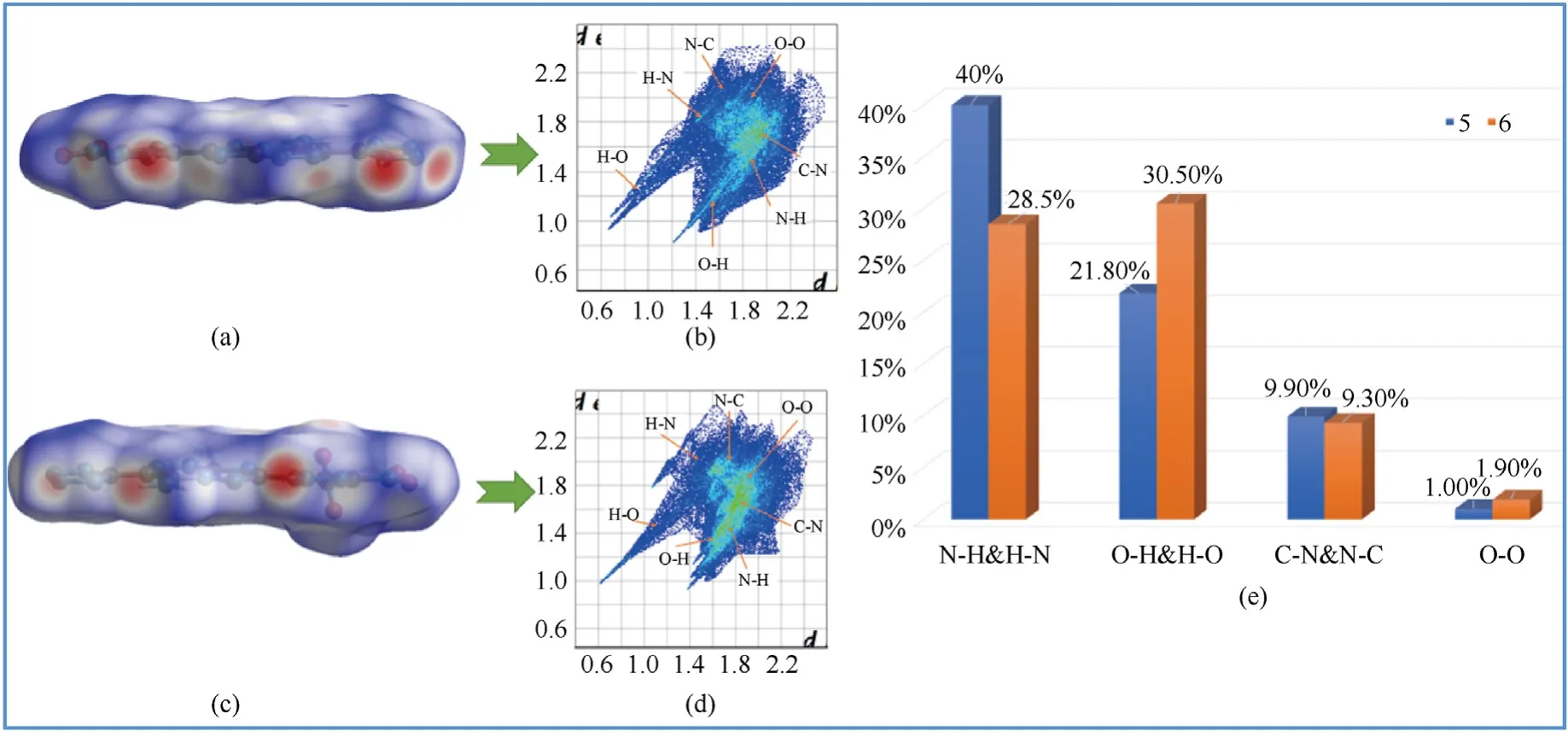
Fig.3.Hirshfeld surfaces,2D-fingerprint plots and percentage contribution for (a) 5 and (c) 6,(b) 5 and (d) 6,(e) (5 and 6).
In order to study the effect of the weak intermolecular and intramolecular interactions on crystal packing of5and6,the noncovalent interaction (NCI) plots were applied [28].Generally,the blue region represents the hydrogen bond interaction,while the green flakes represent π-π interactions.As shown in Fig.4(a),the clear green layer between the two molecules indicates that the compound5own strong π-π interaction.Moreover,the larger green flakes indicate the stronger π-π interaction in compound5(Figs.4(a) and 4(b)).The extensive π-π interactions may suggest that compound5own low impact and friction sensitivity.In addition,there are obvious intramolecular hydrogen bonds in both compounds5and6(Figs.4(a)and 4(b)).The existence of hydrogen bond and strong π -π interaction contribute to the satisfactory density and low sensitivity of compounds5and6,which is consistent with the experimental results.
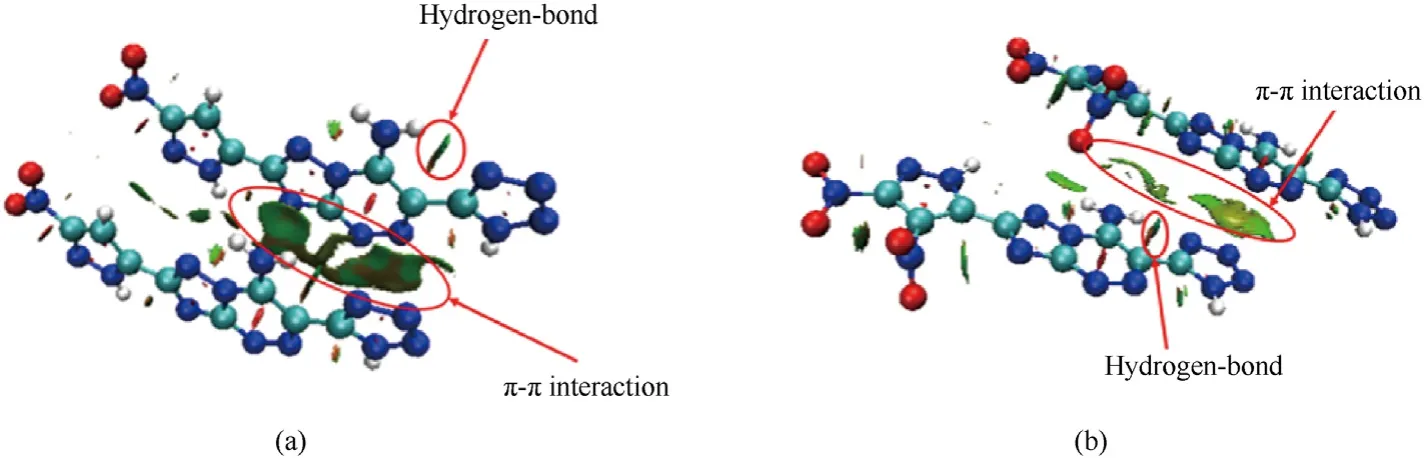
Fig.4.NCI plots for compound (a) 5 and (b) 6.
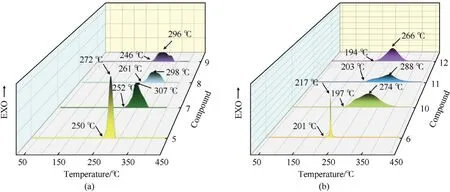
Fig.5.3D-DSC curve of all energetic compounds.
The thermodynamic stability is the focus of the test to evaluate the explosives [29].The thermal stability of all the synthesized compounds5-12was determined by differential scanning calorimetry measurement.As shown in Fig.5,all compounds exhibit an exothermic peak,which indicates that they decompose without melting point.Compounds5and6began to decompose from 250 ℃ to 201 ℃,while their energetic salts5-12have the onset decomposition temperature between 194 ℃ and 261 ℃.It is noteworthy that compound5and6has the high peak temperature of 272 ℃ and 217 ℃,which is higher than the peak temperature of RDX (Td=204 ℃) (Fig.5).
Compared with compound5,a concentrated red region near the pyrazole ring of compound6changes to a blue region,indicating that positive charge accumulation begins to change to negative charge accumulation.In order to further explore the difference of thermodynamic stability between mononitro fused compound5and dinitro fused compound6,the molecular electrostatic potential(ESP) analysis were drawn [30].Different color depths in the ESP diagram represent the ESP values in the molecules which was drawn by the Multiwfn software.As shown in Fig.6,compared with compound5,a concentrated red region near the pyrazole ring changes to the blue region of compound6,which indicates that the positive charge accumulation begins to change to negative charge accumulation.At the same time,with the introduction of nitro,the maximum positive charge in the red region of N-H position of pyrazole ring increases to 68.51 kcal/mol.In addition,the maximum electrostatic potential near amino group and N-H of tetrazole in compound6increases significantly compared with that of compound5(58.64 kcal/mol),which can be used to explain the worse stability of compound6(Figs.6(a)and 6(b)).
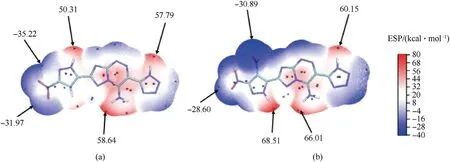
Fig.6.Electrostatic potential (ESP) of (a) 5 and (b) 6.
Compatibility refers to the ability when explosives are mixed or contacted with other materials,the physical and chemical properties of the system will not exceed the allowable range compared with the original components.It is necessary to consider the compatibility closely related to the safety and reliability of explosives.The DSC diagram at the heating rate of 2,5,10 and 20 K/min is displayed in Supporting Information,so as to obtain the decomposition peak temperature of the individual(mixed)system(Table 2).The peak temperature deviation value(ΔTp)is calculated according to the decomposition peak at different heating rates in Table 2.According to the Ozawa formula,the apparent activation energy (ΔE)with the changing rate ΔE/Eais calculated [31].The compatibility of samples can be judged according to ΔTpand ΔE/Ea.As shown in Table 3,the compatibility between RDX and compound 6,NC and compound 5 has reached compatibility level 1,which indicates that they have good compatibility respectively.At the same time,the compatibility of RDX with compound 5,NC and compound 6 reached compatibility level 2 respectively.The above analysis can be used to prove that compounds 5 and 6 have good safety and reliability when mixed with traditional energetic compounds.
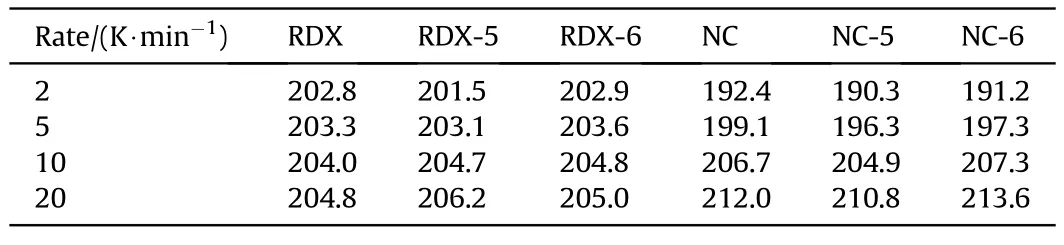
Table 2 Decomposition peak temperature under different beating rates.
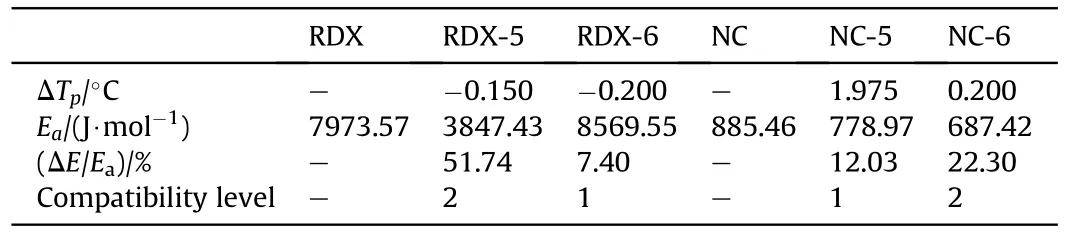
Table 3 Opinion of explosive’s compatibility.
3.Conclusions
In summary,with the combination of 5,6-fused triazolo-triazine and nitropyrazole-tetrazole,the energy-stability balanced compounds 5 and 6 were synthesized in this work.Meanwhile,a series of divalent cationic salts 7-9 and 10-12 were synthesized based on neutral compounds 5 and 6,respectively.Notably,all these fused compounds demonstrate low sensitivity(IS>40 J;FS ≥342 N)and the high detonation velocity (D: 8322-9075 m/s).Among them,energetic salt (11) exhibits better integrated properties(D=9075 m/s,IS >40 J,ρ=1.83 g/cm3) than that of the RDX(D=8795 m/s,IS=7.5 J,ρ=1.80 g/cm3).In order to study the relationship between structure and properties,the NCI,ESP,twodimensional fingerprint and Hirshfeld surface were applied.Through the analysis of compatibility,it can be proved that compounds 5 and 6 have better safety and reliability when mixed with traditional energetic compounds(RDX and NC).Based on the above analysis,it is obvious that the combination of 5,6-fused triazolotriazine and nitropyrazole-tetrazole is an effective method to design energetic materials with high energy and low sensitivity.
4.Experimental section
4.1.Synthesis of 3-carbonitrile-4-amino-7-(5-nitro-1H-pyrazol-3-yl)-[1,2,4]triazolo [5,1-c] [1,2,4]triazine (3)
Compound1(1.97 g,10.0 mmol) was slowly added to the mixture of 3 ml of concentrated hydrochloric acid in 10 ml of water.The sodium nitrite(0.75 g,11.0 mmol)and water(6 ml)was added to the mixture at -5 ℃ for 1 h.Then,a mixture of malononitrile(0.50 g,10.5 mmol) and sodium acetate (3.97 g,11.7 mmol) was added to the system.The reaction system was stirred at -5 ℃ for 2 h,and then raised to 30 ℃ for overnight reaction.The precipitate was filtered,washed with ethanol and dried at 30 ℃ to give a brown solid(2.40 g,89.0%).1H NMR(500 MHz,DMSO-d6):6.05(br),8.92 (s) ppm.13C NMR (125 MHz,DMSO-d6) δ 111.6,115.5,132.6,134.2,136.8,144.6,155.85,158.1 ppm.Elemental analysis for C8H4N10O2(272.19):calcd.C 35.30,H 1.48,N 51.46%.Found:C 35.35,H 1.49,N 51.40%.
4.1.1.Synthesis of 4-amine-3-(1h-tetrazol-5-yl)-7-(5-nitro-1Hpyrazol-3-yl)-[1,2,4]triazolo [5,1-c] [1,2,4]triazin (5)
0.35 g of NaN3and compound3(1.36 g,5.0 mmol)was added to the 40 ml of N,N-dimethylformamide at 0 ℃ and stirred for half an hour.Slowly heat to 105 ℃ and react for 24 h.After that,a large number of solid precipitates out.The precipitate was filtered,washed with ethanol and dried at 30 ℃ to give a brown black solid(1.44 g,92.5%).1H NMR (500 MHz,DMSO-d6): 7.54 (s),8.99 (s),10.03(s)ppm.13C NMR(125 MHz,DMSO-d6):δ 101.9,120.8,136.3,140.40,152.5,156.2,156.4,156.8 ppm.IR (KBr): 3608,3426,3209,3153,3016,2970,2480,1641,1580,1567,1529,1469,1431,1385,1369,1305,1277,1256,1200,1174,1068,997,930,820,752,700,626,587 cm-1.Elemental analysis for C8H5N13O2(315.22):calcd.C 30.48,H 1.60,N 57.77%.Found: C 30.58,H 1.60,N 57.67%.
Procedure for prepare salts 7-9.
Add the 28% ammonia (0.02 g,1.4 mmol),98% hydrazine monohydride (0.71 g,1.4 mmol) and 50% hydroxylamine water(0.92 mg,1.4 mmol) to the mixed solution of compound5(0.31 g,1.0 mmol)in acetonitrile(15 ml)under stirring at 25 ℃,while the reaction was maintained for half an hour.The precipitate obtained by filtration was washed with acetonitrile.
Dark brown solid,yield: 0.32 g,91.2%.1H NMR (500 MHz,DMSO-d6): 7.45 (s),7.66 (br) ppm.13C NMR (125 MHz,DMSO-d6):δ 102.3,126.8,139.6,140.4,156.0,157.7,158.4,159.2 ppm.IR (KBr):3741,3562,3146,2988,2813,1861,1620,1580,1518,1477,1475,1259,1196,1179,1112,1011,937,902,822,778,732,707,626,580 cm-1.Elemental analysis for C8H11N15O2(349.12): calcd.C 27.51,H 3.17,N 60.15%.Found: C 27.55,H 3.19,N 60.09%.
Hydrazinium salt (8).
Dark yellow solid,yield: 0.34 g,90.7%.1H NMR (500 MHz,DMSO-d6):7.27 (s),7.50 (s) ppm.13C NMR (125 MHz,DMSO-d6):δ 102.4,126.1,139.5,142.2,155.52,156.1,158.1,160.6 ppm.IR(KBr):3401,3307,3142,2845,2603,2169,1641,1585,1501,1486,1536,1486,1361,1291,1158,1070,1025,997,972,944,825,784,749,623,560 cm-1.Elemental analysis for C8H13N17O2(379.31): calcd.C 25.33,H 3.45,N 62.78%.Found: C 25.33,H 3.46,N 62.77%.
Brown solid,yield:0.35 g,89.3%.1H NMR(500 MHz,DMSO-d6):7.31 (s),8.04 (br) ppm.13C NMR (125 MHz,DMSO-d6): δ 101.9,126.9,137.8,139.7,155.9,156.9,157.2,158.2 ppm.IR (KBr): 3674,3559,3121,2985,2897,2495,1861,1631,1571,1518,1455,1342,1243,1204,990,937,906,829,766,693,623,570 cm-1.Elemental analysis for C8H11N15O4(381.28): calcd.C 25.20,H 2.91,N 55.51%.Found: C 25.25,H 2.92,N 55.45%.
4.1.1.1.Synthesis of 3-carbonitrile-4-amino-7-(4,5-dinitro-1H-pyrazol-3-yl)-[1,2,4]triazolo[5,1-c][1,2,4]triazine(4).Compound 4 was synthesized by using a synthetic method similar to that of compound 3,but compound 2 (2.40 g,10 mmol) was used instead of compound 1.Yellow solid,yield: 2.42 g,77.8%.1H NMR (500 MHz,DMSO-d6): 8.39 (s) ppm.13C NMR (125 MHz,DMSO-d6): δ 110.8,115.1,128.0,135.9,144.3,149.2,155.9,159.2 ppm.Elemental analysis for C8H3N11O4(317.79): calcd.C 30.29,H 0.95,N 48.58%.Found: C 30.38,H 0.96,N 48.48%.
4.1.1.2.Synthesis of 4-amine-3-(1h-tetrazol-5-yl)-7-(4,5-dinitro-1Hpyrazol-3-yl)-[1,2,4]triazolo [5,1-c] [1,2,4]triazin (6).0.35 g of NaN3and compound 4 (2.02 g,6.0 mmol) were suspended in 40 ml of DMF.The solution was heated to 105 ℃ and reacted for 12 h.The solution is concentrated and then diluted with an aqueous solution of sodium chloride until the maximum amount of sediment is present.The precipitate was filtered,washed with ethanol and dried at 30℃ to obtain brownish black solid,yield:3.24 g,90.6%.1H NMR (500 MHz,DMSO-d6):7.75 (s),8.31 (s) ppm.13C NMR(125 MHz,DMSO-d6):δ 120.9,127.3,133.4,140.9,148.2,152.6,156.2,156.4 ppm.IR (KBr): 3653,3562,3205,2985,2987,2904,2141,1644,1557,1476,1399,1393,1340,1238,1193,1106,1018,944,902,850,759,679,644,580,490 cm-1.Elemental analysis for C8H4N14O4(360.21): calcd.C 26.28,H 1.12,N 54.44%.Found: C 26.30,H 1.15,N 54.39%.
Procedure for prepare salts 10-12.
Add the 28% ammonia (0.02 g,1.4 mmol),98% hydrazine monohydride (0.71 g,1.4 mmol) and 50% hydroxylamine water(0.92 mg,1.4 mmol) to the mixed solution of compound6(0.36 g,1.0 mmol) in acetonitrile(15 ml)under stirring at 25 ℃,while the reaction was maintained for half an hour.The precipitate obtained by filtration was washed with acetonitrile.
Brown solid,yield:0.35 g,88.5%.1H NMR(500 MHz,DMSO-d6):δ 6.32 (s),7.66 (br) ppm.13C NMR (125 MHz,DMSO-d6): δ 125.9,128.2,137.4,139.5,149.5,155.7,156.3,156.8 ppm.IR (KBr): 3569,3167,2985,2127,1693,1567,1411,1492,1418,1322,1256,1151,1105,1067,1032,951,848,763,693,667,558 cm-1.Elemental analysis for C8H10N16O4(394.28): calcd.C 24.37,H 2.56,N 56.84%.Found: C 24.40,H 2.60,N 56.77%.
4.1.1.3.Hydrazinium salt (11).Brown solid,yield: 0.39 g,92.4%.1H NMR (500 MHz,DMSO-d6): δ 6.33 (s),7.73 (br) ppm.13C NMR(125 MHz,DMSO-d6):δ 126.1,128.2,137.4,139.6,149.5,155.8,156.3,156.8 ppm.IR (KBr): 3664,3328,2985,2901,2614,2162,1630,2585,1480,1378,1350,1319,1249,1154,1105,1028,994,846,808,752,693,616,581 cm-1.Elemental analysis for C8H12N18O4(424.31):calcd.C 22.65,H 2.85,N 59.42%.Found:C 22.70,H 2.86,N 59.36%.
Hydroxylammonium salt (12).
Brown solid,yield:0.38 g,90.2%.1H NMR(500 MHz,DMSO-d6):δ 7.18 (s),7.78 (s) ppm.13C NMR (125 MHz,DMSO-d6): δ 125.3,128.2,137.3,139.7,149.5,155.8,157.6,159.7 ppm.IR (KBr): 3681,2995,2971,2897,2185,1637,1570,1518,1508,1445,1396,1329,1245,1165,1046,1009,1011,948,860,780,630,574 cm-1.Elemental analysis for C8H10N16O6(426.27):calcd.C 22.54,H 2.36,N 52.57%.Found: C 22.59,H 2.41,N 52.47%.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No.21875110,22075143) and the Science Challenge Project.H.Y thanks the Qing Lan Project for the grant.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2022.10.003.
杂志排行
Defence Technology的其它文章
- An energetic nano-fiber composite based on polystyrene and 1,3,5-trinitro-1,3,5-triazinane fabricated via electrospinning technique
- Dynamic response of UHMWPE plates under combined shock and fragment loading
- Aerial multi-spectral AI-based detection system for unexploded ordnance
- Model-based deep learning for fiber bundle infrared image restoration
- Robust design and analysis for opto-mechanical two array laser warning system
- Combustion behavior and mechanism of molecular perovskite energetic material DAP-4-based composites with metal fuel Al
