Fabrication of electrostatic safety copper azide film with in situ growing MOF
2023-10-09ShungWngLiYngZhenzhnYnJiminHnXiotingRenWeiLi
Shung Wng ,Li Yng ,* ,Zhenzhn Yn ,Jimin Hn ,Xioting Ren ,Wei Li
a State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing,100081,China
b Science and Technology on Aerospace Chemical Power Laboratory,Hubei Institute of Aerospace Chemotechnology,Xiangyang,441003,Hubei,China
Keywords:Carbon nanofilm Metal-organic framework Copper azide (CA)Electrostatic sensitivity Electrospinning
ABSTRACT Due to its extremely low electrostatic sensitivity,copper azide primary explosive is greatly limited in practical applications.In this study,a composite film with Cu-MOF in-situ growth on carbon nanofilm was prepared by electrospinning and solvothermal methods,and CNF@Cu-N3 film with electrostatic safety was obtained by carbonization and azide later.Its electrostatic sensitivity (E50) was greatly increased from 0.05 mJ of raw materials to 4.06 mJ,and still maintained a good detonation performance which could successfully detonate the CL-20 secondary explosive.This is mainly due to the synergistic effect of the carbon film and the MOF structure,which greatly improves the conductivity of the entire system and the uniform distribution of copper particles,providing a new preparation strategy for metal azide film that is suitable for the micro-initiator device.
1.Introduction
With the development of new ammunition,fuze and other weapon systems for miniaturization,integration and intelligence,technologies related to microelectromechanical systems (MEMS)have also been widely used in the field of energy.Due to the miniaturization of ignition devices,lead-based primary explosives have gradually been unable to meet the energy requirements of MEMS micro-detonation devices.Instead,copper-based primary explosives with higher energy and more environmental protection are used.Among them,the detonation ability of copper azide(CA)is 8 times that of lead azide(LA),and the limit charge of detonating PETN is only 1/6 of that of LA.In addition,it is also environmentfriendly,and is the best candidate to replace traditional primers such as lead azide in recent years[1-5].If CA is used as a primary in MEMS fireworks,it can not only reduce the volume occupied by energetic materials,but also effectively increase the output energy of energetic devices,while reducing the required input energy to achieve high reliable detonation of quality.Unfortunately,CA has an extremely high sensitivity,and only 0.05 mJ of static electricity can cause its explosion,which greatly limits its practical application.
To solve this problem of CA,many scholars tried adding carbon nanotubes,graphene,and other carbon-based materials to it to enhance the internal conductivity [6-12],thereby reducing the static accumulation and reducing its electrostatic sensitivity.Such as using electrodeposition technology to deposit nano-copper dendrites in carbon nanotubes,and then constructing copper azide-based energetic materials bound by carbon nanotubes through azide [13].Or prepared spherical copper azide/carbon/reduced graphene oxide (CA/C/rGO) composites [14].The CA nanoparticles were uniformly distributed on the carbon framework and got good electrostatic stability and ignition ability.Besides,researchers also used some template agents to prepare uniformly distributed porous copper precursors [15-18],Yu [17] used CuO nanorod arrays and porous copper films as precursors to in-situ synthesize copper azide films in NaN3solution by electricassisted azide method,which reduced the electrostatic sensitivity to a certain extent.Wang[18]has further strengthened the uniform distribution of copper particles by introducing MOF materials,and obtained copper azide encapsulated inside the MOF skeleton through the in-situ azide process,effectively reducing its electrostatic sensitivity.Most of the above reports received better sensitivity properties copper azide-based composites by introducing carbon skeletons with good electrical conductivity;or preparing uniformly distributed porous copper.However,these methods reduced the electrostatic sensitivity of CA to a certain extent,the effective combination of carbon materials and copper particles is still a big problem,and it is also not conducive to subsequent microcharge in the MEMS system.Therefore,it is imperative to develop a new strategy that is more suitable for MEMS and can more efficiently solve the problem of excessive electrostatic sensitivity caused by particle agglomeration.
Carbon nanofilm (CNF),one-dimensional carbon supports prepared by electrospinning technology,has begun to receive great attention in the field of photoelectric catalysis [19-23].Especially when it is combined with metal-organic frameworks materials with high specific surface area,MOF-driven carbon-based composites exhibit excellent catalytic performance [24,25].These studies showed that the use of CNF in combination with MOF materials can not only play the role of the high specific surface area of MOFs,but also effectively and uniformly load metal particles in MOFs,further enhancing the electrical and thermal conductivity of the composites and the uniformly distributed of nanoparticles[26-33].What's more,the film material prepared by electrospinning is flexible and can be cut into any shape,which is conducive to matching with the MEMS charging cavity,and is safer than the filling of powder primary explosive.
Herein,a novel MOF-driven carbon nanofilm (CNF@Cu-MOF)was fabricated by a simple electrospinning technique and solvothermal method.The copper azide-based primary explosive with good thermal stability and electrostatic safety was prepared by further carbonization and in-situ azide technology.The final prepared CNF@Cu-N3benefits from the uniform distribution of copper particles,and the excellent thermal and electrical conductivity of the carbon skeleton,with good thermal stability and significantly reduced electrostatic sensitivity.
2.Experimental
2.1.Materials
PAN,stearic acid,copper acetate monohydrate(Cu(OOCCH3)2∙H2O,Cu(OAc)2)and Sodium hydroxide(NaOH)were purchased from Beijing Chemical Industry with analytically pure.Sodium azide of analytical grade was purchased from Xiya Reagent.Trimesic acid (H3BTC),N,N-dimethylformamide (DMF) and absolute ethanol were purchased from Aladdin.
2.2.Preparation of PAN and CNF
The preparation process of the sample was shown in Fig.1.Firstly,1 g PAN was dissolved in 10 mL DMF with vigorous stirring at 80 ℃ for 2 h and obtained the precursor solution of electrospinning.Then,transferred the solution to a syringe equipped with a G20 needle (din=0.6 mm,dout=0.9 mm) and adjusted the electrospinning parameters:the output positive voltage was 15 kV,collection distance was 15 cm,and the spinning jet speed was 0.1 mm/min under room temperature.After the electrospinning was completed,the PAN-based membrane was gently peeled off,and the film was raised from room temperature to 800 ℃ at a heating rate of 5 ℃/min under a nitrogen atmosphere,carbonized at high temperature for 15 min,and then slowly lowered to room temperature,the black CNF sample was obtained.
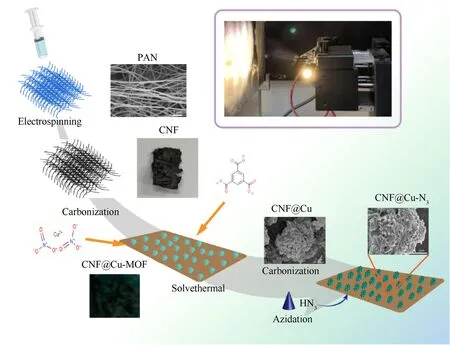
Fig.1.Schematic representation of the preparation of CNF@Cu-N3.
2.3.Preparation of CNF@Cu-MOF and CNF@Cu
CNF@Cu-MOF was prepared by a facile solvothermal method.Dissolved 0.25 g Cu(NO3)2∙3H2O and 0.14 g of H3BTC in 20 mL deionized water and 20 mL DMF,respectively,and sonicate for 10 min.Then added 20 mL ethanol and the previously prepared CNF to continue sonication for 20 min.After that,the above solution was placed in a high-temperature reaction kettle made of polytetrafluoroethylene for reaction at 120℃ for 12 h.After cooling to room temperature,it was repeatedly washed with deionized water and DMF,and the remaining residues outside the film were filtered off,and finally a blue film material grown by Cu-MOF in situ was obtained.
The CNF@Cu-MOF sample grown with Cu-MOF was carbonized at a high temperature of 600 ℃ for 15 min under a nitrogen atmosphere,and finally obtained the CNF@Cu.
2.4.Preparation of CNF@Cu-N3
The gaseous HN3generated by the reaction of stearic acid and sodium azide continuously and slowly passed through the CNF@Cu sample for 24 h,and NaOH was dissolved in water as a tail gas collection device to collect the remaining azide gas from the reaction,and finally CNF@Cu-N3was obtained.Caution!The prepared azide has high energy and is very sensitive,the whole reaction device should be carried out behind the explosion-proof device in the fume hood,and the prepared azide material should avoid shaking and friction as much as possible.
2.5.Characterization
The surface morphology,mean size,and size distribution of the prepared samples was characterized using scanning electronic microscopy(SEM,S-4800,HITACHI,Japan)at 15 kV equipped with an EDS system.Powder X-ray diffraction(XRD)pattern and Fourier transform infrared spectra (FT-IR) were used to analyze the composition of samples.And the thermal property of CNF@Cu-N3was analyzed using the differential scanning calorimeter(DSC),and the conditions of DSC were as follows: sample mass,0.5 mg;heating rate,10 ℃/min;nitrogen atmosphere,30 mL/min.
Electrostatic sensitivity test: Test conditions: the electrode gap length is 0.12 mm and the charge capacitance is 250 μF.Primary explosives prepared were tested using the up and down method for each condition,and the electrostatic sensitivity (E50) for 50% probability of ignition was calculated.Flame sensitivity test: Place 20 mg of primary explosive prepared in a copper cap,and ignite it with a black powder column.Primary explosives prepared were tested using the up and down method for each condition,and the flame sensitivity (H50) for 50% probability of ignition was calculated.
3.Results and discussion
3.1.Characterization of CNF@Cu-MOF and CNF@Cu
The sample maintained the original film shape during the whole preparation process.At first,a white PAN film was obtained by electrospinning,and its SEM showed a uniform fibrous shape(Fig.2).After high-temperature annealing,it became a black film with some wrinkles on the surface,and the carbonized sample became a layered carbon skeleton film from a micro perspective.After the solvothermal reaction,the surface of the CNF@Cu-MOF film gradually turned blue,and the Cu-MOF bulk with a particle size of about 5 μm-10 μm grew on the surface of the carbon film.After further carbonization,the surface of the sample turned black again,the MOF skeleton was carbonized,and the copper particles of 100 nm-200 nm are uniformly distributed on the carbonized MOF skeleton.
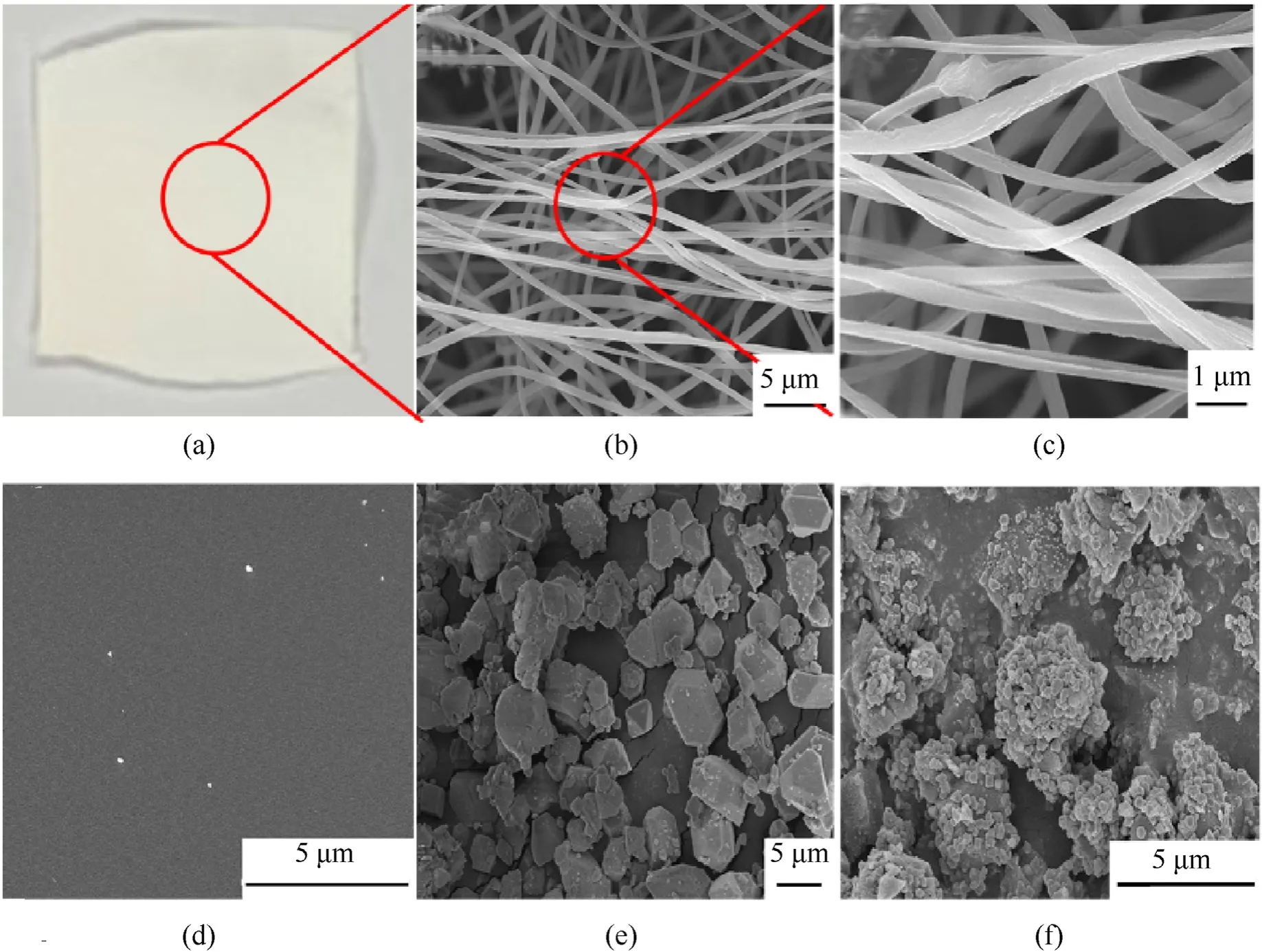
Fig.2.Digital photograph of the (a) PAN,and SEM images of (b) and (c) PAN,(d) CNF,(e) CNF@Cu-MOF and (f) CNF@Cu.
In addition,the CNF@Cu-MOF and CNF@Cu were further analyzed by elemental scanning using EDS equipped with SEM(Fig.3).It can be seen from the figure that the two samples are mainly composed of C,O,and Cu elements.After high-temperature carbonization,the MOF framework was carbonized,and more copper particles were exposed.It can be clearly seen that the copper distribution of CNF@Cu is much higher and evenly distributed.
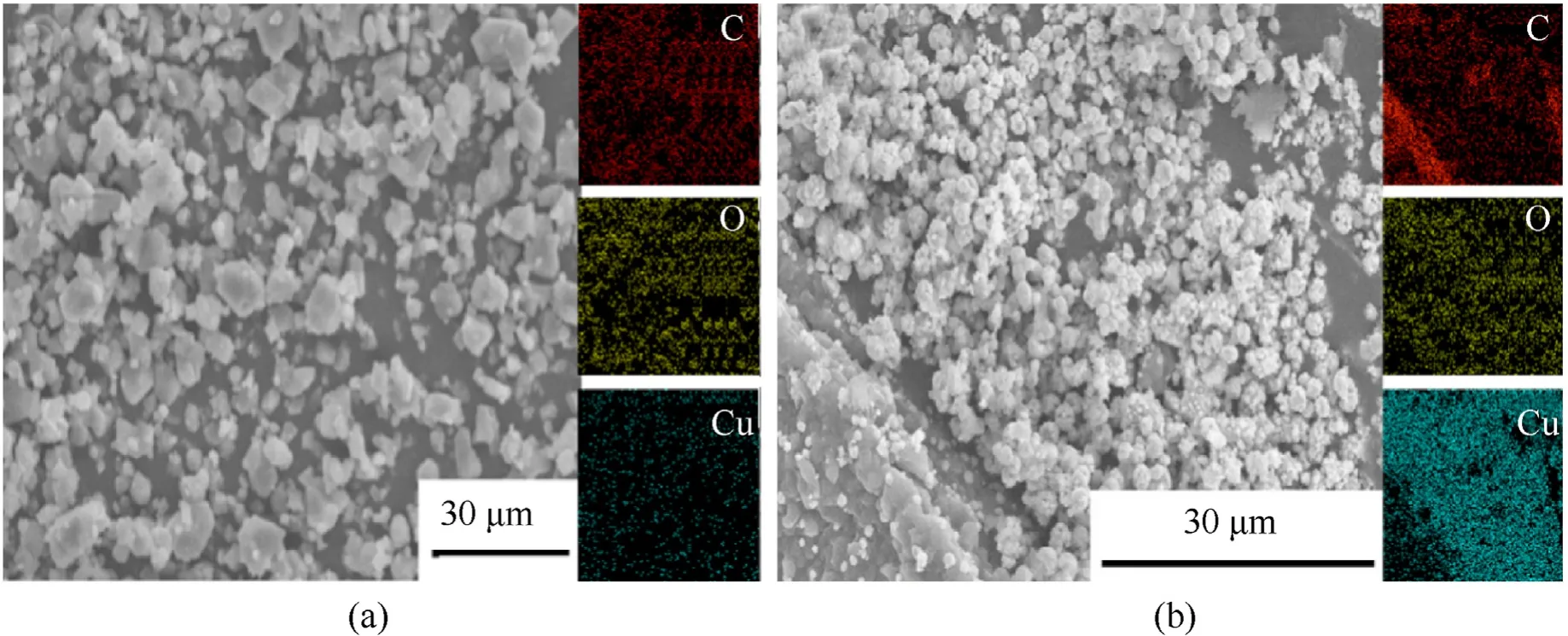
Fig.3.Energy dispersive X-ray spectroscopy (EDS) mapping of (a) CNF@Cu-MOF and CNF@Cu.
To further elucidate the transformation relationship between these membranes,additional in-depth studies with XRD and FTIR were carried out.Fig.(4)a showed the XRD patterns of three samples of CNF,CNF@Cu-MOF and CNF@Cu.The peaks at 25.6°observed on the pattern of CNF was reflected the typical peak of graphitic materials,and both CNF@Cu-MOF and CNF@Cu samples retained this characteristic peak,indicating that the carbon skeleton has always existed.For the XRD pattern of the CNF@Cu-MOF,the main diffraction peaks were located at (2θ=) 6.6°,9.5°,11.7°,13.5°,14.8°,17.6°,19.1°,24.1°,26.1°,29.4°and 35.2°,which correspond to the diffraction characteristic peaks of Cu-MOF on the(200),(220),(222),(401),(331),(440),(600)(731),(751)(773)and(882),indicating the successful in-situ synthesis of MOF materials on carbon films.At the same time,there are still two obvious diffraction peaks at 43.4°and 53.5°,which correspond to the two characteristic peaks of copper respectively.After further carbonization,the characteristic fronts of Cu-MOF basically disappeared,leaving the characteristic peaks of copper and copper oxide,indicating that CNF@Cu mainly contains copper oxide and copper,which corresponds to the result of EDS element scanning.
As presented in Fig.4(b),typical FTIR spectra were obtained for these three samples.The vicinity of 2200 cm-1corresponds to the absorption band of the nitrile group in CNF,and the 700 cm-1-750 cm-1corresponds to the C-H bending vibration of the benzene ring.After in situ growth of MOF,it can be clearly seen that asymmetric and symmetric stretching peaks of BTC carboxylic acid groups appear at 1372 cm-1-1444 cm-1and 1553 cm-1-1658 cm-1,and the stretching vibration peaks of Cu-O appeared at 521 cm-1and 600 cm-1indicating the success of in-situ synthesis of Cu-MOF on CNF film.After carbonization,the stretching peak of the BTC carboxylic acid group basically disappeared,while the vibration peaks of Cu-O at 624 cm-1and 668 cm-1were more obvious,indicating that CuO and other substances were left after the MOF framework was carbonized,which was consistent with the XRD results.
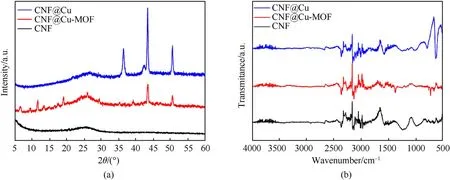
Fig.4.(a) XRD patterns and (b) FTIR spectra of samples.
3.2.Characterization of CNF@Cu-N3
As shown in Fig.5(a)and Fig.5(b),after HN3gas was introduced into the CNF@Cu-N3sample,short columnar materials with a diameter of about 500 μm were formed in situ on the original carbon film and carbon framework,which still retained the pore structure of the MOF framework.The XRD spectra (Fig.5(c)) of CNF@Cu-N3exhibited typical Cu(N3)2patterns,suggesting the successful azide reaction process for CNF@Cu-N3.In addition,DSC thermal analysis technique was used to characterize the thermal properties of CNF@Cu-N3,and the heating rate of 10 ℃/min was used for testing.CNF@Cu-N3has a sharper downward exothermic peak at 210.4 ℃,which is the exothermic peak of CA,proving the success of the azide process again.It also indicated a very fast reaction of CA in the CNF@Cu-N3.And CNF@Cu-N3didn't show any decomposition even heated at 180 ℃,which means CNF@Cu-N3has a good long-term thermal stability that is critical during the transportation and storage of explosives.
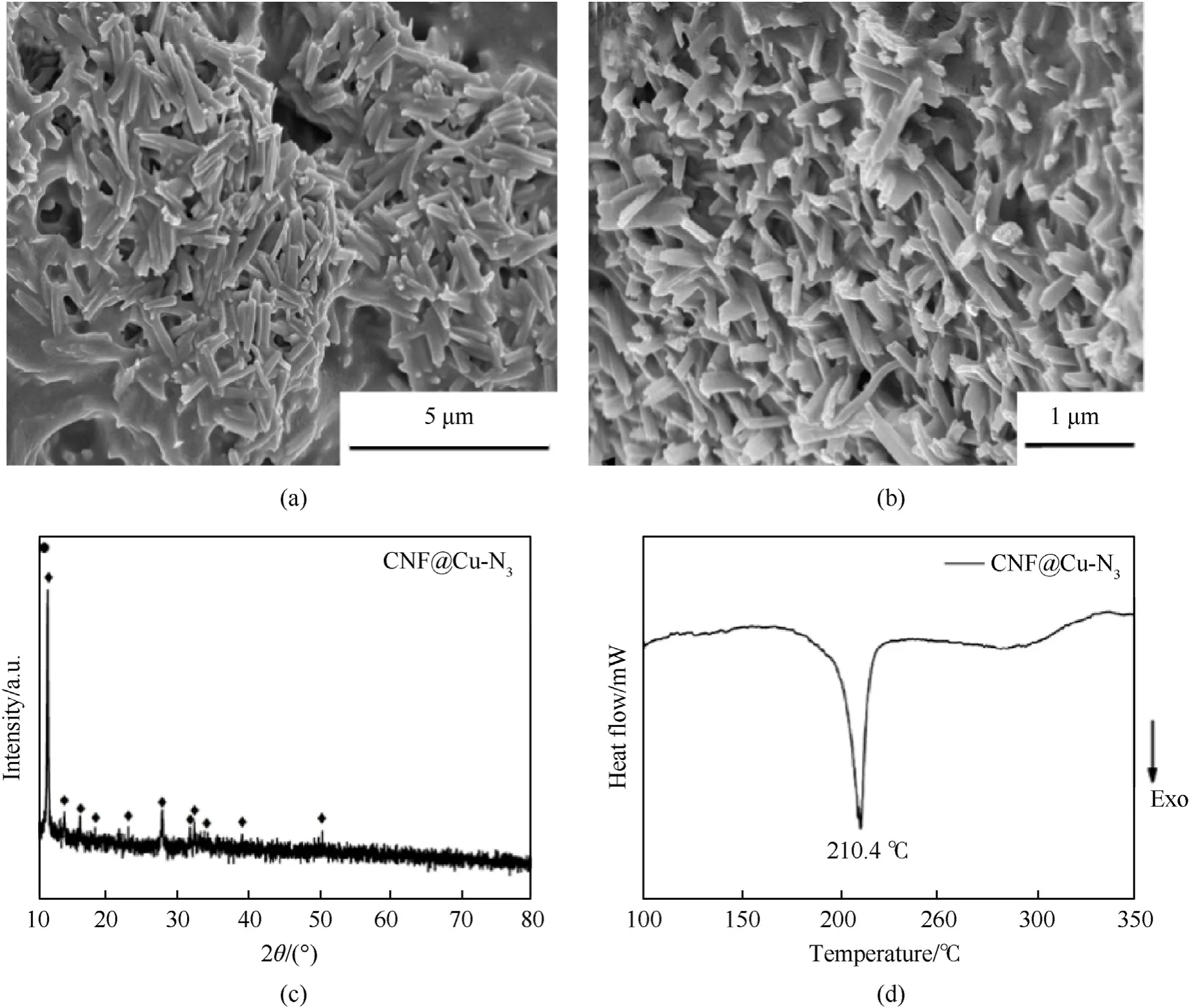
Fig.5.(a),(b) SEM images;(c) XRD pattern,and (d) DSC curve of CNF@Cu-N3.
3.3.Sensitivity properties of CNF@Cu-N3
To evaluate the stability and ignition performance,the electrostatic and flame sensitivities of CNF@Cu-N3were investigated(Fig.6).For comparison,the commonly used primary explosives and other modifications of copper azide samples were also shown in the figure.It can be clearly seen from Fig.6(b)that the prepared CNF@Cu-N3has good electrostatic sensitivity and moderate flame sensitivity.The electrostatic sensitivity was increased from 0.05 mJ of the raw material to 4.06 mJ,which is about 80 times that of the raw material,and is generally better than that of the modified samples in some studies,while the flame sensitivity of 46 cm,remained moderate.Compared with the other copper azide-based composites modified by carbon-based materials and MOF materials,CNF@Cu-N3can still stand out and have better sensitivity performance.This is mainly due to the synergistic use of carbon nanofilms and MOF materials.The CNF@Cu-N3not only has the excellent thermal conductivity and electrical conductivity of carbon nanofilms,but also has the characteristics of a large specific surface area of MOF.The copper particles are evenly distributed in the carbon skeleton,which effectively avoids the classic accumulation caused by agglomeration and greatly reduces the sensitivity.
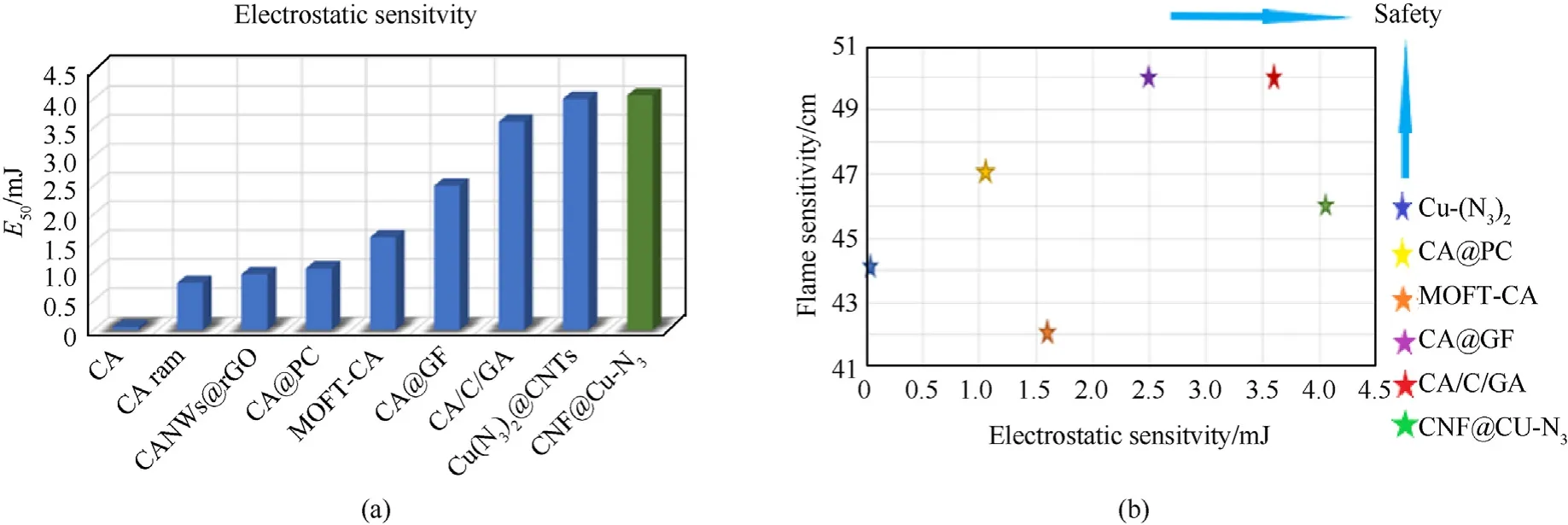
Fig.6.(a) Electrostatic sensitivities of CA-based samples and (b) the map of their electrostatic and flame sensitivities of them.
3.4.Sensitivity change mechanism of CNF@Cu-N3
As shown in Fig.7,the mechanism of electrostatic sensitivity change in CNF@Cu-N3and other modified samples by carbonbased materials (CA/C) was proposed.When the needle-like CA is gathered,the electrostatic charge cannot be taken away in time,and when the accumulated electrostatic charge is large enough,the CA will explode,which is the main reason for the extremely high electrostatic sensitivity of CA.When carbon nanotubes,graphene and other carbon-based materials are doped into CA raw materials,due to the excellent electrical conductivity of carbon materials,when electrostatic accumulation occurs in CA/C,most of the charges are exported by carbon-based materials,which reduces its electrostatic sensitivity and is not easy to be easily detonated,and greatly enhances the safety performance.However,during the combined process of CA and carbon materials,there will be agglomeration,and the Cu particles in the CA/C composites have uneven distribution,which will still lead to the local accumulation of electrostatic charges,and eventually lead to an explosion.CNF@Cu-N3was prepared by in-situ growth of Cu-MOF on carbon thin films,followed by carbonization and in-situ azide.The existence of the MOF framework ensures the uniform distribution of copper particles on the one hand,and on the other hand,it can also form a faraday cage to effectively avoid the accumulation of charges on the CA.In addition,the existence of the carbon film is equivalent to adding a layer of double insurance to the CNF@Cu-N3,fixing the MOF and effectively reducing the electrostatic potential energy generated by friction,it also further increases the effective current flow in the entire system.The accumulation of electrostatic charges is greatly reduced,which also greatly reduces the electrostatic sensitivity of the composites.Moreover,the sample CNF@Cu-N3remained a film eventually,which is of great significance for the subsequent application to MEMS microcharges.
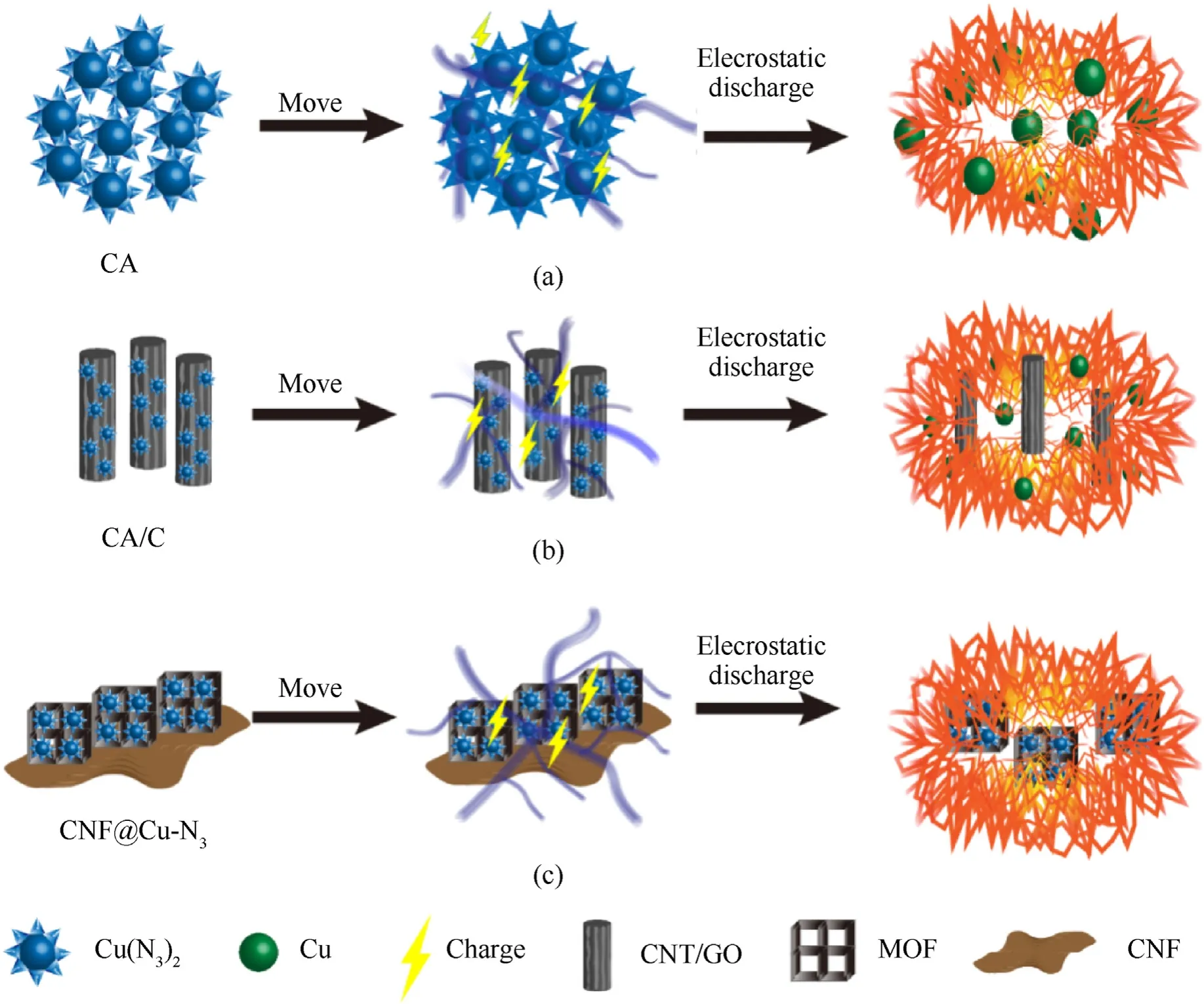
Fig.7.Schematics illustration of the mechanism of electrostatic sensitivity of (a) CA,(b) CA/C and (c) CNF@Cu-N3.
3.5.Detonation performance of CNF@Cu-N3
To further study the detonation performance of CNF@Cu-N3film,a micro-initiator device(Fig.8(a))using Ni-Cr bridge wire for electrical ignition was used to demonstrate its detonation performance.The CNF@Cu-N3film was cut into a suitable shape(ɸ=1 m×1 m,m=0.0048 g),and loaded into the charge cavity in a micro-initiator device.Electric ignition is carried out using Ni-Cr bridge wire under the ignition capacitor of 35 μF and the ignition voltage of 30 V.After CNF@Cu-N3was ignited,it quickly changed from deflagration to detonation and detonated the CL-20 secondary explosive (m=0.006 g) in an instant,leaving obviously explosion traces on the charging plate (Fig.8(b)).The greater power of the explosion could also be seen from the dents and circular explosion marks on the identification block at the bottom.The explosion test demonstrated that CNF@Cu-N3film is a flexible energetic film that can maintain good detonation properties,and is also an attractive candidate for primary explosive.
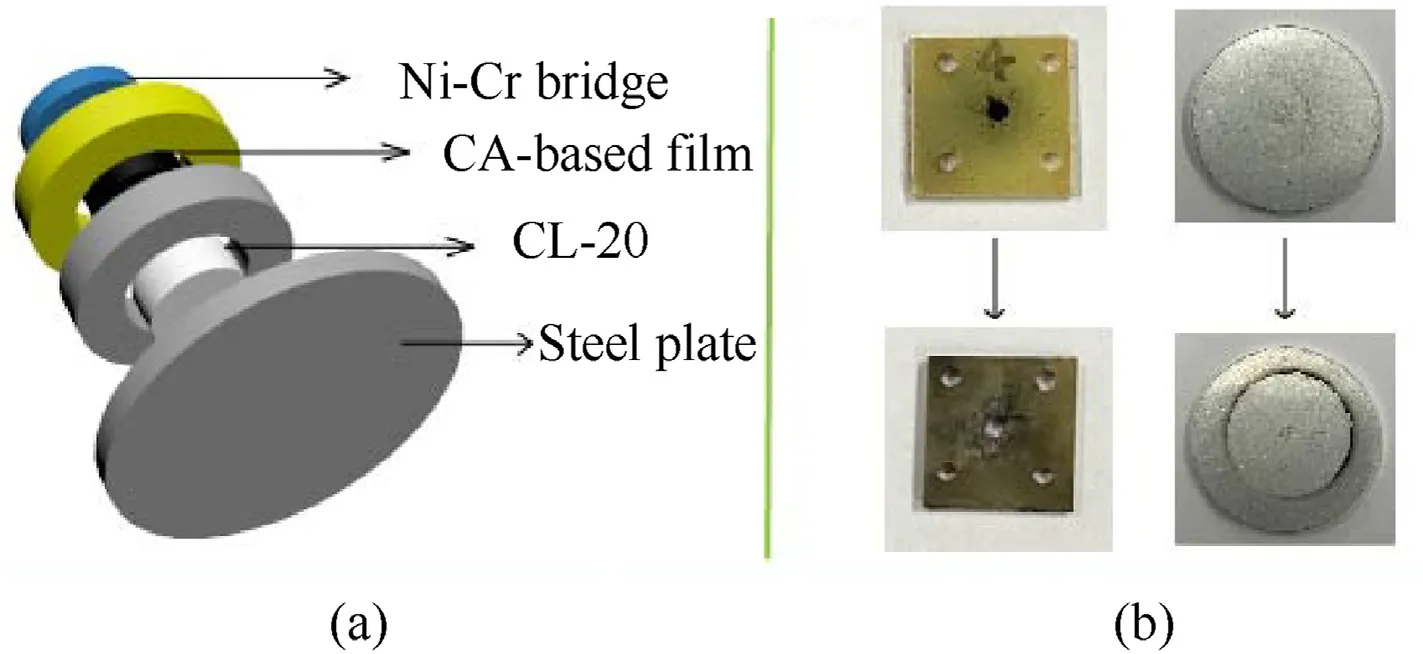
Fig.8.(a) The micro-initiator device and (b) the charge plate before and after detonation.
4.Conclusions
In summary,a flexible thin film material was prepared for use in MEMS micro-initiators.Carbon nanofilms with excellent thermal and electrical conductivity were used as flexible substrate materials,which can effectively enhance the thermal stability of CNF@Cu-N3and facilitate electron transport.At the same time,the existence of porous MOF structure in the precursor also effectively avoided the agglomeration of copper particles and charge reduction.Their synergistic effect of them effectively improved CNF@Cu-N3film's electrostatic safety (0.05 mJ-4.06 mJ).And the flexible substrate has the advantages of better safety,easy processing and molding than powder materials.In addition,the film material(0.0048 g)can successfully detonate the CL-20 secondary explosive,which not only improves the safety,but also maintains a good detonation performance.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge financial support from the National Natural Science Foundation of China (Grant No.12102051),the State Key Laboratory of Explosion Science and Technology (Grant No.QNKT2022-04).
杂志排行
Defence Technology的其它文章
- An energetic nano-fiber composite based on polystyrene and 1,3,5-trinitro-1,3,5-triazinane fabricated via electrospinning technique
- Dynamic response of UHMWPE plates under combined shock and fragment loading
- Aerial multi-spectral AI-based detection system for unexploded ordnance
- Model-based deep learning for fiber bundle infrared image restoration
- Robust design and analysis for opto-mechanical two array laser warning system
- Combustion behavior and mechanism of molecular perovskite energetic material DAP-4-based composites with metal fuel Al
