丙戊酸对双峰驼成纤维细胞重编程影响
2023-07-09张启冉李宗帅马甜李怡娜赵兴绪张勇
张启冉,李宗帅,马甜,李怡娜,赵兴绪,,张勇,
丙戊酸对双峰驼成纤维细胞重编程影响

1甘肃农业大学生命科学技术学院/甘肃农业大学甘肃省动物生殖生理及繁殖调控重点实验室,兰州 730070;2甘肃农业大学动物医学院,兰州 730070
【目的】探讨提高双峰驼成纤维细胞重编程效率,减少因原癌基因引入造成致瘤风险。试验在成纤维细胞重编程过程中添加丙戊酸(valproic acid,VPA),探索小分子物质对双峰驼成纤维细胞重编程的影响,为双峰驼发病机制研究提供参考依据。【方法】采用3月龄双峰驼胎儿成纤维细胞,结合经典诱导组合OSKM(Oct4、Sox2、Klf4和c-Myc及治疗)和EGFP等5种逆转录病毒对双峰驼成纤维细胞进行重编程(OSKM组),并在第二次病毒感染后添加VPA处理7 d(OSKM+VPA组)收集细胞。利用PCR技术对所得细胞进行内、外源基因检测以证实逆转录病毒对双峰驼成纤维细胞的修饰作用,根据RNA-seq数据从VPA影响较为明显的基因中随机挑选8个基因,检验其在添加VPA前后的变化趋势是否与RNA-seq数据趋势一致,以验证RNA-seq数据的准确性。通过GO分析对转录组样本基因进行分类,并使用KEGG通路富集分析和超几何验证分析明确目的基因显著富集通路。对收集到的细胞进行总RNA提取并结合RNA-seq技术和实时荧光定量PCR(Real time-quantitative interpretation,RT-qPCR)技术检测VPA对双峰驼成纤维细胞重编程的影响。【结果】使用PCR技术检测内、外源基因在不同分组的表达,结果显示、、、、在OSKM和OSKM+VPA组中均有表达,且OSKM+VPA组表达量高于OSKM组,而其在BCEFs组不表达。随机挑选8个基因检测,结果表明与细胞周期信号通路有关的、、这3个基因在添加VPA后表达量下调;与癌症表型特征相关的、、、表达下调;PI3k-Akt信号通路中表达上调;此表达趋势与组学数据趋势一致。结果显示在添加VPA后,增殖基因和表达下调,而凋亡基因表达上调。对转录组数据进行KEGG和超几何验证分析,根据分析结果筛选出959个差异表达基因,富集在276个信号通路中,其中Q值小于0.05的信号通路有八条:类固醇生物合成、细胞周期、PPAR信号通路、孕酮介导的卵母细胞成熟、脂肪酸代谢、ECM-受体相互作用、细胞黏附分子和胆固醇代谢。经筛选获得与细胞周期、脂肪酸代谢、细胞黏附分子和胆固醇代谢等相关的26个差异表达基因,并随机选取其中四个基因进行检测,结果表明:VPA可使双峰驼成纤维细胞黏附分子信号通路中、、三个基因表达上调,细胞间互作增强,同时上调脂肪酸信号通路中基因的表达。【结论】VPA可将细胞阻滞在分裂期之前,以减少重编程过程中分化几率。同时,VPA对双峰驼成纤维细胞重编程过程中多条信号通路均具有影响,调节信号通路中相关基因表达趋势,有效提高细胞重编程效率,在双峰驼成纤维细胞重编程中发挥重要作用。
诱导多能干细胞;双峰驼;丙戊酸;细胞重编程
0 引言
【研究意义】双峰驼是荒漠特有物种,主要生活在我国西北地区,近年来由于人口数量不断增长,开垦范围进一步扩大,导致野生双峰驼的水源地被人为破坏,造成其数量急剧下降,现已被列为濒危动物。双峰驼不仅在历史文化中有着重要作用,在科研方面也具有很好的研究前景。因此,运用科学技术手段对其种质资源进行保护具有重要意义。【前人研究进展】日本京都大学Takahashi等[1]成功将与细胞多能性相关的4个基因、、和随机整合到小鼠胚胎成纤维细胞中,从而将其重编程为一类具有类似于胚胎干细胞的诱导多能干细胞(induced pluripotent stem cells,iPSCs),此过程被称为iPS经典诱导途径。iPSCs在细胞形态、增殖能力、分化能力及形成畸胎瘤等方面均与胚胎干细胞相似,且可以避免因使用胚胎干细胞而造成的伦理问题,这对于干细胞、表观遗传学及生物医学等研究领域具有深远影响。此外,研究人员还利用此技术成功得到人类和多种动物的iPSCs[2-10]。【本研究切入点】然而,到目前为止有关双峰驼iPSCs的报道屈指可数,获得双峰驼iPSCs对其种质资源保护显得尤为重要。在经典诱导途径中外源诱导因子参与调控多条癌症信号通路,且其易与细胞基因组发生整合,因此减少细胞重编程中外源诱导因子的使用,可提高细胞重编程的安全性。随着不断探索,研究人员发现许多小分子化合物,如丙戊酸(valproic acid,VPA)、5-氮杂氧胞苷(5-Azadeoxycytidine,Aza)、维生素C等可有效提高细胞重编程效率[11-13]。其中,丙戊酸是一种组蛋白去乙酰化酶抑制剂(histone deacetylase inhibitors,HDACi),可代替小鼠成纤维细胞重编程中的c-Myc诱导因子[14],甚至可以在人成纤维细胞重编程中代替c-Myc和Klf4完成细胞重编程过程[12]。不仅如此,VPA还可将iPSCs的诱导效率提高100倍以上,并保持其稳定性[14-16]。【拟解决的关键问题】本试验通过在双峰驼成纤维细胞重编程过程中添加VPA,并运用RNA-seq、RT-qPCR技术对收集到的细胞进行分析,以阐明VPA在双峰驼成纤维细胞重编程过程中的作用及iPSCs早期诱导过程中的分子机制,为双峰驼发病机制研究及细胞替代性治疗、新药筛选等提供参考依据。
1 材料与方法
本研究于2019年8月至2021年6月在甘肃省甘肃农业大学甘肃省动物生殖生理及繁殖调控重点实验室完成。
1.1 质粒的制备
制备经典诱导途径中PMXS-Oct4、PMXS-Sox2、PMXS-Klf4、PMXS-cMyc和PMXS-EGFP质粒,同时制备用于病毒包装的pCMV-VSV-G和PumV质粒(甘肃省动物生殖生理及繁殖调控重点实验室)。
1.2 细胞系和细胞培养条件
双峰驼胎儿成纤维细胞(BCFFs)来源于三月龄双峰驼胎儿(甘肃省张掖市骆驼养殖场),组织块贴壁法消化分离后培养,冻存F1—F3细胞。高转染293细胞由中国科学院兰州兽医研究所捐赠。两种细胞都在含10%胎牛血清(BI,Israel)的DMEM/F12葡萄糖(Hyclone,USA)培养基中,并置于37 ℃、5% CO2培养箱中进行培养。
1.3 逆转录病毒感染和VPA处理
293T包装细胞以每60 mm培养皿1×106个细胞接种,孵育过夜。次日,根据产品说明书,使用 Lipofectamine 2000(Invitrogen,USA)用5 µg pMXs载体(OSKMG)和5 µg逆转录病毒包装混合物转染细胞。48 h后,收集培养基作为第一支含病毒上清液并为细胞更换新培养基,24 h后再次收集培养基作为第二支含病毒上清液。
转导前一天,将BCFFS接种至六孔板(每孔2×105个细胞)。转导过程中,将含病毒的上清液通过0.45 mm孔径滤器过滤,滤液等比例混合并补充8 mg·mL-1聚凝胺,置于成纤维细胞培养皿中孵育过夜。转导24 h后,用第二支含病毒上清液替换已有培养基。
细胞随机分为对照组和处理组,每组重复3次。两次转导后,将培养基更换为iPSC诱导培养基,该培养基由DMEM/F12培养基(Gibco,USA)、20%胎牛血清(Gibco,USA)、2 mmol·L-1非必需氨基酸(Gibco,USA)、0.1 mmol·L-1β-巯基乙醇(Sigma- Aldrich,USA)、20 ng·mL-1FGF2 (Thermo,USA)和10 ng·mL-1白血病抑制因子(Thermo,USA)组成;处理组细胞额外补充1 mmol·L-1丙戊酸(Sigma,USA)。7 d后,通过胰蛋白酶消化处理收集细胞样品[17],加入TRIzol试剂(Sigma,USA)重悬,液氮速冻后储存于-80 ℃。
1.4 RNA提取、文库构建和RNA测序
对照组(n=3)随机命名为CK-1、CK-2、CK-3;处理组(n=3)随机命名为T-1、T-2、T-3。根据产品说明,使用TRIzol试剂从6个样品中提取总RNA。通过Agilent 2100生物分析仪,在去除rRNA后进行无RNase琼脂糖凝胶电泳检测RNA质量和浓度。随后,使用oligo-dT珠子分离Poly (A) mRNA,并通过超声波中断mRNA,以片段化mRNA为模板,随机寡核苷酸为引物,采用M-MuLV逆转录酶系统合成第一条cDNA链,紧接着使用RNaseH降解RNA链,在DNA聚合酶I的作用下,以dNTPs为模板合成第二条cDNA链。纯化的双链cDNA在末端被修复,连接至poly A尾并连接测序接头。使用AMPure XP微珠筛选200 bp的cDNA并进行PCR扩增。最后,使用AMPure XP珠对PCR产物进行纯化和富集,以构建最终的cDNA文库。
使用双末端技术,在Genedenovo Biotechnology Co., Ltd(中国广州)Illumina测序平台(Illumina HiSeq™ 2500)上对cDNA文库进行测序。用fastp[18]程序删除低质量序列(一个序列中有超过50%的碱基质量低于20)、N碱基超过10%的读数(碱基未知)和包含适配器的序列(表1)。

表1 引物序列及参数
、、、:转录因子;:肿瘤蛋白p53;:细胞周期蛋白B1;:基质金属肽酶9;:细胞分裂周期20;:S100钙结合蛋白A4;:血管内皮生长因子C;:纹状蛋白;:CDC28蛋白激酶调节亚基2;:单克隆抗体Ki 67鉴定抗原;:增殖的细胞核抗原;:半胱天冬酶7;:脂肪酸转位酶;:神经纤维素;:L1细胞黏附分子;:接触蛋白1
,,,: Transcription factors;: tumor protein p53;: cyclin B1;: matrix metallopeptidase 9;: cell division cycle 20;: S100 calcium binding protein A4;: vascular endothelial growth factor C;: vimentin;: CDC28 protein kinase regulatory subunit 2;: antigen identified by monoclonal antibody ki 67;: proliferating cell nuclear antigen;: caspase7;: fatty acid translocation enzyme;: neurofascin;: L1 cell adhesion molecule;: contactin 1
1.5 转录本组装和表达值估计
利用HISAT2[19]对RNA测序读数和双曲线Camelus bactrianus Ca_bactrianus_MBC_1.0基因组组装进行对比。
已知双峰驼基因(https://www.ncbi.nlm.nih.gov/ genome/?term=Camelus+bactrianus)作为基因组参照组。在HISAT2比较的基础上,使用Stringtie[20]重建转录本并计算每个样本中全部基因的表达水平,校正测序深度以及基因或转录本长度,以确保后续分析的准确性。在此之前,计算得到每个基因的FPKM值。
基于基因表达信息,使用R(http://www.r-project. org/)对主成分进行分析(PCA,Principal Component Analysis)并计算皮尔逊相关系数。随后使用DESeq2[21]软件进行差异基因表达分析,结合KEGG(//www. kegg.jp/)和GO(//geneontology.org/)研究分析相关途径。
1.6 引物设计及实时荧光定量PCR
利用NCBI和primer 3.0网站设计特异性引物,以作为内参基因对所选目的基因进行RT- qPCR分析,以检验测序结果的准确性并进一步分析转录组数据。
使用RevertAid™ First Strand cDNA Synthesis Kit (Thermo FisherScientific Inc., USA) 产品说明制备cDNA。RT-qPCR反应体积为20 μL,其中包含10 μLReal Master Mix SYBR、8 μL ddH2O、正向和反向引物各1 μL,以及1 μL cDNA,使用ΔΔCT方法计算所有基因的相对表达量。
1.7 重编程进度检测
为区分对照组与处理组,将未添加VPA组命名为OSKM,将添加VPA组命名为OSKM+VPA。通过PCR技术测试BCFFs、OSKM和OSKM+VPA此3组中的特定基因,其中包含3组外源基因、干细胞特异性基因和3个胚层标记基因。检测成纤维细胞中特定基因的表达水平以验证外源基因对成纤维细胞的修饰作用及重编程进度。
2 结果
2.1 VPA处理
使用293T细胞包装含有经典组合Oct3、c-Myc、Klf4、Sox2和EGFP逆转录病毒载体,在病毒转导后72 h,观察到EGFP逆转录病毒包装结果(图1-A),利用EGFP预测其余4种逆转录病毒的转录效率。第二次逆转录病毒感染后,在iPSC培养基中添加1 mmol·L-1VPA对细胞进行处理,第二次病毒感染后第8天观察到转染效果(图1-B),光学显微镜观察第二次病毒感染后8 d的细胞形态(图1-D),及未处理的空白对照组细胞形态(图1-C)。光学显微镜观察VPA处理7 d后的细胞状态(图1-F)及其对照组(图1-E)。
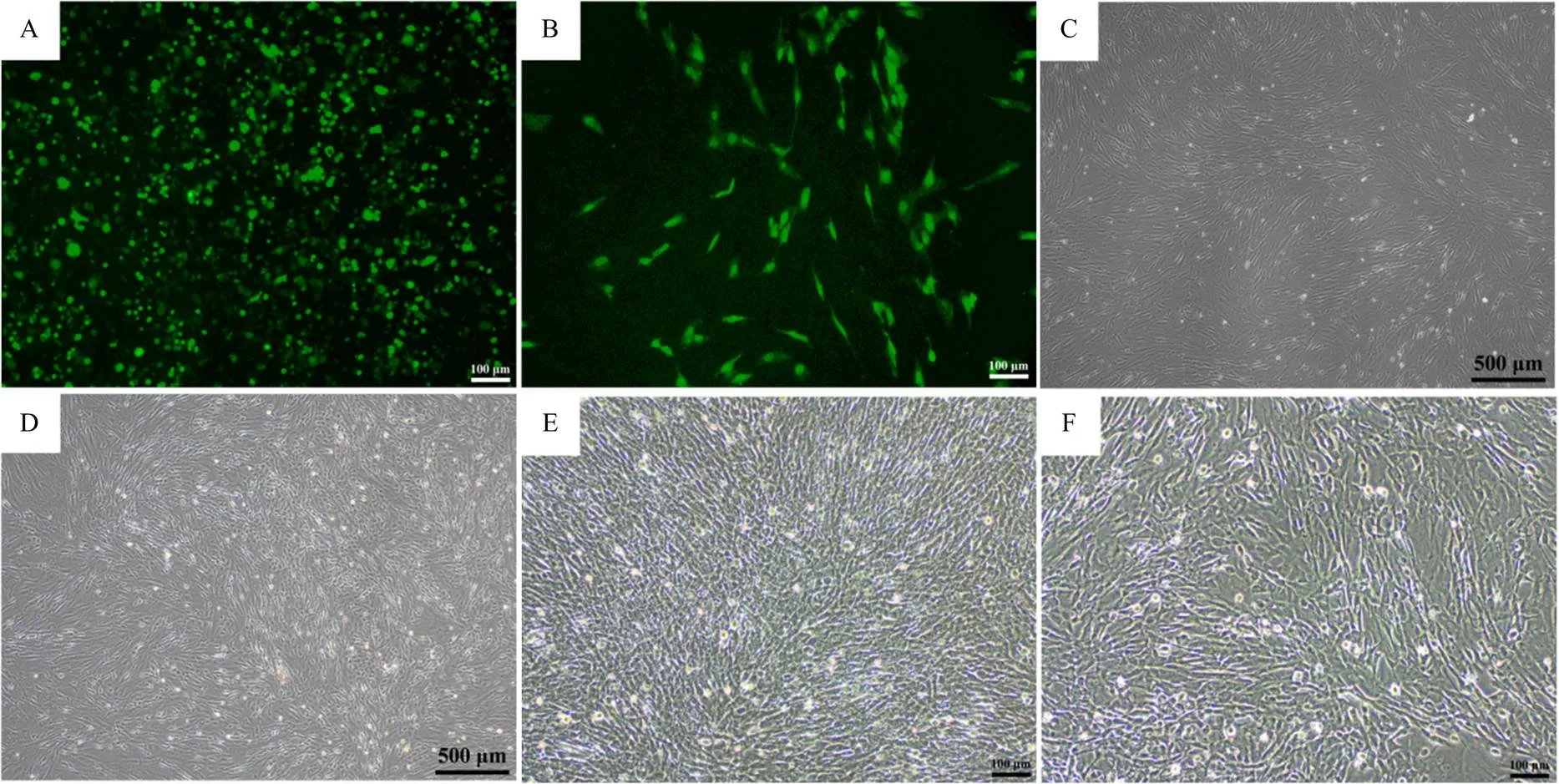
A. 转导后72 h的EGFP逆转录病毒包装;B. 第二次病毒感染后8 d;C. 培养8 d的成纤维细胞;D. 第二次病毒感染后8 d的细胞形态;E. 培养基中未添加VPA处理7 d的细胞形态;F. 培养基中添加VPA并处理7 d的细胞形态
2.2 基因转移验证和组学数据验证
重编程后双峰驼成纤维细胞中内、外源基因的表达规律如图2所示,、、、、在OSKM和OSKM+VPA组中均有表达(图2-A),且OSKM+VPA组表达量高于OSKM组,而其在BCEFs组不表达。为了验证组学数据的可靠性,随机选取8个基因进行检测,结果显示、、、、、在OSKM+VPA组中表达下调,且差异极显著(<0.01);基因表达呈上调趋势,且差异极显著(<0.01)。在OSKM+VPA组中表达量无明显差异(图2-B),8个基因变化趋势与RNA-seq组学数据一致,表明组学数据准确可信,可用于后续分析。
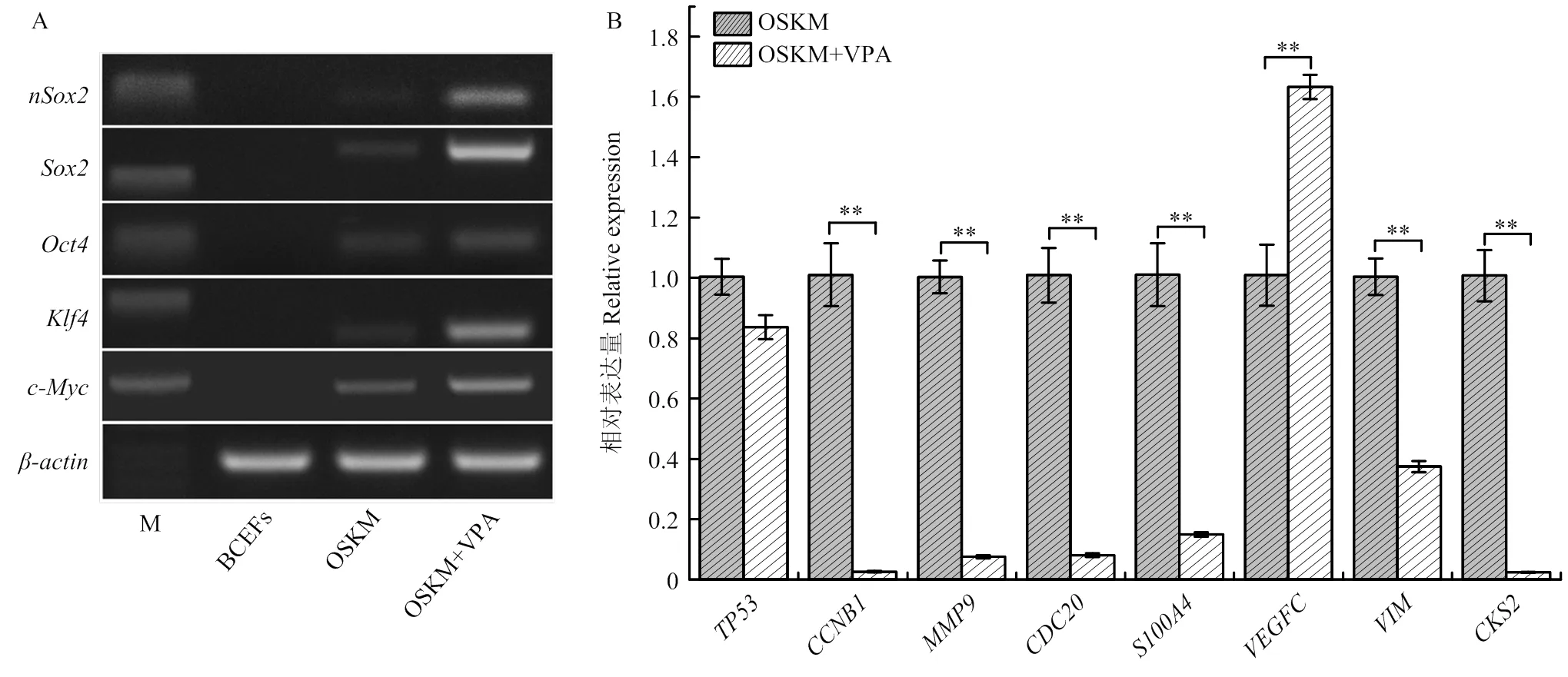
A. 半定量PCR检测BCFFs、OSKM和OSKM+VPA组中内、外源基因mRNA表达水平;B. TP53、CCNB1、MMP9、CDC20、S100A4、VEGFC、VIM和CKS2 mRNA表达水平(内参基因:β-actin)。**表示差异极显著(P<0.01)
2.3 转录组测序分析
为确定VPA在成纤维细胞重编程中的作用,通过RNA-seq技术对处理组和对照组的6个样本进行转录组分析。从6个样本中分别获得了61 256 236、65 376 850、66 062 928、69 282 900、68 014 520和67 418 570个clean reads(表2)。其中90.40%、90.12%、90.32%、90.39%、90.12%和90.75%的clean reads被比对到参考基因组(Table 2),并注释了14 377、14 332、14 399、14 367、14 427和14 427个基因(表3)。
根据每个样本基因表达谱,使用PCA(图3-A)对样本进行分析,了解样本重复性并排除异常值。此外,通过小提琴图将每个样本中基因丰度的表达可视化(图3-B)。基因总数是19 748个,根据基因丰度≥1进行筛选后,基因数是11 780个,其中处理组独有的318个基因、对照组独有的260个基因,两者共11 202个基因。如图3-C所示。基于差异分析结果,在添加VPA处理7d后,FDR<0.05和| log2FC |>1为显著差异表达基因。因此,根据每个对照组的差异表达基因(469个上调基因和490个下调基因)建立火山图;基因越接近两端,差异程度越大(图3-D)。并且假设具有相似表达模式的基因有共同功能或参与共同代谢途径和信号传导途径,以此为基础进行了进一步分析。
利用GO分析对样本基因进行功能分类。基于序列同源性,unigenes分为三大功能类别:生物学过程,细胞组分和分子功能(图3-E)。为确定目的基因显著富集通路,使用KEGG通路富集分析和超几何检验分析。筛选获得959个差异表达基因,富集在276个信号通路中。其中值小于0.05的信号通路有42条,值小于0.05的信号通路有8条。这8条信号通路分别为类固醇生物合成、细胞周期、PPAR信号通路、孕酮介导的卵母细胞成熟、脂肪酸代谢、ECM-受体相互作用、细胞黏附分子和胆固醇代谢(图3-F)。
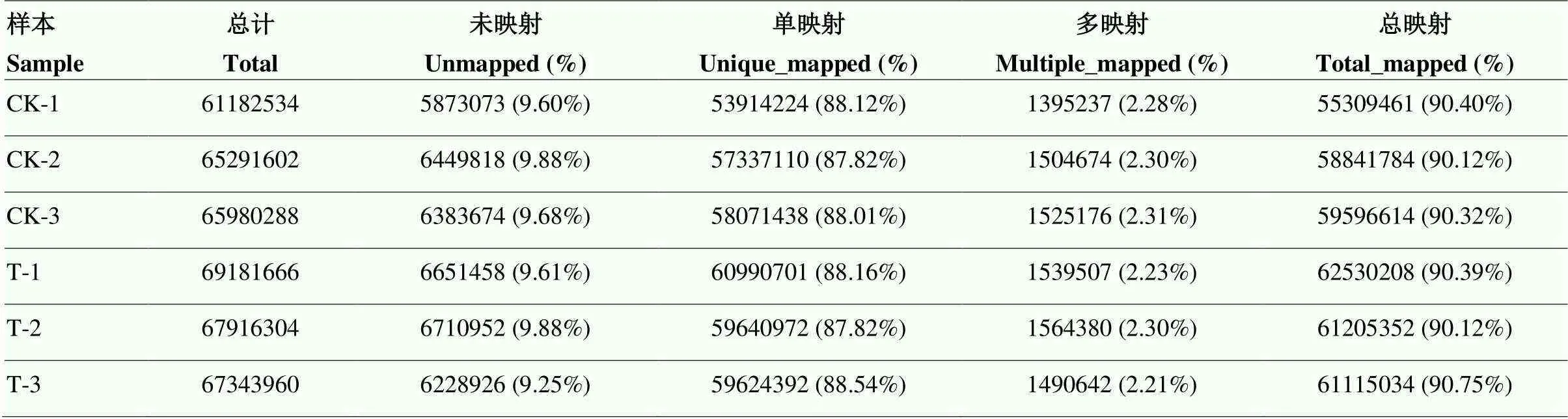
表2 计数据比较
Sample:样本名;Total:过滤核糖体后reads数量,称为有效reads;Unmapped (%):未比对上参考基因组的reads数及占有效reads比例;Unique_Mapped (%):唯一对比上参考基因组的reads数及占有效reads比例;Multiple_Mapped (%):多处比对上参考基因组的reads数及占有效reads比例;All_Mapped:全部的可以定位到基因组上的reads数量及占有效reads比例
Sample: sample name; Total: number of reads after filtering ribosomes, called valid reads; Unmapped (%): number of reads that are not matched to the reference genome and the proportion of valid reads; Unique_Mapped (%): number of reads that are uniquely matched to the reference genome and the proportion of valid reads; Multiple_Mapped (%): number and proportion of effective reads in the reference genome compared at multiple locations; All_Mapped: the number of reads that can be localized to the genome and the proportion of valid reads

表3 基因检测统计
Sample:样本名;Refer genes:参考基因组(或参考基因集合)的基因总数,当不存在新基因时,无该列结果;Sequenced refer genes (%):测序结果所检测到的Refer genes总数及占Refer genes的百分比,当不存在新基因时,无该列结果;Novel genes:项目发现的新基因数目,当不存在新基因时,无该列结果;Sequenced novel genes (%):测序结果检测到的Novel genes总数及占Novel genes的百分比,当不存在新基因时,无该列结果;Total genes:所有基因总数,包含参考基因组及新基因;Sequenced total genes (%):测序结果所检测到的基因总数及占All genes的百分比
Sample: sample name; Refer genes: total number of genes in the reference genome (or reference gene collection), no result in this column when no new genes are present; sequenced Refer genes (%): total number and percentage of Refer genes detected by sequencing results, no result in this column when no new genes are present; Novel genes: number of new genes discovered by the project, no result in this column when no new genes are present; sequenced_Novel genes (%): total number of Novel genes detected by sequencing results and percentage of Novel genes , when no new genes are present, no result in this column; Total_Genes: total number of all genes, when no new genes are present, no result in this column; Total_Genes: total number of all genes, including reference genome and novel genes; sequenced_Total genes (%): total number of genes detected by sequencing results and percentage of All_Genes
2.4 增殖和凋亡基因的影响
增殖和凋亡基因在BCEF、OSKM和OSKM+VPA组的表达规律如图4所示。iPSC培养基中加入VPA后,增殖基因和表达呈下降趋势,差异极显著(<0.01);而凋亡基因表达极显著上调(<0.01)。

A. 主成分分析:PC1坐标代表第一主成分;PC2坐标代表第二主成分;图中的彩色点代表每个样本;B. 样本的相关热图:图中横坐标和纵坐标分别为样本,颜色深度(强度)表示两个样本之间的相关系数;C. 样本或组间基因丰度表达的可视化;D. 维恩图显示了两组的共同和独特基因;E. 横坐标代表两组差异倍数的对数,纵坐标代表两组差异FDR的负Log10值,红色(group_2向上,相对于group_1的调节表达)和蓝色(下调表达)表示基因表达水平的差异;F. 用不同颜色表示不同样本基因,颜色越红,表达越高;颜色越蓝,表达越低
A. Principal Component Analysis: the PC1 coordinate represents the first principal component; the PC2 coordinate represents the second principal component; while, the colored dots in the figure represent each sample; B. Correlation heat map of the samples: In the figure, the abscissa and ordinate are the respective samples and the color depth (intensity) indicates the correlation coefficient between the two samples; C. Visualization of gene abundance expression between samples or groups; D. Venn diagram showing the common and unique genes of the two groups; E. The abscissa represents the logarithm of the multiple of the difference between the two groups, the ordinate represents the negative Log10 value of the FDR of the difference between the two groups, and the red (group_2 up-regulated expression relative to group_1) and blue (down-regulated expression) indicate a difference in the gene expression levels; whereas, the black dots represent no difference; F. Genes from different samples are expressed in different colors; the redder the color, the higher the expression; whereas, the bluer the color, the lower the expression
图3 转录组测序、相关性的基本分析和组间差异
Fig. 3 Transcriptomic sequencing, basic analysis of correlations, and differences between groups
2.5 VPA处理影响的路径分析
在本研究中,根据值小于0.05的8个KEGG通路,筛选出与细胞周期、脂肪酸代谢、细胞黏附分子和胆固醇代谢等相关的26个差异表达基因,根据这些基因构建热图,分析其在不同分组的表达差异。结果显示,在OSKM和OSKM+VPA组之间这些差异表达基因在重编程细胞中表达具有显著性差异,尤其是脂肪酸合酶和细胞黏附分子(图5-A)。在OSKM组中表达量显著下调(<0.01),经VPA处理后(OSKM+VPA组)较OSKM组表达量上调,且差异显著(<0.01)(图5-B);在OSKM组中表达量下调,且差异显著(<0.01),其在VPA处理后(OSKM+VPA组)表达量无明显差异(图5-C);和,在OSKM+VPA组中表达量显著上调(<0.01),而在BCEF组中不表达(图5-D、E);和基因在OSKM和OSKM+VPA组均有表达,且OSKM+VPA组表达量高于OSKM组,而其在BCEFs组不表达(图5-F)。
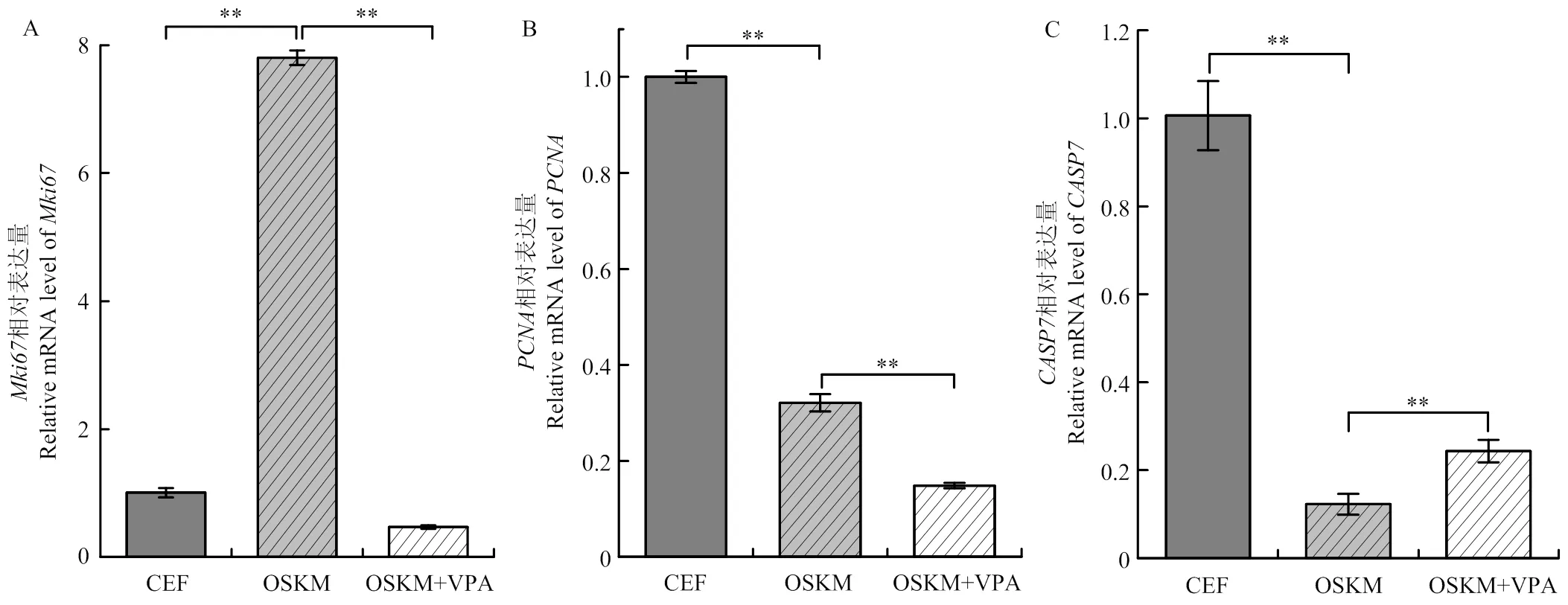
A. Mki67 mRNA表达水平(内参基因:β-actin);B. PCNA mRNA表达水平(内参基因:β-actin);C. CASP7 mRNA表达水平(内参基因:β-actin)。**表示差异极显著(P<0.01)
3 讨论
细胞重编程技术在种质资源保护、再生医学和新药筛选等方向都具有潜在的应用价值。但因其重编程效率较低,使得该技术很难由实验室向临床应用发展,导致重编过程中因原癌基因引入造成的安全问题限制了其在临床上的应用[22]。随着对重编程技术的探索,研究人员发现小分子物质可以提高重编程的效率以及减少原癌基因的使用。本研究以双峰驼成纤维细胞为研究对象,结合RNA-seq、RT-qPCR和半定量PCR等方法,探讨小分子物质丙戊酸对双峰驼成纤维细胞重编程的影响。结果表明,丙戊酸可抑制细胞周期信号通路,阻滞细胞分化,并通过影响细胞黏附分子信号通路增强细胞间互作,在脂肪酸代谢途径中发挥调控作用。
3.1 丙戊酸对细胞周期信号通路的影响
细胞衰老是OSKM因子感染细胞使其进入重编程的阻碍之一,而VPA可通过将细胞阻滞在G2/M期来提高细胞重编程的效率[23-24]。本研究检测了与细胞周期信号通路相关的、、、、和等基因,其中效应凋亡蛋白是参与细胞凋亡的重要成员之一[25],有研究表明的缺乏会减弱大鼠细胞凋亡程度[26];在细胞复制和DNA修复过程中表达,从细胞G1期开始合成,S期达到峰值,G2和M期表达量开始下降[27]。研究发现在添加VPA后增殖相关基因、、和表达下调,而凋亡基因则呈上调趋势。这一结果提示VPA通过调控重编程中增殖和凋亡基因的表达趋势将细胞阻滞在未分化阶段,以促进双峰驼成纤维细胞重编程效率。
3.2 丙戊酸对脂肪酸代谢相关基因的影响
脂肪酸作为脂质组成中最基础同时也是最重要的组成部分,是机体主要能量来源之一,其合成对于人类多能干细胞的生存有重要意义[28]。Abumrad等[29]通过克隆大鼠脂肪细胞膜蛋白,发现大鼠脂肪酸转位酶(fatty acid translocase,FAT)与人有同源性,证明了的FAT活性,从而明确了其在脂肪酸摄取中的作用。本研究结果显示VPA可使脂肪酸代谢途径中的表达量上调,提示VPA可上调表达从而促进长链脂肪酸的吸收,调节脂肪酸代谢,以期获得质量更优的重编程细胞。
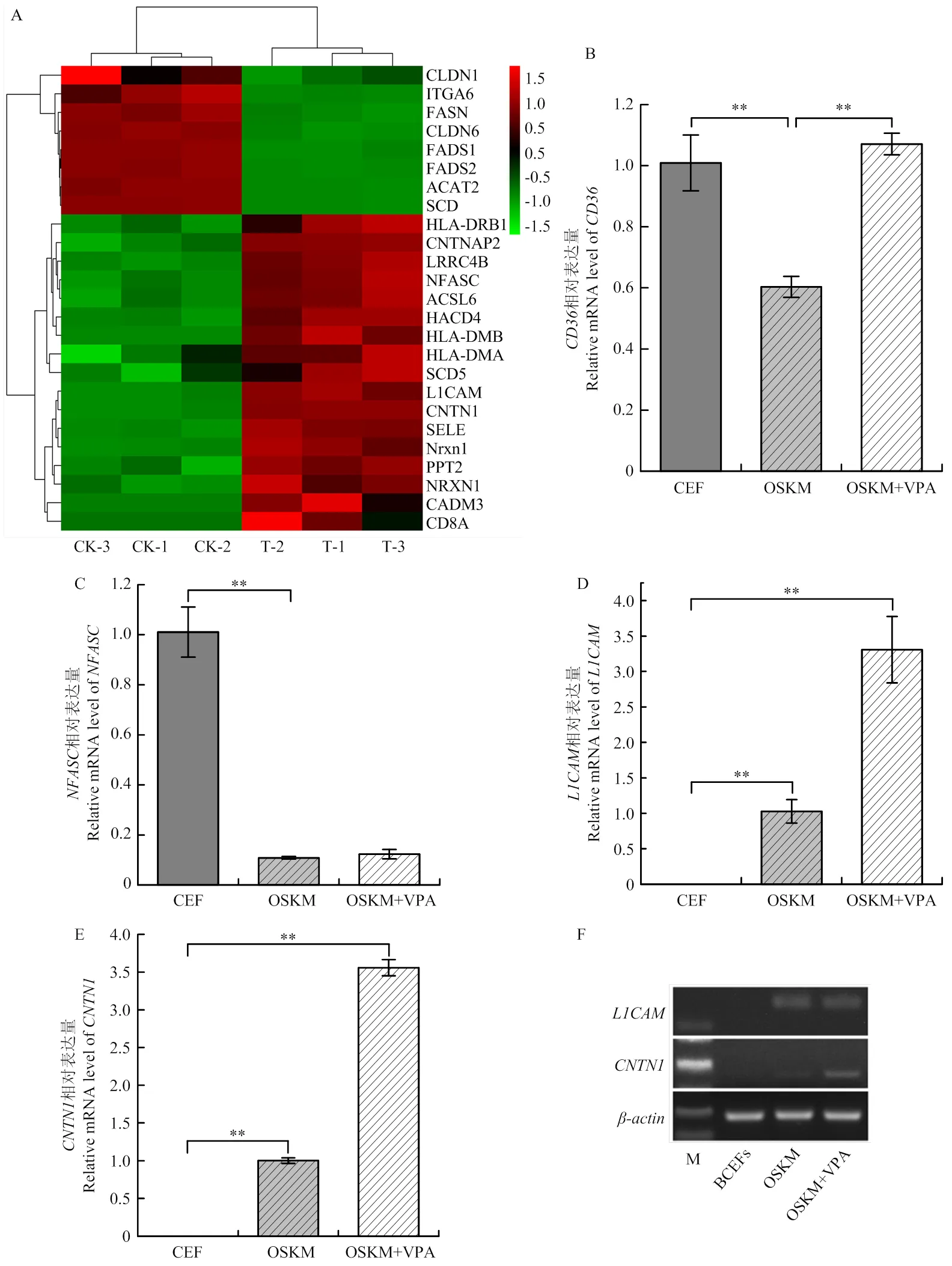
A. 26个差异表达基因的热图;B. CD36 mRNA表达水平(内参基因:β-actin);C. NFASC mRNA表达水平(内参基因:β-actin);D. L1CAM mRNA表达水平(内参基因:β-actin);E. CNTN1 mRNA表达水平(内参基因:β-actin);F. 半定量PCR检测BCFFs、OSKM和OSKM+VPA三组中L1CAM、CNTN1 mRNA表达水平。**表示差异极显著(P<0.01)
3.3 丙戊酸对细胞黏附分子信号通路的影响
细胞黏附分子介导的细胞间互作,对人类多能干细胞的自我更新和多能干细胞状态有重要贡献[30]。细胞黏附分子一般分为5类,包括整合素、选择素、钙黏素、免疫球蛋白超家族(IgSF)和黏蛋白血管地址素[31]。研究发现,人类干细胞表面存在很多CAM家族成员,它们对干细胞的自身更新和多能性具有调节作用[1,32-36]。试验检测了、和这3个与细胞黏附分子信号通路相关的基因,其中和属于免疫球蛋白超家族成员(IgSF)且已被证明由未分化的人类胚胎干细胞表达,但其作用机制尚不完全清楚[36]。结果表明,丙戊酸可以促进、和的表达,使其表达量上调,这一结果提示丙戊酸可以促进与细胞黏附分子信号通路相关的基因表达,增强细胞重编程过程中互作作用,从而促进重编程过程中细胞的自我更新。
4 结论
丙戊酸可抑制细胞周期信号通路,减少重编程中细胞分化几率;同时在脂肪酸代谢中发挥调控作用;上调细胞黏附分子信号通路中相关基因表达,增强细胞间互作。丙戊酸在双峰驼成纤维细胞重编程中通过调控相关信号通路从而对重编程起到促进作用。这将为丙戊酸促进双峰驼成纤维细胞重编程作用机制的研究提供试验基础,同时为双峰驼种质资源保护提供理论参考。
[1] TAKAHASHI K, YAMANAKA S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006, 126(4): 663-676.
[2] YU J, VODYANIK M A, SMUGA-OTTO K, ANTOSIEWICZ- BOURGET J, FRANE J L, TIAN S, NIE J, JONSDOTTIR G A, RUOTTI V, STEWART R, SLUKVIN I I, THOMSON J A. Induced pluripotent stem cell lines derived from human somatic cells. Science, 2007, 318(5858): 1917-1920.
[3] EZASHI T, TELUGUA B P V. L., ALEXENKO A P., SACHDEV S, SINHA S, ROBERTS M. Derivation of induced pluripotent stem cells from pig somatic cells. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(27): 10993-10998.
[4] HAN X, HAN J, DING F, CAO S, LIM S S, DAI Y, ZHANG R, ZHANG Y, LIM B, LI N. Generation of induced pluripotent stem cells from bovine embryonic fibroblast cells. CellResearch, 2011, 21(10): 1509-1512.
[5] LIAO J, CUI C, CHEN S, REN J, CHEN J, GAO Y, LI H, JIA N, CHENG L, XIAO H, XIAO L. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell, 2009, 4(1): 11-15.
[6] NAGY K, SUNG H, ZHANG P, LAFLAMME S, VINCENT P, AGHA-MOHAMMADI S, WOLTJEN K, MONETTI C, MICHAEL I P, SMITH L C, NAGY A. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Reviews and Reports, 2011, 7(3):693-702.
[7] LIU J, BALEHOSUR D, MURRAY B, KELLY J M, SUMER H, VERMA P J. Generation and characterization of reprogrammed sheep induced pluripotent stem cells. Theriogenology, 2012, 77(2): 338-346.
[8] LIU H, ZHU F, YONG J, ZHANG P, HOU P, LI H, JIANG W, CAI J, LIU M, CUI K, QU X, XIANG T, LU D, CHI X, GAO G, JI W, DING M, DENG H. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell, 2008, 3(6): 587-590.
[9] BUEHR M, MEEK S, BLAIR K, YANG J, URE J, SILVA J, MCLAY R, HALL J, YING Q, SMITH A. Capture of authentic embryonic stem cells from rat blastocysts. Cell, 2008, 135(7): 1287-1298.
[10] LI P, TONG C, MEHRIAN-SHAI R, JIA L, WU N, YAN Y, MAXSON R E, SCHULZE E N, SONG H, HSIEH C, PERA M F, YING Q. Germline competent embryonic stem cells derived from rat blastocysts. Cell, 2008, 135(7): 1299-1310.
[11] WERNIG M,LENGNER C J, HANNA J, LODATO M A, STEINE E, FOREMAN R, STAERK J, MARKOULAKI S, JAENISCH R. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nature Biotechnol, 2008, 26(8): 916-924.
[12] HUANGFU D, OSAFUNE K, MAEHR R, GUO W, EIJKELENBOOM A, CHEN S, MUHLESTEIN W, MELTON D A. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nature Biotechnol, 2008, 26(11): 1269-1275.
[13] ESTEBAN M A, WANG T, QIN B, YANG J, QIN D, CAI J, LI W, WENG Z, CHEN J, NI S, CHEN K, LI Y, LIU X, XU J, ZHANG S, LI F, HE W, LABUDA K, SONG Y, PETERBAUER A, WOLBANK S, REDL H, ZHONG M, CAI D, ZENG L, PEI D. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell, 2010, 6(1): 71-79.
[14] HUANGFU D, MAEHR R, GUO W, EIJKELENBOOM A, SNITOW M, CHEN A E, MELTON D A. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature Biotechnol, 2008, 26(7): 795-797.
[15] GURVICH N, TSYGANKOVA O M, MEINKOTH J L, KLEIN P S. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Research, 2004, 64(3): 1079-1086.
[16] PHIEL C J, ZHANG F, HUANG E Y, GUENTHER M G, LAZAR M A, KLEIN P S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. The Journal of Biological Chemistry, 2001, 276(39): 36734-36741.
[17] GIORGETTI A, MONTSERRAT N, AASEN T, GONZALEZ F, RODRíGUEZ-PIZà, VASSENA R, RAYA A, BOUé S, BARRERO M J, CORBELLA B A, TORRABADELLA M, VEIGA A, BELMONTE J C I. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell, 2009, 5(4): 353-357.
[18] CHEN S, ZHOU Y, CHEN Y, GU J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics, 2018, 34(17): i884-i890.
[19] KIM D, LANGMEAD B, SALZBERG S L. HISAT: A fast spliced aligner with low memory requirements. Nature Methods, 2015, 12(4): 357-360.
[20] LOVE M I, HUBER W, ANDERS S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology, 2014, 15(12): 550-571.
[21] SUN J, LI W, SUN Y, YU D, WEN X, WANG H, CUI J, WANG G, HOFFMAN A R, HU J. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Research, 2014, 42(15): 9588-9601.
[22] 苗向阳, 陈晓瑛. 诱导性多能干细胞的研究及应用. 中国农业科学, 2012, 45(2): 369-375. doi: 10.3864/j.issn.0578-1752.2012. 02.020.
MIAO X Y, CHEN X Y. Study and application of induced pluripotent stem cells. Scientia Agricultura Sinica, 2012, 45(2): 369-375. doi: 10.3864/j.issn.0578-1752.2012.02.020. (in Chinese)
[23] ZHAI Y, CHEN X, YU D, LI T, CUI J, WANG G, HU J, LI W. Histone deacetylase inhibitor valproic acid promotes the induction of pluripotency in mouse fibroblasts by suppressing reprogramming- induced senescence stress. Experimental Cell Research, 2015, 337(1): 61-67.
[24] 魏如雪, 郝海生, 赵学明, 杜卫华, 朱化彬. 抑制体细胞衰老促进诱导性多潜能干细胞(iPSC)生成的研究进展. 中国农业科学, 2015, 48(16): 3258-3265. doi: 10.3864/j.issn.0578-1752.2015. 16.015.
WEI R X, HAO H S, ZHAO X M, DU W H, ZHU H B. Studies of improved efficiency of induced pluripotent stem cell generation by restraining somatic cell senescence. Scientia Agricultura Sinica. 2015, 48(16): 3258-3265. doi: 10.3864/j.issn.0578-1752.2015.16.015. (in Chinese)
[25] YANG Y B, PANDURANGAN M, HWANG I. Targeted suppression of mu-calpain and caspase 9 expression and its effect on caspase 3 and caspase 7 in satellite cells of Korean Hanwoo cattle. Cell Biology International, 2012, 36(9):843-849.
[26] LAKHANI S A, MASUD A, KUIDA K, JR G A P, BOOTH C J, MEHAL W Z, INAYAT I, FLAVELL R. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science, 2006, 311(5762): 847-851.
[27] WERNIG M, MEISSNER A, FOREMAN R, BRAMBRINK T, KU M, HOCHEDLINGER K, BERNSTEIN B E, JAENISCH R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature, 2007, 448(7151): 318-324.
[28] TANOSAKI S, TOHYAMA S, FUJITA J, SOMEYA S, HISHIKI T, MATSUURA T, NAKANISH H, OHTO-NAKANISH T, AKIYAMA T, MORITA Y, KISHINO Y, OKADA M, TANI H, SOMA Y, NAKAJIMA K, KANAZAWA H, SUGIMOTO M, KO M S H, SUEMATSU M, FUKUDA K. Fatty acid synthesis is indispensable for survival of human pluripotent stem cells. iScience, 2020, 23(9): 101535-101568.
[29] ABUMRAD N A, EL-MAGHRABI M R, AMRI E Z, LOPEZ E, GRIMALDI P A. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. The Journal of Biologic Chemistry, 1993, 268(24): 17665-17668.
[30] LI L, BENNETT S A L, WANG L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adhesion Migration, 2012, 6(1): 59-70.
[31] SAMANTA D, ALMO S C. Nectin family of cell-adhesion molecules: structural and molecular aspects of function and specificity. Cellular and Molecular Life Sciences, 2015, 72(4): 645-658.
[32] PROKHOROVA T A, RIGBOLT K T G, JOHANSEN P T, HENNINGSEN J, KRATCHMAROVA I, KASSEM M, BLAGOEV B. Stable isotope labeling by amino acids in cell culture (SILAC) and quantitative comparison of the membrane proteomes of self-renewing and differentiating human embryonic stem cells. Molecular Cellular Proteomics, 2009, 8(5): 959-970.
[33] LI L, WANG S, JEZIERSKI A, MOALIM-NOUR L, MOHIB K, PARKS R J, RETTA S F, WANG L. A unique interplay between Rap1 and E-cadherin in the endocytic pathway regulates self-renewal of human embryonic stem cells. Stem Cells, 2010, 28(2): 247-257.
[34] ROWLAND T J, MILLER L M, BLASCHKE A J, DOSS E L, BONHAM A J, HIKITA S, JOHNSON L V, CLEGG D O. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells and Development, 2010, 19(8): 1231-1240.
[35] CHEN T, YUAN D, WEI B, JIANG J, KANG J, LING K, GU Y, LI J, XIAO L, PEI G. E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells, 2010, 28(8): 1315-1325.
[36] SON Y S, SEONG R H, RYU C J, CHO Y S, BAE K, CHUNG S J, LEE B, MIN J, HONG H J. Brief report: L1 cell adhesion molecule, a novel surface molecule of human embryonic stem cells, is essential for self-renewal and pluripotency. Stem Cells, 2011, 29(12): 2094-2099.
Effect of Valproic Acid on Reprogramming of Bactrian Camel Fibroblasts

1College of Life Science and Technology/Gansu Key Laboratory of Animal Reproductive Physiology and Reproductive Regulation, Gansu Agricultural University, Lanzhou 730070;2College of Veterinary Medicine, Gansu Agricultural University, Lanzhou 730070
【Objective】To improve the efficiency of the reprogramming process of Bactrian camel fibroblasts and to reduce the risk of tumorigenesis caused by the introduction of proto-oncogenes. In this experiment, valproic acid (VPA) was added to the fibroblast reprogramming process to explore the effect of small molecules on the reprogramming of Bactrian camel fibroblasts. 【Method】Given this, March-aged Bactrian camel fetal fibroblasts were used as test materials, combined with classic induction combination OSKM (Oct4, Sox2, Klf4, and c-Myc) and EGFP five retrovirus reprogramming of Bactrian camel fibroblasts (OSKM group), and the cells by adding VPA treatment for 7 days after the second viral infection (OSKM+VPA group) were collected. Endogenous and exogenous genes were examined by using PCR to confirm the modification effect of retrovirus on Bactrian camel fibroblasts. Eight genes were randomly selected from those more significantly affected by VPA. According to RNA-seq data, whether their trends before and after VPA addition were consistent with the trends of RNA-seq data was checked to verify the accuracy of RNA-seq data. The transcriptome sample genes were classified by GO analysis and significant enrichment pathways for target genes were clarified by using KEGG pathway enrichment analysis and hypergeometric validation analysis. Total RNA was extracted from the collected cells, and then, combined with RNA-seq and Real time-quantitative interpretation (RT-qPCR) techniques to detect the effect of VPA on the reprogramming of Bactrian camel fibroblasts. 【Result】 It was detected by using PCR that the expression of endogenous and exogenous genes in different groups. The results showed that,,,, andgenes were expressed in both OSKM and OSKM+VPA groups, and the expression in OSKM+VPA group was higher than that in the OSKM group, while they were not expressed in BCEFs group. Eight genes were randomly selected for testing, and the results showed that: three genes of,, andwere down-regulated in expression after the addition of VPA, which were related to the cell cycle signaling pathway.,,, andgenes signaling were down-regulated in expression, which was related to the phenotypic characteristics of cancer;gene expression was up-regulated in the PI3k-Akt signaling pathway. This expression trend was consistent with the trend of the histological data. The results showed that the expression of proliferation genesandwere down-regulated, while the expression of apoptosis genewas up-regulated after the addition of VPA. KEGG and hypergeometric validation analyses of the transcriptome data were performed, and 959 differentially expressed genes were screened according to the analysis results, which were enriched in 276 signaling pathways, including eight signaling pathways with Q values less than 0.05: steroid biosynthesis, cell cycle, PPAR signaling pathway, progesterone-mediated oocyte maturation, fatty acid metabolism, ECM-receptor interactions, cell adhesion molecules, and cholesterol metabolism. The 26 differentially expressed genes related to cell cycle, fatty acid metabolism, cell adhesion molecule, and cholesterol metabolism were screened, and four of which were randomly selected for testing, showing that VPA upregulated the expression of,andgenes in the Bactrian camel fibroblast adhesion molecule signalling pathway and enhanced intercellular interactions. It was also upregulated that the expression ofgene in the fatty acid signaling pathway. 【Conclusion】 The results showed that the VPA blocked cell before the split phase to reduce risk differentiation during the process of reprogramming. Meanwhile, VPA affected several signaling pathways in the reprogramming process of Bactrian camel fibroblasts, and regulated the expression trend of related genes in the signaling pathways, which effectively improved the reprogramming efficiency of the cells and played an important role in the reprogramming of Bactrian camel fibroblasts.
induced pluripotent stem cells; Bactrian camel; valproic acid; cell reprogramming

10.3864/j.issn.0578-1752.2023.12.014
2022-03-14;
2022-05-19
国家自然科学基金(31560638,31960725)
张启冉,E-mail:1074671622@qq.com。通信作者张勇,E-mail:zhychy@163.com
(责任编辑 林鉴非)
