Development and validation of dynamic nomogram of frailty risk for older patients hospitalized with heart failure
2023-05-14QinLiYnpingChenDehunQinShumeiLiShiyuZhngLiuFngJifengZhuYinghoWngYnnMoLneZhng
Qin Li ,Ynping Chen ,Dehun Qin ,Shumei Li ,Shiyu Zhng ,Liu Fng ,Jifeng Zhu ,Yingho Wng ,Ynn Mo ,Lne Zhng ,*
a School of Nursing,Weifang Medical University,Weifang,China
b Department of Nursing,Weifang People’s Hospital,Weifang,China
c Department of Anesthesiology,Weifang People’s Hospital,Weifang,China
Keywords: Aged Frailty Heart failure Patients Nomogram
ABSTRACT Objective:This study aimed to establish and validate a dynamic online nomograph for predicting the risk of frailty in older patients hospitalized with heart failure in China.Methods:A total of 451 older adults with heart failure hospitalized were selected between December 2021 and November 2022 at the Department of Cardiovascular Medicine in a Class A tertiary hospital in Shandong,China.The data of patients were obtained by using Barthel Index,instrumental activity of daily living scale,mini nutrition assessment-short form,Pittsburgh sleep quality index scale,Morse fall risk assessment scale and general information scale.The brain natriuretic peptide and echocardiographic indexes of patients were collected by electronic medical records.All participants were randomly divided into the training set(n=319)and the validation set(n=132)at the ratio of 7:3.The training set is used for model construction,and the validation set is used for internal validation.Using the Least Absolute Shrinkage and Selection Operator (LASSO) regression method to filter modeling variables,while the multivariable logistic regression was used to establish the nomogram based on the screened optimal variables.The performance of the model was evaluated by the area under the curve(AUC)of the receiver operator characteristic(ROC)curve,Hosmer-Lemeshow test,calibration plot,and decision curve analysis(DCA).Results:The prevalence of frailty in 451 patients was 50.6%,51.4%,and 48.5% in the training and validation sets,respectively.Drinking,grip strength,New York Heart Association (NYHA) class,multimorbidity,hospitalization history of heart failure,Barthel Index,the instrumental activities of daily living,nutritional status,sleep,fall,and left atrial end-diastolic diameter were used for LASSO regression analysis as the significant predictors of frailty.According to internal validation,the AUC of the ROC curve for the nomogram was 0.920,with a sensitivity of 86.8% and specificity of 84.4%.Moreover,in the validation set,the P-values of the H-L test were 0.742,and the calibration curve had good concordance between the estimated frailty risk and actual observation,indicating the model was well-calibrated.The DCA results confirmed that the nomogram had a well-performance in clinical suitability.Conclusions:An online dynamic nomogram predicting frailty for older patients hospitalized for heart failure in China was well-established and identified in this study.This model benefits medical professionals in identifying high-risk frailty in older hospitalized patients with heart failure,which could reduce the medical and disease burden of heart failure to a certain extent.However,further verification is needed in the future.
What is known?
·Older patients hospitalized with heart failure have a high rate of frailty,hindering prognosis and accelerating the progression.
·If recognized and treated early,frailty may be reversible,decelerating aging and restoring resilience.
·Few studies have included clinical indicator variables in the assessment of frailty,but these indicators are irreplaceable for hospitalized patients.
What is new?
·In this study,we established an online nomogram model and validated its effectiveness for predicting frailty in older hospitalized patients with heart failure.
·The prediction model benefits medical professionals in identifying high-risk frailty populations in older hospitalized patients,which could reduce the medical and disease burden of heart failure.
1.Introduction
Heart failure (HF) is a severe clinical syndrome associated with substantial morbidity and mortality[1].It is caused by structural or functional damage to the heart that reduces its ability to fill with or pump blood [2].An estimated 64.3 million people live with this syndrome worldwide [3].Although the age-adjusted incidence of heart failure is reducing,possibly owing to improved management of cardiovascular diseases,its overall incidence is increasing due to aging [4].In China,for example,large population sizes,prolonged life expectancy,and more intensive treatments are changing the epidemiology of HF towards older persons who are more likely to be frail and have comorbidities.Thus,health management of older patients with HF remains a considerable challenge for medical professionals and imposes a substantial financial burden on economies [5].
Frailty is a biological or geriatric syndrome characterized by an age-related decline in physiological reserve and function,including multiple physiological systems,resulting in enhanced vulnerability to endogenous and exogenous stressors [6].Older people with frailty are susceptible to adverse events,such as falls,malnutrition,multi-morbidity hospitalizations,and even sudden death [7].Additionally,the frailty of older patients with HF raises the need for long-term care,leading to an increasing medical care cost [8].If recognized and treated in an early stage,frailty is potentially reversible,which can delay the progression of aging and restore resilience[7].The European Society of Cardiology (ESC)guidelines on HF suggests that healthcare professionals should monitor frailty and seek and address reversible causes of deterioration in frailty score in elderly patients [2].This is beneficial to reduce disability,hospitalization rate,comorbidity,and medical burden.HF and frailty are interdependent syndromes.However,the predisposition to either condition is complex due to overlaps in physiological underpinnings,symptoms,and prognoses[9].Frailty is common in older patients with HF and significantly increases with aging.The overall prevalence of frailty is about 45%in patients with HF,which is 6-fold higher than those without suffering from HF.This rate is much higher in hospitalized patients with HF (56%-76%) [10].Consequently,frailty is a major contributor to hospital admission in older populations,related to worse clinical outcomes and complexity of care[11].HF's inherent multiple morbidity and frailty risks increase the medical burden on individuals and society.
Tools for identifying frailty have been developed over the past decades.Currently,the classic and commonly used tools in the HF population are mainly as the followings: Fried’s frailty phenotype[6],the Edmonton Frail Scale[12],the Rockwood Frailty Index[13],and the FRAIL Scale recommended by the International Association of Nutrition and Aging[14].However,these scales have limitations.For example,these represent assessment/screening tools that can only identify participants who suffer from frailty rather than predicting mathematical frailty risks.Further,these screening tools mainly apply to community people in developed countries.Although these tools have been validated for screening the frailty of hospitalized patients in recent years,their practical application in developing countries is still limited.
Moreover,these tools can be used in various diseases,and their diagnosis is universal.This diagnostic efficacy focuses on a single disease rather than a certain system (such as the cardiovascular system).Additionally,although these tools have high reliability,they are determined by the clinician’s judgment of the patient’s functional status and independence and the complex clinical operation.Conversely,some simple tools are obtained from the participants’ answers to the questions.Hence,this has obvious subjectivity and variability.In recent studies,some clinical indicators such as brain natriuretic peptide(BNP)[15],left ventricular ejection fraction(LVEF)[16],and left atrial internal diameter at enddiastole (LAIDd) [17],was reported to be closely related to frailty.We suppose incorporating these clinical indicator variables may be more effective and available for frailty in older patients with HF.
In this study,we aimed to incorporate comprehensive data such as physical measurement,questionnaire evaluation,and clinical indicators to build an applicable risk prediction model in the frailty of hospitalized HF patients.To this end,we established a dynamic online nomogram with visible and mathematical superiority.This prediction model can integrate diverse prognostic and determinant variables to aid clinical decision-making and generate a numerical probability of clinical events to assess patients’ frailty.More importantly,we verified that the nomogram is beneficial to help medical professionals to identify and treat high-risk frailty in older patients with HF as early as possible and reduce the burden of the disease.
2.Methods
2.1.Study design and participants
This study followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis(TRIPOD).All participants were older patients with HF who were hospitalized at the Department of Cardiovascular Medicine,Weifang People’s Hospital,between December 2021 and November 2022.The inclusion criteria for participants were: 1) conform to diagnostic criteria for HF issued by the ESC in 2021[2],2)age ≥60 years,3) stable condition,4) ability to complete the questionnaire unassisted or with assistance,and 5) informed consent and voluntary participation.Exclusion criteria were 1) severe mental disorder,language communication disorder,and cognitive impairment,and 2)acute attack state.According to the empirical criteria of events per variable(EPV)of logistic,the EPV was at least 5-10 of the included variables,and the results are stable and effective[18].The prevalence of frailty in hospitalized patients with HF was 36.2%-76.0% [10,11].According to the incidence rate of 40%,we calculated and added 10% invalid samples;the sample size was 165-330.
2.2.Data collection
All data were collected after the participants were admitted to the hospital and in stable condition.The questionnaire evaluation of the participants was performed at the bedside.Researchers read the questions and filled in the questionnaire for participants with difficulty reading and writing.Echocardiography and laboratory examination results were obtained through the hospital’s information system.All researchers involved in the study received uniform instructions before starting the survey.The data quality entered into the computer system was inferred based on completeness and accuracy.Data completeness was evaluated with on-site verification after questionnaire completion,while data accuracy was with double-person checking.
2.2.1.Frailty assessment
The frailty of participants was assessed with the FRAIL scale[14].It consisted of 5 domains: fatigue,resistance (ability to climb 1 flight of stairs),ambulation(ability to walk 1 block),illness(greater than 5),and loss of weight(>5%).The total score ranged from 0 to 5,with higher scores indicating severe frailty.None of the 5 items were diagnosed as robust;1 to 2 items were considered pre-frailty,while 3 or more were frailty.The Cronbach’s α coefficient of the Mandarin version is 0.826,and the content validity index is 0.93-1.00 [19].
2.2.2.Candidate predictors
A total of 31 candidate predictors were enrolled,including age,gender,marital status,living arrangement,education,number of children,monthly income,medical reimbursement,activity time,smoking,drinking,body mass index(BMI),grip strength,New York Heart Association (NYHA) functional class,multiple drugs (≥5),multimorbidity,hospitalization history with HF (within a year),activities of daily living (ADL) and instrumental activities of daily living(IADL),nutritional status,quality of sleep,and risk of falls.
A handgrip dynamometer obtained grip strength (EH101,CAMRY,China).The seated participants were instructed to squeeze the dynamometer as hard as possible.They performed three times with their left and right hands,respectively,and ultimately,the highest value of these tests was regarded as the final result.
According to the 10th revision of the International Classification of Diseases (ICD-10),this study investigated 11 types of chronic diseases except for HF,including hypertension,diabetes,coronary heart disease,chronic obstructive pulmonary disease (COPD),chronic cerebrovascular disease (CCVD),bone and joint disease,chronic kidney disease (CKD),chronic gastrointestinal disease,chronic liver disease,cancer,and depression.As the study population was older patients with HF,any combination of one or more chronic conditions in an individual was defined as multimorbidity[20].
Activities of Daily Living (ADL) was evaluated using the Barthel Index(BI),which included a 10-item instrument(feeding,bathing,grooming,dressing,controlling bowels and bladder,toilet use,transfers,mobility,and climbing stairs) measuring disability in terms of a person’s level of functional independence in personal ADL [21].A higher score represents a well-performed capacity in daily living activity.The Mandarin version of the BI had good reliability and validity (Cronbach’s α=0.916) [22].The Instrumental Activities of Daily Living Scale (IADL) was used to measure IADL[23].Commonly,a more complex activity requires more autonomy and cognitive function.Participants were categorized into groups with scores of 8 (Normal) and <8 (Impaired).
The Mini Nutrition Assessment-Short Form(MNA-SF)was used to evaluate nutrition status,which has been identified as wellvalidated for malnutrition screening in older populations with frailty [24].A total score of 12-14 points indicates normal nutritional status,and 12 points indicate malnutrition or at risk of malnutrition.
Pittsburgh Sleep Quality Index (PSQI) was used to assess the sleep quality of hospitalized patients [25].The score of PSQI was between 0 and 21 points if an adult scored >7 points who were diagnosed as impaired sleep quality.
The Morse Fall Risk Assessment Scale(MFS)measures the risk of falls for hospitalized patients,which is well-identified and performed in medical institutions worldwide [26].The higher scores indicate a greater risk of falls.
The accessible clinical predictors were echocardiographic parameters,including aortic diameter (AoD),main pulmonary artery diameter(MPAD),LAIDd,left ventricular internal diameter at enddiastole(LVIDd),left ventricular posterior wall thickness at diastole(LVPWTd),interventricular septal thickness at diastole (IVSTd),LVEF,fractional shortening (FS),and BNP levels.
2.3.Statistical analysis
Statistical analysis was performed using SPSS version 26.0(IBM Corp,Armonk,NY,USA) and R version 4.2.1 (R Foundation for Statistical Computing,Vienna,Austria) software.All participants were randomly divided into the training and validation set groups at a ratio of 7:3,respectively[27].The training set was adopted for variable filtering and model construction,and the validation set was used to validation the prediction efficacy of the nomogram for the frailty risk.
Continuous variables were described by mean and standard deviation or median and interquartile range,and categorical variables were described by frequency and percentage.Data were assessed for normality with the Shapiro-Wilk test.Thet-test or Mann-WhitneyUtest was used to compare differences between continuous variables.The Pearson’s Chi-square or Fisher’s test was used to compare differences between categorical variables.
The Least Absolute Shrinkage and Selection Operator (LASSO)regression method was incorporated for dimensional reduction,determining the optimal predictors to prevent overfitting[28].The value of the regularization parameter (lambda) was determined with 10-fold cross-validation in R (“glmnet” package) by minimizing the sum of the least square and shrinkage penalty.The model performance was evaluated using receiver operator characteristic(ROC)curves,the area under the curve(AUC)of the ROC,Hosmer-Lemeshow (H-L) test,calibration plot,and decision curve analysis (DCA).An online dynamic nomogram application was generated using Shiny,a package from RStudio.Statistical significance was inferred when two-sidedP<0.05.
2.4.Ethical considerations
This study was approved by the ethics committee of Weifang Medical University(No.2022YX74).All participants signed written informed consent before inclusion.
3.Results
3.1.Participants characteristics and frailty assessment
A total of 506 patients were enrolled,of which 55 were excluded because of age <60 years (n=26),acute stage of HF (n=18),organic heart disease (n=3),cognitive impairment (n=3),and inability to cooperate(n=5).Finally,451 participants with HF were included in the study,of which 319(70%)were randomly assigned to a training set and 132 (30%) to a validation set using random sampling.
Of the 451 elderly hospitalized HF patients,262 were male(58.1%)and 189 were female(41.9%),with a mean age of 73(68-80)years.Two hundred twenty-eight were diagnosed with frailty,accounting for 50.6%of the included patients.Conversely,223(49.4%)participants were diagnosed without frailty.The prevalence of frailty in the two sets was 51.4%and 48.5%,respectively.There was no statistical difference in the demographic characteristics of the participants and the prevalence of frailty between the two sets(Table 1).Besides,in the analysis consisting of three types of EF,theincidence of three EF types of frailty was HF with mid-range ejection fraction (HFmEF): 47.4% (54/114);HF with reduced ejection fraction (HFrEF): 60.1% (86/143);In HF with preserved ejection fraction(HFpEF),the incidence of frailty was 45.4%(88/194),which was lower than the other two types.
3.2.Construction of the nomogram
A total of 11 factors were screened with non-zero coefficients calculated by LASSO regression analysis.These were drinking,grip strength,NYHA class,multimorbidity,hospitalization history of HF,BI,IADL,MNA-SF,PSQI,MFS,and LAIDd.The results of the multivariable logistic regression analysis are shown in Table 2.A nomogram model incorporating the above 11 independent predictors was constructed (Fig.1).Its dynamic version was available online (https://liqian.shinyapps.io/DynNomapp/).
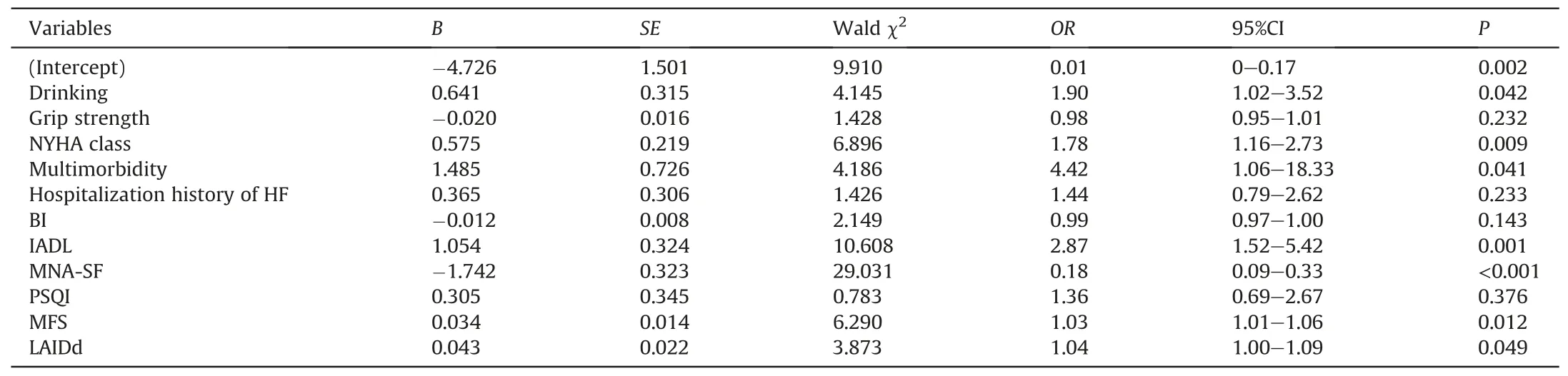
Table 2 Multivariate logistic regression analysis of risk factors for frailty in older patients hospitalized with heart failure(n=319).
3.3.Validation of the nomogram
The AUC of the ROC for the nomogram was 0.873 in the training set with a specificity of 73.5%and sensitivity of 87.2%.In the internal bootstrap method (with 1,000 resamples),the AUC of the ROC curve for the validation set was 0.920 with a specificity of 86.8%and sensitivity of 84.4% (Fig.2),suggesting good discriminative ability.Moreover,in the validation set,theP-value of the H-L test was 0.742,indicating that the model was well-calibrated.Collectively,these results indicate that the nomogram model is available for predicting the risk of frailty for older patients who were hospitalized with HF.
To evaluate the clinical utility of the model,we used decision curve analysis (DCA) to verify.As expected,the nomogram for frailty prediction increases more benefit at a threshold probability between 50% and 100% than all-intervene or no-intervene (Fig.3).The y axis represents the net benefit and the x axis represents the high risk threshold that was chosen here between 0 and 1/0.8 depending on the clinical relevance.The red/blue line represents the net benefit value of the nomogram,and the gray slash line assumes that all patients experienced weakness;the black line assumes that no patients experienced weakness.Within the threshold range(abscissa),the net benefit rate(red/blue line)of the prediction model was above both extreme lines (black and gray lines),indicating that the model had good clinical applicability.
4.Discussion
The exact and timely diagnosis of frailty is of great importance for patients with HF because it allows effective intervention,such as therapy.Therefore,establishing a model for identifying the risk of frailty and aiding its management is necessary [7].Factors for predicting frailty involve age,grip strength [29],multimorbidity[30],nutrition [31],sleep [32],hospitalization,and falls [33].However,it is unclear how accurately these factors predict the development of frailty and the underlying mechanisms.In this study,we selected 11 variables for predicting the frailty risk among 451 hospitalized older patients with HF using LASSO regression analysis.We used these factors to establish a comprehensive,easy-to-use nomogram model.We demonstrated its good discriminative ability and clinical practicability with a net benefit.In addition to a static nomogram,widely used as a simple tool to screen frailty,we also generated online a dynamic nomogram to enable more personalized medicine with a user-friendly digital interface.
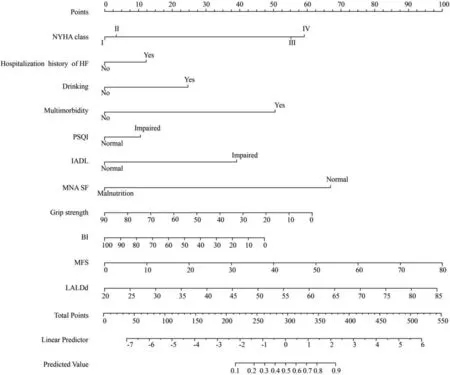
Fig.1.A nomogram to predict frailty for older patients hospitalized with heart failure.NYHA=New York Heart Association.HF=heart failure.PSQI=Pittsburgh Sleep Quality Index.IADL=instrumental activities of daily living.MNA-SF=mini nutrition assessment-short form.BI=Barthel Index.MFS=Morse Fall Scale.LAIDd=Left atrial end diastolic diameter.
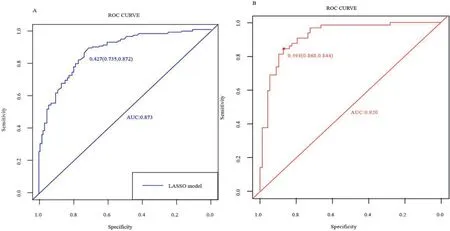
Fig.2.The receiver operator characteristic (ROC) curve of the frailty risk prediction model.A.Training set.B.Validation set.
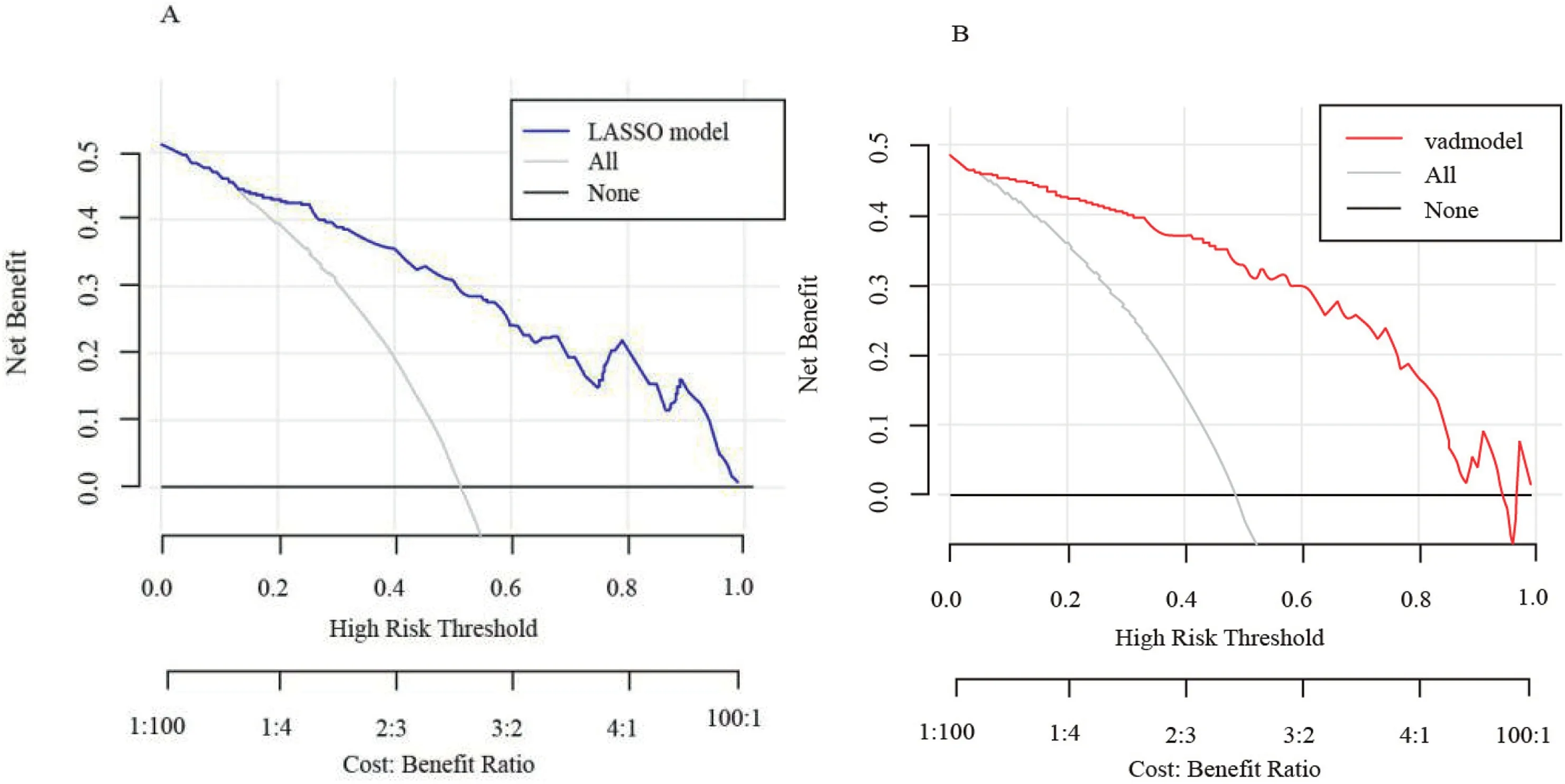
Fig.3.The decision curve analysis (DCA) of the frailty risk prediction model.A is the DCA of the nomogram,and B is the DCA of the validation set model.
Our study’s prevalence of frailty was high overall among hospitalized patients with HF,which agrees with other studies on hospitalized patients[11].It was also substantially higher than the prevalence in communities and other institutions [34].Because mechanisms of frailty and HF overlap,patients with HF are approximately three times more likely to become frail than those without [35].Thus,the high prevalence of frailty in HF indicates frailty is more common in hospitalized patients with HF than we may have previously thought,having vital implications for practitioners caring for an older patient with HF.
In this study,it was different from the previous studies that the prevelance of frailty in HFpEF is the lowest among the three categories of HF.A previous study has shown that HF patients with HFpEF are more likely to have frailty and higher frailty states [36].Compared with the previous studies,the age of participants in this study is relatively low (73 [68-80]),which may be the possible reason for the difference between this study and previous studies.A recent study demonstrated that the proportion of HFpEF patients increases with age,reaching 52% among HF patients over 75,and HFpEF patients were older than HFrEF patients [37].However,any HF was associated with neuroendocrine pathway activation and chronic tissue perfusion insufficiency.These events jointly lead to structural and functional abnormalities of cardiovascular and various other organ systems,reduce physiological reserves,increase physical vulnerability,and ultimately lead to frailty [11].With the progress of global aging,the health management of HF with frailty could be an important issue in the super-aged society,which needs further study.
The results of this study showed that the risk of frailty in patients with more than two multimorbidities was 4.42-fold higher and ranked first among all independent factors,which were consistent with previous studies.A study has demonstrated that the prevalence of both frailty and pre-frailty increased with increasing multimorbidity,and multimorbidity was also more common in frail participants [38].Increasing evidence suggests they are the chief cause of adverse clinical outcomes and frequent hospitalizations.Hospitalizations occur at least once a year for 65%of patients with HF[30],and readmissions are often due to reasons other than HF,reflecting the high comorbidity burden among these patients.Altogether,multimorbidity is a predisposing factor for the development of frailty in the older population and an important cause of aggravated frailty,underscoring the importance of investigating frailty in patients with HF.Multiple clinical problems and pathophysiological changes caused by different diseases affect the normal physiology of an individual.Nonetheless,we encourage a multidisciplinary approach to manage these conditions and minimize their negative effects.
The NYHA functional class is routinely used to assess cardiac function in the clinical setting and directly predicts the severity of frailty [39].Indeed,our findings show that a higher NYHA functional class is a good predictor of frailty in hospitalized older patients with HF.Adults in the frailty group had NYHA III or higher class,while most of those in the no-frailty group had NYHA I and II.While controversies remain on the credibility of NYHA classification in discriminating functional impairments [40],we confirm it provides guidance for recognizing changes in cardiac function in older patients with HF to determine frailty risk.
Few studies that evaluate cardiac function in HF with frailty are available.Thus,we assessed it noninvasively using the echocardiographic characteristics of patients with HF and frailty.We further determined the predictive effect of each cardiac functionrelated indicator on frailty.The cardiac function index related to frailty was neither LVEF nor FS.Notably,LAIDd was proved to be an independent influencing factor of frailty in patients with HF.The cause of frailty due to cardiac structural and functional changes are still elusive,but this finding demonstrates that changes in cardiac structure and function worsen frailty and underscore the importance of incorporating cardiac function evaluation into the frailty assessment of these patients.Some scholars [41] evaluated echocardiograms and frailty phenotypes in people aged 65 or older.They showed that increased stroke volume was an independent protective factor for frailty(OR=0.87),whereas increased left atrial volume was an independent risk factor for frailty (OR=1.06).Hence,cardiac function and structural parameters may predict frailty.
One advantage of this study was that compared with existing studies,it integrated comprehensive data collection and evaluation to build a specific screening tool for frailty for elderly inpatients.Another advantage was that the prediction model established in this study performed well in identification,calibration,and clinical application.Our dynamic nomograph is equivalent to a web calculator.By inputting the corresponding variable value,we can get the risk of frailty.The nomogram can available online at any time and easy to use,allowing a quick frailty assessment of hospitalized patients and timely diagnosis of high-risk people without complications,which has an important clinical impact on patient management.
Admittedly,this study has some limitations.First,it was monocentric and performed only with internal validation in a single Class A tertiary hospital in China,without external validation.Therefore,multicentered large-sample clinical studies and validations are central for future investigations.Second,the LASSO regression algorithm does not fundamentally solve the multicollinearity problem but limits the influence of multicollinearity to select the characteristics of the optimal model.In the future,we will perform clinical studies and validations of multicentered largesample.
5.Conclusion
In conclusion,we established a simple-to-use dynamic online nomogram to assess frailty in older patients with HF.It combines 11 demographic and clinical characteristics of patients with HF with commonly used evaluation items to predict the risk of frailty in populations of older hospitalized patients.Our model provides valuable information and a basis for designing quick frailty screening tools that should help improve intervention plans for Chinese hospitalized patients.
Funding
This study was supported by the Nursing Research Fund of Weifang Medical University (2022MS001).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Qian Li:Conceptualization,Methodology,Software,Visualization.Yanping Chen:Investigation,Reviewing,Project administration.Dechun Qin:Investigation,Reviewing,Project administration.Shumei Li:Investigation,Reviewing,Project administration.Shiyu Zhang:Writing-original draft,Reviewing and editing.Liu Fang:Data curation,Reviewing and editing.Jiafeng Zhu:Data curation,Reviewing and editing.Yingchao Wang:Software,Validation,Reviewing and editing.Yanan Mao:Software,Validation,Reviewing and editing.Lane Zhang:Supervision,Writing-reviewing and editing.
Declaration of competing interest
The authors have declared no conflict of interest.
Acknowledgments
We would like to express our gratitude to all members and patients of this study for their effort,support and understanding.Additionally,we thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2023.03.014.
杂志排行
International Journal of Nursing Sciences的其它文章
- A feasibility study on home-based kyphosis-specific exercises on reducing thoracic hyperkyphosis in older adults
- Validation of the Portuguese version of the social isolation scale with a sample of community-dwelling older adults
- Effects of pre-operative education tailored to information-seeking styles on pre-operative anxiety and depression among patients undergoing percutaneous coronary intervention: A randomized controlled trial
- Factors influencing the quality of sexual life in the older adults: A scoping review
- Comparison of the effects of three kinds of hand exercises on improving limb function in patients after transradial cardiac catheterization
- The trajectories of physical growth in 4 months postnatal corrected age among preterm infants discharged from neonatal intensive care units and associated factors: A prospective study
