The trajectories of physical growth in 4 months postnatal corrected age among preterm infants discharged from neonatal intensive care units and associated factors: A prospective study
2023-05-14WenyingGoTomeiZhngQihuiWngXioliTngYingZhng
Wenying Go ,Tomei Zhng ,Qihui Wng ,Xioli Tng ,Ying Zhng ,*
a Shanghai Jiao Tong University, School of Nursing, Shanghai, China
b Shanghai Jiao Tong University, School of Medicine, Shanghai Children’s Medical Center, Department of Neonatology, Shanghai, China
c Shanghai Jiao Tong University, School of Medicine, Shanghai Children’s Medical Center, Department of Nursing, Shanghai, China
Keywords: Growth Latent growth model Mother-infant interaction Neonatal intensive care unit Postpartum depression Premature infant
ABSTRACT Objectives: Growth retardation is a risk for premature infants.In addition to demographic and perinatal factors,preterm infants’ physical growth may be affected by neonatal intensive care unit (NICU) stress,maternal postpartum depression,and mother-infant interaction.This study aimed to investigate the trajectories of physical growth in 4 months corrected age among preterm infants discharged from the NICU and the impactors on these trajectories. Methods:A prospective study was conducted among 318 preterm infants from September 2019 to April 2021 in Shanghai,China.Latent growth modeling was applied to identify the weight,length,and head circumference growth trajectories in 4 months corrected age and explore the effects of demographic and medical characteristics,infant stress during NICU stay,maternal postpartum depression,and motherinfant interaction on each trajectory. Results:Unconditional latent growth models showed curve trajectories with increasingly slower growth in weight,length,and head circumference until 4 months of corrected age.Conditional latent growth models showed that a longer length of stay in the NICU and more skin punctures were negatively associated with weight at 40 weeks corrected gestational age (β=-0.43 and -0.19,respectively,P<0.05).The maternal postpartum depression between 40 weeks corrected gestational age and 1 month corrected postnatal age was associated with a lower growth rate of length (β=-0.17,P=0.040),while between 2 and 3 months corrected postnatal age,there were lower growth rates of weight and head circumference (β=-0.15 and -0.19,respectively,P<0.05).The mother-infant interaction scores between 40 weeks corrected gestational age and 1 month corrected postnatal age negatively predicted the growth rate of weight (β=-0.19,P=0.020).Conclusion:The physical growth trajectories of preterm infants discharged from the NICU were influenced by infant stress during the NICU stay,maternal postpartum depression and mother-infant interaction.
What is known?
·It is suggested that the period between birth and 4 months of corrected age is important for catch-up growth of preterm infants.
·Preterm infants’ neurodevelopment might be strongly affected by stress during neonatal intensive care unit (NICU) stay while its effect on physical growth still lacks in evidence.
·Postpartum depression is common in mothers of preterm infants,which might be related to poor quality mother-infant interaction at the same time;however,both of these can lead to negative infant care,which in turn leads to growth retardation in preterm infants.
What is new?
·It is indicated that the period between birth and 4 months of corrected age is important for catch-up growth.Preterm infants in this study did not lag in terms of weight,length,and head circumference growth compared with the World Health Organization (WHO) growth standard for term infants.
·Our study found that infant stress during the NICU stay,maternal postpartum depression and mother-infant interaction all affected the physical growth trajectories,including levels and rates among preterm infants in the 4 months corrected age.
1.Introduction
Preterm birth(at gestational age <37 weeks)is a major factor in maternal and child health worldwide,and the associated complications are the leading cause of infant and under-5 mortality [1].Reports from the World Health Organization (WHO) indicate that,globally,approximately 15 million infants (10.6% of all live births)were born prematurely in 2014[2].In China,which has the second largest population of preterm infants worldwide,the incidence of preterm births is approximately 7%,with an increasing trend in the past 30 years (annual growth rate: 1.1%-1.3%) that could continue for a long period [3] due to the three-child policy and advances in assisted reproductive technology.Rapid advances in perinatal and neonatal medicine have decreased mortality and increased the survival of preterm infants.However,efforts to monitor and improve the long-term prognosis,including the growth and development of preterm infants,are equally important.To date,numerous studies have shown that a large proportion of preterm infants lag behind full-term infants in physical growth.A multicenter study [4] showed that 26.1% of preterm infants presented with weight retardation a few days after birth.In a previous study[5],the incidences of growth retardation in weight,length,and head circumference among preterm infants at 12 months corrected postnatal age were 16.3%,10.3%,and 18.3%,respectively.
As reported,the level of weight,length,and head circumference among preterm infants were approaching or exceeding the 10th percentile of standards at 4 months of corrected age,then with gentle changes at a later time [6].It is indicated that the period between birth and 4 months corrected age is critical for the catchup growth of preterm infants,and most preterm infants might achieve catch-up growth at 4 months corrected age [7].Recently,several studies suggested that the physical growth status during this period was closely related to long-term outcomes,including cognitive and motor development[8-11].Therefore,it is necessary to explore the crucial factors in physical development before 4 months of corrected age.Considering the consensus on starting to add complementary foods at 4-6 months of corrected age,which might bring great differences in growth due to the diversity of complementary foods,we focused on the physical growth in 4 months of corrected age among preterm infants.
Most of the existing studies focused on the effect of perinatal characteristics on the physical growth of offspring.However,these characteristics prove challenging to regulate for better growth.Therefore,it is crucial to identify contributory factors that can be regulated during neonatal intensive care unit (NICU) hospitalization and follow-up.
During hospitalization,infant stress during the NICU stay might be a potential negative factor in the early growth and development of preterm infants.To our knowledge,infant stress during NICU stay has not been clearly defined,although the condition can be typified as a cause of physical stress or mental tension due to environmental factors in the NICU,such as invasive procedures,pain,and motherinfant separation[12].Moreover,preterm infants are more likely to be affected strongly by stress than full-term infants because of their immature pain-perceptive neuronal connections and function right after birth,which confers an inability to distinguish between harmful and non-harmful stress to enable successful coping [13].However,few studies have investigated the impact of infant stress during NICU stays on the physical growth of preterm infants rather than on neurodevelopment.
Usually,mothers are the primary caregivers for preterm infants at home,and their depressive symptoms can profoundly impact infants’development through caregiving,which has been proven in several studies.A systematic review of 17 original studies [14]showed that infants exposed to maternal postpartum depression had a 1.4-1.5 times higher risk of weight and length retardation;additionally,the risk of growth retardation might increase from 24% to 29% with exposure to maternal postpartum depression.In the first postpartum year,maternal postpartum depression is more common among mothers after preterm birth [15,16] than among those after term birth(incidence: ≤40%vs.10%-15%,respectively)[17].That is to say,those preterm infants exposed to maternal depression were at a high risk of growth retardation and failure to catch up with growth.Furthermore,the effect of maternal postpartum depression on the growth of infants was considered related to the poor quality of mother-infant interaction.These mothers with maternal depressive symptoms are likely to perform poorer mother-infant interactions because of their lower maternal sensitivity,which is characterized by a failure to identify infant cues of need and to respond immediately[18,19].Besides,preterm infants,especially those born before 32 weeks of gestation,are more likely to perform worse in interactions within the mother-infant dyad[20].In a conceptual model [21],maternal behavioral care (e.g.,skin-to-skin contact) and nutritional care (lactation),which constitute the mothers’ care tasks during feeding,are interrelated and contribute to both nutritional and non-nutritional growth.Thus,not only nutrition but the feeding-related interaction in the mother-infant dyad contributes to early growth and development[22].There are,however,very few investigations of the relationship between mother-infant interaction and physical growth since it is challenging to observe mother-infant interaction in large samples.The 10-item Mother and Baby Interaction Scale(MABIS)may serve as a potential measure for accessing the mother-infant interaction for screening large population samples [23].
Therefore,this study was conducted to clarify the factors associated with the growth of preterm infants by 1) identifying the dynamic physical growth trajectories of preterm infants in 4 months of corrected age and 2) determining potential critical contributors,including infant stress during NICU stay,maternal postpartum depression,and mother-infant interaction,after controlling for confounders.
2.Methods
2.1.Study design and participants
This prospective survey with a 4-month follow-up period was conducted from September 2019 to April 2021 in two inner-city maternity and neonatal hospitals in Shanghai,China.We included mother-infant dyads who met the following criteria by convenience sampling at outpatient follow-up clinics: 1) infants were prematurely born before 37 weeks of gestation;2) mothers self-identify as primary caregivers for infants;3) mothers can read Chinese.Infants with severe congenital disorders or malformations,critical illnesses,or those who were transferred or died during their NICU stay were excluded.Those mothers with psychotic disorders were excluded.
2.2.Data collection
The data on weight,length,and head circumference were collected at five time points:40 weeks of corrected gestational age(T1),1 month(T2),2 months(T3),3 months(T4),and 4 months(T5)of corrected postnatal age.In total,318 mother-infant dyads with at least two follow-up data records were included.Some sample attrition occurred when the research was paused during the COVID-19 pandemic,and consequently,294,279,244,221,and 191 mother-infant dyads were included at corrected ages of 40 weeks gestation and 1,2,3,and 4 months,respectively.Maternal postpartum depression and mother-infant interaction were measured during two time intervals: T1-T2 (n=280) and T3-T4 (n=233).Data collection was conducted by two researchers(T.Z.and Q.W.).
2.3.Measures
2.3.1.Demographic and medical characteristics
Data on the demographic and medical characteristics,including sex,gestational age at birth and birth weight of infants,maternal age,educational background,singleton pregnancy,delivery mode,gestational history,pregnancy-related complications,and so on,were collected from the medical records in the Hospital Information System(HIS).
2.3.2.Physical growth
Weight,length,and head circumference were used to determine the physical growth of premature infants.Weight and length were measured using standard calibrated weight and length scales,accurate to 10 g and 1 cm,respectively,in a special clinic room.Infants were laid down on the board in a supine position without shoes and with minimal clothing during the measurements.Head circumference was measured to the nearest 0.1 cm using a non-elastic tape that encircled the most prominent part of the occiput and around the forehead.The assessments were performed and uniformly recorded in the HIS by trained staff.
2.3.3.Infant stress during the NICU stay
Infant stress during the NICU stay was described by several variables,including the length of stay(LOS,days)in the NICU,whether the infant received mechanical ventilation(MV),and the total counts of skin punctures (the sum of heel lances,peripheral venous and central vascular punctures).LOS comprised the days from NICU admission to transfer out of the NICU.Whether the infant received MV was determined based on medical records for tracheal tubes.Details of skin punctures were collected from medical records.
2.3.4.Maternal postpartum depression
Maternal postpartum depression was assessed using the Chinese version of the Edinburgh PostnatalDepression Scale(EPDS),a 10-item screening scale for the early detection of depressive symptoms[24,25].Each itemwas scored by mothers from 0(never)to 3(always)according to their feelings over the past 7 days,and higher summed scores(range 0-30)indicated more severe depressive symptoms.The Chinese-version scale had a sensitivity of 82%and a specificity of 86%at a cut-off score of 9/10[25].In this study,a total score of 10 or more indicated depressive symptoms[26].The reliability coefficient of the EPDS was high(Cronbach’s α=0.85)in this study.
2.3.5.Mother-infant interaction
The mother-infant interaction was measured on the MABIS,wherein the responses represented the situation in the preceding month[27].The scalecomprises 10 items and the score foreach item is selected from 0(always),1(most of the time),2(occasionally),3(not often),or 4(never).Especially,items 3,7,and 9 are reverse-scored.The total score of the scale ranges from 0 to 40,with lower scores indicating greater mother-infant interaction.The Cronbach’s α for MABIS was 0.69 in a Norwegian study [23].In the present study,with the authorization of the original authors,the MABIS was translated into Chinese through translation,back translation,cultural debugging,and evaluation procedures by the whole study group,which includes two candidates for a master of science in nursing,three clinical nursing specialists,a professor studying pediatric nursing,and a lecturer of psychology.The Chinese version of the MABIS in this study has good internal consistency(Cronbach’s α=0.76).
2.4.Data analysis
To achieve the first aim of this study,latent growth modeling(LGM) was used to identify the trajectories of change for weight,length,and head circumference among infants over time.LGM is a powerful analytical approach for determining the growth process from repeated measurement outcomes by using a series of latent factors,such as intercept (representing the initial level of the outcome),slope (representing the rate of change over time),and quadratic(representing the change in the rate of change over time).Three candidate unconditional LGMs were constructed: 1) linear model with intercept and slope factors describing a linear pattern of change over time;2)quadratic model with intercept,slope,and quadratic factors describing a quadratic growth curve for change over time;3) freely estimated model with intercept and slope factors,while its slope loadings were fixed at 0 and 1 at T1 and T2 and freely estimated for T3,T4,and T5.The best-fit unconditional LGMs would be chosen for further analysis.
The second aim of the study was to explore critical contributors to the changes in physical growth over time.Those potential covariates (i.e.,sex,birth weight,skin punctures,maternal postpartum depression,mother-infant interaction) identified by the primary analysis,includingt-test and Spearman correlation analysis,entered the unconditional LGMs to examine the influences on the changes in physical growth.To obtain the final models with the best fit,we simplified the models by removing the most nonsignificant paths one by one to improve the model fits.
We used SPSS software (version 24.0) to conduct primary analysis,including description,t-test,and correlation analysis,and AMOS software(version 23.0)to construct LGMs.Full-information maximum-likelihood estimation (FIML) was performed in LGM analyses,which is a great technique for dealing with missing data based on all available information.Various model-fit indices were used to evaluate the goodness of LGMs as follows: chi-square statistics (χ2/df),comparative fit index (CFI),normed fit index (NFI),root mean square error of approximation (RMSEA) and Akaike information index (AIC).Those models with higher CFI and NFI as well as lower RMSEA and AIC performed better fit.All tests were performed at the level of significance of α=0.05.
2.5.Ethical consideration
This study was approved by the Public Health and Nursing Research Ethics Committee of Shanghai Jiao Tong University(SJUPN-202021).Before enrollment in the study,we obtained written informed consent from mothers of preterm infants.Information such as names was excluded from the data analysis for privacy protection.
3.Results
3.1.Descriptive analysis of mother-infant dyads
In total,318 mother-infant dyads were included in the survey,and their detailed characteristics are presented in Table 1.In this study cohort,56(17.6%)preterm infants were born before 32 weeks and 262(82.4%)were born between 32 and 37 weeks of gestation.The mean birth weight was 2,075.61 g (SD=501.82,range 735-3,680 g),and 255 (80.2%) weighed less than 2,500 g at birth.Only 37 (11.6%) infants were born small for gestational age (SGA);14.2% of the participants received MV during hospitalization.Each infant stayed in the NICU for 14.20 days and experienced 20 skin punctures.Between the corrected ages of 40 weeks gestational age and 1 month,36.1% of mothers experienced maternal postpartum depression,whereas,between 2 and 3 months,30.5% reported maternal postpartum depression.As for the mother-infant interaction,280 mothers reported a mean MABIS score of 11.81(SD=5.33)between the corrected ages of 40 weeks gestational age and 1 month,and 233 mothers reported a mean MABIS score of 10.18(SD=4.72)between 2-and 3-months corrected age.Changes in weight,length,and head circumference of preterm infants over time were summarized in Supplementary Table 1,and the corresponding trajectories of weight,length and head circumference were shown in Fig.1 in comparison with growth standards published by WHO.
3.2.Unconditional LGMs for trajectories of physical growth
To investigate the change in weight,length,and head circumference among the sample,we tested linear,quadratic,and freely estimated trajectories for each growth indicated.The linear models for weight,length and head circumference were initially excluded from our consideration for their poor model fit with extremely low CFI and NFI (all <0.7).Though quadratic models for each growth indicated that all performed better than freely estimated models interms of model fit,several parameters showed standardized factor loadings of more than 1,which violated estimation,indicating a lack of modeling ability.Therefore,freely estimated LGMs with acceptable fits(CFI,NFI,all >0.85,and RMSEA <0.2)were chosen to be used in subsequent analysis (details shown in Supplemental Table 2).
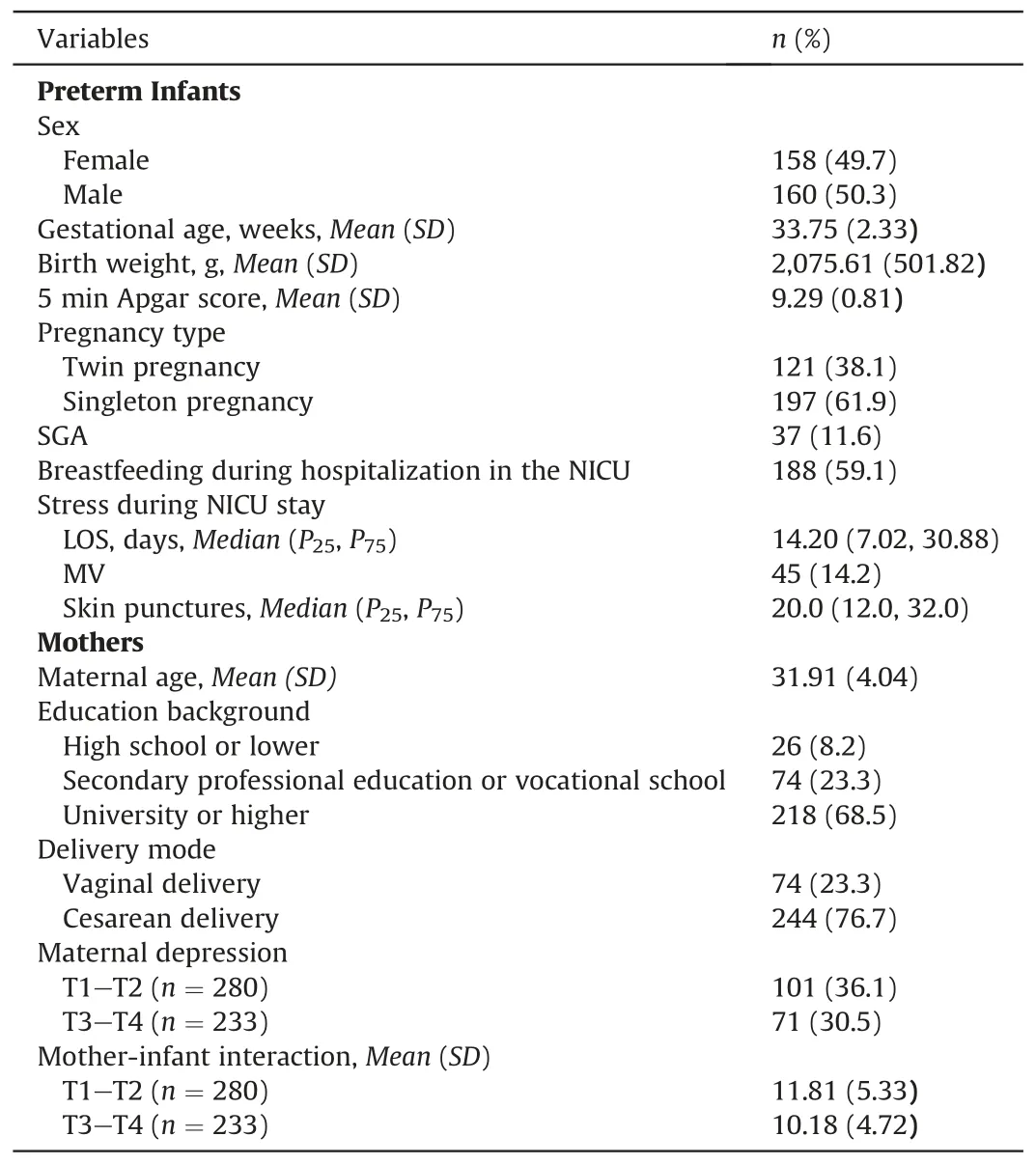
Table 1 Mother-infant characteristics (n=318).
For the weight model,the slope factor loadings were 0,1.0,1.8,2.4,and 2.9,respectively,presenting the relative increment in weight at T1-T5 compared with the weight level at T1.There were significant initial level and growth increases in weight (intercept factor mean=3,333.86,P<0.001;slope factor mean=1,383.38,P< 0.001) and interindividual variability (intercept factor variance=266,740.24,P<0.001;slope factor variance=69,229.02,P<0.001) during the study period.No significant correlation between weight level at the 40-week corrected age and weight growth rates was observed.
For the length model,the estimated slope factor loadings were 0,1.0,2.0,2.7,and 3.3,respectively,at the five predefined time points.The intercept and slope factors were statistically significant(intercept factor mean=50.50,P<0.001;slope factor mean=4.12,P<0.001).Furthermore,there was significant variance in the intercept and slope (intercept factor variance=3.53,P<0.001;slope factor variance=0.23,P<0.001).The covariance between the intercept and slope was statistically non-significant (r=-0.09,P=0.297).
For the head circumference model,the estimated loadings for slope factors were 0,1.0,2.0,2.7,and 3.2.The intercept and slope factors were statistically significant(intercept factor mean=34.67,P<0.001;slope factor mean=2.10,P<0.001).In addition,there were significant variances in the intercept and slope factors(intercept factor variance=1.38,P< 0.001;slope factor variance=0.09,P<0.001).The covariance between the intercept and slope showed a non-significant correlation (r=-0.06,P=0.100).
3.3.Conditional LGMs for trajectories of physical growth
Firstly,potential predictors were selected according to results in single-covariate conditional LGMs,the independent samplest-test,and Spearman’s rank correlation test (detailed results are presented in Supplemental Tables 3-9).Secondly,these potential contributors were incorporated together into the LGMs for weight,length,and head circumference,as presented in Fig.2.Thirdly,the models were simplified in an attempt to improve the fit by reducing the non-significant paths.The final models for weight,length and head circumference all had a good fit: 1) for the weight model,χ2=135.62,P<0.001,CFI=0.97,NFI=0.96,RMSEA=0.08,AIC=351.62;2) for the length model,χ2=86.11,P<0.001,CFI=0.98,NFI=0.97,RMSEA=0.06,AIC=248.11;3)for the head circumference model,χ2=175.38,P<0.001,CFI=0.95,NFI=0.94,RMSEA=0.10,AIC=337.38.
The results showed that demographic and medical characteristics such as maternal age,male,gestational age and birth weight might contribute to the final models for weight,length,and head circumference.Tables 2-4,respectively,show the details of the final models for weight,length,and head circumference.Longer LOS and more skin punctures might contribute to a lower level of weight gain at 40 weeks’ corrected age (β=-0.43 and -0.19,P=0.004 and 0.048).Otherwise,preterm infants with maternal postpartum depression between 40-week and 1-month corrected age might have a lower growth rate of length(β=-0.17,P=0.040),whereas those with maternal postpartum depression between 2-and 3-month corrected age might have a lower rate of weight and head circumference (β=-0.15 and -0.19,P=0.037 and 0.049).Furthermore,the slope of the weight model was negatively predicted by the score of mother-infant interaction (β=-0.19,P=0.020),which means that a worse mother-infant interaction may lead to a slower increase in weight for preterm infants.
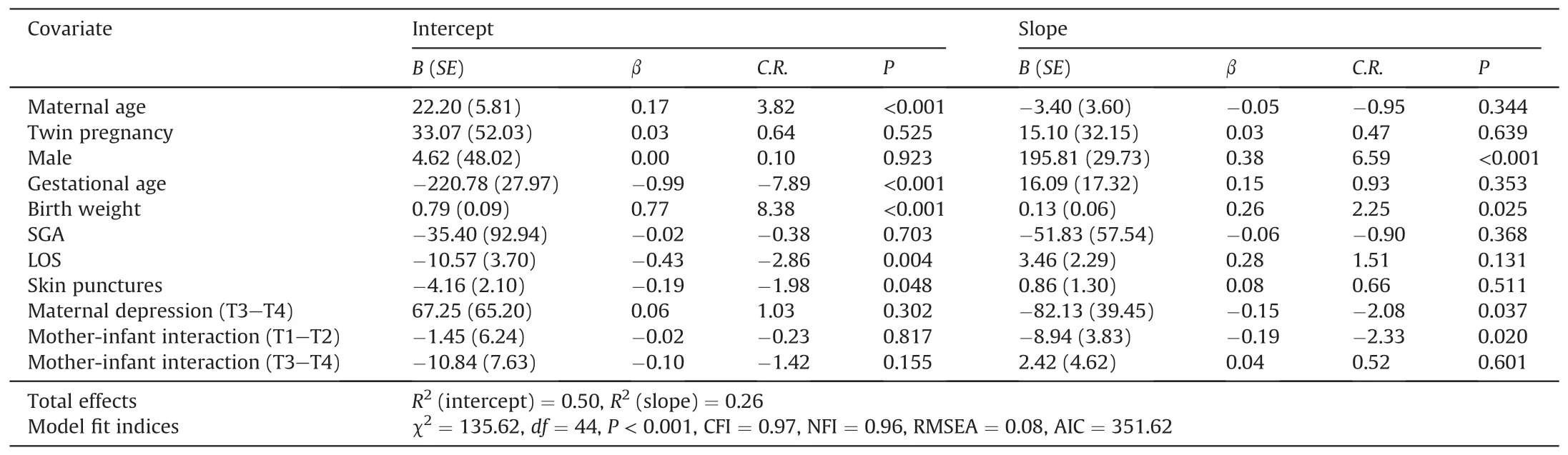
Table 2 Effects of demographic and medical characteristics,infant stress during NICU stay,maternal depression and mother-infant interaction on the weight growth trajectory.
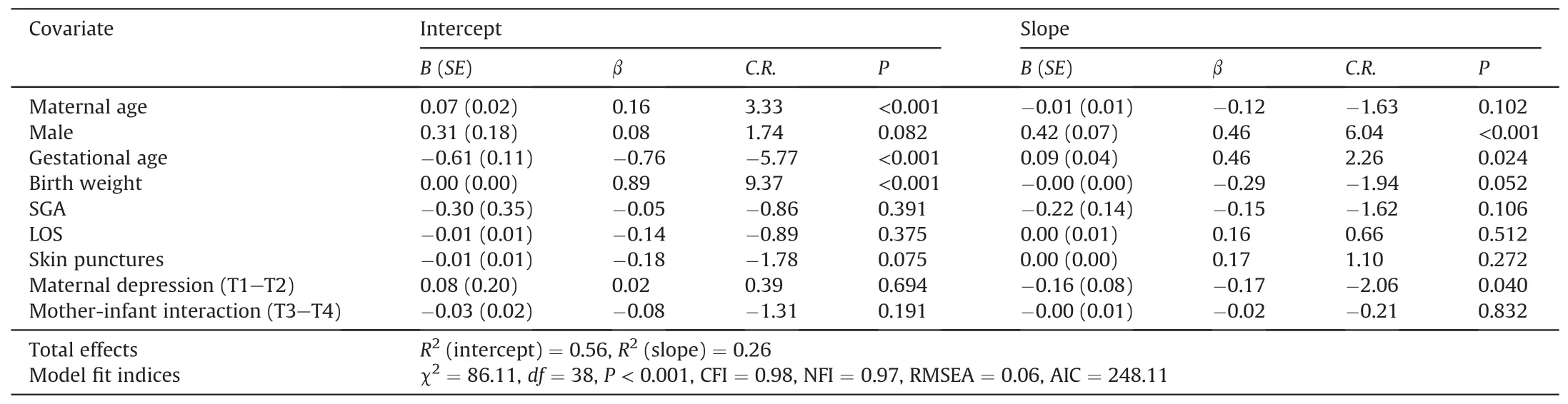
Table 3 Effects of demographic and medical characteristics,infant stress during NICU stay,maternal depression and mother-infant interaction on length growth trajectory of premature infants.
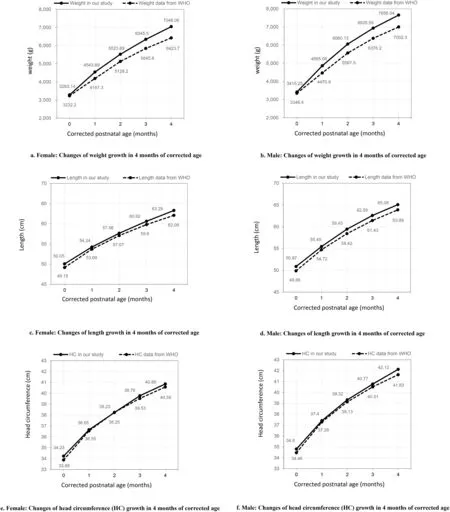
Fig.1.Growth curve of preterm infants in this study and the WHO standards.
4.Discussion
In this longitudinal study,the change in weight,length,and head circumference in 4 months of corrected age among preterm infants discharged from the NICU all showed a curved trajectory with increasingly slower growth rates.There were individual differences in both initial levels and growth rates of weight,length,and head circumference among the participants.Besides,the growth rates were not associated with the initial level at 40 weeks corrected age.Compared with the WHO growth standards for the same age and sex,the preterm infants in this study did not lag in terms of weight,length,and head circumference growth and showed better growth with regard to weight,contrary to the result of a previous study [5].This difference is probably a result of the higher proportion of moderate-to-late preterm infants with better growth status in our study and the increasing focus on monitoring as well as nutritional support guided by follow-up clinics for preterm infants in Shanghai,China,in the past 2 years.Moreover,recent studies have shown that preterm infants grow better than the WHO standards but worse than term infants in China [28,29].The WHO standards describe the growth of children worldwide,and country-specific growth references should be paid more attention to.Considering that the healthy,full-term infants indicate the optimal growth potential rather than preterm infants in equal conditions,it might be more instructive to include a full-term cohort in further research,which was a pity in this study.
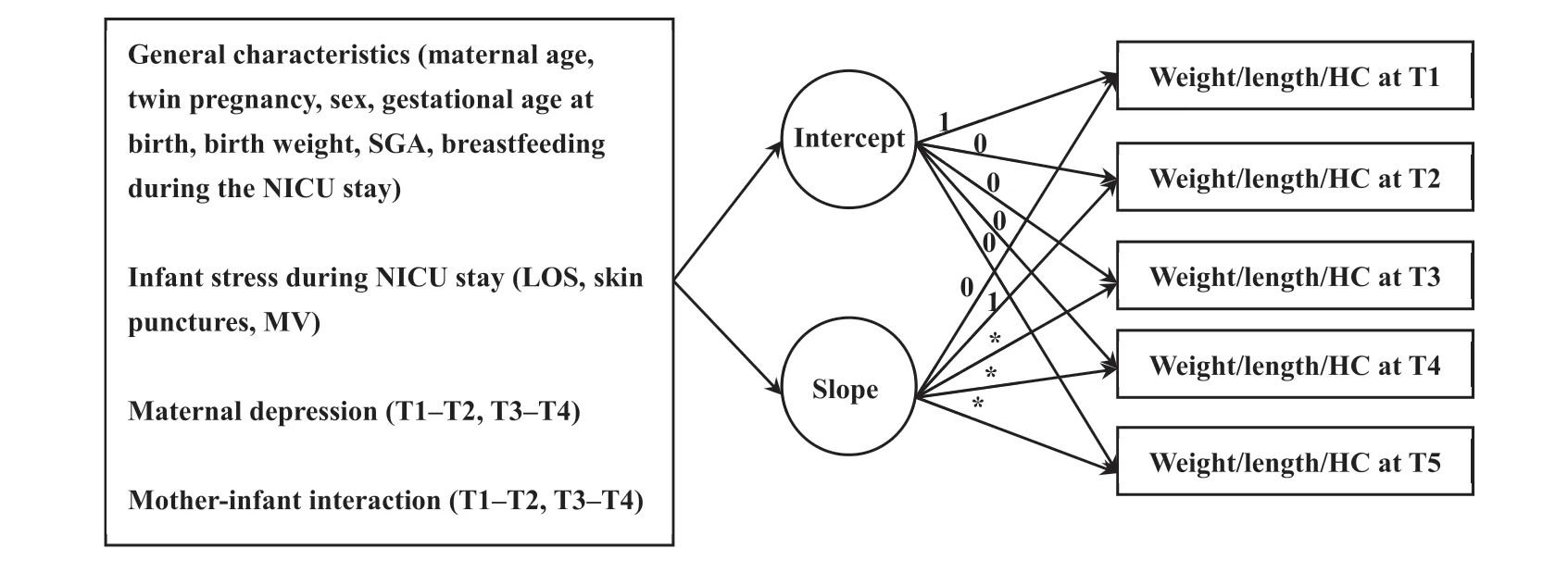
Fig.2.The measurement of conditional Latent Growth Models with covariates for the trajectories of weight,length,and head circumference of preterm infants.The covariates were maternal age,twin pregnancy,sex,gestational age at birth,birth weight,SGA,breastfeeding during the NICU stay,LOS,skin punctures,MV,maternal depression during T1-T2 and T3-T4,and mother-infant interaction during T1-T2 and T3-T4.For the conditional LGM of weight,the slope factor loadings were set as 0,1.0,1.8,2.4,and 2.9 at T1-T5,respectively;for the length model,the slope factor loadings were set as 0,1.0,2.0,2.7,and 3.3 at T1-T5,respectively;for the HC model,the slope factor loadings were set as 0,1.0,2.0,2.7,and 3.2 at T1-T5,respectively.Abbreviation:HC=head circumference.LGM=Latent Growth Model.LOS=length of stay in the NICU.MV=mechanical ventilation.SGA=small for gestational age infant.T1=40 weeks of corrected gestational age.T2=1 month of corrected postnatal age.T3=2 months of corrected postnatal age.T4=3 months of corrected postnatal age.T5=4 months of corrected postnatal age.
In this study,each preterm infant experienced 20 skin punctures as painful operations during the mean 14.2-day NICU stay,which translates to 1.4 skin punctures per day.Compared with the frequencies,5[30]and 10[31]painful operations,for each infant per day in previous studies,we found that the average frequency of painful operations in this cohort was lower due to the lack of records of repeated punctures.We found that infants with a shorter LOS were likely to perform better in the weight status at 40 weeks corrected age.In addition,the results showed that frequent skin punctures were a risk factor that influenced the weight status at 40 weeks corrected age for preterm infants because worse growth was observed in infants with more skin punctures during the NICU stay.These findings can be explained from two perspectives.Firstly,those preterm infants with a longer LOS or experiencing more skin punctures might have a greater demand for and consumption of nutrition to recover from their serious diseases and unstable physiological conditions,which might lead to worse nutritional growth.The second perspective is supported by a behavioral epigenetics model,wherein early-life cumulative exposure to stress resulted in epigenetic modifications of glucocorticoid-related genes and changes in cortisol reactivity that might disrupt the neurobehaviors that are critical for the achievement of oral feeding skills[32].
During follow-up investigations,potential factors,including maternal postpartum depression and mother-infant interaction,were mainly evaluated.As suggested by the results,a risk of slower growth rates in weight,head circumference,or length was noted for preterm infants whose mothers had depressive symptoms,and the incidence of maternal postpartum depression in this study was high.To the best of our knowledge,few studies have revealed the impact of maternal postnatal depression on the dynamic change of early growth among preterm infants,as most studies only evaluated the effect of maternal postnatal depression on the growth status at specific time points.These findings were most likely attributable to two main factors.Firstly,it was suggested that mothers with postpartum depression have lower serum prolactin levels [33],which may result in reduced lactation and supply for breastfeeding.Secondly,mothers with postpartum depression are more likely to develop worse infant-feeding behaviors and outcomes due to their lower sensitivity.A recent study[34]supported the negative impact of maternal postpartum depression on responsive feeding,which involves identifying and responding to infant cues of hunger or satiety.Moreover,numerous studies have demonstrated that mothers with depression might have shorter breastfeeding duration for their lack of confidence and self-efficacy[35,36].Another important follow-up factor is the mother-infant interaction.As expected,preterm infants with lower-quality of mother-infant interactions showed slower weight gain,which is similar to the finding in normal infants[37].Though we did not find clear evidence for this relationship,it can be partly explained by the shortened duration of breastfeeding that was related to worse mother-infant interaction [38].As mentioned earlier,the physical growth of preterm infants might be associated with successful feeding due to effective mother-infant interactions in the feeding context.A randomized controlled trial [39] revealed that the specific intervention conducted during a feeding interaction significantly improved maternal sensitivity,which was thought to lead to better infant outcomes.In this study,the 10-item MABIS still lacks in interaction details,and the discussion on the mechanism requires more nuanced microscopic observations to provide a basis for the further intervention.
A few demographic and medical characteristics contributed to the physical growth trajectories of preterm infants,and maternal age,the infant’s sex,gestational age,and birth weight were the major independent predictors.It is indicated that premature infants with younger mothers were likely to exhibit smaller weight,length and head circumference changes at 40 weeks corrected age,which is consistent with the results reported from other studies[40].This finding may be attributed to the fact that young mothers are likely to have difficulties feeding and caring for babies compared with experienced and well-prepared older mothers.Another possible explanation was that the higher fat content in the breastmilk of older mothers (age ≥35 years) may be more beneficial to growth[41].Furthermore,we found that male infants grew faster in all three aspects of physical growth than female infants in the 4 months of corrected age duration,which is similar to the result from another study [41],indicating the extensive impact of sex from birth to discharge.The difference in preterm infant sex should be taken into account when developing a future trend of sex-specific nutritional intervention and guidance [42].Another finding was unexpected and suggested that the levels of body weight,length,and head circumference for preterm infants at 40 weeks corrected age were negatively associated with decreased gestational age at birth.A multicenter study [4] revealed that a higher incidence of extrauterine growth restriction was associated with higher gestational age.This finding might be partly related to the nutrition-enhancing interventions that are conducted more frequently as well as body growth monitoring during follow-up for those preterm infants with a lower gestational age at birth,which resulted in the poorer growth performance of preterm infants who were born at a higher gestational age.Therefore,it is necessary to remain attentive to this group of preterm infants with a nearly fullterm gestational age.Furthermore,we found that birth weight was positively associated with the initial level of the growth trajectory,and this finding was in agreement with the result of a previous study [43].The above-mentioned demographic and medical characteristics may facilitate the identification of preterm infants who are at a high risk of delayed growth.
5.Limitations
As this longitudinal study was conducted prospectively during a period overlapping the coronavirus disease outbreak,there were missed visits that cannot be ignored and may have affected the reliability and stability of the results.Additionally,there are some limitations in the selection of outcome measures.Firstly,we employed only three indices,including LOS,skin punctures,and MV,for the evaluation of infant stress during the NICU stay;this neglected other invasive operations,such as gastric tube retention,which can increase the infant exposure stressors.Secondly,the detailed data on skin punctures in our study were collected from medical records,where repeated punctures would have been missed and cannot be taken into account.As the family-centered units have shown significant influences on feeding practices and good outcomes in infant recovery and development,we cannot deny the potential impact of mother-infant interaction during parental engagement in the NICU.In this study,during follow-ups,we only focused on mother-infant interaction in the context of the family due to the restriction of parental visits and engagement during the coronavirus disease outbreak.Moreover,we did not consider maternal postpartum depression and mother-infant interaction as time-varying covariates.Studies of the dynamics of maternal postpartum depression and mother-infant interaction over time and their impact on the growth of preterm infants are needed to explore the underlying mechanisms that deeply influence this relationship.
6.Conclusion
In summary,according to the WHO growth criteria,the physical development of preterm infants who were released from the NICU did not lag behind that of term infants at the corrected age of 4 months.We found that infant stress during the NICU stay,maternal postpartum depression and mother-infant interaction affected the physical growth trajectories of preterm infants at 4 months of corrected age.We stressed the need for less stressful care in the NICU to prevent harm to the nutritional status and growth of preterm neonates.In addition,it is essential to add the screening of maternal postpartum depression and guidance for parental skills related to mother-infant interaction to safeguard the physical growth of preterm infants.
Funding
This study was funded by a research project from the Shanghai Municipal Health Commission (No.201940056 &No.202040024),Clinical Research Plan of Shanghai Hospital Development Center(No.SHDC2020CR4098),Nursing Development Program from Shanghai Jiao Tong University School of Medicine,and Pilot Educational Project for Application-Oriented Nursing Baccalaureate Program (No.HLDC21-06).
CRediT authorship contribution statement
Wenying Gao:Conceptualization,Methodology,Validation,Formal analysis,Investigation,Data curation,Writing -original draft,Writing -review &editing.Taomei Zhang:Conceptualization,Methodology,Validation,Formal analysis,Investigation,Data curation,Writing -review &editing,Supervision,Project administration.Qihui Wang:Conceptualization,Methodology,Investigation,Resources,Data curation,Writing -review &editing.Xiaoli Tang:Conceptualization,Methodology,Writing-Review&Editing,Supervision,Funding acquisition.Ying Zhang:Conceptualization,Methodology,Validation,Formal analysis,Writing -Review &Editing,Supervision,Project administration,Funding acquisition.
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the privacy and ethical restrictions but are available from the corresponding author upon reasonable request.
Declaration of competing interest
The authors have declared no conflict of interest.
Acknowledgments
We would like to thank the paediatricians,nurses and staffs who completed measurements and recordings of physical growth for preterm infants.Moreover,we would like to appreciate the support of all the mothers who cooperated with the investigation.
Appendices.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2023.03.016.
杂志排行
International Journal of Nursing Sciences的其它文章
- A feasibility study on home-based kyphosis-specific exercises on reducing thoracic hyperkyphosis in older adults
- Development and validation of dynamic nomogram of frailty risk for older patients hospitalized with heart failure
- Validation of the Portuguese version of the social isolation scale with a sample of community-dwelling older adults
- Effects of pre-operative education tailored to information-seeking styles on pre-operative anxiety and depression among patients undergoing percutaneous coronary intervention: A randomized controlled trial
- Factors influencing the quality of sexual life in the older adults: A scoping review
- Comparison of the effects of three kinds of hand exercises on improving limb function in patients after transradial cardiac catheterization
