蓝藻生物活性提取物的研究进展
2023-04-29田龙龙张闻定金小霞冯杰李婷婷
田龙龙 张闻定 金小霞 冯杰 李婷婷

摘要:蓝藻又称蓝绿藻,是可以进行光合作用的原核生物,被认为是新的生物活性物质的重要来源。蓝藻的多种天然产物在抗菌、杀虫、抗病毒以及抗癌等方面展现出不同的生物活性。本文介绍了抗菌、杀虫、抗病毒、抗癌等蓝藻提取物的研究进展,以期为蓝藻活性产物的进一步研究与开发提供帮助和新思路。
关键词:蓝藻;天然产物;抗菌;杀虫;抗病毒;抗癌
中图分类号:R978.1 文献标志码:A 文章编号:1001-8751(2023)03-0202-09
Research Progress of Bioactive Extracts from Cyanobacteria
Tian Long-long1, Zhang Wen-ding1, Jin Xiao-xia1, Feng Jie1,2, Li Ting-ting1
(1 School of Basic Medicine, Lanzhou University, Lanzhou 730000;
2 State Key Laboratory of Veterinary Etiological Biology,
College of Veterinary Medicine, Lanzhou University, Lanzhou 730000)
Abstract: Cyanobacteria, also known as blue-green algae, are photosynthesizing prokaryotes and are considered to be in great potential for providing substances with unique bioactivities. In this paper, the research progress of antibacterial, insecticidal, antiviral and anticancer extracts of cyanobacteria was reviewed, in order to provide help and new ideas for further research and development of cyanobacteria active products.
Key words: cyanobacteria; natural product; antibacterial; disinsection; antiviral; anticancer
蓝藻是可以进行光合作用的原核生物,又称蓝绿藻或蓝细菌[1]。大多数蓝藻的细胞壁外面有胶质衣,因此又名粘藻。蓝藻主要包括蓝球藻、颤藻、念珠藻和发菜等。蓝藻在自然界中分布十分广泛,可在多种环境中生存,在生态系统中发挥着独特的作用[2]。蓝藻营养需求低,易于培养,某些菌株具有固氮的能力[3]。蓝藻富含肽、蛋白质、脂质、维生素、色素、碳水化合物、萜类化合物、多不饱和脂肪酸、类黄酮、酚类化合物等多种化合物,是潜在的新型生物活性化合物的“生产工厂”[4]。蓝藻所产的多种活性物质在抗菌、杀虫、抗病毒、抗癌中都有极大的应用前景[5]。
细菌、寄生虫、病毒感染以及肿瘤细胞侵袭严重危害着人类健康,同时由于耐药问题,现有的抗感染、抗癌药物治疗效果有不同程度的下降。随着抗菌药物的大量使用,细菌耐药的问题日趋严重,2022年世界卫生组织报告显示,大肠埃希菌对环丙沙星的耐药率高达92.9%,肺炎克雷伯菌对环丙沙星的耐药率高达79.4% [6]。寄生虫感染引起的疾病也在全球范围蔓延,2019年至2020年期间,疟疾感染人数达1400万,死亡人数达6.9万[7]。然而传统使用的抗疟药物已出现了耐药问题[8]。病毒也对人类健康造成了巨大的挑战,2020年全球艾滋病死亡人数约150万[9],埃博拉病毒(Ebola virus,EBOV)可引起致死性的中风、心肌梗塞、低血容量休克或多发性器官衰竭,患者病死率高达50%至90%[10],严重急性呼吸系统综合征冠状病毒2型(Severe acute respiratory syndrome coronavirus 2,SARS-CoV-2)导致约660万人死亡[11]。此外,肿瘤细胞侵袭是人类死亡的主要原因,而且新型癌症的发病率正在迅速增加,但肿瘤细胞对目前可用的药物(如长春花生物碱和紫杉烷类)产生耐药[12],因此也需要开发新的抗癌药物。综上所述,随着细菌、寄生虫、病毒以及肿瘤细胞对药物的耐药问题日趋严重,开发新的抗感染、抗癌药物迫在眉睫,然而活性化合物资源枯竭导致新药的研发速度越来越慢。目前的研究已从蓝藻中分离发现了多种具有抗感染及抗癌活性的化合物[13],蓝藻有望成为活性化合物药物开发的新来源。本综述将对蓝藻天然产物的抗菌、杀虫、抗病毒以及抗癌作用的最新研究成果进行归纳和总结,以利于进一步研究和开发蓝藻活性物质。
1 蓝藻提取物的抗菌及抗炎活性研究
抗菌肽(Antimicrobial peptides , AMPs)是一类具有抗菌活性的多肽。研究表明,它们主要通过破坏细菌细胞膜完整性对细菌起到杀伤作用[14-15]。从淡水菌株鞘丝藻(Lyngbya sp.)中鉴定出的环肽Lyngbyazothrins C和Lyngbyazothrins D对革兰阴性菌(大肠埃希菌、铜绿假单胞菌和黏质沙雷菌)和革兰阳性菌枯草芽孢杆菌都具有杀菌作用[16]。研究者还从鞘丝藻属中发现了Pahayokolide A和Pitipeptolide A-F这七种抗菌肽[17]。Pahayokolide A可以抑制枯草芽孢杆菌、巨大芽孢杆菌、蜡样芽孢杆菌的生长[18]。Pitipeptolide A-F对结核分枝杆菌有杀菌作用[17],2020年,全球范围内约有1000万人罹患结核病,估计有150万人死于结核病[9],而Pitipeptolide A-F有望进一步开发结核病防治新药物。相较于化学合成的抗菌肽,蓝藻产生的多肽绿色环保,合成成本低。另外,与核糖体多肽相比,蓝藻产生的非核糖体多肽常被非核糖体肽合成酶修饰,结构更稳定,不易被蛋白酶降解,不易产生耐药[15]。且蓝藻营养需求低,易于培养,能够产生的肽类丰富,合成可塑性强 ,因此蓝藻有望成为更好的抗菌肽新来源。
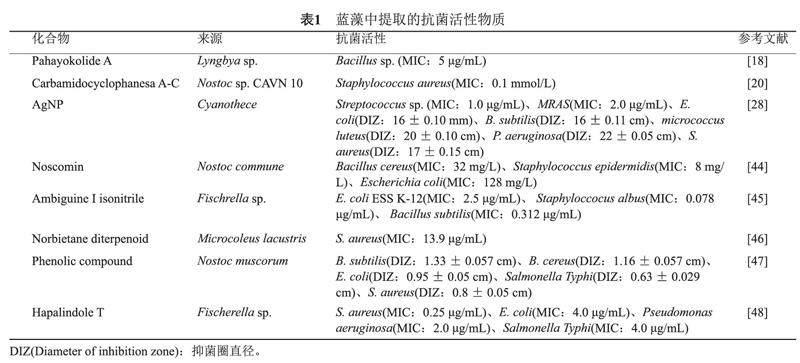
我们对多种来源于念珠藻(Nostoc sp.)、费氏藻(Fischrella sp.)和鞘丝藻的抗菌活性物质进行了总结(见表1)。近年来,耐甲氧西林金黄色葡萄球菌(Methicillin-resistant Staphylococcus aureus,MRSA)的感染不断增多,2020年全国细菌耐药监测报告显示MRSA全国平均检出率为29.4%[19]。而从念珠藻 CAVN 10中分离出来的Carbamidocyclophanes对MRSA表现出抗菌活性,其最小抑制浓度MIC为0.1 mmol/L[20]。此外,研究者利用丝状蓝藻VPG 16-59的天然产物合成了新的抗菌药物Anaephenes A和B,该药物对MRSA也具有抗菌活性(MIC:8 μg/mL)[21]。这些化合物可能为MRSA的临床治疗提供新的方案。据报道,从费氏藻属中分离出的Ambiguine-I isonitrile对枯草芽孢杆菌和白葡萄球菌表现出比链霉素更强的抗菌活性,其MIC分别为0.312 μg/mL、0.078 μg/mL[5]。此外,研究人员对蓝藻提取物的活性成分进一步分析,发现蓝藻产生的脂质α-亚麻酸(α-Linolenic acid)对金黄色葡萄球菌具有抗菌活性(MIC:15.6 mg/L)[22]。
另一方面,目前发现的300多种海洋蓝藻生物碱中有很多非核糖体多肽或混合聚酮—非核糖体多肽生物合成途径的产物[15],通过该途径合成的天然产物包括许多目前临床使用的药物,例如抗菌药物万古霉素(糖肽类)、免疫抑制剂环孢霉素(非核糖体多肽)和抗癌剂博来霉素(糖肽类)等[23]。海洋蓝藻中这些独特的天然产物的发现,为此类新药开发提供了除放线菌和真菌以外的又一重要来源。
不同于传统的药物,金属纳米颗粒是一种新型抗菌材料。金属纳米颗粒的主要抗菌机制是破坏细胞膜或细胞壁以及产生活性氧使蛋白质和核酸等生物大分子失活[24]。在金属和金属氧化物纳米颗粒中,纳米银粒子(Argentum nanoparticles,AgNP)对各种病原体特别是耐药细菌表现出独特的抗菌活性[25]。金属银离子可以在蓝藻细胞内与藻蓝蛋白结合形成AgNP,也可以在蓝藻细胞外与藻类多糖结合形成AgNP[26]。相比于传统纳米材料,蓝藻AgNP体积更大,更易于提取使用[27]。蓝藻合成的AgNP对巨大芽孢杆菌、大肠埃希菌、枯草芽孢杆菌、金黄色葡萄球菌、铜绿假单胞菌和MRSA均具有抗菌作用[26,28]。在一项比较研究中,利用海洋蓝藻Phormidium formosum合成的AgNP 对金黄色葡萄球菌的抑菌圈值为2.2 cm,而抗菌药物头孢丙烯与多黏菌素对金黄色葡萄球菌的抑菌圈值分别为1.5 cm及2.0 cm [29],蓝藻AgNP表现出更好的抗菌效果,由此可见蓝藻AgNP的抗菌优势。
广谱β-内酰胺酶(Extended-spectrum β-lactamases,ESBL)是一类能水解多种青霉素类、头孢菌素类以及单环β-内酰胺类抗菌药物的酶。ESBL(+)菌可对上述抗菌药物产生广泛的耐药性,从而导致难以治愈的菌血症、腹腔内感染、尿路感染和呼吸道感染[30]。5%至25%的呼吸机相关性肺炎患者感染ESBL(+)菌后,会影响治疗、预后甚至危及生命[31]。研究人员从海洋蓝藻Accillatoria acuminata NTAPC05中提取的生物活性物质MGDG-palmitoyl对ESBL(+)的大肠埃希菌、嗜麦芽窄食单胞菌和阿氏肠杆菌B938的最低杀菌浓度MBC均为100 μg/mL,表现出较高的杀菌活性[32],这为抗ESBL(+)菌的药物开发提供了新的方向。
此外,具有杀细菌、杀真菌作用的蓝藻活性化合物也在开发外用杀菌剂以及农业杀菌剂方面有巨大的优势和应用前景。一项研究发现鱼腥藻属(Anabaena)蓝藻胞外蛋白酶对念珠镰刀菌等植物病原真菌具有杀菌作用,可以作为植物病原真菌生物防治剂[33]。从蓝藻中提取的Cryptophycin是对抗隐球菌的有效杀菌剂,目前已投入使用[34]。蓝藻产生的杀菌化合物凭借高活性、易分解的特点,在有效杀灭细菌和真菌的同时能够避免杀菌剂残留,防止毒性物质在食物链中的富集和扩散。而且由于蓝藻营养需求低,无须添加碳源,易于大规模培养,利用蓝藻生产天然杀菌剂的方法明显优于昂贵且不环保的化学合成方法[35]。
蓝藻活性物质不仅具有抗菌活性,还可以调节炎症反应。例如,海洋蓝藻产物中的脂多糖分子CyP可以调节细菌感染引起的炎症。人体细胞中的Toll样受体4(Toll-like receptor 4,TLR4)和MD-2受体可以通过识别革兰阴性菌的脂多糖(Lipopolysaccharide,LPS)激活机体免疫应答[36-37],合成促炎因子,诱发机体产生炎症[38]。CyP可与LPS竞争性结合MD-2受体,进而抑制LPS与TLR4-MD-2受体复合物结合[36],从而选择性抑制TLR4通路介导的细胞因子表达和一氧化氮(NO)的产生,调节炎症反应。牙龈卟啉单胞菌的LPS(Pg-LPS)能够促进牙周的慢性炎症。研究发现,CyP可以抑制Pg-LPS诱导的炎症,具体作用机制是阻断人单核细胞中TLR4-MD-2通路,促进miR-146a(促炎反应的负调节因子)的表达,从而抑制肿瘤坏死因子(Tumor necrosis factor, TNF)-α、白细胞介素(Interleukin,IL)-1β和IL-8等促炎因子生成[39]。牙龈卟啉单胞菌也在口腔癌的发病中发挥作用[40],CyP的上述抗炎活性可能对牙龈卟啉单胞菌相关的口腔癌的治疗具有重大意义。又如,Malyngamides是一类衍生自海洋蓝藻鞘丝藻属巨大鞘丝藻(Lyngbya majuscula)的酰胺类化合物,该类化合物选择性地抑制TLR4和TNF-α介导的髓样分化因子依赖性通路中的蛋白质的活化和磷酸化,抑制了IL-1β、IL-6、IL-10、TNF-α和诱导型一氧化氮合酶转录,进而抑制巨噬细胞中NO等免疫因子参与的炎症反应[40-41]。鞘丝藻酰胺的抑制炎症作用使其成为潜在的免疫调节剂。从海洋蓝藻鞘丝藻属获得的生物活性物质Grassystatins A可以与外源性抗原(如破伤风毒素)竞争性结合主要组织相容性复合体Ⅱ类分子,阻碍外源性抗原进入辅助性T淋巴细胞,从而抑制T细胞的增殖,抑制炎性细胞因子(IL17和干扰素-γ)的生成,减轻炎症反应[42-43]。
2 蓝藻提取物的杀虫活性研究
2.1 蓝藻提取物的杀伤寄生虫活性研究
从蓝藻菌株中发现的多种生物活性物质如Viridamide A、Venturamides、Dragomabin、Ambigol C可以有效杀伤寄生虫(见表2)。研究发现Nostocarboline及其二聚体是恶性疟原虫的有效抑制剂,其半数抑制浓度(IC50)为194 nmol/L[49],此外,该化合物对布氏锥虫、克氏锥虫、杜氏利什曼原虫均表现出杀虫作用,且对大鼠L6细胞毒性极弱,有良好的药物开发的潜力[49-50]。Clark等[50]从海洋蓝藻中分离出6种新的酰基脯氨酸衍生物Tumonoic acids D-I均在抗疟试验中有效,其中,Tumonoacid I效果最好(IC50:2 μmol/L)。从蓝藻束藻属(Symploca sp.)获得的Symplocamide A也对疟原虫有杀虫效果(IC50:0.95 μmol/L)[51]。此外,Sanchez等[52]从巨大鞘丝藻中分离并鉴定出了一系列脂肽Almiramids A-C,这些脂肽在体外实验中杀利什曼原虫作用与杀锥虫药物硝呋替莫相同(IC50:3.0 μmol/L)。2015年,研究者从热带蓝藻Okeania hirsuta中分离出一种新的化合物Bastimolide A,它对氯喹、甲氟喹、乙胺嘧啶和阿托伐醌耐药的疟原虫有抑制活性[53],有望用于抗耐药恶性疟原虫的药物开发。
2.2 蓝藻提取物杀伤蚊虫活性的研究
蚊子作为疟原虫虫媒可以传播疟疾,2021年世界范围内通过按蚊传播的疟疾导致约61.9万人死亡[54]。研究发现蓝藻活性物质可以对多种蚊子起到强效杀虫作用,进而减少疟原虫等寄生虫的传播。研究筛选了多种蓝藻分离株及其提取物,发现蓝藻Westiellopsis sp.提取物可以杀伤多种蚊子幼虫,例如埃及伊蚊及致倦库蚊等[55]。蓝藻费氏藻ATCC 43239产生的吲哚生物碱Hapalindole在低剂量下对摇蚊幼虫表现出杀伤作用[56]。来自鞘丝藻属的化合物Pahayokolides对蚊子幼虫也有杀伤作用[35, 57]。此外,从项圈藻(Anabaenopsis)和微囊藻属(Microcytis sp.)的蓝藻中获得的蓝藻毒素(Cyanobacterial toxins)、柱孢藻毒素(Cylindrospermopsin)和微囊藻毒素(Microcystins)等对蚊子幼虫的杀灭率超过50%[35]。不同于传统农药,部分蓝藻毒素与微囊藻毒素属于肽类化合物,这些化合物容易被水解,在环境中不易长期残留[58],因此该类化合物在杀灭蚊子幼虫的同时能够有效避免毒素残留,属于环境友好型杀虫剂。
3 蓝藻提取物抗病毒活性的研究
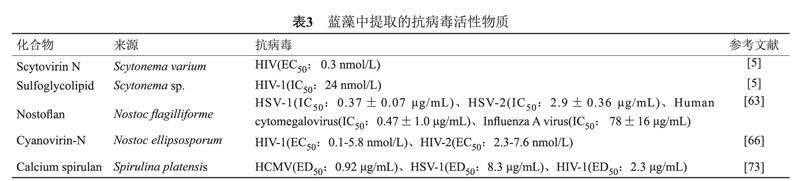
蓝藻提取物对人类免疫缺陷病毒(Human immunodeficiency virus,HIV)、单纯疱疹病毒(Herpes simplex virus,HSV)、丙型肝炎病毒(Hepatitis C virus,HCV)、EBOV和一系列其他包膜病毒显示出抗病毒活性[63-66](见表3)。从念珠藻中提取的两种糖结合蛋白,蓝藻抗病毒蛋白N(Cyanovirin-N,CV-N)和Scytovirin N(SVN)有望成为新型的杀病毒药物。CV-N在体外和体内均表现出抗HIV和其他慢病毒的活性[67-68]。此类糖结合蛋白可以干扰病毒感染细胞过程中的多个步骤,如通过干扰HIV gp120蛋白与CD4+受体、靶细胞趋化因子受体的结合来抑制HIV病毒与CD4+细胞的吸附[67]。CV-N在发挥抗HIV病毒作用的同时对人体毒性小,有望开发成为抑制HIV性传播的阴道凝胶,从而减少HIV-1的全球传播[69]。此外,CV-N还被发现可在体外抑制单纯疱疹病毒-6与靶细胞的吸附;抑制麻疹病毒的包膜糖蛋白与血凝素的结合;强烈阻断猫类免疫缺陷病毒感染免疫细胞[67]。CV-N具有SARS-CoV-2的S糖蛋白的聚糖类型表征,利用分子对接和分子动力学模拟研究发现CV-N与SARS-CoV-2 S糖蛋白对接时形成了稳定的分子间氢键[70],被认为是SARS-CoV-2的潜在阻断剂[71]。SVN具有广泛的抗病毒活性,其主要机制是SVN与许多病毒的包膜糖蛋白上富含甘露糖的寡糖具有高亲和力,可以与靶细胞上的受体分子竞争性结合病毒的包膜糖蛋白,从而阻止病毒进入靶细胞[65]。从伪枝藻属(Scytonema varium)中提取分离出来的Scytovirin N可以与HIV的包膜糖蛋白(gp120、gp160和gp41)结合,在低浓度下就可使HIV病毒失活[69]。研究发现SVN可以与扎伊尔型埃博拉病毒(Zaire Ebola virus,ZEBOV)包膜糖蛋白上的黏蛋白区域特异性结合,抑制ZEBOV复制[65]。来自蓝藻伪枝藻属的天然硫糖脂也可以抑制HIV逆转录酶和DNA聚合酶的活性[72],从而起到抗HIV病毒的作用。
从蓝藻钝顶螺旋藻(Spirulina platensis)中分离出的Calcium spirulan(Ca-SP)是一种具有广谱抗病毒活性的硫酸化多糖,可强烈抑制多种人类病毒如人巨细胞病毒(Human cytomegalovirus,HCMV)、HSV-1、麻疹病毒、腮腺炎病毒、甲型流感病毒和HIV-1的复制[73-74]。Ca-SP可抑制病毒附着并入侵宿主细胞;还可以抑制感染HIV和未感染HIV的CD4 +淋巴细胞之间的融合,从而极大减弱病毒的传染性[63]。Hayashi等[74-75]评估了Ca-SP对HIV-1和HSV-1的抗病毒潜力,发现使用Ca-SP的艾滋病模型小鼠的血清样品在24 h后没有观察到病毒诱导的合胞体形成。相较于其他硫酸化多糖,Ca-SP抗病毒效果强,抗凝血活性低,药物动力学半衰期更长,Ca-SP可以作为抗HIV治疗药物的候选药物。此外,从念珠藻中分离出的抗病毒多糖Nostoflan可以抑制病毒与细胞的相互作用,对HSV-1、HSV-2、人巨细胞病毒和甲型流感病毒显示出有效的抗病毒活性[63]。
4 蓝藻提取物的抗癌及抗肥胖活性研究
有研究显示,从念珠藻属GSV224中分离出来的念珠藻素-1可以干扰微管的组装,导致有丝分裂停滞,阻断G2或M期的细胞增殖,诱导癌细胞死亡[34]; 现已发现它对KB人鼻咽癌细胞、人结直肠癌LoVo细胞、阿霉素耐药的M17乳腺癌细胞系和DMS 273肺癌细胞系均表现出有效的抗癌活性[34]。化合物Curacin A首先由Gerwick等从鞘丝藻属巨大鞘丝藻中分离出来[76],该化合物可导致真核细胞的有丝分裂停滞[77],未来有成为抗癌药物的潜力。从束藻属中分离出来的海兔毒素-10是有效的抗细胞增殖剂[78-79],海兔毒素-10的类似物TZT-1027在小鼠癌症模型中可以有效抑制癌细胞的增殖[80]。目前,海兔毒素类似物ILX-651已成功完成I期临床试验[75]。Apratoxin A是从鞘丝藻属中分离出的环肽,已发现其可诱导人癌细胞的细胞周期停滞和凋亡[81];Somocystinamide A是从海洋蓝藻鞘丝藻属巨大鞘丝藻中分离出来的化合物,可以破坏内皮细胞增殖和内皮细胞小管形成,导致真核细胞的有丝分裂停滞,抑制癌细胞增殖[77]。
研究发现,通过抑制蛋白酶的活性可抑制人癌细胞的生成并诱导细胞凋亡,且对正常细胞的毒性较小[82],因此多种蛋白酶(如胰蛋白酶-3)被认为是癌症治疗的靶点[83],蛋白酶抑制剂在抗癌领域有良好前景。已经有人发现了一些蓝藻来源的蛋白酶抑制剂,如圆柱菌素、铜氯菌素[84-85]。来自圆柱菌素的柱胞藻毒素(Cylindrospermopsin,CYN)是由许多淡水蓝藻产生的一种聚酮类化合物。CYN可以诱导DNA聚合物形成、DNA链断裂,引起纺锤体破坏,导致染色体非整倍性丢失,进而抑制细胞有丝分裂,抑制癌细胞增殖[85]。胰蛋白酶-3可以介导前列腺癌细胞的增殖和转移[86],来自铜氯菌素的Suomilide可以抑制胰蛋白酶-3的产生,进而抑制前列腺癌细胞的增殖,同时不影响正常前列腺细胞的增殖[87],可以作为前列腺癌治疗的候选药物进行进一步的开发。
肥胖被认为是一种低度炎症状态[88]。钝顶螺旋藻中的藻蓝蛋白和β-胡萝卜素是有效的抗炎和抗氧化成分,可以通过清除自由基和活性氧来抑制促炎细胞因子(IL-2、TNF-α)的释放,进而降低血脂、减少脂肪因子的生成,从而有效改善肥胖或超重[89-90]。目前蓝藻中的螺旋藻已被开发为膳食补充剂(如螺旋藻片)用于改善肥胖[91-92],未来可能有更多从蓝藻中开发的改善肥胖的药物走向市场。
5 小结与展望
蓝藻是天然活性化合物的“合成工厂”和资源宝库,从蓝藻中已发现了丰富的抗菌、杀虫、抗病毒、抗癌及抗肥胖活性化合物。对蓝藻生物活性化合物资源的进一步发掘和研究预期将发现更多的高效、低毒、低成本的新药物前体,为人类的生命健康作出贡献。
参 考 文 献
Singh J S, Kumar A, Rai A N, et al. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability[J]. Front Microbiol, 2016, 7: 529.
Lin J Y, Tan S I, Yi Y C, et al. High-level production and extraction of C-phycocyanin from cyanobacteria Synechococcus sp. PCC7002 for antioxidation, antibacterial and lead adsorption[J]. Environ Res, 2022, 206: 112283.
Fay P. Oxygen relations of nitrogen fixation in cyanobacteria[J]. Microbiol Rev, 1992, 56(2): 340-373.
Khalifa S A M, Shedid E S, Saied E M, et al. Cyanobacteria-from the oceans to the potential biotechnological and biomedical applications[J]. Mar Drugs, 2021, 19(5): 241.
Singh R K, Tiwari S P, Rai A K, et al. Cyanobacteria: an emerging source for drug discovery[J]. J Antibiot (Tokyo), 2011, 64(6): 401-412.
Geneva. Antimicrobial resistance fact sheet.[EB/OL]. [2022-5-3] https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
World malaria report Geneva: World Health Organization[EB/OL]. [2022-5-3] https://www.who.int/publications/i/item/9789240040496.
Wicht K J, Mok S,Fidock D A. Molecular mechanisms of drug resistance in plasmodium falciparum malaria[J]. Annu Rev Microbiol, 2020, 74: 431-454.
Organization W H. Global tuberculosis report Geneva: World Health Organization [EB/OL]. [2022-5-3] https://apps.who.int/iris/rest/bitstreams/1398397/retrieve.
Jacob S T, Crozier I, Fischer W A, et al. Ebola virus disease[J]. Nat Rev Dis Primers, 2020, 6(1): 13.
Weekly epidemiological update on COVID-19 - 30 November 2022[EB/OL]. [2022-11-30] https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-30-november-2022.
Haider T, Pandey V, Banjare N, et al. Drug resistance in cancer: mechanisms and tackling strategies[J]. Pharmacol Rep, 2020, 72(5): 1125-1151.
Dixon R A, Al-Nazawi M, Alderson G. Permeabilizing effects of sub-inhibitory concentrations of microcystin on the growth of Escherichia coli[J]. FEMS Microbiol Lett, 2004, 230(2): 167-170.
Lazzaro B P, Zasloff M,Rolff J. Antimicrobial peptides: application informed by evolution[J]. Science, 2020, 368(6490): eaau5480.
Rojas V, Rivas L, Cárdenas C, et al. Cyanobacteria and eukaryotic microalgae as emerging sources of antibacterial peptides[J]. Molecules, 2020, 25(24): 5804.
Zainuddin E N, Jansen R, Nimtz M, et al. Lyngbyazothrins A-D, antimicrobial cyclic undecapeptides from the cultured cyanobacterium Lyngbya sp.[J]. J Nat Prod, 2009, 72(8): 1373-1378.
Xue Y, Zhao P, Quan C, et al. Cyanobacteria-derived peptide antibiotics discovered since 2000[J]. Peptides, 2018, 107: 17-24.
Berry J P, Gantar M, Gawley R E, et al. Pharmacology and toxicology of pahayokolide A, a bioactive metabolite from a freshwater species of Lyngbya isolated from the Florida Everglades[J]. Comp Biochem Physiol C Toxicol Pharmacol, 2004, 139(4): 231-238.
2020年全国细菌耐药监测报告(简要版)[EB/OL]. [2021-11-17] http://www.carss.cn/Report/Details/808.
Bui H T, Jansen R, Pham H T, et al. Carbamidocyclophanes A-E, chlorinated paracyclophanes with cytotoxic and antibiotic activity from the vietnamese cyanobacterium Nostoc sp.[J]. J Nat Prod, 2007, 70(4): 499-503.
Kukla D L, Canchola J,Mills J J. Synthesis of the Cyanobacterial Antibiotics Anaephenes A and B[J]. J Nat Prod, 2020, 83(6): 2036-2040.
Cepas V, Gutiérrez-Del-Río I, López Y, et al. Microalgae and cyanobacteria strains as producers of lipids with antibacterial and antibiofilm activity[J]. Mar Drugs, 2021, 19(12): 675.
Schwarzer D, Finking R, Marahiel M A. Nonribosomal peptides: from genes to products[J]. Nat Prod Rep, 2003, 20(3): 275-287.
Karthikeyan C, Tharmalingam N, Varaprasad K, et al. Biocidal and biocompatible hybrid nanomaterials from biomolecule chitosan, alginate and ZnO[J]. Carbohydr Polym, 2021, 274: 118646.
Alavi M. Bacteria and fungi as major bio-sources to fabricate silver nanoparticles with antibacterial activities[J]. Expert Rev Anti Infect Ther, 2022, 20(6): 897-906.
Patel V, Berthold D, Puranik P, et al. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity[J]. Biotechnol Rep (Amst), 2015, 5: 112-119.
Brayner R, Barberousse H, Hemadi M, et al. Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route[J]. J Nanosci Nanotechnol, 2007, 7(8): 2696-2708.
El Semary N A,Bakir E M. Multidrug-resistant bacterial pathogens and public Health: The antimicrobial effect of cyanobacterial-biosynthesized silver nanoparticles[J]. Antibiotics (Basel), 2022, 11(8): 1003.
Elkomy R G. Antimicrobial screening of silver nanoparticles synthesized by marine cyanobacterium Phormidium formosum[J]. Iran J Microbiol, 2020, 12(3): 242-248.
Dhillon R H P, Clark J. ESBLs: A Clear and Present Danger?[J]. Critical Care Research and Practice, 2012: 1-11.
Pilmis B,Zahar J R. Ventilator-associated pneumonia related to ESBL-producing gram negative bacilli[J]. Ann Transl Med, 2018, 6(21): 424.
Parveez Ahamed A A, Rasheed M U, Peer Muhamed Noorani K, et al. In vitro antibacterial activity of MGDG-palmitoyl from Oscillatoria acuminata NTAPC05 against extended-spectrum β-lactamase producers[J]. J Antibiot (Tokyo), 2017, 70(6): 754-762.
Prasanna R, Nain L, Tripathi R, et al. Evaluation of fungicidal activity of extracellular filtrates of cyanobacteria--possible role of hydrolytic enzymes[J]. J Basic Microbiol, 2008, 48(3): 186-194.
Patterson G M L, Baldwin C L, Bolis C M, et al. Antineoplastic activity of cultured blue-green algae (cyanophyta)1[J]. J Phycology, 1991, 27(4): 530-536.
Berry J P, Gantar M, Perez M H, et al. Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides[J]. Mar Drugs, 2008, 6(2): 117-146.
Macagno A, Molteni M, Rinaldi A, et al. A cyanobacterial LPS antagonist prevents endotoxin shock and blocks sustained TLR4 stimulation required for cytokine expression[J]. J Exp Med, 2006, 203(6): 1481-1492.
Medzhitov R, Preston-Hurlburt P, Janeway C A. A human homologue of the drosophila Toll protein signals activation of adaptive immunity[J]. Nature, 1997, 388(6640): 394-397.
Roy A, Srivastava M, Saqib U, et al. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways[J]. Int Immunopharmacol, 2016, 40: 79-89.
Molteni M, Bosi A, Rossetti C. The effect of cyanobacterial LPS antagonist (CyP) on cytokines and micro-RNA expression induced by porphyromonas gingivalis LPS[J]. Toxins (Basel), 2018, 10(7): 290.
Tuominen H,Rautava J. Oral microbiota and cancer development[J]. Pathobiology, 2021, 88(2): 116-126.
Villa F A, Lieske K,Gerwick L. Selective MyD88-dependent pathway inhibition by the cyanobacterial natural product malyngamide F acetate[J]. Eur J Pharmacol, 2010, 629(1-3): 140-146.
Kwan J C, Eksioglu E A, Liu C, et al. Grassystatins A-C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation[J]. J Med Chem, 2009, 52(18): 5732-5747.
Cunningham C, Appleman L J, Kirvan-Visovatti M, et al. Phase I and pharmacokinetic study of the dolastatin-15 analogue tasidotin (ILX651) administered intravenously on days 1, 3, and 5 every 3 weeks in patients with advanced solid tumors[J]. Clin Cancer Res, 2005, 11(21): 7825-7833.
Jaki B, Orjala J,Sticher O. A novel extracellular diterpenoid with antibacterial activity from the cyanobacterium Nostoc commune[J]. J Nat Prod, 1999, 62(3): 502-503.
Raveh A,Carmeli S. Antimicrobial ambiguines from the cyanobacterium Fischerella sp. collected in Israel[J]. J Nat Prod, 2007, 70(2): 196-201.
Pérez Gutiérrez R M, Martínez Flores A, Vargas Solís R, et al. Two new antibacterial norabietane diterpenoids from cyanobacteria, Microcoleous lacustris[J]. J Nat Med, 2008, 62(3): 328-331.
El-Sheekh M M, Osman M E, Dyab M A, et al. Production and characterization of antimicrobial active substance from the cyanobacterium Nostoc muscorum[J]. Environ Toxicol Pharmacol, 2006, 21(1): 42-50.
Asthana R K, Srivastava A, Singh A P, et al. Identification of an antimicrobial entity from the cyanobacterium Fischerella sp. isolated from bark of Azadirachta indica (Neem) tree[J]. Journal of Applied Phycology, 2006, 18(1): 33-39.
Barbaras D, Kaiser M, Brun R, et al. Potent and selective antiplasmodial activity of the cyanobacterial alkaloid nostocarboline and its dimers[J]. Bioorg Med Chem Lett, 2008, 18(15): 4413-4415.
Clark B R, Engene N, Teasdale M E, et al. Natural products chemistry and taxonomy of the marine cyanobacterium Blennothrix cantharidosmum[J]. J Nat Prod, 2008, 71(9): 1530-1537.
Linington R G, Edwards D J, Shuman C F, et al. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp[J]. J Nat Prod, 2008, 71(1): 22-27.
Sanchez L M, Lopez D, Vesely B A, et al. Almiramides A-C: discovery and development of a new class of leishmaniasis lead compounds[J]. J Med Chem, 2010, 53(10): 4187-4197.
Shao C L, Linington R G, Balunas M J, et al. Bastimolide A, a potent antimalarial polyhydroxy macrolide from the marine cyanobacterium Okeania hirsuta[J]. J Org Chem, 2015, 80(16): 7849-7855.
Despite continued impact of COVID-19, malaria cases and deaths remained stable in 2021[EB/OL]. [2022-12-8] https://www.who.int/news/item/08-12-2022-despite-continued-impact-of-covid-19--malaria-cases-and-deaths-remained-stable-in-2021.
Rao D R, Thangavel C, Kabilan L, et al. Larvicidal properties of the cyanobacterium Westiellopsis sp. (blue-green algae) against mosquito vectors[J]. Trans R Soc Trop Med Hyg, 1999, 93(3): 232.
Becher P G, Keller S, Jung G, et al. Insecticidal activity of 12-epi-hapalindole J isonitrile[J]. Phytochemistry, 2007, 68(19): 2493-2497.
Gantar M, Berry J P, Thomas S, et al. Allelopathic activity among cyanobacteria and microalgae isolated from florida freshwater habitats[J]. FEMS Microbiol Ecol, 2008, 64(1): 55-64.
Gerwick W H, Tan L T,Sitachitta N. Nitrogen-containing metabolites from marine cyanobacteria[J]. Alkaloids Chem Biol, 2001, 57: 75-184.
Simmons T L, Engene N, Ure?a L D, et al. Viridamides A and B, lipodepsipeptides with antiprotozoal activity from the marine cyanobacterium oscillatoria nigro-viridis[J]. J Nat Prod, 2008, 71(9): 1544-1550.
Linington R G, Gonzalez J, Ure?a L D, et al. Venturamides A and B: antimalarial constituents of the panamanian marine cyanobacterium Oscillatoria sp.[J]. J Nat Prod, 2007, 70(3): 397-401.
Mc Phail K L, Correa J, Linington R G, et al. Antimalarial linear lipopeptides from a panamanian strain of the marine cyanobacterium Lyngbya majuscula[J]. J Nat Prod, 2007, 70(6): 984-988.
Wright A D, Papendorf O,K?nig G M. Ambigol C and 2,4-dichlorobenzoic acid, natural products produced by the terrestrial cyanobacterium Fischerella ambigua[J]. J Nat Prod, 2005, 68(3): 459-461.
Kanekiyo K, Lee J B, Hayashi K, et al. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme[J]. J Nat Prod, 2005, 68(7): 1037-1041.
Takebe Y, Saucedo C J, Lund G, et al. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus[J]. PLoS One, 2013, 8(5): e64449.
Garrison A R, Giomarelli B G, Lear-Rooney C M, et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against zaire ebola virus[J]. Antiviral Res, 2014, 112: 1-7.
Boyd M R, Gustafson K R, McMahon J B, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development[J]. Antimicrob Agents Chemother, 1997, 41(7): 1521-1530.
Dey B, Lerner D L, Lusso P, et al. Multiple antiviral activities of cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses[J]. J Virol, 2000, 74(10): 4562-4569.
Klasse P J, Shattock R,Moore J P. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission[J]. Annu Rev Med, 2008, 59: 455-471.
Xiong C, OKeefe B R, Byrd R A, et al. Potent anti-HIV activity of scytovirin domain 1 peptide[J]. Peptides, 2006, 27(7): 1668-1675.
Lokhande K B, Apte G R, Shrivastava A, et al. Sensing the interactions between carbohydrate-binding agents and N-linked glycans of SARS-CoV-2 spike glycoprotein using molecular docking and simulation studies[J]. J Biomol Struct Dyn, 2022, 40(9): 3880-3898.
Barre A, Van Damme E J M, Simplicien M, et al. Man-specific lectins from plants, fungi, algae and cyanobacteria, as potential blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) coronaviruses: biomedical perspectives[J]. Cells, 2021, 10(7): 1619.
Loya S, Reshef V, Mizrachi E, et al. The inhibition of the reverse transcriptase of HIV-1 by the natural sulfoglycolipids from cyanobacteria: contribution of different moieties to their high potency[J]. J Nat Prod, 1998, 61(7): 891-895.
Hayashi T, Hayashi K, Maeda M, et al. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis[J]. J Nat Prod, 1996, 59(1): 83-87.
Hayashi K, Hayashi T,Kojima I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: in vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities[J]. AIDS Res Hum Retroviruses, 1996, 12(15): 1463-1471.
Rechter S, K?nig T, Auerochs S, et al. Antiviral activity of arthrospira-derived spirulan-like substances[J]. Antiviral Res, 2006, 72(3): 197-206.
Nagle D G, Geralds R S, Yoo H D, et al. Absolute configuration of curacin A, a novel antimitotic agent from the tropical marine cyanobacterium Lyngbya majuscula[J]. Tetrahedron Letters, 1995, 36(8): 1189-1192.
Iwasaki S,Shirai R. Natural organic compounds that affect to microtubule functions: syntheses and structure-activity relationships of combretastatins, curacin A and their analogs as the colchicine-site ligands on tubulin[J]. Yakugaku Zasshi, 2000, 120(10): 875-889.
Vaishampayan U, Glode M, Du W, et al. Phase Ⅱ study of dolastatin-10 in patients with hormone-refractory metastatic prostate adenocarcinoma[J]. Clin Cancer Res, 2000, 6(11): 4205-4208.
Luesch H, Moore R E, Paul V J, et al. Isolation of dolastatin 10 from the marine cyanobacterium symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1[J]. J Nat Prod, 2001, 64(7): 907-910.
Kobayashi M, Natsume T, Tamaoki S, et al. Antitumor activity of TZT-1027, a novel dolastatin 10 derivative[J]. Jpn J Cancer Res, 1997, 88(3): 316-327.
Luesch H, Chanda S K, Raya R M, et al. A functional genomics approach to the mode of action of apratoxin A[J]. Nat Chem Biol, 2006, 2(3): 158-167.
Daniel K G, Kuhn D J, Kazi A, et al. Anti-angiogenic and anti-tumor properties of proteasome inhibitors[J]. Curr Cancer Drug Targets, 2005, 5(7): 529-541.
Cohen I, Kayode O, Hockla A, et al. Combinatorial protein engineering of proteolytically resistant mesotrypsin inhibitors as candidates for cancer therapy[J]. Biochem J, 2016, 473(10): 1329-1341.
Jaspars M,Lawton L A. Cyanobacteria - a novel source of pharmaceuticals[J]. Curr Opin Drug Discov Devel, 1998, 1(1): 77-84.
Shen X, Lam P K, Shaw G R, et al. Genotoxicity investigation of a cyanobacterial toxin, cylindrospermopsin[J]. Toxicon, 2002, 40(10): 1499-1501.
Diederichs S, Bulk E, Steffen B, et al. S100 family members and trypsinogens are predictors of distant metastasis and survival in early-stage non-small cell lung cancer[J]. Cancer Res, 2004, 64(16): 5564-5569.
Ahmed M N, Wahlsten M, Jokela J, et al. Potent inhibitor of human trypsins from the aeruginosin family of natural products[J]. ACS Chem Biol, 2021, 16(11): 2537-2546.
Bulló M, Casas-Agustench P, Amigó-Correig P, et al. Inflammation, obesity and comorbidities: the role of diet[J]. Public Health Nutrition, 2007, 10(10A): 1164-1172.
Deng R,Chow T J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina[J]. Cardiovasc Ther, 2010, 28(4): e33-45.
Riss J, Décordé K, Sutra T, et al. Phycobiliprotein C-phycocyanin from spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters[J]. J Agricultural Food Chemistry, 2007, 55(19): 7962-7967.
Grosshagauer S, Kraemer K,Somoza V. The True Value of Spirulina[J]. J Agric Food Chem, 2020, 68(14): 4109-4115.
Lafarga T, Fernández-Sevilla J M, González-López C, et al. Spirulina for the food and functional food industries[J]. Food Res Int, 2020, 137: 109356.
收稿日期:2022-12-13
作者简介:田龙龙,本科生,主要从事病原生物学研究。
*通讯作者:李婷婷,博士,研究员,硕士研究生导师,主要从事病原微生物与宿主互作和免疫机制工作。
