两个棉蚜胰岛素受体生物信息学分析及其在三种有翅型棉蚜中的表达模式比较
2023-04-29王柳玉王丽朱香镇张开心李东阳姬继超崔金杰马伟华
王柳玉 王丽 朱香镇 张开心 李东阳 姬继超 崔金杰 马伟华
摘要:【目的】明確棉蚜2个胰岛素受体基因的序列结构及其在3种有翅型棉蚜翅型分化与发育过程中的表达模式。【方法】基于棉蚜全基因组数据获取2个胰岛素受体基因(insulin receptor 1, AgInR1和insulin receptor 2, AgInR2)序列并进行氨基酸序列分析、系统发育分析,而后对InR1和InR2进行蛋白保守结构域分析以及基序分析。通过实时荧光定量聚合酶链式反应(quantitative real-time polymerase chain reaction, qRT-PCR)技术分析2种胰岛素受体基因在棉蚜孤雌无翅蚜以及3种有翅蚜不同发育时期的表达模式。【结果】理化性质分析结果显示AgInR1和AgInR2均为亲水性跨膜蛋白。系统发育分析结果显示17种昆虫的InR1聚为1簇,14种昆虫的InR2聚为1簇。半翅目蚜科的InR1、InR2分别以极高的自展值聚为一支,亲缘关系较近。保守结构域和基序分析表明棉蚜InR1和InR2保守结构排列较为相似,但相较于AgInR1,AgInR2缺少1个FN3结构域。qRT-PCR结果显示,AgInR1在孤雌有翅蚜、性母、雄蚜这3种有翅蚜的4龄、成虫期的表达量均显著高于同龄期的孤雌无翅蚜,而AgInR2在3种有翅蚜中的表达模式与AgInR1不同。【结论】AgInR1可能参与调控3种有翅蚜在4龄及成虫期的翅发育过程。AgInR2与AgInR1的表达模式不同,可能具有不同的生物学功能。该研究为进一步探究胰岛素信号通路在棉蚜翅型分化中的作用提供理论支持。
关键词:棉蚜;翅多态性;胰岛素受体;表达模式;翅型分化
Bioinformatics analysis of two insulin receptors in Aphis gossypii and comparison of their expression patterns in three winged phenotypes
Wang Liuyu1, Wang Li2, Zhu Xiangzhen2, Zhang Kaixin2, Li Dongyang2, Ji Jichao2*, Cui Jinjie2*, Ma Weihua1*
(1. College of Plant Science and Technology, Huazhong Agricultural University, Wuhan 430070, China; 2. Institute of Cotton Research, Chinese Academy of Agricultural Sciences/National Key Laboratory of Cotton Bio-breeding and Integrated Utilization, Anyang, Henan 455000, China)
Abstract: [Objective] This study aims to clarify the sequence structure and expression pattern of two insulin receptor genes in three kinds of winged aphids of Aphis gossypii. [Methods] Based on the whole genome data of A. gossypii, amino acid sequence analysis of two insulin receptor genes (insulin receptor 1, AgInR1; insulin receptor 2, AgInR2) was identified, and phylogenetic analysis of insect InR1 from 17 insects and InR2 from 14 insect was performed. The protein conserved structural domains of insulin receptors were analyzed and motif analysis was conducted using online software. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to analyze the expression patterns of AgInR1 and AgInR2 in wingless female and three kinds of winged aphids at different developmental stages. [Results] The physicochemical analysis showed that AgInR1 and AgInR2 were hydrophilic transmembrane proteins. Phylogenetic analysis showed that InR1 and InR2 of the analyzed insects were clustered into two distinctive groups. The InR1 and InR2 of Hemiptera Aphididae were clustered together with very high bootstrap values, showed close affinity. Conserved domains and motif analyses showed that conserved domain arrangement of InR1 and InR2 from A. gossypii were relatively similar. Compared with AgInR1, AgInR2 lacked one FN3 domain. The results of qRT-PCR showed that the expression level of AgInR1 in the 4th instar and adult stages of the three winged aphids, namely, parthenogenetic wing female, gynoparae, and male was significantly higher than that of parthenogenetic wingless female at the same instar. Meanwhile the expression pattern of AgInR2 in the three kinds of winged aphids was different from that of AgInR1. [Conclusion] AgInR1 might involve in regulating the wing development process in the 4th instar and adult stages of three winged aphids. AgInR2 had a different expression pattern from AgInR1 and might have different biological functions. The study provides theoretical support for further investigation of the role of insulin signaling pathway in wing differentiation of A. gossypii.
Keywords: Aphis gossypii; wing polymorphism; insulin receptor; expression patterns; wing dimorphism
棉蚜(Aphis gossypii)属于半翅目(Hemiptera)蚜科(Aphididae),是棉田及温室种植蔬菜的主要害虫[1]。棉蚜为异寄主全周期蚜虫,在春夏季节进行孤雌生殖,秋末冬初产生性母,并飞至冬寄主产下性雌,进入有性生殖阶段。在棉蚜的生活史中存在3种有翅型形态,即孤雌有翅蚜、性母、雄蚜。春夏季节,孤雌有翅蚜在不同寄主间迁移,种群快速扩张;秋冬季节,性母、雄蚜迁飞至冬寄主,雄蚜与性雌交配产卵,而卵可抵御寒冬[2]。棉花病虫害的发生直接影响棉花的产量和纤维品质,有翅型棉蚜在棉蚜种群扩张、病毒传播中起重要作用。
昆虫翅的多态性是昆虫在长期进化中适应环境的策略,也是昆虫对环境变化的响应[3]。翅多态性的昆虫通过优化资源分配,平衡飞行与繁殖需求以适应复杂多变的生存环境,对稳定种群以及增加种群遗传多样性具有重要意义[4-5]。半个世纪以来,研究者以果蝇(Drosophila)、家蚕(Bombyx mori)、蟋蟀(Gryllulus)等为模式昆虫,对昆虫翅的多态性展开了广泛的研究。对家蚕翅发育的研究主要集中于翅发育基因的功能以及相关信号通路的调控作用[6]。对果蝇翅的发育机制已有比较透彻的研究,其发育与形成由多条信号通路的协调作用共同决定,包括Dpp信号通路和Hedgehog信号通路等[7-10]。昆虫种内最常见的翅型分化有长翅型和短翅型、有翅型和无翅型[11]。在半翅目飞虱科(Hemiptera: Delphacidae)昆虫类群中,翅型分化为长/短类型。然而,在半翅目蚜科(Hemiptera: Aphididae)昆虫类群中,翅型分化为有/无类型。影响翅型分化的因素有很多,如温度、光照、种群密度、寄主营养等[12-14]。此外,多项研究表明蜕皮激素、保幼激素(juvenile hormone)参与调控昆虫翅型分化。例如,蜕皮激素通过TORC1依赖机制调节果蝇翅盘大小。在豌豆蚜(Acyrthosiphon pisum)与甜菜蚜(A. fabae)中,在1龄期局部应用保幼激素可以使有翅蚜发育为无翅蚜[15-17]。
近些年,胰岛素信号通路成为翅型分化分子机制研究的热点。从果蝇到哺乳动物,胰岛素信号途径高度保守,在生长发育、代谢平衡、翅二态性以及衰老过程等方面发挥重要作用[14, 18]。胰岛素受体是胰岛素信号通路的上游因子,与胰岛素结合后触发信号转导级联反应,实现对机体的调控[19]。胰岛素受体一般包括配体结合L结构域(Recep_L)、半胱氨酸富集区Furin样区(Furin-like)、纤连蛋白III型结构域(FN3)和酪氨酸激酶催化结构域(TyrKc),各结构域行使的功能不同[20]。2个Recep_L及其中间的1个Furin-like作为配体结合位点,在胰岛素与胰岛素受体结合过程中起重要作用。FN3对α-亚基和β-亚基结合时产生的二硫键起到重要作用,TyrKc自身磷酸化可能会充分激活胰岛素受体的激酶活性[21-22]。昆虫胰岛素和胰岛素样生长因子信号的功能可塑性可能来自于胰岛素受体的多样性[23]。有研究表明在果蝇突变体中,改变胰岛素受体激酶活性或蛋白结构域相互作用能延缓其衰老[24]。
2015年,Xu等[25]在褐飞虱(Nilaparvata lugens)翅型分化分子机理方面取得了突破进展,发现褐飞虱体内有2个胰岛素受体基因(insulin receptor 1,InR1和insulin receptor 2,InR2),通过调节叉头转录因子FoxO的活性,在控制长翅型和短翅型的发育中起着相反的作用。在之后的研究中发现褐飞虱长/短翅的发育取决于Zfh1-FoxO通路和IIS-FoxO途径的平衡[26]。研究表明miR-9b和ABCG4调控胰岛素信号途径在柑橘蚜虫(Aphis citricidus)中介导翅两型分化,且该研究结果在豌豆蚜中得到证实。在柑橘蚜虫中,拥挤条件下aci-miR-9b负调控AcABCG4的表达,从而激活胰岛素和胰岛素类似肽信号通路,增加有翅后代的比例[27]。在4龄的柑橘蚜虫中沉默胰岛素受体基因会出现翅发育畸形、翅发育不良以及若虫阶段之后无法发育的蚜虫[28]。由于棉蚜体型小、生活史复杂,加上前人对性母、雄蚜的研究相对较少,胰岛素信号通路在棉蚜翅型分化的系统性研究进展缓慢。
本研究通过生物信息学手段分析2个棉蚜胰岛素受体的基因序列结构与保守结构域,探究其与其他昆虫胰岛素受体蛋白的序列相似性,并用实时荧光定量聚合酶链式反应(quantitative real-time polymerase chain reaction, qRT-PCR)技术测定2个基因在3种有翅型棉蚜不同发育时期的表达量,以期为进一步探究胰岛素信号通路在棉蚜翅型分化中的作用奠定基础。
1 材料与方法
1.1 供试昆虫
以3种有翅型棉蚜以及孤雌无翅蚜作为供试虫源,棉蚜为中国农业科学院棉花研究所棉花生物育种与综合利用全国重点实验室连续饲养多代种群,遗传背景一致。棉蚜以棉花品种中棉所49的幼苗为食,孤雌有翅蚜的饲养条件为温度(25±1)℃,相对湿度75%,光周期(光照时间/黑暗时间)14 h/10 h,性母与雄蚜饲养条件为温度18 ℃,相对湿度75%,光周期(光照时间/黑暗时间)8 h/16 h[2]。
1.2 试验处理
根据形态学特征,分别收取1龄、2龄、3龄、4龄以及成虫期的孤雌有翅蚜、性母及雄蚜样品用于RNA提取,其中1龄孤雌有翅蚜与1龄孤雌无翅蚜为同种试虫[29]。每个龄期设置3个生物学重复。
1.3 总RNA的提取及cDNA合成
收集的棉蚜加液氮研磨充分后,按照TRIzol试剂(Life Technologies,美国)说明书步骤提取RNA,利用TaKaRa公司的PrimeScriptTM RT reagent Kit(Perfect Real Time)反转录试剂盒合成第一链cDNA。
1.4 qRT-PCR
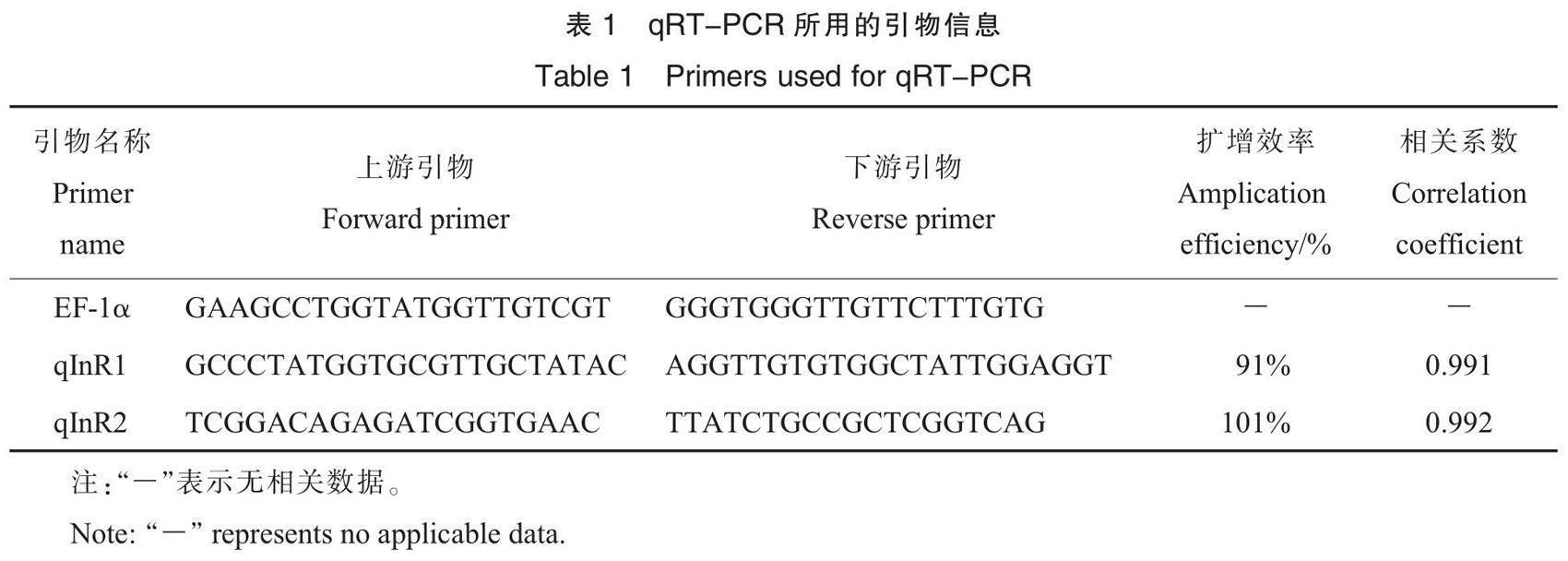
采用Primer Premier 6.0,根据棉蚜基因组序列信息设计引物(表1),由生工生物工程(上海)股份有限公司合成引物。以棉蚜cDNA为模板,棉蚜EF-1α基因为内参基因[30],采用TransStart Top Green qPCR SuperMix(+Dye I)荧光定量试剂盒进行qRT-PCR,反应体系及条件按照说明书进行,试验所用仪器为eppendorf Mastercycler epgradient S,数据分析软件为realplex Properties。qRT-PCR引物验证,以cDNA为模板,梯度稀释后配制反应体系,得到相应的标准曲线。从数据分析软件导出扩增效率和相关系数,根据扩增效率和相关系数选择合适的引物。基因表达量根据2-△△Ct公式计算[31],每个生物学重复设置3个技术重复,采用单因素方差分析,用Duncan氏新复极差法进行多重比较(P<0.05)。
1.5 生物信息学分析
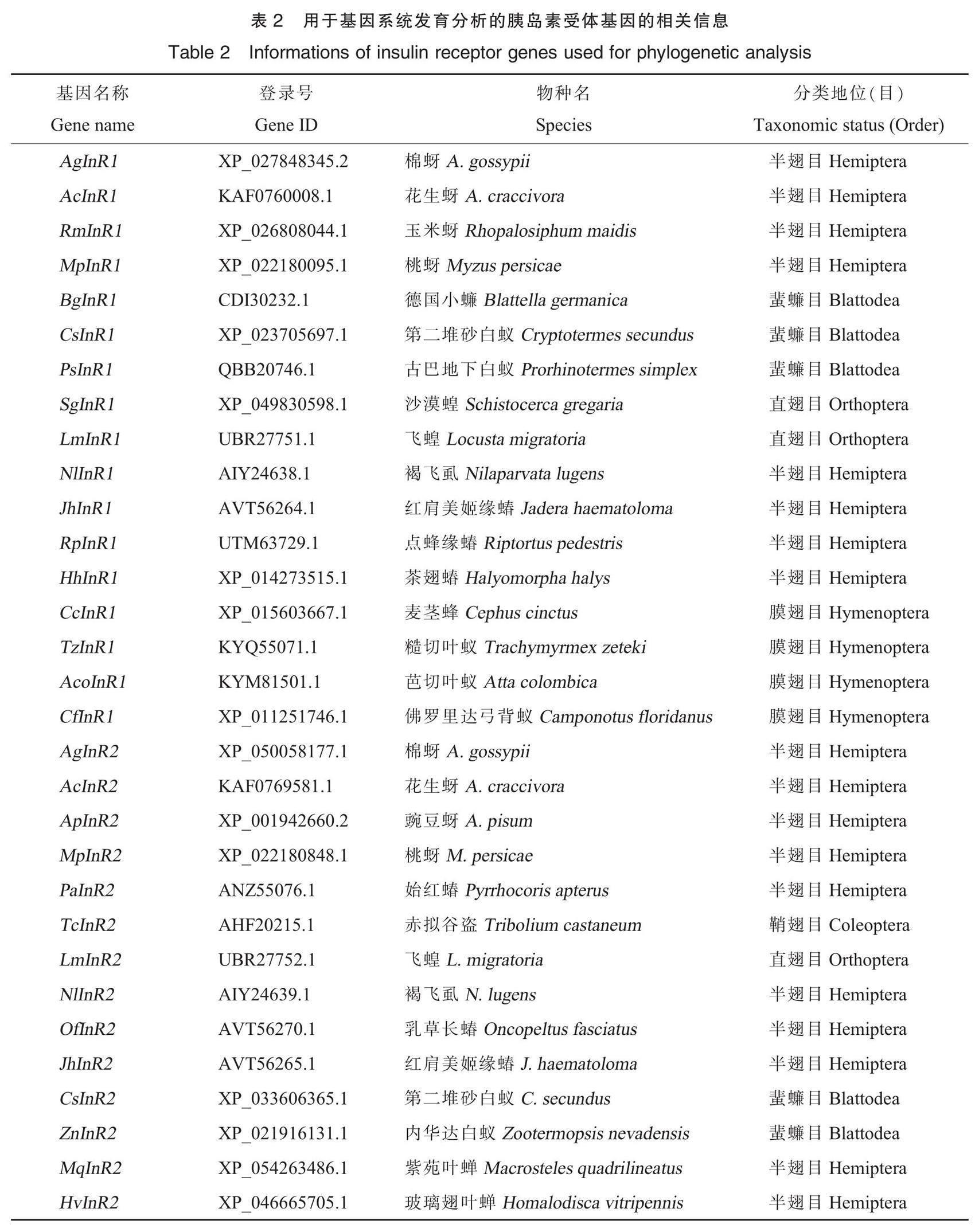
对AgInR1(XP_027848345.2)和AgInR2(XP_050058177.1)运用在线软件Expasy-ProtParam tool(https://web.expasy.org/protparam/)进行理化性质分析,用在线软件TMHMM-2.0分析跨膜结构,在https://services.healthtech.dtu.dk/services/SignalP-5.0/网页预测信号肽。通过在线网页SOPMA(https://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html)分析蛋白的二级结构,采用NetPhos 3.1程序(http://www.cbs.dtu.dk/services/NetPhos-3.1/)预测蛋白的磷酸化位点。在美国国立生物技术信息中心(National Center for Biotechnology Information, NCBI)下载24种昆虫的胰岛素受体蛋白的氨基酸序列全长,包括17条昆虫的InR1氨基酸序列以及14条昆虫的InR2氨基酸序列(表2),利用MEGA 7.0 软件采用邻接法(neighbour joining)对棉蚜以及其他昆虫的InR1和InR2进行系统发育分析,设自展值(bootstrap)为1 000次,其他参数为默认值。并通过在线工具iTOL对进化树进行美化;使用MEME(https://meme-suite.org/meme/doc/meme.html)在线分析保守基序(motif),设置基序数量为12,其他参数为默认值。采用CD-Search(https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi)分析保守结构域,并通过TBtools对系统发育分析、保守结构域分析以及保守基序分析结果进行可视化[32-33]。
2 结果与分析
2.1 AgInR1和AgInR2蛋白理化性质分析
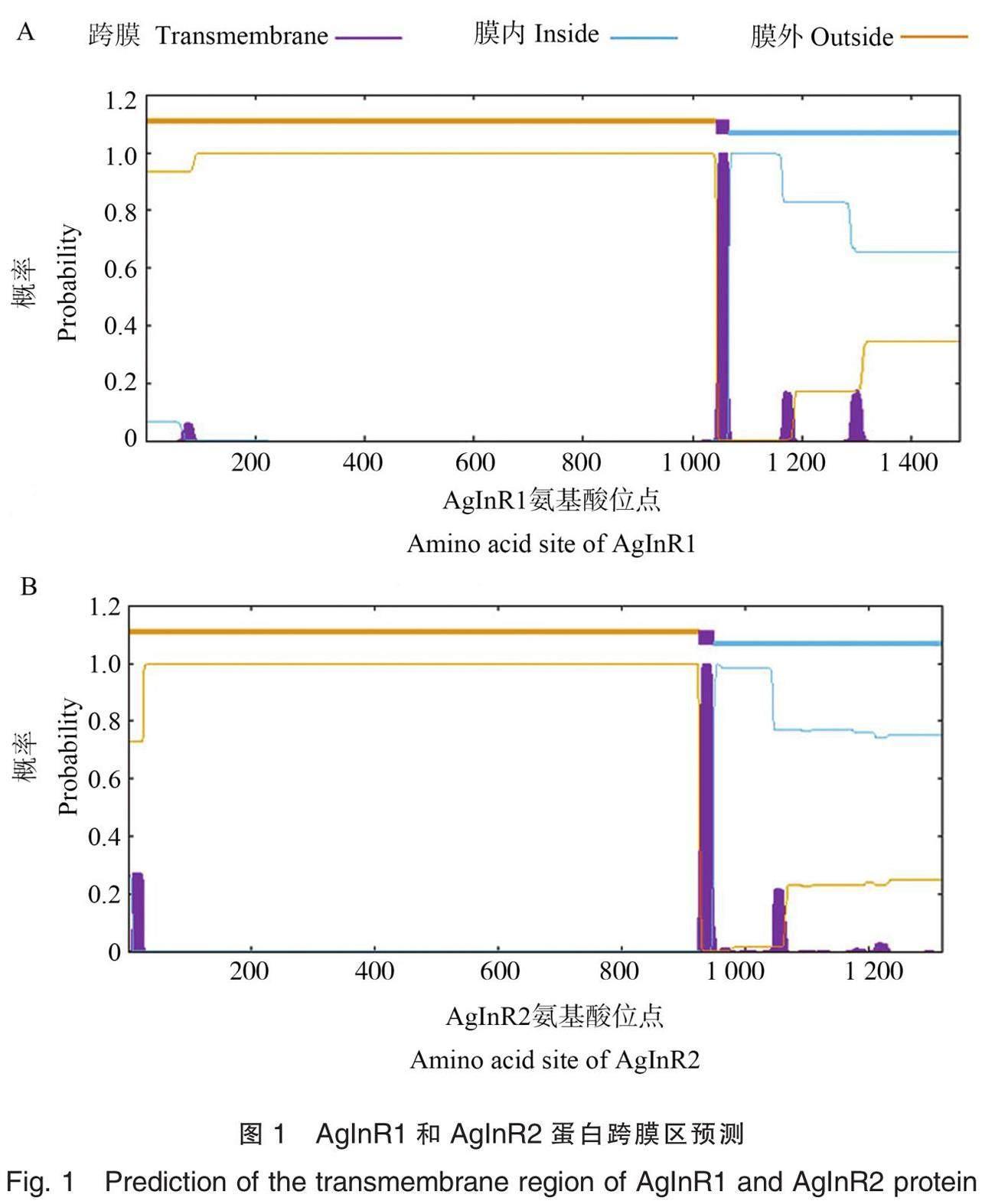
AgInR1基因编码1 490个氨基酸(amino acid, aa),预测其分子量和理论等电点分别为168.745 KDa和5.79。AgInR1亲水性平均系数(grand average of hydropathicity,GRAVY)为-0.413,是亲水性蛋白。预测显示,AgInR1有1个跨膜区域,位于1 044~1 066 aa处(图1A);对AgInR1进行信号肽预测,结果显示无信号肽(图2A)。通过二级结构分析,发现AgInR1蛋白主要包括α-螺旋(alpha helix)、延伸链(sheet)、β-转角(beta-turn)和不规则卷曲(random coil),占比分别为27.03%、19.73%、4.86%和48.38%(图3A)。磷酸化位点分析显示丝氨酸(S)磷酸化位点71个,酪氨酸(Y)磷酸化位点24个,苏氨酸(T)磷酸化位点43个。
AgInR2基因编码1 320个氨基酸,预测其分子量和理论等电点分别为150.162 KDa和6.01,GRAVY为-0.261,是亲水性蛋白。预测AgInR2在926~948 aa有1个跨膜区域(图1B);信号肽预测分析显示其N端存在信号肽,剪切位点在第23、24残基处(图2B)。通过二级结构分析,发现AgInR1蛋白主要包括α-螺旋、延伸链、β-转角和不规则卷曲,占比分别为25.30%、21.29%、5.61%和47.80%(图3B)。磷酸化位点分析显示丝氨酸(S)磷酸化位点76个,酪氨酸(Y)磷酸化位点24个,苏氨酸(T)磷酸化位点32个。
2.2 氨基酸序列同源性分析
如图4和图5A所示,系统进化分析表明昆虫的InR1(蓝色分支)与InR2(绿色分支)各聚为一支。棉蚜与花生蚜的InR1以极高的自展值聚在一起,亲缘关系最近,其次是玉米蚜(Rhopalosiphum maidi)和桃蚜(Myzus persicae)。同样地,棉蚜的InR2与花生蚜的InR2也以极高的自展值聚为一小支,亲缘关系最近,其次是豌豆蚜和桃蚜。
结构域分析结果(图5B)显示,文中比较的17种昆虫InR1均含有Furin-like,Recep_L和FN3结构域,其中4种蚜虫(棉蚜、玉米蚜、桃蚜和花生蚜)InR1中的TyrKc结构域为PKc_like_superfamily,其他昆虫的InR1的TyrKc结构域为PTKc_InsR_like。PKc_like_superfamily结构域和PTKc_InsR_like结构域都属于蛋白激酶超家族,能够催化γ-磷酸基团从ATP转移到蛋白质底物中的酪氨酸残基[34]。除蜚蠊目(Blattodea)的2种昆虫的InR2包含PTKc_InsR_like结构域外,文中分析的InR2均含有PKc_like_superfamily、Recep_L和FN3结构域。蚜虫InR1和InR2的保守结构域较为相似,均有PKc_like_superfamily,Recep_L和FN3结构域。在棉蚜中FN3结构域的数量存在差异,AgInR1有3个FN3,AgInR2有2个FN3。
将MEME中得到的基序用Pfam预测相对应的结构域,结果显示motif 1、motif 2、motif 3、motif 7、motif 9、motif 10均存在于TyrKc结构域,motif 4、motif 5、motif 6对应Recep_L结构域的部分序列,motif 8、motif 12均对应FN3结构域的部分序列,而motif 11没有匹配到结构域。Motif分析结果显示(图5C),所列17种昆虫的InR1均含有12种相同的基序,位置排列基本一致。而InR2预测的motif较为复杂,半翅目中4种蚜虫棉蚜、花生蚜、桃蚜、豌豆蚜的InR2均没有motif 11,且motif 11在其他半翅目昆虫InR2中位置不同。乳草长蝽(Oncopeltus fasciatus)和红肩美姬缘蝽(Jadera haematoloma)的InR2中没有motif 5和motif 6,而结构域分析显示它们较其他InR2蛋白少1个Recep_L结构域。对于棉蚜和花生蚜,InR2比InR1缺少1个motif 11以及1个motif 8,即缺乏1个FN3,与保守结构域预测结果相对应。
2.3 AgInR1和AgInR2基因在3种有翅蚜中的表达模式
对于棉蚜来说,1龄时,孤雌有翅蚜和孤雌无翅蚜在外观上无法区分,2龄时,在体视镜下观察可见有翅蚜的翅原基略有突起,3龄时肉眼可观察到翅原基突起,4龄时翅芽发育明显,在成虫期发育为完整的翅。通过qRT-PCR检测AgInR1和AgInR2在孤雌无翅蚜和3种有翅蚜不同发育时期的表达水平,均以AgInR1在孤雌蚜1龄期中的表达量为对照(图6)。在孤雌无翅蚜中,与1龄、2龄相比,3龄时AgInR1的表达量显著下降。在孤雌有翅蚜中,与1龄相比,AgInR1在3龄时的表达量下降但未达到显著水平,在4龄及成虫期的表达量显著上升。在性母中,与1龄性母相比,AgInR1的表达量在4龄和成虫期显著升高;且在3龄、4龄、成虫期AgInR1的表达量均显著高于同龄期的孤雌无翅蚜,在2龄期显著低于同龄期的孤雌无翅蚜。在雄蚜发育过程中AgInR1的表达量整体呈现出上升趋势,其在成虫期的表达量显著高于各龄期;在3龄、4龄、成虫期雄蚜中AgInR1的表达量均显著高于同龄期的孤雌无翅蚜。在孤雌有翅蚜、性母、雄蚜这3种有翅蚜的4龄期和成虫期,AgInR1的表达量均显著高于同龄期的孤雌无翅蚜。
AgInR2在孤雌无翅蚜与孤雌有翅蚜中均呈现先下降后上升的趋势。在孤雌无翅蚜中,与1龄相比,在2龄和3龄期AgInR2的表达量显著下降。在4龄及成虫期,孤雌有翅蚜中AgInR2的表达量均显著低于同龄期的孤雌无翅蚜。在性母中,与1龄性母相比,AgInR2的表达量在4龄和成虫期显著上升,且在2龄、3龄、4龄以及成虫期AgInR2的表达量均显著高于同龄期的孤雌无翅蚜。在雄蚜中,与1龄相比,AgInR2的表达量在4龄明显下降但并不显著,在成虫期显著上升;AgInR2的表达量在1龄和4龄显著低于同龄期的孤雌无翅蚜。
3 讨论
本研究针对棉蚜胰岛素受体的理化性质、蛋白结构域等进行分析,为深入探究其功能奠定基础。对AgInR1和AgInR2蛋白基本理化性质分析结果显示AgInR1和AgInR2均为亲水性跨膜蛋白。进化分析结果显示,昆虫的InR1和InR2分别聚为一簇,在进化中较为保守。
此前大量研究已证实,许多昆虫类群飞行肌与生殖系统之间存在资源权衡[35]。在翅多态昆虫中,长翅形态具有飞行能力,但以牺牲生殖力为代价;短翅及无翅飞行力较弱或无飞行能力,但具有更强的生殖力[36]。相较于无翅蚜,棉蚜中的有翅蚜形态相似,头部、翅膀和胸部变黑硬化[2]。棉蚜InR1和InR2在3种有翅蚜不同发育时期的表达模式显示,相较于同龄期孤雌无翅蚜,3种有翅蚜AgInR1的表达量在4龄以及成虫期均显著上升。这与柑橘蚜虫(A. citricidus)后期InR1的表达相似,而且在A.citricidus中干扰InR1导致23%的蚜虫翅发育畸形,暗示AgInR1可能在3种有翅蚜的翅发育后期起重要作用[24]。AgInR2在3种有翅蚜中的表达模式与AgInR1不同,说明AgInR2在3种有翅蚜生长发育中可能具有不同于AgInR1的功能。光周期和温度对棉蚜两性形态有明确的影响[37]。已有研究表明光周期的变化影响胰岛素信号通路相关基因表达量的变化,在中短光照条件下,豌豆蚜的胰岛素受体基因表达量下降[38]。AgInR2在孤雌无翅蚜和孤雌有翅蚜中的表达量整体呈现出先下降后上升趋势,而在性母和雄蚜中略有差异,可能是由于胰岛素信号通路对光周期产生了响应。胰岛素受体在胰岛素信号通路中发挥重要作用,参与调控许多昆虫的繁殖[20]。在雄蚜和性母4龄时,AgInR2的表达趋势相反,可能是由于AgInR2参与了性母的生殖调控。
4 结论
本研究分析了棉蚜胰岛素受体基因AgInR1和AgInR2在3种有翅蚜不同发育时期的表达模式。AgInR1的表达量在3种有翅蚜的4龄及成虫期显著上升,AgInR2的表达量在孤雌无翅蚜和孤雌有翅蚜中整体呈现出先下降后上升趋势。本研究为进一步探究胰岛素信号通路在棉蚜生长发育及翅型调控中的作用奠定基础。
参考文献:
[1] Li J H, Wu Y K, Zhang Q, et al. Aphid parasitism and parasitoid diversity in cotton fields in Xinjiang, China[J/OL]. PLoS ONE, 2018, 13(11): e0207034[2023-07-16]. https://doi.org/10.1371/journal.pone.0207034.
[2] Ji J C, Huangfu N B, Luo J Y, et al. Insights into wing dimorphism in worldwide agricultural pest and host-alternating aphid Aphis gossypii[J/OL]. Journal of Cotton Research, 2021, 4: 5[2023-07-16]. https://doi.org/10.1186/s42397-021-00080-w.
[3] Yang X W, Liu X X, Xu X L, et al. Gene expression profiling in winged and wingless cotton aphids, Aphis gossypii (Hemiptera: Aphididae)[J/OL]. International Journal of Biological Sciences, 2014, 10(3): 257-267[2023-07-16]. https://doi.org/10.7150/ijbs.7629.
[4] Tigreros N, Davidowitz G. Flight-fecundity tradeoffs in wing-monomorphic insects[J/OL]. Advances in Insect Physiology, 2019, 56: 1-41[2023-07-16]. https://doi.org/10.1016/BS.AIIP.2019.02.001.
[5] Renault D. A review of the phenotypic traits associated with insect dispersal polymorphism, and experimental designs for sorting out resident and disperser phenotypes[J/OL]. Insects, 2020, 11(4): 214[2023-07-16]. https://doi.org/10.3390/insects11040214.
[6] Yin J, Zhang J, Li T, et al. BmSd gene regulates the silkworm wing size by affecting the Hippo pathway[J/OL]. Insect Science, 2020, 27(4): 655-664[2023-07-17]. https://doi.org/10.1111/1744-7917.12702.
[7] Zhang X, Luo D, Pflugfelder G O, et al. Dpp signaling inhibits proliferation in the Drosophila wing by Omb-dependent regional control of bantam[J/OL]. Development, 2013, 140(14): 2917-2922[2023-07-16]. https://doi.org/10.1242/dev.094300.
[8] Wang W N, Peng J, Li Z, et al. Transcription factor E93 regulates wing development by directly promoting Dpp signaling in Drosophila[J/OL]. Biochemical and Biophysical Research Communications, 2019, 513(1): 280-286[2023-07-16]. https://doi.org/10.1016/j.bbrc.2019.03.100.
[9] Tripathi B K, Irvine K D. The wing imaginal disc[J/OL]. Genetics, 2022, 220(4): iyac020[2023-07-16]. https://doi.org/10.1093/genetics/iyac020.
[10] Bray S. Drosophila development: Scalloped and Vestigial take wing[J/OL]. Current Biology, 1999, 9(7): R245-R247[2023-07-16]. https://doi.org/10.1016/s0960-9822(99)80154-7.
[11] 王小艺, 杨忠岐, 魏可, 等. 昆虫翅型分化的表型可塑性机制[J]. 生态学报, 2015, 35(12): 3988-3999.
Wang Xiaoyi, Yang Zhongqi, Wei Ke, et al. Mechanisms of phenotypic plasticity for wing morph differentiation in insects[J]. Acta Ecologica Sinica, 2015, 35(12): 3988-3999.
[12] Lees A D. The production of the apterous and alate forms in the aphid Megoura viciae Buckton, with special reference to the role of crowding[J/OL]. Journal of Insect Physiology, 1967, 13(2): 289-318[2023-07-16]. https://doi.org/10.1016/0022-1910(67)90155-2.
[13] Gudmunds E, Narayanan S, Lachivier E, et al. Photoperiod controls wing polyphenism in a water strider independently of insulin receptor signaling[J/OL]. Proceedings of the Royal Society B: Biological Sciences, 2022, 289(1973): 20212764[2023-07-16]. https://doi.org/10.1098/rspb.2021.2764.
[14] Zhang C X, Brisson J A, Xu H J. Molecular mechanisms of wing polymorphism in insects[J/OL]. Annual Review Entomo-logy, 2018, 64: 297-314[2023-07-16]. https://doi.org/10.1146/annurev-ento-011118-112448.
[15] Strassburger K, Lutz M, Müller S, et al. Ecdysone regulates Drosophila wing disc size via a TORC1 dependent mechanism[J/OL]. Nature Communications, 2021, 12(1): 6684[2023-07-16]. https://doi.org/10.1038/s41467-021-26780-0.
[16] Vellichirammal N N, Gupta P, Hall T A, et al. Ecdysone signa-ling underlies the pea aphid transgenerational wing polyphenism[J/OL]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(6): 1419-1423[2023-07-16]. https://doi.org/10.1073/pnas.1617640114.
[17] Jim H. Juvenile hormone mimics the photoperiodic apterization of the alate gynopara of aphid, Aphis fabae[J/OL]. Nature, 1980, 286: 602-604[2023-07-16]. https://doi.org/10.1038/286602a0.
[18] Tanabe K, Itoh M, Tonoki A. Age-related changes in insulin-like signaling lead to intermediate-term memory impairment in Drosophila[J/OL]. Cell Reports, 2017, 18(7): 1598-1605[2023-07-16]. https://doi.org/10.1016/j.celrep.2017.01.053.
[19] Wente S R, Rosen O M. Insulin-receptor approaches to studying protein kinase domain[J/OL]. Diabetes Care, 1990, 13(3): 280-287[2023-07-16]. https://doi.org/10.2337/diacare.13.3.280.
[20] Wu S, Tang Y, Su S J, et al. RNA interference knockdown of insulin receptor inhibits ovarian development in Chilo suppressalis[J/OL]. Molecular Biology Reports, 2022, 49(12): 11765-11773[2023-07-16]. https://doi.org/10.1007/s11033-022-07948-3.
[21] De Meyts P. Insulin and its receptor: structure, function and evolution[J/OL]. BioEssays, 2004, 26(12): 1351-1362[2023-07-16]. https://doi.org/10.1002/bies.20151.
[22] 卢博. 褐飞虱胰岛素受体基因分析与功能研究[D]. 杭州: 浙江大学, 2015.
Lu Bo. Genetic and functional analysis of insulin receptor genes of brown planthopper (Nilaparvata lugens)[D]. Hangzhou: Zhejiang University, 2015.
[23] Sm?kal V, Pivarci M, Provazník J, et al. Complex evolution of insect insulin receptors and homologous decoy receptors, and functional significance of their multiplicity[J/OL]. Molecular Biology and Evolution, 2020, 37(6): 1775-1789[2023-07-16]. https://doi.org/10.1093/molbev/msaa048.
[24] Yamamoto R, Palmer M, Koski H, et al. Aging modulated by the Drosophila insulin receptor through distinct structure-defined mechanisms[J/OL]. Genetics, 2021, 217(2): iyaa037[2023-07-16]. https://doi.org/10.1093/genetics/iyaa037.
[25] Xu H J, Xue J, Lu B, et al. Two insulin receptors determine alternative wing morphs in planthoppers[J/OL]. Nature, 2015, 519(7544): 464-467[2023-07-16]. https://doi.org/10.1038/nature14286.
[26] Zhang J L, Chen S J, Liu X Y, et al. The transcription factor Zfh1 acts as a wing-morph switch in planthoppers[J/OL]. Nature Communications, 2022, 13(1): 5670[2023-07-16]. https://doi.org/10.1038/s41467-022-33422-6.
[27] Shang F, Niu J, Ding B Y, et al. The miR-9b microRNA mediates dimorphism and development of wing in aphids[J/OL]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(15): 8404-8409[2023-07-16]. https://doi.org/10.1073/pnas.1919204117.
[28] Ding B Y, Shang F, Zhang Q, et al. Silencing of two insulin receptor genes disrupts nymph-adult transition of alate brown citrus aphid[J/OL]. International Journal of Molecular Sciences, 2017, 18(2): 357[2023-07-16]. https://doi.org/10.3390/ijms18020357.
[29] Ji J C, Zhang S, Luo J Y, et al. Comparative transcriptional analysis provides insights of possible molecular mechanisms of wing polyphenism induced by postnatal crowding in Aphis gossypii[J/OL]. Journal of Cotton Research, 2019, 2: 17[2023-07-16]. https://doi.org/10.1186/s42397-019-0036-z.
[30] Ma K S, Li F, Liang P Z, et al. Identification and validation of reference genes for the normalization of gene expression data in qRT-PCR analysis in Aphis gossypii (Hemiptera: Aphididae)[J/OL]. Journal of Insect Science, 2016, 16(1): 17[2023-07-16]. https://doi.org/10.1093/jisesa/iew003.
[31] Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT method[J/OL]. Methods, 2001, 25(4): 402-408[2023-07-16]. https://doi.org/10.1006/meth.2001.1262z.
[32] 晁毛妮, 董洁, 胡根海, 等. 陆地棉AGPase基因家族的鉴定及表达分析[J/OL]. 棉花学报, 2022, 34(4): 299-312[2023-07-16]. https://doi.org/10.11963/cs20220026.
Chao Maoni, Dong Jie, Hu Genhai, et al. Identification and expression analysis of AGPase gene family in upland cotton (Gossypium hirsutum L.)[J/OL]. Cotton Science, 2022, 34(4): 299-312[2023-07-16]. https://doi.org/10.11963/cs20220026.
[33] 吴翠翠, 肖水平, 夏芝, 等. 陆地棉LTPG基因家族的全基因组鉴定及表达分析[J/OL]. 棉花学报, 2023, 35(1): 1-16[2023-07-16]. https://doi.org/10.11963/cs20220064.
Wu Cuicui, Xiao Shuiping, Xia Zhi, et al. Genome-wide identification and expression analysis of LTPG gene family in Gossypium hirsutum L.[J/OL]. Cotton Science, 2023, 35(1): 1-16[2023-07-16]. https://doi.org/10.11963/cs20220064.
[34] Lu S N, Wang J Y, Chitsaz F, et al. CDD/SPARCLE: the conserved domain database in 2020[J/OL]. Nucleic Acids Research, 2020, 48(D1): D265-D268[2023-07-16]. https://doi.org/10.1093/nar/gkz991.
[35] Braendle C, Davis G K, Brisson J A, et al. Wing dimorphism in aphids[J/OL]. Heredity, 2006, 97(3): 192-199[2023-07-16]. https://doi.org/10.1038/sj.hdy.6800863.
[36] Chang H H, Guo X Q, Guo S L, et al. Trade-off between flight capability and reproduction in Acridoidea (Insecta: Orthoptera)[J/OL]. Ecology and Evolution, 2021, 11(23): 16849-16861[2023-07-16]. https://doi.org/10.1002/ece3.8317.
[37] Kwon S H, Kim D S. Effects of temperature and photoperiod on the production of sexual morphs of Aphis gossypii (Hemiptera: Aphididae) in Jeju, Korea[J/OL]. Journal of Asia-Pacific Entomology, 2017, 20(1): 53-56[2023-07-16]. http://dx.doi.org/10.1016/j.aspen.2016.11.006.
[38] Barberà M, Ca as-Ca as R, Martínez-Torres D. Insulin-like peptides involved in photoperiodism in the aphid Acyrthosiphon pisum[J/OL]. Insect Biochemistry and Molecular Biology, 2019, 112: 103185[2023-07-17]. https://doi.org/10.1016/j.ibmb.2019.103185.
(责任编辑:付毓 责任校对:王小璐)
收稿日期:2023-08-23 第一作者简介:王柳玉(1998―),女,硕士研究生,liuyuwang2021@163.com。 *通信作者:姬继超,hnnydxjc@163.com;崔金杰,aycuijinjie@163.com;马伟华,weihuama@mail.hzau.edu.cn
基金项目:国家自然科学基金(32102214)
