基于网络药理学和分子对接技术的含笑内酯缓解痛风性关节炎作用机制研究
2023-04-29王冰王兰秦向阳
王冰 王兰 秦向阳
摘要:為了探讨含笑内酯(MCL)缓解痛风性关节炎(GA)的潜在靶点及相关作用机制.运用网络药理学技术,检索数据库获取MCL和GA相关靶点,筛选两者交集靶点,构建蛋白互作图并筛选核心靶点.进行基因本体(GO)和信号通路(KEGG)富集分析,绘制“MCL-靶点-通路-GA”网络图并对MCL和核心靶点进行分子对接验证.结果共筛选出MCL相关靶点778个,GA相关靶点351个,交集靶点58个.预测TNF和IL-1Β(IL-1β)等24个靶点为MCL缓解GA的核心靶点.GO分析确定生物过程(BP)相关条目294条,细胞组分(CC)相关条目38条,分子功能(MF)相关条目57条.KEGG分析得到105条信号通路,涉及炎症、代谢、感染及肿瘤等多种相关通路.分子对接结果表明MCL可通过氢键与TNF和IL-1Β(IL-1β)等核心靶点紧密结合,其中MCL与IL-1Β(IL-1β)结合最紧密.初步预测了MCL缓解GA的核心靶点及涉及的生物学过程和信号通路,并进行了分子对接验证,为进一步体内外实验提供了思路.
关键词:网络药理学; 分子对接; 含笑内酯; 痛风性关节炎
中图分类号:R285.5文献标志码: A
Study on mechanism of micheliolide in alleviating gouty arthritis based on
network pharmacology and molecular docking technology
WANG Bing WANG Lan QIN Xiang-yang(1.School of Food Science and Engineering, Shaanxi University of Science & Technology, Xi′an 710021, China; 2.The 986th Hospital, Xijing Hospital, The Air Force Military Medical University, Xi′an 710032, China; 3.School of Pharmacy, The Air Force Military Medical University, Xi′an 710032, China)
Abstract:To investigate the potential targets and related mechanisms of micheliolide (MCL) in alleviating gouty arthritis (GA).The network pharmacology technology was used to retrieve MCL and GA related targets,screen their intersection targets,construct protein interaction maps and screen core targets.Enrichment analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) was conducted,the ″MCL-target-pathway-GA″ network diagram was drawn,and molecular docking verification of MCL and core target was conducted.Results A total of 778 MCL related targets,351 GA related targets and 58 intersection targets were screened out.Twenty-four targets were predicted to be the core targets of MCL inalleviating GA,such as TNF,IL-1B(IL-1β) and so on.GO analysis confirmed 294 items related to biological process (BP),38 items related to cellular components (CC) and 57 items related to molecular function (MF).KEGG analysis revealed 105 signaling pathways,including inflammation,metabolism,infection and tumor.The molecular docking results showed that MCL binds tightly to the core target of TNF and IL-1B (IL-1β) by hydrogen bonding,and the MCL binds most tightly to the core target of IL-1B (IL-1β).The core targets and related biological processes and signaling pathways of MCL alleviating GA were preliminarily predicted,and molecular docking verification was carried out,providing ideas for further in vivo and in vitro experiments.
Key words:network pharmacology; molecular docking; micheliolide; gouty arthritis
0引言
痛风性关节炎(Gouty Arthritis,GA)是一种代谢性免疫疾病,机体内血尿酸水平过高引起尿酸盐(Monosodiumurate,MSU)结晶过饱和析出,沉积于关节腔导致关节红肿热痛,活动受限,严重者还可出现关节畸形残疾[1,2].随着生活水平的提高,患病率明显上升,已成为临床多发病[3,4].中医药有着悠久的历史和丰富的临床经验,中西医结合治疗GA被越来越广泛的应用于临床[5],疗效显著的相关药物有待进一步研究其作用机制.
含笑内酯(Micheliolide,MCL)是一种倍半萜类的天然产物,主要从台湾含笑(Michelia compressa)[6]、云南含笑(Michelia yunnanensis Franch)[7]、黄兰(Michelia champaca)[6]和广玉兰(Magnolia grandiflora)[8,9]等木兰科植物中提取分离得到.《中药大辞典(第二版)》[10]和《中华本草》[11]中记载云南含笑清热解毒,主治咽喉炎,鼻炎,结膜炎和脑漏;黄兰祛风湿,利咽喉,主治风湿痹痛和咽喉肿痛;广玉兰祛风散寒,行气止痛,主治头痛和脘腹胀痛.近代研究表明 MCL对炎症[12,13]和癌症[14,15]具有良好的疗效,本课题组前期研究也发现MCL能抑制急性腹膜炎小鼠血清中多种炎症因子的表达,改善肺和肝损伤[16].MCL结构改良后得到的衍生物66PR可改善小鼠炎症性肠病[17].
网络药理学(Network Pharmacology)由英国药理学家Andrew L Hopkins首次提出并系统阐述[18].通过网络药理学分析可提高新药临床试验的成功率,大大节约药物开发成本[19].分子对接技术(Molecular Docking)通过计算机模型小分子配体和受体生物大分子的匹配程度,预测他们之间的相互作用,筛选出最优构象[20].在药物设计与筛选、药理分析中有广泛应用.
研究发现MCL可治疗类风湿关节炎[21]和强直性脊柱炎[22],说明MCL对关节炎类疾病具有治疗效果,本课题组进行了初步的预实验,发现MCL可缓解小鼠痛风性关节炎.为了提高后期药理实验成功率,本研究拟采用网络药理学的方法初步探索含笑内酯缓解痛风性关节炎的作用机制,并结合分子对接技术进行验证,为进一步的体内外实验提供参考.
1材料与方法
1.1查询与预测MCL相关靶点
在PubChem(https://pubchem.ncbi.nlm.nih.gov)数据库中检索MCL的2D和3D化学结构.通过TargetNet (http://targetnet.scbdd.com),Swiss Target Prediction ( http://www.swisstargetprediction.ch ),SuperPred (https://prediction.charite.de)和ChEMBL (https://www.ebi.ac.uk/chembl)数据库预测MCL相关靶点,并用Uniport(https://www.uniprot.org/)数据库对筛选出的靶点进行标准化.
1.2查询与预测GA相关靶点
在DisGeNET(https://www.disgenet.org)和GeneCards (https://www.genecards.org)数据库中输入关键词“Arthritis,Gouty”和“Gouty Arthritis”检索GA相关靶点,并用Uniprot数据库对筛选出的靶点进行标准化.
1.3获取MCL-GA交集靶点
将获取的MCL和GA相关靶点分别输入Venny (https://bioinfogp.cnb.csic.es/tools/venny/)数据库绘制韦恩图,获取两者的交集靶点.
1.4构建蛋白互作网络图(PPI)及确定核心靶点
将在韦恩图中筛选得到的交集靶点输入String(https://string-db.org/)平台,构建蛋白互作(Protein-Protein Interaction,PPI)网络图.运用Cytoscape(https://cytoscape.org/)软件对PPI网络图进行美化并选出核心靶点.
1.5GO与KEGG通路富集分析
在DAVID(https://david.ncifcrf.gov/)数据库进行核心靶点基因本位 (Gene Ontology,GO)富集分析和京都基因和基因组百科全书 (Kyoto Encyclopedia of Genes and Genomes,KEGG)通路富集分析.
1.6“化學成分-靶点-通路-疾病”网络构建
使用Cytoscape软件构建“MCL-靶点-通路-GA”网络关系图.
1.7分子对接验证
从PDB (https://www.rcsb.org/)数据库和ZINC (https://zinc.docking.org/) 数据库分别下载核心靶点蛋白质结构和MCL结构.对结构进行前处理后,用AutoDock (https://autodock.scripps.edu/)软件进行对接,并用Pymol (https://pymol.org/2/)软件对最优结合构象进行可视化分析.
2结果与讨论
2.1MCL的2D和3D结构
将含笑内酯(micheliolide,MCL)输入 PubChem数据库,获取其2D结构(图1)和3D结构(图2).
2.2筛选 MCL、GA相关靶点及交集靶点
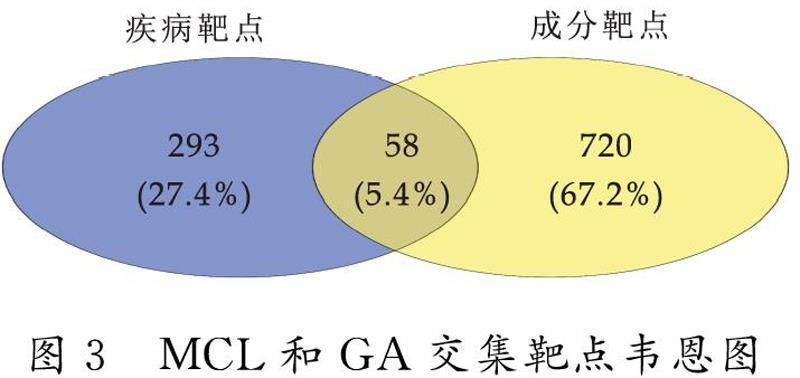
从数据库TargetNet,SuperPred,ChEMBL和Swiss Target Prediction中筛选去重后收集到与MCL相关的靶点共778个.从数据库DisGeNET和GeneCards中筛选去重后收集到与GA相关的靶点共351个.导入Venny平台做韦恩图,获取两者交集靶点共58个(图3).
2.3构建 PPI 网络并确定MCL缓解GA的核心靶点将58个交集靶点提交至String数据库,得到PPI网络图(图4),该网络共有58个节点,328条边,平均度值(Degree)为11.3.为了更加清楚地体现核心靶点在PPI中的调控作用,通过Cytoscape软件进行可视化分析,得到美化后的PPI网络图(图5).根据度值大小进行排序,度值较高的则可能为核心靶点,TNF,IL-1Β(IL-1β)等24个靶点度值大于平均度值,在PPI网络中发挥关键调控作用,预测为核心靶点.核心靶点及其度值如表1所示.
2.4GO功能富集分析
GO分析包括生物过程(Biological Process,BP)、细胞组分(Cellular Component,CC) 和分子功能(Molecular Function,MF)分析.共获得BP相关条目294条,涉及细胞对脂多糖的反应,对炎症反应的调节,对细菌来源分子的反应,对含嘌呤化合物的反应,对有机磷的反应等.CC相关条目38条,涉及膜筏,膜微结构域,膜区等.MF相关条目57条,涉及RNA聚合酶II特异性DNA结合转录因子,DNA结合转录因子,药物结合,核受体活性,配体激活转录因子活性等.根据富集度(Enrichment Score)筛选每项前10条绘制柱状图(图6).结果显示MCL缓解GA的机制主要与细胞受炎性刺激物刺激、生物合成过程调控、细胞周期调控等有关.其中炎症反应是GA患者的主要问题,缓解炎症反应是治疗GA的主要方式.
2.5KEGG 信号通路分析
对MCL缓解GA的核心靶点进行KEGG分析,得到105条通路途径,取排名靠前的30条通路做气泡图(图7).前30条通路中涉及炎症、代谢、感染及肿瘤等多种相关通路,其中涉及炎症的主要通路有IL-17信号通路(IL-17 signaling pathway)、Nod样受体信号通路(Nod-like receptor signaling pathway)、Toll样受体信号通路(Toll-like receptor signaling pathway)、NF-κB信号通路(NF-kappaB signaling pathway)、TNF信号通路(TNF signaling pathway)、低氧诱导因子-1信号通路(HIF-1 signaling pathway)、血管内皮生长因子信号通路(VEGF signaling pathway)等.
2.6构建“成分-靶点-通路-疾病”网络关系图
为了进一步研究MCL、核心靶点、信号通路及GA之间的相互关系,将筛选的24个核心靶点和前30条信号通路导入Cytoscape软件,构建“MCL-核心靶点-信号通路-GA”网络图(图8).绿色菱形代表MCL,黄色圆圈代表核心靶点,蓝色三角形代表相关信号通路,橙色正方形代表GA.灰色连线代表各点之间的作用关系,线条的数量与作用关系呈正相关.由图可得,MCL緩解GA有多个治疗靶点,同一靶点可调节多个信号通路,体现出MCL可多靶点和多信号通路协同缓解GA.
2.7分子对接验证
根据GO富集分析得知炎症反应是GA患者的主要问题,缓解炎症反应是治疗GA的主要方式.
KEGG分析得到与炎症相关的主要通路有IL-17信号通路,Nod样受体信号通路,Toll样受体信号通路,NF-κB信号通路,TNF信号通路等,参与这些炎症通路且蛋白互作度值较大的核心靶点有TNF,IL-1Β(IL-1β),CASP1,MAPK14,MMP9,PTGS2,RELA等.将这些度值大且与炎症相关的靶点与MCL进行分子对接,以验证预测结果的准确性.
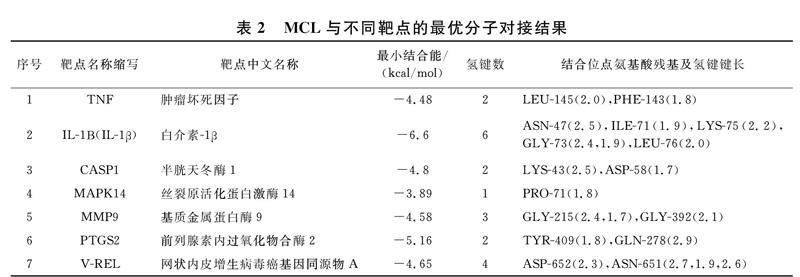
结合能力用自由结合能大小评价,自由结合能越小,结合越紧密.对每个靶点最优的对接构象进行可视化分析(图9).MCL与不同靶点的最小自由结合能、氢键个数、结合残基位点及氢键键长见表2所示.结果表明MCL可通过氢键与TNF,IL-1Β(IL-1β),CASP1,MAPK14,MMP9,PTGS2,RELA的氨基酸残基紧密结合.MCL与IL-1Β(IL-1β)结合的氢键个数最多,结合能最小,结合最紧密.
白细胞介素-1β(IL-1Β,IL-1β)是促炎细胞因子家族中的重要成员,具有较强的促炎活性,是炎症反应的重要介质[23],在痛风发病机制中发挥核心作用[24].NLRP3炎性小体是一种胞内多蛋白复合体,由Nod样受体蛋白NLRP3,衔接蛋白ASC以及效应蛋白前体半胱天冬酶1 (pro-caspase-1)组成复合体.IL-1β的前体pro-IL-1β不具有生物活性,被半胱天冬酶1 (CASP1,Caspase-1)蛋白水解产生成熟的IL-1β后触发炎症反应并介导细胞焦亡[25].抑制炎症小体NLRP3释放IL-1β可有效缓解急性痛风的炎症表现[26].Wu等[27]发现MCL通过调节NLRP3炎症小体介导的细胞焦亡改善放射性肠炎.Tian等[22]发现MCL通过降低强直性脊柱炎模型小鼠NLRP3,caspase-1等的蛋白表达水平,调节NLRP3 / NF-κB信号通路,治疗强直性脊椎炎.本研究分子对接实验也表明MCL可与IL-1Β(IL-1β)和CASP1紧密结合,MCL与IL-1Β(IL-1β)蛋白结构中的ASN-47,ILE-71,LYS-75,GLY-73,LEU-76五个氨基酸残基形成6个分子间氢键,结合紧密.预测MCL可通过抑制Nod样受体NLRP3信号通路的IL-1Β(IL-1β),CASP1等核心靶点的表达,缓解痛风性关节炎的炎症反应.
肿瘤坏死因子-α (TNF-α) 是细胞凋亡及免疫和炎症反应的重要调节因子,在许多急慢性炎症中发挥关键作用[28].通过调控TNF依赖的膜相关和细胞内蛋白信号复合物分子的泛素化和磷酸化[29],改善类风湿关节炎、强直性脊柱炎和克罗恩病等疾病[30].Toll样受体4(TLR4)可参与识别疾病相关分子,激活促炎细胞因子[31].V-REL网状内皮增生病毒癌基因同源物A(RELA)也是NF-κB通路的关键分子,具有调节炎症,中性粒细胞凋亡的重要作用[32].丝裂原活化蛋白激酶(MAPK)能被多种炎性刺激激活,MAPK14也称作p38-α,通过转录因子磷酸化而参与信息传递,调节炎症反应[33].GA患者Toll样受体信号通路的TLR4等受体通过识别沉积在关节处的过饱和的炎症刺激MSU,进一步激活NF-κB信号通路,调控TNF-α等炎症细胞因子的释放,加速关节炎症反应[34,35].Zhang等[36]发现MCL可抑制IL-1Β(IL-1β),TNF-a的分泌,影响PI3K/Akt/NF-κB通路和NLRP3炎症小体的调控,从而缓解结核分枝杆菌诱导的炎症反应,辅助治疗结核病.Kalantary等[37] 发现MCL可降低TNF-α的基因表达,调节PI3K/Akt/NF-κB信号通路,对阿霉素诱导的心脏毒性具有保护作用.Lei等[38]发现MCL可抑制TNF-α,IL-1β,caspase-1和ROS的分泌,调节mROS/NF-κB/NLRP3信号通路,抑制脂多糖诱导的肾炎.本研究分子对接实验也表明MCL可与TNF,MAPK14,RELA紧密结合,预测MCL可通过抑制Toll样受体信号通路的TNF等核心靶点的表达,调节NF-κB信号通路,缓解痛风性关节炎的炎症反应.
前列腺素内过氧化物合酶2(PTGS2)又叫环氧合酶2(COX-2),是前列腺素合成的关键酶,其介导的花生四烯酸代谢在炎症性疾病中发挥着重要作用,密切参与炎症反应[39].基质金属蛋白酶9(MMP9)在骨发育和修复中发挥重要作用[40],也在炎症细胞中表达并参与炎症反应[41].白细胞介素17(IL-17)信号通路不仅参与炎症调节,还可通过诱导MMP9和COX-2,直接造成关节软骨和滑膜破坏[42,43].Sun等[44]发现MCL可降低COX-2,TNF-a,IL-6和IL-1β的表达,激活IkBa/NF-κB/Akt通路,在神经炎症相关的神经退行性疾病中发挥神经保护剂的作用.本研究分子对接实验表明MCL可与PTGS2和 MMP9紧密结合,预测MCL可通过抑制PTGS2(COX2)等靶点的表达,参与IL-17信号通路,调控炎症反应和软骨细胞增殖分化与凋亡,减轻痛风性关节炎的炎症反应和关节软骨退化情况.
3结论
痛风性关节炎(GA)是一种炎症性自身免疫性疾病,关节炎症反应是GA患者的主要问题,控制炎症反应是治疗GA的可行方法.本研究借助网络药理学初步探索了MCL缓解痛风性关节炎的作用机制.筛选出TNF,IL-1Β(IL-1β)等24个靶点为MCL缓解GA的核心靶点,并用分子对接技术验证了MCL可通过氢键与核心靶点TNF,IL-1Β(IL-1β),CASP1,MAPK14,MMP9,PTGS2和RELA的氨基酸残基紧密结合,其中MCL与IL-1Β(IL-1β)的结合最紧密.预测到MCL缓解GA涉及炎症、代谢、感染及肿瘤等多种相关通路,与调控炎症相关的主要有IL-17,Nod样受体,Toll样受体,NF-κB和TNF信号通路等.为后续更深层次的体内外实验研究验证及临床治疗提供了思路及理论依据.
参考文献
[1] 徐鹏,刘树民,于栋华,等.痛风性关节炎治疗的研究进展[J].中国医药导报,2022,19(5):44-47.
[2] Wilson L,Saseen J J.Gouty arthritis:A review of acute management and prevention[J].Pharmacotherapy,2016,36(8):906-922.
[3] Keyer G.Gout arthritis:Pathogenesis,diagnostics and treatment[J].Deutsche Medizinische Wochenschrift,2020,145(14):991-1 005.
[4] Keller S F,Mandell B F.Management and cure of gouty arthritis[J].Rheumatic Disease Clinics of North America,2022,48(2):479-492.
[5] 郑颖,唐红珍.中医药治疗痛风性关节炎临床研究进展[J].国际中医中药杂志,2021,43(9):941-945.
[6] Gach K,Janecka A.α-Methylene-γ-lactones as a novel class of anti-leukemic agents[J].Anticancer Agents in Medicinal Chemistry,2014,14(5):688-694.
[7] 丁林芬,程彬,晏通,等.云南含笑果實中倍半萜类化学成分研究[J].中草药,2017,48(13):2 608-2 613.
[8] Ding L F,Su J,Pan Z H,et al.Cytotoxic sesquiterpenoids from the leaves of Magnolia grandiflora[J].Phytochemistry,2018,155:182-190.
[9] 丁林芬,郭亚东,潘争红,等.荷花玉兰叶中2个新的倍半萜[J].中草药,2017,48(17):3 463-3 468.
[10] 南京中医药大学.中药大辞典[M].2版.上海:上海科技出版社,2018.
[11] 国家中医药管理局《中华本草》编委会.中华本草第二册[M].1版.上海:上海科技出版社,2006:879-895.
[12] Liu W T,Chen X W,Wang Y X,et al.Micheliolide ameliorates diabetic kidney disease by inhibiting Mtdh-mediated renal inflammation in type 2 diabetic db/db mice[J].Pharmacological Research,2019,150:104 506.
[13] Wu D M,Li J,Shen R,et al.Autophagy induced by micheliolide alleviates acute irradiation-induced intestinal injury via inhibition of the NLRP3 inflammasome[J].Frontiers in Pharmacology,2022,12:773 150.
[14] Tang X,Ding Q,Chen C,et al.Micheliolide inhibits gastric cancer growth in vitro and in vivovia blockade of the IL-6/STAT3 pathway[J].Pharmazie,2019,74(3):175-178.
[15] Viennois E,Xiao B,Ayyadurai S,et al.Micheliolide,a new sesquiterpene lactone that inhibits intestinal inflammation and colitis-associated cancer[J].Laboratory Investigation,2014,94(9):950-965.
[16] Qin X Y,Jiang X R,Jiang X,et al.Micheliolide inhibits LPS-induced inflammatory response and protects mice from LPS challenge[J].Scientific Reports,2016,6:23 240.
[17] Chen Y Z,Qin X Y,An Q X,et al.Mesenchymal stromal cells directly promote Inflammation by canonical NLRP3 and non-canonical caspase-11 inflammasomes[J].EBioMedicine,2018,32:31-42.
[18] Hopkins A L.Network pharmacology[J].Nature Biotechnology,2007,25(10):1 110-1 111.
[19] 蘇瑶,王兰,常相娜,等.基于网络药理学及分子对接技术探讨绞股蓝防治肥胖的作用机制[J].食品工业科技,2022,43(4):12-23.
[20] 张金,王良鹏,赵佳文,等.1-苯基-1′H-螺[吲哚-3,2′-喹唑啉]-2,4′(H)-二酮及其衍生物抗炎活性及分子对接研究[J].陕西科技大学学报(自然科学版),2015,33(3):94-98.
[21] Xu H,Wang J,Wang C,et al.Therapeutic effects of micheliolide on a murine model of rheumatoid arthritis\[J\].Molecular Medicine Reports,2015,11(1):489-493.
[22] Tian Z G,Yao M,Chen J.Micheliolide alleviates ankylosing spondylitis (AS) by suppressing the activation of the NLRP3 inflammasome and maintaining the balance of Th1/Th2 via regulating the NF-κB signaling pathway[J].Annals of Translational Medicine,2020,8(16):991.
[23] Weber A,Wasiliew P,Kracht M.Interleukin-1beta (IL-1βeta) processing pathway[J].Science Signaling,2010,19(3):105.
[24] So A,Dumusc A,Nasi S.The role of IL-1 in gout:From bench to bedside[J].Rheumatology(Oxford),2018,57(suppl_1):i12-i19.
[25] Zhang J,Liu X,Wan C,et al.NLRP3 inflammasome mediates M1 macrophage polarization and IL-1β production in inflammatory root resorption[J].Journal of Clinical Periodontology,2020,47(4):451-460.
[26] Renaudin F,Orliaguet L,Castelli F,et al.Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages[J].Annals of the Rheumatic Diseases,2020,79(11):1 506-1 514.
[27] Wu D M,Li J,Shen R,et al.Autophagy induced by micheliolide alleviates acute irradiation-induced intestinal injury via inhibition of the NLRP3 inflammasome[J].Frontiers in Pharmacology,2022,18(12):773 150.
[28] Holtmann M H,Neurath M F.Differential TNF-signaling in chronic inflammatory disorders[J].Current Molecular Medicine,2004,4(4):439-444.
[29] Varfolomeev E,Vucic D.Intracellular regulation of TNF activity in health and disease[J].Cytokine,2018,101:26-32.
[30] Holbrook J,Lara Reyna S,Jarosz Griffiths H,et al.Tumour necrosis factor signalling in health and disease[J].F1000 Research,2019,8:111.
[31] Campolo M,Paterniti I,Siracusa R,et al.TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson′s diseases in vivo model[J].Brain Behavior Immunity,2019,76:236-247.
[32] 耿云峰,杜鸿斌,刘琳琳,等.NF-κB家族成员RelA的翻译后修饰及其生理病理作用的研究进展[J].生命科学,2020,32(5):431-438.
[33] Sanz Ezquerro J J,Cuenda A.p38 signalling pathway[J].International Journal of Molecular Sciences,2021,22(3):1 003.
[34] Liu Bryan R,Scott P,Sydlaske A,et al.Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation[J].Arthritis Rheumatology,2005,52(9):2 936-2 946.
[35] 蔣莉,周京国,青玉凤,等.Toll样受体2和Toll样受体4及其信号通路在原发性痛风性关节炎发病机制中作用的研究[J].中华风湿病学杂志,2011,15(5):300-304.
[36] Zhang Q,Jiang X,He W,et al.MCL plays an anti-inflammatory role in mycobacterium tuberculosis-induced immune response by inhibiting NF-κB and NLRP3 inflammasome activation[J].Mediators of Inflammation,2017,2017:2 432 904.
[37] Kalantary Charvadeh A,Sanajou D,Hemmati Dinarvand M,et al.Micheliolide protects against doxorubicin-induced cardiotoxicity in mice by regulating PI3K/Akt/NF-kB signaling pathway[J].Cardiovascular Toxicology,2019,19(4):297-305.
[38] Lei X H,Li S T,Luo C W,et al.Micheliolide attenuates lipopolysaccharide-induced inflammation by modulating the mROS/NF-κB/NLRP3 axis in renal tubular epithelial cells[J].Mediators of Inflammation,2020,2020:3 934 769.
[39] Simon L S.Role and regulation of cyclooxygenase-2 during inflammation[J].The American Journal of Medicine,1999,106(5B):37S-42S.
[40] Burrage P S,Mix K S,Brinckerhoff C E.Matrix metalloproteinases:Role in arthritis[J].Frontiers in Bioscience,2006,11:529-543.
[41] Corry D B,Kiss A,Song L Z,et al.Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines[J].Faseb Journal,2004,18(9):995-997.
[42] Shui X L,Lin W,Mao C W,et al.Blockade of IL-17 alleviated inflammation in rat arthritis and MMP-13 expression[J].European Review for Medical and Pharmacological Sciences,2017,21(10):2 329-2 337.
[43] Du B,Zhu M,Li Y,et al.The prostaglandin E2 increases the production of IL-17 and the expression of costimulatory molecules on γδ T cells in rheumatoid arthritis[J].Scandinavian Journal of Immunology,2020,91(5):e12 872.
[44] Sun Z,Li G,Tong T,et al.Micheliolide suppresses LPS-induced neuroinflammatory responses[J].PLOS One,2017,12(10):e0 186 592.
【责任编辑:蒋亚儒】
