Hydrodeoxygenation of lignin derived bio-oil into aromatic hydrocarbons over Ni-Cu-Ru/HZSM-5 catalyst
2023-03-11LIBingshuoFENGBixuanWUKaiyeYANGTianhua
LI Bing-shuo,FENG Bi-xuan,WU Kai-ye,YANG Tian-hua
(Shenyang Aerospace University, College of Energy and Environment, Key Laboratory of Clean Energy of Liaoning,Shenyang 110136, China)
Abstract: The complexity and diversity of lignin derived bio-oil (LDB) has posed a great challenge to the subsequent processing and utilization.In this work,HZSM-5 was modified by sodium hydroxide and followed by Ni,Cu and Ru species.LDB was used as the raw biocrude to prepare bio-oil rich in aromatic hydrocarbons with modified HZSM-5 catalysts under supercritical ethanol conditions (320 °C,14 MPa).Results showed that the desilicated HZSM-5 with the loading of Ni,Cu and Ru (Ni-Cu-Ru/DeHZSM-5) exhibited the best catalytic performance with a high relative amount of aromatic hydrocarbons of 28.95%.After catalytic hydrodeoxygenation (HDO) of LDB,80.40% upgraded bio-oil (UBO) with 96.32% energy recovery was obtained in the presence of Ni-Cu-Ru/DeHZSM-5.Demethoxylation and dehydration were the main reactions in the catalytic HDO process.Potential reaction pathways of guaiacol,syringol and creosol were also proposed in this paper.The heating value of UBO reached 35.22 MJ/kg compared with LDB,which was increased by 19.80%.The water content and viscosity of UBO were also significantly improved.The micro-mesoporous structure of modified HZSM-5 with loading of Ni,Cu and Ru was beneficial to promote the yield of the aromatic hydrocarbons.
Key words: lignin;bio-oil;hydrodeoxygenation;catalyst;mechanism;aromatic hydrocarbons
Lignin is a kind of main component of plant,which accounts for about 15%-35% of the dry weight varies with different types of lignocellulosic biomass[1].Commercially,lignin is produced as a kind of waste or byproduct from the paper and pulp industry or cellulosic bioethanol plants,which is usually used for combustion to generate heat as low-quality solid fuel.Valorization of lignin into high-value products has been received extensive attention for the sustainable development of industries.Because of its aromatic structure in nature,the production of transportation fuels (e.g.,jet fuel) from lignin has become a hotspot[2].
Several methods have been reported in literature that include thermochemical,biological,electrochemical,etc.,for the depolymerization and conversion of lignin into value-added products[3].Currently,thermochemical methods include two routes.One is the direct conversion of lignin into hydrocarbon liquid fuels via catalytic pyrolysis[4]or solvolysis[5],and the other is the depolymerization of lignin to bio-oil and subsequent bio-oil catalytic hydrodeoxygenation(HDO) to biofuels.The depolymerization product of lignin is usually called lignin-derived bio-oil (LDB),which contains various phenolic compounds,such as phenolic monomers,dimers,oligomers,etc[6].However,LDB has low calorific value,poor stability and high viscosity,which limit its direct use as a transportation fuel. Therefore,further deoxygenation and hydroprocessing are required to produce liquid hydrocarbon fuels.The route of on-line conversion lignin to biofuels is a promising strategy in a heterogeneous system.This strategy combines the lignin depolymerization into LDB and the catalytic HDO of LDB in one pot.However,after reaction the heterogeneous catalyst is mixed in the solid residues,which is difficult to be separated and recycled.Thus,catalytic HDO of LDB is widely studied in literature[7-9],including upgrading of lignin model compounds[10,11],which has been the most promising approach of lignin valorization[12].
Metal-based catalysts are the key factors in heterogeneous catalysis,and the metal site and catalyst support are of great importance for the catalytic HDO of LDB.Ni-based catalysts as non-precious metal catalysts are considered to be the HDO catalysts with the great development potential owing to their cheap price and excellent catalytic performance for the production of aromatic hydrocarbons[13,14].However,Ni-based catalysts are prone to rapid deactivation due to coke deposition,which will greatly affect the conversion yield and product selectivity.The addition of promoters is an interesting option for decreasing the coke formation.Bykova et al.[15]observed that Cu addition to Ni/SiO2reduced the carbon formation from 15.8% to 2.3%.Li et al.[16]also proved that Ni-Cu/HZSM-5 could prevent deactivation more effectively than Ni/HZSM-5 when reaction temperature increased from 250 to 330 °C.Besides,Ni-based catalysts modified with different promoters had different catalytic effect on the HDO process,whereas noble metals Ru showed better activity on the hydrogenation reaction of the aromatic rings in the HDO process due to the high hydrogen adsorption and dissociation[17].Lin et al.[14]studied the conversion of lignin into monophenols via Ni-Ru/ZSM-5 catalyst,and the results showed that the metal Ru provided the possibility to modify the properties of Ni-based catalysts and improved its hydrogenation reaction performance.
Various catalyst supports have been studied in the HDO process,such as ZrO2,SiO2,activated carbon(AC),Al2O3,and CNTs.Among them,zeolites have good thermal stability and provide suitable Lewis acid and Brønsted acid sites,which show great potential for the HDO reactions[12].For example,PdNi/HZSM-5 catalyst showed the good HDO catalytic activity for lignin model compounds[13].Bifunctional catalysts showed better catalytic activity on deoxygenation and hydrogenation reaction for the LDB[12].But in the presence of water,the catalysts suffered from serious catalyst deactivation such as leaching,sintering and collapse of catalyst support[18].On the whole,further improvements of Ni-based zeolites catalysts and the reaction environment are still needed to enhance the activity,selectivity and stability of the catalysts.
In this article,the Ni/HZSM-5 catalysts modified by Cu and Ru were investigated on the HDO reaction of LDB in supercritical ethanol system.And the effects of promoters (Cu and Ru) on the catalyst were also studied in the HDO process of LDB for aromatic hydrocarbons.This work was our continued research on the valorization of lignin and would further improve the conversion from lignin to liquid biofuels by twostep including hydrogenolysis and HDO process.
1 Materials and methods
1.1 Materials
LDB sample was obtained from rice husk lignin,which was the same as the one reported in our previous study[6].The lignin was extracted in ethanol at 180 °C for 2 h from 30-50 mesh rice husk.And then,converting the extracted lignin to phenolic-rich oil in sub-supercritical ethanol system in CO2atmosphere.And the liquid product was the LDB sample.
Sodium hydroxide (NaOH),ammonium nitrate,nickel (II) nitrate hexahydrate (Ni(NO3)2·6H2O),copper (II) nitrate trihydrate (Cu(NO3)2·3H2O) and ruthenium (III) chloride trihydrate (RuCl3·3H2O) were purchased from Kermel Chemical Reagent Co.,Ltd,China.HZSM-5 (Si/Al=25) was purchased from Nankai University catalyst Co.,Ltd,China.Ethanol and acetone were both analytical grade.
1.2 Preparation of catalysts
Wetness impregnation method was used to prepare the catalysts.Firstly,the HZSM-5 was added in 0.5 mol/L NaOH solution by stirring at 80 °C for 2 h and then filtered.After drying the solid parts,the HZSM-5 was ion-exchanged with a 0.8 mol/L NH4Cl solution at 80 °C for 10 h together with filtering.After ion-exchange,the HZSM-5 were calcined at 600 °C for 6 h.And then,the HZSM-5 was washed with distilled water to neutrality.After drying at 105 °C for 12 h,the desilication of HZSM-5 (DeHZSM-5) was obtained.Typically,Cu/DeHZSM-5 was prepared by impregnating DeHZSM-5 with a solution of nickel Cu(NO3)2·3H2O (4.5% Cu was loaded).Ni modified Cu/DeHZSM-5 was prepared by impregnation method with the aqueous solutions of Ni(NO3)2·6H2O (3% Ni was loaded).Ru modified Ni-Cu/DeHZSM-5 was prepared by impregnation method with the aqueous solutions of RuCl3·3H2O (1.5% Ru was loaded).
1.3 Hydrodeoxygenation of LDB
Catalytic HDO of LDB was conducted in a 100 mL autoclave.In each run,5 g LDB,50 mL ethanol and 1 g catalyst were placed into the reactor.The system was sealed and the air inside the reactor was removed by purging nitrogen gas (99.999%) for 3 times.Subsequently,the reactor was purged with pure hydrogen gas (99.999%) for 3 times and pressurized to 5.0 MPa.The HDO of LDB was carried out at 320 °C and 300 r/min for 60 min.After the reaction,the reactor was cooled down to room temperature by a fan and then the exhaust valve was opened to release the noncondensable gas.The solid-liquid mixed products were collected,separated by vacuum filtration and the filter cake was extracted repeatedly with acetone until it was colorless.Finally,the ethanol and acetone were removed by rotary evaporation and the upgraded bio-oil (UBO)was obtained.The filter cake was dried in the oven for 12 h at 105 °C to obtain solid residue.The yields of UBO and solid products were determined by measuring the weight of the collected liquid and solid phase.The gas yield was estimated by difference. All the experiments were repeated at least three times under the same conditions and the mean values were reported.
Yields of UBO,solid residue,and gas were calculated according to the following formulas:
where UBO (g) was the weight of UBO;LDB (g) was the weight of LDB;solid residue (g) was the weight of solid residue;catalyst (g) was the weight of catalyst.
The energy recovery from LDB to UBO was calculated as following:
where HHVUBOand HHVLDBwere the HHV of UBO and LDB,respectively.
The H/C and O/C mole ratio were calculated as following:
wheremHand HMwere the percentage (%) and the relative atomic mass of hydrogen,respectively;mCand CMwere the percentage (%) and the relative atomic mass of carbon,respectively;mOand OMwere the percentage (%) and the relative atomic mass of oxygen,respectively.
1.4 Characterizations of catalysts
X-ray diffraction (XRD) spectra of the catalysts were recorded with a PW3040/60X X ’Pert MPD diffractometer using CuKα (40 kV,40 mA,λ=0.154 nm)radiation.The 2θangle was scanned from 10 to 75° at a speed of 3(°)/min.The surface morphology of catalyst was observed by scanning electron microscopy (SEM,FEI Inspect F50) coupled with energy dispersive X-ray spectroscopy (EDX) to determine the elemental composition.The specific surface area and pore size distribution of the catalysts were determined by N2isothermal adsorption on Micrometric Acusorb 2100E physical adsorber.The Brunauer-Emmett-Teller (BET)equation was used to calculate the specific surface area.The mesopore size was calculated using the Barrett-Joyner-Halenda (BJH) method.The micropore size was calculated using thet-plot method.
1.5 Characterizations of oils
The elemental compositions of oils were analyzed by the elemental analyzer (EA3000,Italy).The functional groups of oils were characterized by attenuated total reflectance Fourier transfer infrared spectroscopy (ATR-FTIR,Nicolet IS50,Thermo Fisher Nicolet,America).The chemical compositions of oils were characterized using gas chromatography-mass spectrometry (GC-MS,Agilent 7890A-5975C)equipped with a HP-5MS capillary column (30 m ×25 mm × 0.25 μm).Helium was used as the carrier gas(flow rate 1 mL/min).The sample was injected 1 μL with split ratio of 50∶1.The temperature of oven was increased from 28 (hold for 3 min) to 180 °C at 5 °C/min,and then to 280 °C at 10 °C/min (hold for 10 min).The ionization was conducted in EI mode,and the electron energy was 70 eV.The compounds were recognized by comparing the spectra of sample component with those in the electron impact mass spectrum from a NIST-14 Database.The water content and viscosity of the oils were determined using Karl Fischer moisture titrator (SYD-2122C,Shanghai Changji Geological Instrument CO.,LTD) and kinematic viscosity tester (SYD-265H,Shanghai Changji Geological Instrument CO.,LTD),respectively.
2 Results and discussion
2.1 Characterization of catalysts
2.1.1 XRD results
Figure 1 showed XRD patterns of modified and fresh HZSM-5 catalysts.Characteristic diffraction peaks located at 23.14°,23.97° and 24.44° indicated the typical MFI topological structure of HZSM-5[19].The characteristic diffraction peaks of HZSM-5 and DeHZSM-5 indicated that the structure of HZSM-5 might not change after desilication,but the crystal structure of the HZSM-5 was destroyed to some extent as a result of partial dissolution with the diffraction peak intensity of DeHZSM-5 significantly weakened.X-ray reflections at 2θvalues of 38.04° and 43.19°were assigned to the NiO phase,suggesting the formation of nickel particles on the surface of the catalyst.X-ray reflections at 2θvalues of 35.5° and 38.7° results of Cu-impregnated catalysts belonged to the CuO phase,and X-ray reflections at 2θvalues of 28° and 54° results of Ru-impregnated catalysts belonged to the Ru2O phase.The relative dispersion characteristics peaks of the active components indicated that the metals (Ni,Cu and Ru) were well dispersed on the surface of the catalysts.
2.1.2 SEM-EDX results
Figure 2 showed the SEM-EDX images of modified and fresh HZSM-5 catalysts.SEM images showed the surface structure of the catalysts.The DeHZSM-5 surface was rougher than that of HZSM-5,which increased the surface area and total pore volumes (Table 1).From the EDX images,Ni,Cu and Ru were detected and the mass percentage of a random dot on the catalyst surface were 1.92%,4.38% and 0.86%,respectively.

Figure 1 XRD patterns of modified and fresh HZSM-5 catalysts

Figure 2 SEM images and EDS mapping of modified and fresh HZSM-5 catalysts
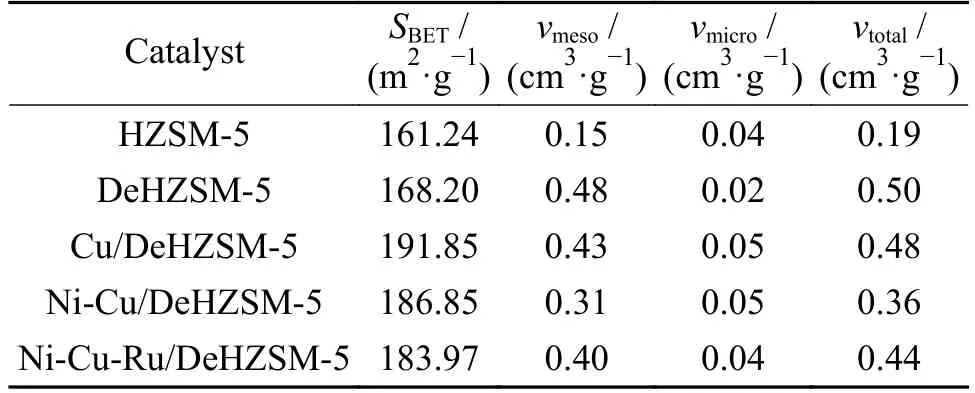
Table 1 Textural properties of modified and fresh HZSM-5 catalysts
2.1.3 BET results
Table 1 listed the textural properties of modified and fresh HZSM-5 catalysts determined by nitrogen adsorption-desorption isotherms. The mesopore volumes were significantly increased by desilication.The formation of mesoporosity was accompanied by the expense of microporosity,which resulted in the decrease of micropore volumes.The large BET surface area of Cu/HZSM-5 was helpful for the metal sites (Ni and Ru) to disperse well over the catalyst surface.The surface area and pore volume were reduced with incorporation NiO and Ru2O on Cu/HZSM-5,indicating that the pore inside was filling of metals[5].
2.2 Effect of catalysts on the products distribution
The products yields from catalytic HDO of LDB were shown in Figure 3.Compared with the noncatalytic HDO process (blank),the UBO yield increased with the addition of catalysts mainly due to the reduction of solid residue.The highest UBO yield of 80.40% and the lowest solid residue yield of 1.80%were obtained with Ni-Cu-Ru/DeHZSM-5 followed by Ni-Cu/DeHZSM-5.This indicated that the trimetallic Ni-Cu-Ru/HZSM-5 catalyst performed well in the suppression of coke formation than that of bimetallic Ni-Cu/DeHZSM-5 catalyst.Adding metallic Ru to the Ni-Cu/DeHZSM-5 could effectively increase the total conversion of LDB mainly due to the excellent catalytic hydrogenation performance of Ru.Ignoring the effects of ethanol and hydrogen on the contribution to the UBO yield,the energy recovery was significantly enhanced by the addition of the catalysts.A relatively higher energy recovery of 96.32% was obtained with Ni-Cu-Ru/DeHZSM-5 catalyst.
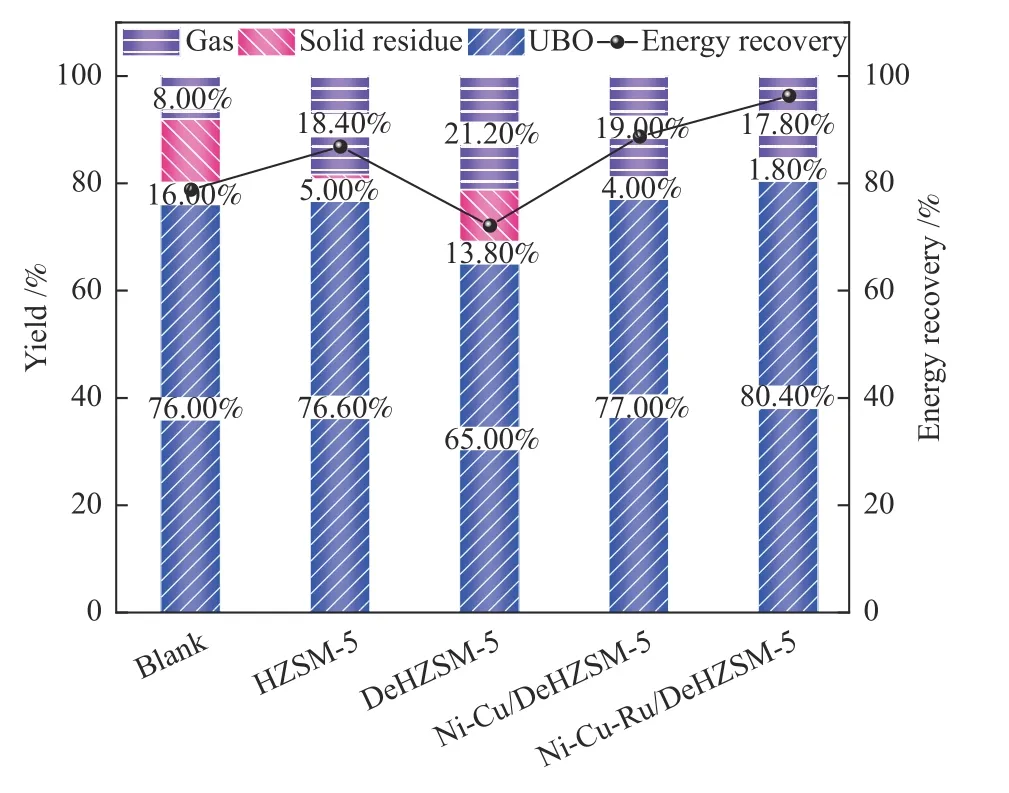
Figure 3 Products yields from HDO of LDB under different catalytic conditions
2.3 Characterization of oils
2.3.1 Physicochemical analysis
The results of the elemental analysis and properties of LDB and UBO were shown in Table 2.As expected,with the presence of catalysts,much lower oxygen content,higher carbon content and higher hydrogen content were obtained in the UBO.The HHVs of UBO were remarkably increased from 29.29 to 35.28 MJ/kg due to the lower atomic O/C,suggesting that Ni-Cu-Ru/DeHZSM-5 contributed more to the deoxygenation (47.01%) and hydrogenation reactions.The UBO showed superior properties related to water content and viscosity than that of LDB.The higher content of hydrophobic products such as hydrocarbons might contribute to the water removal in the UBO.Generally,the higher oxygen content of UBO was responsible for the high viscosity[20].The viscosity of UBO was decreased from 38.95 to 16.82 mm2/s with the assisted of Ni-Cu-Ru/DeHZSM-5,which was also attributed to the decrease of long carbon chain groups (e.g.,esters).

Table 2 Elemental analysis and properties of LDB and UBO
2.3.2 FT-IR analysis
The functional groups of UBO obtained at 320 °C with and without catalysts were detected by FT-IR,and the absorption spectra were shown in Figure 4.The broad band between 3200-3450 cm-1was O-H stretching vibrations of carboxylic,phenolic and water in the oils[22].The peaks at 3000-2870 cm-1and 1500-1300 cm-1were attributed to C-H stretching (alkanes and aromatics) and C-H bending (alkanes),respectively[22].The intense peak around 1710 cm-1was ascribed to C=O stretching indicating the presence of ketones,aldehydes,carboxylic acids or esters in the oils.Absorption peaks at 1300-1000 cm-1were mainly caused by the C-O stretching vibration of the esters,alcohols or ethers[22].With the intensity of C=O and C-O stretching weakened,it was postulated that the catalysts promoted the HDO reactions,and the Ni-Cu-Ru/DeHZSM-5 performed the best of all the modified HZSM-5 catalysts.The C=C vibration absorption peaks at 1600 and 1515 cm-1indicated the presence of alkenes and aromatics,respectively[23].The peaks appearing in the range of 900-700 cm-1were attributed to the aromatic stretching vibrations of aromatic compounds[23],and the increased intensity of aromatic stretching of UBO indicated that the content of aromatics in the UBO was higher than that in the LDB.

Figure 4 FT-IR spectra of oils obtained under different catalytic conditions
2.3.3 GC-MS analysis
The compositions of UBO obtained at 320 °C with and without catalysts were qualitatively analyzed by GC-MS.The main components (area peak >0.2%) in the UBO could be divided into acids,ketones,esters,alcohols,phenolics,aromatic and alkanes (Figure 5).The details of detected compounds were shown in supplementary Table S1.The main compounds in the LDB were phenolic compounds(56.78%),which were derived from the lignin decomposition,and the same results were found in our previous study[24].Comparing to the LDB,the content of phenolics in the UBO was much higher when HZSM-5 was used as catalyst.The results were consisted with Hita et al.[25],who demonstrated that less acidic HZSM-5 zeolite was preferred to enhance the yields of phenol and aromatic in raw bio-oil.After desilication,the content of phenolics was significantly decreased mainly due to the formation of mesopores induced by NaOH.The presence of using HZSM-5 in combination with an active metallic phase Ni in the HDO of phenolics was evidenced[26,27].Compared with Ni/DeHZSM-5,the synergistic effect of the Ni-Cu and Ni-Cu-Ru contributed to the production of aromatic hydrocarbons.Li et al.[16]reported that Ni-Cu/HZSM-5 accelerated the HDO of biocrude more effectively because Cu could reduce the coke formation.Ru as a kind of very active noble metallic phase had outstanding performance for both hydrogenation and deoxygenation reactions[28]. Therefore,Ni-Cu-Ru/DeHZSM-5 was the most active catalyst among them for HDO of LDB.The content of carboxyl compounds including acids and esters in the UBO was reduced obviously compared to the LDB. The decarboxylation reactions contributed to the formation of hydrocarbons,as a result,the content of long chain hydrocarbons (C11-C25) was increased from 0.45% in the LDB to 7.56% in the UBO over the Ni-Cu-Ru/DeHZSM-5 catalyst. Decarbonylation reaction was enhanced with catalysts as the content of ketones decreased from 4.47% to 1.54%.These results revealed that the trimetallic Ni-Cu-Ru/DeHZSM-5 catalyst was beneficial to upgrade LDB to aromatic hydrocarbons than bimetallic Ni-Cu/DeHZSM-5 catalyst.The GC-MS results were also consisted with the FT-IR results.
2.4 Possible catalytic mechanism
The functional groups of LDB mainly included phenolic hydroxyl,methoxyl and unsaturated groups.Reaction schemes for the catalytic HDO of guaiacol,syringol and creosol were established by early studies using metal-supported HZSM-5 catalysts[26,29-31].Ni/HZSM-5 could promote the aromatics saturation in the biocrude upgrading processes[30,32],whereas metal Cu could active hydrogen[16]and metal Ru could lower the energy barrier of C-O bond cleavage[33].This work revealed that the Ni-Cu-Ru/HZSM-5 exhibited much higher HDO activity than Ni-Cu/HZSM-5 catalyst.A possible reaction mechanism was proposed as shown in Figure 6. One potential pathway,2-methoxy-4-methylphenol (creosol) underwent methoxy group removal and dehydration to form toluene.Syringol underwent methoxy group removal to form guaiacol,and guaiacol underwent methoxy group removal to form phenol and subsequent benzene formed by dehydration.

Figure 5 Compositions of UBO obtained under different catalytic conditions
3 Conclusions
Upgrading of LDB by catalytic HDO over modified HZSM-5 catalysts for aromatic hydrocarbons production was conducted.The modified catalysts were characterized and their catalytic activity was evaluated.It was demonstrated that the trimetallic Ni-Cu-Ru/DeHZSM-5 was beneficial to upgrade LDB to aromatic hydrocarbons than bimetallic Ni-Cu/DeHZSM-5 catalyst.The properties of the UBO were enhanced with a relatively lower water content,lower viscosity and higher calorific value.The relative content of phenolics significantly decreased from 56.78% to 35.29%,and the relative content of aromatic hydrocarbons was sharply increased from 0.81% to 28.95% in the UBO.The higher heating value of UBO reached 35.22 MJ/kg with energy recovery of 96.32%.Hydroxyl and methoxyl groups removal were the main reactions occurred in the catalytic system based on the proposed mechanism.It could be concluded that an integrated modification of HZSM-5 combined desilication and metal-loaded (Ni,Cu and Ru) favored the HDO ability.
