FSCN1在乳腺癌发生发展中的作用及其调控机制
2023-03-03常栋刘享享刘睿孙建伟
常栋,刘享享,刘睿,孙建伟
综 述
FSCN1在乳腺癌发生发展中的作用及其调控机制
常栋1,刘享享1,刘睿2,孙建伟1
1. 云南大学生命科学学院,生命科学研究中心,昆明 650504 2. 昆明医科大学第三附属医院乳腺外科,昆明 650118
FSCN1是一种肌动蛋白结合蛋白,能够将肌动蛋白丝集成一束。FSCN1在几乎所有的转移性肿瘤中高表达,并与大部分肿瘤的不良预后密切相关。在基底样和三阴性乳腺癌中高度表达。近年来关于FSCN1的报道愈发频繁,随着深入研究发现,FSCN1除了促进癌细胞的迁移、侵袭和转移定植,维持癌细胞自我更新和增强耐药性,还具有调控癌细胞的糖脂代谢及线粒体重塑等功能。本文从FSCN1的结构和调节形式,促进乳腺癌发生和转移的分子机制,以及其在乳腺癌中的作用及功能展开介绍,最后对FSCN1在临床上的价值进行了总结,为FSCN1在乳腺癌领域的研究提供重要的借鉴和参考。
FSCN1;肌动蛋白集束;乳腺癌;肿瘤发生;肿瘤进展
据国际癌症研究机构2021年发布的癌症统计数据分析,乳腺癌目前在全球的癌症发病率中高居首位,是危害女性健康的主要疾病之一[1]。尽管对于乳腺癌(主要是激素受体阳性和人表皮生长因子受体阳性的乳腺癌)的治疗已取得一定进展,但仍有相当比例的乳腺癌患者复发[2]并且经历转移[3],尤其是恶性程度最高的三阴性乳腺癌(triple negative breast cancer,TNBC)患者。因此,寻找乳腺癌的新型标志物和有效的治疗靶点,对改善患者的生存质量尤为关键。
肿瘤转移是一个复杂、多步骤的过程。原发性肿瘤经过上皮–间质转化(epithelial-mesenchymal transition, EMT)获得向周围组织和循环系统的迁移和侵袭能力,EMT的激活导致癌细胞间连接破坏、基质重组和基底膜的降解,并经历细胞骨架的广泛重塑等一系列的变化[4]。具有EMT特征的癌症干细胞(cancer stem cells,CSCs)内渗到附近血管或淋巴,通过循环系统散播到远处组织的微血管,少数“幸运”的癌细胞在适宜的条件下从微血管壁处逃逸并成功定植,形成转移性肿瘤[5]。乳腺癌细胞更容易向骨、肝脏、肺、脑等部位转移[6],但不同乳腺癌亚型的转移行为也各有特点[3]。
肌动蛋白作为细胞骨架蛋白,可以为细胞提供内部的机械支撑,驱动细胞运动并控制胞内物质运动的轨迹,对大多数细胞的生存至关重要[7]。FSCN(fascin actin-bundling protein)最开始在海胆提取物中被鉴定为丝状肌动蛋白(F-actin)集束蛋白[8],与在鲎()精子中发现的细丝束成分中大小为55 kDa的蛋白同源[9]。作为一种关键的特异性肌动蛋白交联剂,FSCN可以将肌动蛋白丝捆绑成平行的一束,直接参与细胞运动和迁移、细胞–基质黏附、伪足形成等过程[10]。家族有三个亚型:表达于神经细胞、内皮细胞、间充质细胞和树突状细胞,而在正常的上皮细胞中不表达或低水平表达[11],并在几乎所有类型的转移性癌症中高表达[12];在视网膜和耳中特异性表达,小鼠中的无义突变会导致进行性听力损失和视网膜变性[13];在睾丸及精子头部特异性表达[14]。近年来,有关FSCN1在乳腺癌发生发展及转移中的作用机制研究越来越多。本实验室及其他实验室的研究表明,高表达介导乳腺癌细胞的迁移、侵袭、转移定植和化疗耐药以及自我更新[15~23],并与乳腺癌的不良预后密切相关,因此,FSCN1可作为某些类型乳腺癌的标志物和潜在的治疗靶点[24~32]。本文将主要围绕FSCN1的结构和调节形式,其促进乳腺癌发生和转移的分子调控机制、在乳腺癌中行使的功能展开综述,最后对FSCN1在临床上的价值进行了探讨和展望。
1 FSCN1的结构
是定位在人7号染色体短臂2区2带(7p22)、由5个外显子组成、大小为13,840 bp的高度保守DNA序列(图1A)。第一个外显子包括5′非翻译区(5′ untranslated region,5′UTR)和大约一半的编码序列,由9.5 kb长的内含子1分隔;下游短外显子2~4在0.8 kb范围内间隔排列,总共编码30%的开放阅读框;外显子5位于下游1.2 kb处,包含剩余的编码部分和3′非翻译区(3′UTR)。
FSCN1是一种由493个氨基酸残基组成的、分子量大小为55 kDa的球状蛋白(图1B)。FSCN1蛋白在进化上是高度保守的,表明FSCN1可能行使某些基本的生物学功能,例如肌动蛋白集束活性的功能[33]。其493个残基通过一系列相互作用构成四个串联的β-三叶结构域(β-Trefoli),N端和C端分别位于β-Trefoli1和β-Trefoli4上[34]。FSCN1具有三个不同的结合肌动蛋白的表面区域,其中两个较大的肌动蛋白结合区域 (actin-bindingsite, ABS)位于β-Trefoli1和β-Trefoli4上以及β-Trefoli1和β-Trefoli2的裂缝中[35],而第三个较小的区域(ABS3)位于β-Trefoli3上[36]。对FSCN1在丝状伪足中捆绑肌动蛋白的低温电子显微镜分析表明,ABS1和ABS2与一个肌动蛋白丝结合,ABS3结合第二个肌动蛋白丝[35,37]。
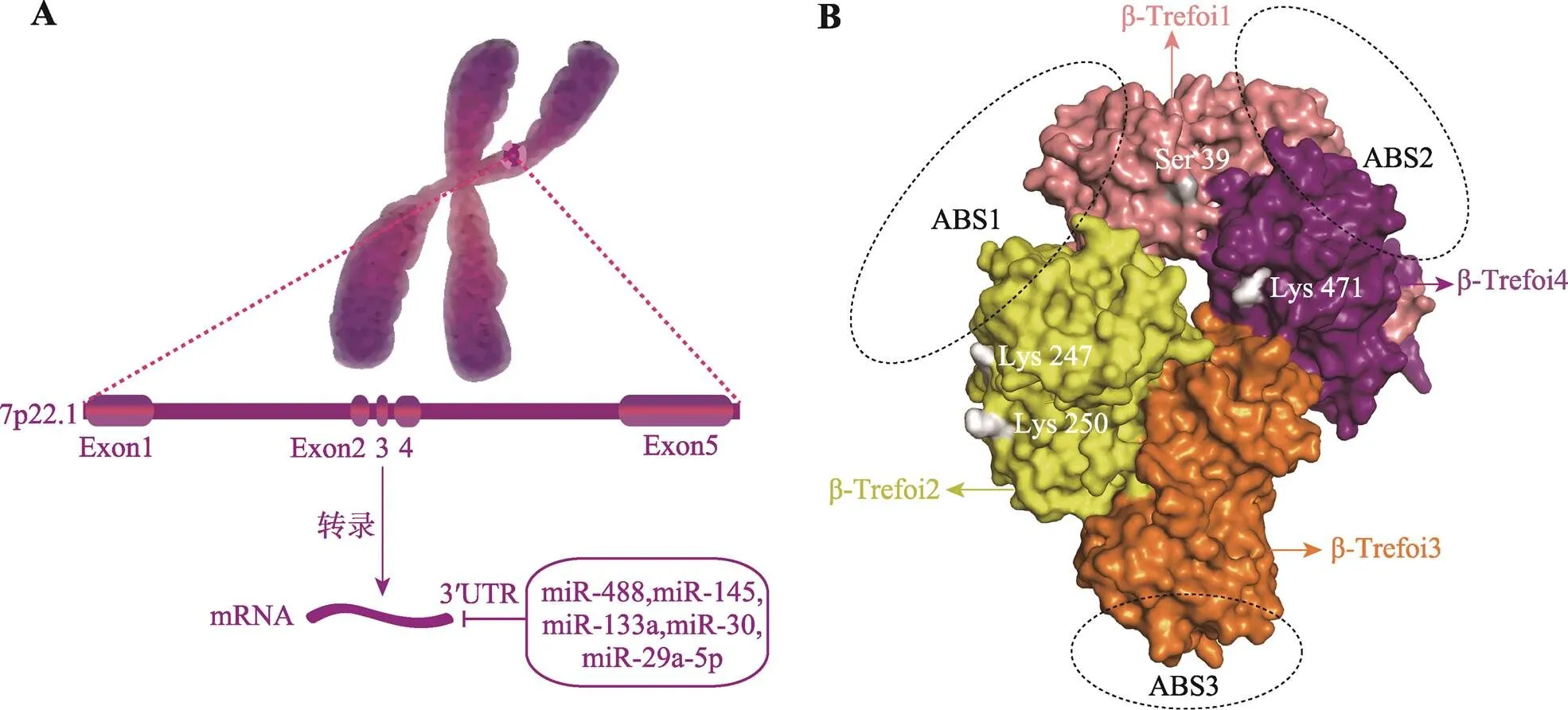
图1 FSCN1的结构
A:FSCN1的基因结构,框内是参与转录后调控的microRNA(miRNA)。B:FSCN1蛋白的三维结构,ABS和β-Trefoli分别代表FSCN1的肌动蛋白结合区域和结构域,内侧标注四个氨基酸残基是FSCN1翻译后修饰的主要位点。
2 FSCN1在乳腺癌中的调控
2.1 FSCN1的转录调控
的核心启动子含有一个TATA盒和一个GC框,以及cAMP反应元件结合蛋白/激活蛋白1(cyclic AMP response element binding protein, CERB/AP-1)复合位点等其他几个转录因子结合位点[38],在乳腺癌细胞中,信号转导和转录激活因子3 (signaltransducerandactivatoroftranscription3, STAT3)、核因子κB(nuclear factor kappa B, NF-κB) 等转录因子可以通过结合到的启动子区调控的表达(图2)。
2.1.1 JAK-STAT通路
炎症是促进肿瘤发生发展的重要因素。癌细胞通过与细胞间质相互作用、招募免疫细胞等方式来制造炎性肿瘤微环境,从而促进自身的增殖和转移[39]。作为经典的炎症通路,炎性因子通过JAK(Januskinase)-STAT途径影响肿瘤发生及进展。在乳腺癌中,STAT3作为转录因子与的启动子特异性结合来调控的表达。
在乳腺癌细胞(4T1、MDA-MB-231)中,STAT3作为转录因子通过响应白介素-6(interleukin6,IL-6)或抑瘤素M (oncostatin M,OSM)与的启动子特异性结合来诱导的表达,并且NF-κB也以STAT3依赖性方式被募集到启动子处[40]。在MDA-MB-231细胞中,经过IL-6或肿瘤坏死因子-α (tumor necrosis factor α, TNF-α)等炎性因子的刺激,STAT3和NF-κB(P50、P65)在细胞核中形成蛋白复合物,并与启动子的160bp保守区域上的STAT和NF-κB共同结合位点GGGGAA重叠序列结合以诱导其表达[41],这说明STAT3/NF-κB复合物的形成有利于乳腺癌细胞中的表达。
最近的研究发现一些化合物可以特异性抑制JAK-STAT通路来下调的表达。例如,从黄孢属植物中鉴定出的氯丹型二萜Crispene E[42],可以与STAT3的SH2结构域(src homology 2 domain)相互作用抑制STAT3的二聚化,进而下调MDA- MB-231细胞中的水平。从南海的白链霉菌SCSIOZH16中分离出来的新型化合物岛霉素 C (Ilamycin C)[43],能够抑制IL-6诱导的JAK2或STAT3的磷酸化来降低STAT3的磷酸化水平,进而抑制的表达和TNBC细胞的迁移和侵袭。
2.1.2 NF-κB通路
NF-κB被认为是慢性炎症引起某些肿瘤进展的关键因子,在肿瘤中NF-κB发挥调控癌细胞的增殖和存活、EMT以及血管生成等多种作用[44]。在乳腺癌中NF-κB也是调控表达的关键转录因子。除了与STAT3协同作用促进的上调外,鞘氨醇(sphingosine, Sph)被鞘氨醇激酶1 (sphingosine kinase1, SPHK1)磷酸化后生成1-磷酸鞘氨醇(sphingosine-1-phosphate, S1P),随后与肿瘤坏死因子受体相关因子2 (TNF receptor associatedfactor2, TRAF2)特异性结合并激活其E3泛素连接酶活性从而启动经典的NF-κB信号通路[45],也上调了的表达。此外,亮氨酸氨肽酶3 (leucineaminopeptidase3, LAP3)通过阻断p38-Hsp27通路促进NF-κB的激活,进而促进的上调[46],因此,来自天然海产品的LAP3抑制剂可高效地抑制该通路引起的效应[47]。
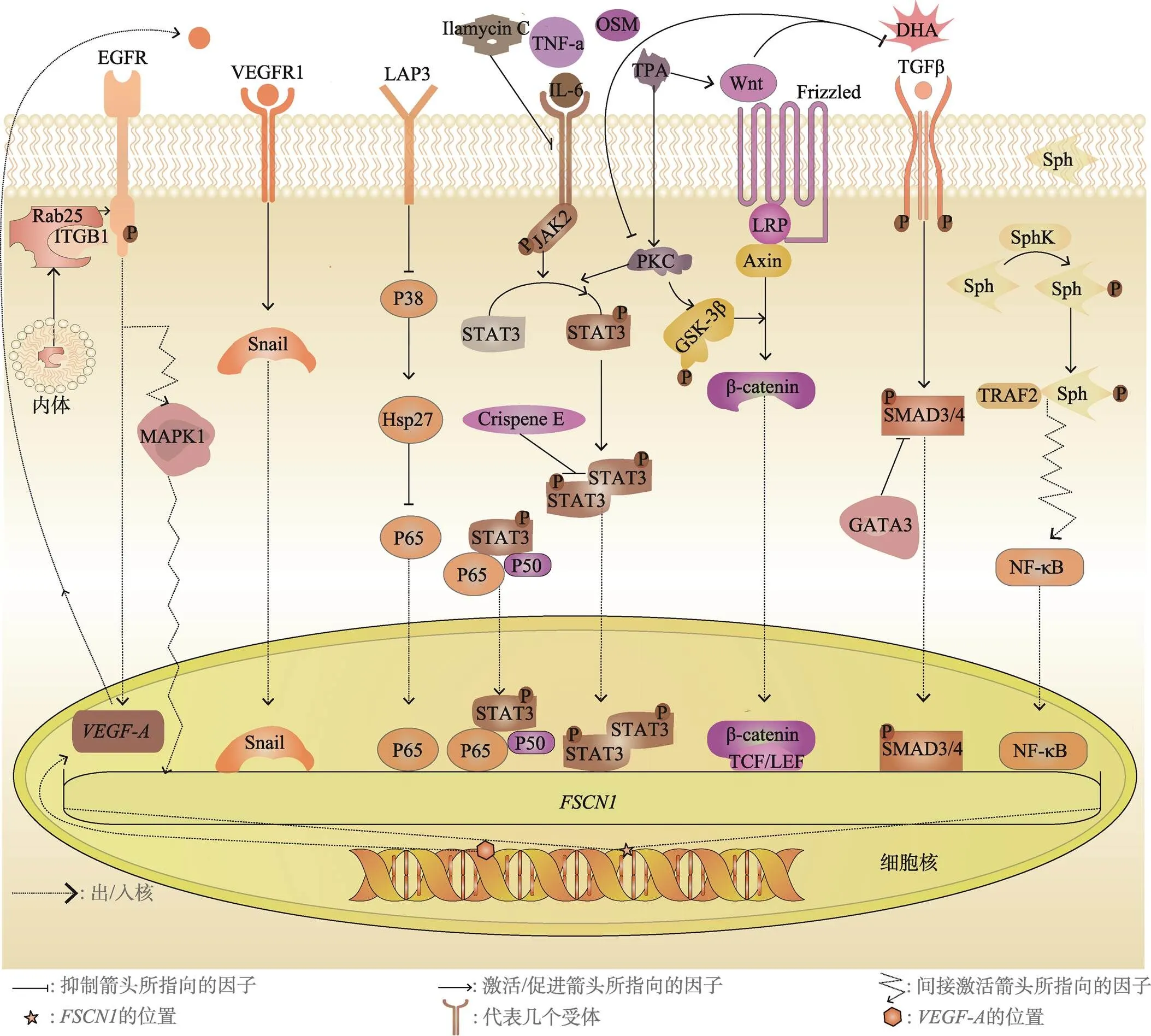
图2 调控FSCN1表达的相关信号通路
2.1.3 TGFβ-SMAD通路
入组标准:1)留守学生组 留守年限>1年的留守学生进入本组;2)非留守学生组 在进入留守学生组的学生同一班级按1:1配对,随机抽取性别、年龄相同的非留守学生进入本组。
转化生长因子β (transforming growth factor beta,TGFβ)在发育、组织动态平衡和再生过程中起调节细胞命运的作用,是免疫功能障碍和各种先天性疾病、纤维化疾病的主要参与者[48]。作为肿瘤微环境中免疫抑制的中心,TGFβ信号的异常是炎症发生的基础,并促进肿瘤的进展。在乳腺癌中,TGFβ信号通路也调控的表达。
TGFβ通过经典的TGFβ-SMAD (mothersagainstdecapentaplegichomolog)途径诱导梭形肿瘤细胞中的表达,并且FSCN1在TGFβ促进的肿瘤转移中发挥核心作用[23]。本实验室的研究表明TGFβ通过激活SMAD3/SMAD4特异性地诱导的表达,在基底样乳腺癌细胞中,TGFβ激活SMAD3后,促进SMAD4直接与启动子的CAGAC结合位点相互作用从而促进的表达,但是异位表达GATA结合蛋白3(GATAbindingprotein3, GATA3)后抑制了TGFβ-SMAD3/4介导的的转录[49],结果证明GATA3抑制了SMAD3/4和启动子区结合进而抑制了的表达。
2.1.4 EGF/EGFR通路
表皮生长因子受体(epidermal growth factor receptor, EGFR)是一种典型的受体酪氨酸激酶,在增殖和迁移、调节细胞内转运等基本细胞功能中起着关键作用;在肿瘤发生中,EGFR与有丝分裂信号和转运失调有密切关联[50],同时EGFR介导的通路或信号轴对乳腺癌中的表达调控起到重要的作用。
在表皮生长因子(epidermalgrowthfactor, EGF)刺激后的TNBC细胞中,的mRNA和蛋白质表达水平均显著增加,随后使用丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)特异性抑制剂U0126可降低的mRNA和蛋白表达水平,并且可阻断EGF对表达的促进作用[51],这说明部分TNBC细胞系通过EGFR-MAPK通路介导FSCN1的上调从而促进TNBC的侵袭能力。
EGFR还介导Rab25对的调控作用:转运蛋白Rab25 (ras-related protein 25)将β1整合素(integrinsubunitbeta1, ITGB1)从内体运输到质膜,二者协调激活EGFR,随后引起 A型血管表皮生长因子(vascularendothelialgrowthfactor A, VEGF-A)和Snail(zincfinger proteinSNAI1)的上调[52]。作为EMT过程的核心转录因子之一,Snail与启动子上的特定位点结合,最终引起的上调,进而促进乳腺癌的侵袭和转移。
作为EGF超家族中的一员,TGFα也可能通过EGFR介导的表达:有报道称,TGFα消除了糖皮质激素对紧密连接形成的刺激,并通过Ras(ratsarcoma)蛋白依赖性途径逆转大鼠乳腺上皮肿瘤细胞中类固醇诱导的的下调[53]。
2.1.5 Wnt/β-catenin通路
Wnt (wingless-typeMMTVintegrationsitefamily)/β-连环蛋白(β-catenin)通路作为发育及肿瘤发生的主要调节途径,在调控肿瘤干细胞活性方面起重要作用[54]。由于癌细胞的干性特征,因此经常发现Wnt信号途径的突变,乳腺癌中异常活跃的Wnt信号下游转录因子也导致了的过表达。
佛波酯蛋白激酶C激活剂(tissue plasminogen activator, TPA)在乳腺癌细胞(MCF-7)中通过蛋白激酶C(protein kinaseC, PKC)间接激活糖原合成酶激酶3β(glycogen synthase kinase 3 beta,GSK3β)以及上调Wnt1表达从而诱导细胞迁移[55],伴随着Wnt信号通路的开启表达也相应的上调,二十二碳六烯酸(docosahexaenoicacid, DHA)通过减弱PKC和Wnt1介导的的表达而有效地抑制TPA诱导的细胞迁移。此外,表皮生长因子受体2(erb-B2 receptor tyrosine kinase 2, ERBB2)过表达可能通过促进Wnt/β-catenin信号通路来增强的转录活性,在此过程中,NF-κB也有一定程度的上调[56]。还有报道称沙枣提取物抑制ERBB2表达和β-catenin的磷酸化,这在一定程度上抑制了的过表达[57]。
2.1.6 RhoA-ROCK1轴
定位在细胞-细胞外基质粘附位点的Ras抑制蛋白1(ras suppressor protein 1,RSU-1)在乳腺癌细胞中以生长分化因子15(growthdifferentiationfactor15, GDF-15)依赖的方式通过RhoA(ras homolog family member A)-ROCK1(rho associated coiled-coil containing protein kinase 1)-轴促进表达[58],并且RSU-1可能通过GDF-15将Ras信号通路与肌动蛋白细胞骨架调节联系起来,但具体的机制仍需进一步探究。
2.2 FSCN1的转录后调控
在乳腺癌中,miRNA参与的转录后调控(图1A)。譬如miR-133a、miR-145、miR488等miRNA与mRNA的3′UTR结合抑制其转录活性或使其mRNA降解[59-61],miR-30a间接调控的转录活性[62],X-连锁凋亡蛋白抑制因子(X-linked inhibitor of apoptosis, XIAP)的3′UTR可以作为内源竞争RNA(competingendogenousRNA, ceRNA)竞争性结合miR-29a-5p,从而将的mRNA从miR-29a-5p的抑制中拯救出来[63]。
2.3 FSCN1的翻译后修饰调控
在蛋白水平上,磷酸化是FSCN1最早被发现的翻译后修饰的方式,也是调控FSCN1最主要的方式。Yamakita和Ono等[64,65]发现PKC在体外能够磷酸化FSCN1的Ser39位点,并且在磷酸化后FSCN1与肌动蛋白结合的能力大大降低,说明Ser39位点磷酸化可以显著抑制FSCN1的肌动蛋白集束活性。Zeng等[66]将Ser39和附近的Ser38及与这两个氨基酸残基具有伪二重对称性的Tyr23和Ser274去磷酸化突变后发现,食管鳞癌细胞(KYSE-150)的增殖、迁移和丝状伪足的形成能力均明显增强,并且Ser38和Ser39残基的双重去磷酸化突变能最大限度地促进食管鳞状细胞癌(esophagealsquamouscellcarcinoma, ESCC)细胞的增殖、迁移和丝足的形成;Villari等[67]证明Ser274是FSCN1介导微管结合的关键残基,该残基的去磷酸化突变导致FSCN1与微管的高度稳定结合。定点去磷酸化突变实验说明FSCN1中可能还存在其他潜在的磷酸化位点,并且这些磷酸化位点会联合调控细胞的行为。关于FSCN1磷酸化的修饰机制仍有待进一步的探讨。Cheng等[68]最近发现FSCN1在人胚胎肾细胞(HEK-293T)和食管鳞癌细胞(KYSE140)中与乙酰基转移酶P300/CBP相关因子(P300/CBP associated factor,PCAF)共定位,并证明FSCN1与PCAF直接相互作用,并被PCAF在保守位点Lys471处乙酰化。FSCN1的Lys471乙酰化能够显著抑制体外某些食管癌细胞迁移和小鼠体内的肿瘤转移,并且在临床上ESCC组织中高水平的FSCN1乙酰化与ESCC患者的总生存期延长和无病生存期密切相关。Lin等[69]揭示了E3泛素连接酶Smurf1 (SMAD specific E3 ubiquitin protein ligase 1)直接催化FSCN1行使集束功能所必需的氨基酸残基(主要是Lys247和Lys250)的单泛素化,Lys247和Lys250单泛素化可能会引入空间位阻从而减弱FSCN1与肌动蛋白相互作用,进而抑制了结直肠癌上皮细胞(DLD-1)的体外迁移。
3 FSCN1在乳腺癌中的功能
3.1 调控迁移、侵袭和转移
EMT是原发肿瘤细胞获得转移能力的核心步骤之一。细胞骨架的广泛重塑、细胞间连接破坏、细胞外基质重组等一系列深刻的变化使癌细胞获得更强的运动能力,从而容易向周围组织和循环系统侵袭和迁移[4]。作为一种主要的肌动蛋白集束蛋白,FSCN1在乳腺癌进展中行使了自身的保守性功能,同样也是最重要的功能:促进乳腺癌细胞的迁移、侵袭和转移。
通过对BALB/c鼠的尾静脉注射4T1细胞,在一段时间后用克隆形成实验检测肺转移4T1细胞的数量发现,相较于对照组而言,敲降组的细胞转移数量更少[16,20]。将携带荧光素酶的MDA-MB-231细胞通过尾静脉注射到一种免疫缺陷鼠(NOD-SCID鼠)中,在40天内通过活体成像观察不同小鼠的肺转移信号强度发现,与FSCN1敲降组小鼠相比,对照组的肺转移信号明显较强,尤其在40天时[16]。一些乳腺癌细胞系的迁移和侵袭试验也是过表达促进乳腺癌细胞迁移和侵袭的佐证[23,45,47,70]。这些证据都表明了过表达增强了不同类型乳腺癌细胞的转移能力,并且FSCN1能与其他肌动蛋白相关蛋白相互协助以进一步的促进乳腺癌细胞的运动[17]。但有报道称高表达促进MDA-MB-231细胞的转移与其肌动蛋白捆绑活性无关[71],这与大多数证据相矛盾,并且只突变Ser39这一肌动蛋白结合位点不足够有说服力。当然FSCN1也可能通过独立于肌动蛋白集束功能的其他功能促进肿瘤细胞转移,这需要更进一步的探索。
3.2 维持癌细胞干性和化疗耐药
大鼠白细胞分化抗原44(CD44)high、大鼠白细胞分化抗原24(CD24)low[72]及乙醛脱氢酶(aldehydedehydrogenase, ALDH)high[73]经常作为鉴定乳腺癌干细胞的标记物。在MDA-MB-231细胞中将敲降后,调节胚胎干细胞多能性的SRY-盒转录因子(SRY-box transcription factor 2, Sox2)等水平下调,乳腺癌中调节细胞自我更新的Notch通路及其下游靶标也有明显的下调[19],FSCN1阳性的乳腺癌细胞集落形成能力更强,在裸鼠中皮下成瘤的体积更大,并且FSCN1与CD44high/CD24low或ALDHhigh干细胞标志物的表达无关,这揭示了FSCN1通过激活Notch通路维持癌症干细胞的自我更新能力。耐药性是肿瘤干细胞的关键特征之一,将敲降和正常的MDA-MB-231细胞注射到裸鼠体内后化疗三个周期后,相对于对照组而言,敲降组的肿瘤体积要小得多。从分子层面来看,FSCN1在部分乳腺癌细胞中通过介导粘着斑激酶(focaladhesionkinase, FAK)的磷酸化激活PI3K(Phosphoinositide 3-kinase)-Akt (AKTserine/threonine kinase)[21]和β-catenin[18]信号级联参与化疗耐药。以上说明FSCN1可能主要通过胚胎信号通路[74]参与乳腺癌细胞的干性维持和化疗耐药。
3.3 癌细胞增殖和肿瘤生长
有报道称FSCN1在乳腺癌细胞(MDA-MB-435和MDA-MB-231)中异位表达增强了细胞的增殖能力[22],并且在胆管癌、黑色素瘤及某些肺癌细胞中[75-77]FSCN1也具有调控细胞增殖的作用。Chen等[16]将4T1细胞的敲降后于完全培养基中培养,发现其生长速度与对照组相当,这说明在体外4T1的增殖似乎与FSCN1无关。Hayashi、Xu等[78,79]在肝细胞癌和胰腺癌中将敲除或异位表达后对细胞的增殖几乎没有影响。当然,鉴于不同肿瘤细胞系间的异质性,在不同的癌症类型及同种类型的不同细胞系中FSCN1表达与细胞增殖的关系也不尽相同,FSCN1与癌细胞增殖的关系仍亟待未来进一步的探索。
4 FSCN1在临床上的价值
针对激素受体阳性和人表皮生长因子受体阳性乳腺癌患者已有相对成熟的治疗体系,但对转移后的乳腺癌患者的治疗仍不容乐观[2]。TNBC作为乳腺癌中恶性程度最高的类型,发病率占乳腺癌的15%,但目前对其具体的分子病理学及生理学细节仍然知之甚少,患者在确诊后3~5年的生存期急剧下降,并且近二十年没有一种新的治疗方法通过临床三期试验评估,传统化疗仍是临床实践中治疗TNBC的唯一有效的手段[80]。因此,寻找合适的生物标志物来改善乳腺癌诊断、预后和治疗监测已成为亟待解决的问题。
通过免疫组化(immunohistochemistry, IHC)及单核苷酸多态性(single nucleotide polymorphism, SNP)基因分型分析了FSCN1在临床样本中作为预测不良预后和肿瘤诊断标记物的价值(表1),同时结合其在肿瘤细胞中高表达的特点[81],以及FSCN1在乳腺癌发生发展中的重要作用,初步说明FSCN1可作为一种有潜力的蛋白生物标志物,以用于乳腺癌的风险评估、诊断、预后,并用于预测治疗效果和复发情况。已有证据表明,针对FSCN1的小分子抑制剂能够有效的阻断FSCN1引起的癌细胞迁移、侵袭和转移(表2)。例如米格拉他汀类似物,作为一种肌动蛋白抑制剂,可以广泛并有效的降低乳腺癌细胞的迁移能力,其通过占据FSCN1的肌动蛋白结合位点来抑制FSCN1的集束活性[16]。在小鼠模型中,米格拉他汀类似物G2的使用使MDA-MB-231细胞的肺定植减少了80%以上,同时也减少了4T1细胞的肺定植[16],并且注射G2超2个月后小鼠也没有体重减轻、嗜睡或其他明显的毒性反应[20]。其他的一些小分子化合物,如LAP3抑制剂[47]、Ilamycin C[43]、Crispene E[42]也具有抑制FSCN1依赖性的肿瘤迁移、侵袭和转移的功能,这些小分子化合物为靶向FSCN1抗肿瘤转移疗法提供了广阔的前景。

表1 FSCN1在临床中的潜在价值
5 结语与展望
FSCN1作为一种重要的肌动蛋白集束蛋白,直接参与细胞运动和迁移等过程[10],并促进了大多数类型癌症的发生发展[12]。在乳腺癌中,JAK-STAT、NF-κB、TGFβ-Smad、Wnt/β-catenin等通路都能不同程度的上调的表达,的过表达会促进乳腺癌细胞迁移和侵袭能力,有利于乳腺癌的远端转移和定植,并赋予乳腺癌细胞维持干性和化疗耐药的能力。最近有研究表明FSCN1增强了铁死亡诱导剂爱拉斯汀(Erastin)诱导的MDA-MB-231细胞铁死亡的易感性[90],这可能会为TNBC患者提供一个有潜力的治疗方向。FSCN1与肿瘤细胞的代谢也存在密切联系,此前本实验室证明了FSCN1介导线粒体重塑及维持线粒体DNA稳定来促进线粒体氧化磷酸化[91];在结直肠癌中,的敲除会导致编码脂肪酸合成关键酶的脂肪酸合酶(fattyacidsynthase, FASN)和硬脂酰–辅酶A去饱和酶(stearoyl-CoAdesaturase,SCD)基因表达下调[92];在非小细胞肺癌和前列腺癌中,FSCN1通过激活YAP1(yes1associated protein 1)-TEAD(TEA domain transcription factor)轴来促进糖酵解[93,94]。另外有研究表明FSCN1通过增强YAP1的机械转导能力以促进胆管癌的发展[95],综合基因组学分析也证明在胃癌中FSCN1与TEAD4存在密切联系[96],以上研究表明FSCN1通过Hippo通路促进肿瘤发生发展。另外,FSCN1还具有介导机械转导、调节核仁形态和染色质修饰的功能[81],最新的研究表明,FSCN1能够动态的进出细胞核,其进入核内会与组蛋白H3直接结合,并促进核F-actin的集束,以及促进癌细胞DNA损伤后的DNA损伤应答[97]。这些研究结果说明FSCN1在肿瘤发生及恶化中具有复杂而重要的作用,这些作用都依赖于FSCN1的肌动蛋白集束活性,至于FSCN1是否存在独立于肌动蛋白集束活性的功能,仍需要进一步的深入研究。
目前大量的研究表明的高表达水平与乳腺癌的不良预后存在密切相关性。基于在肿瘤中高表达并促进其发生发展的重要作用,以及作为乳腺癌的标志物和潜在治疗靶点的重要价值,因此未来开发靶向FSCN1的干预措施,能为肿瘤治疗尤其是乳腺癌的治疗提供可靠的策略。
[1] Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, 2021, 71(3): 209–249.
[2] Waks AG, Winer EP. Breast cancer treatment: a review., 2019, 321(3): 288–300.
[3] Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes., 2010, 28(20): 3271–3277.
[4] Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer., 2019, 20(2): 69–84.
[5] Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells.e, 2016, 529(7586): 298– 306.
[6] Obenauf AC, Massague J. Surviving at a distance: organ-specific metastasis., 2015, 1(1): 76–91.
[7] Pollard TD, Cooper JA. Actin, a central player in cell shape and movement., 2009, 326(5957): 1208– 1212.
[8] Kane RE.Preparation and purification of polymerized actin from sea-urchin egg extracts., 1975, 66(2): 305–315.
[9] Tilney LG. Actin-filaments in the acrosomal reaction of limulus sperm. Motion generated by alterationa in the packing of the filaments., 1975, 64(2): 289–310.
[10] Adams JC. Roles of fascin in cell adhesion and motility., 2004, 16(5): 590–596.
[11] Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function., 2002, 24(4): 350–361.
[12] Lin SC, Taylor MD, Singh PK, Yang SY. How does fascin promote cancer metastasis?, 2021, 288(5): 1434–1446.
[13] Liu X, Zhao MM, Xie Y, Li P, Wang OM, Zhou BX, Yang LL, Nie Y, Cheng L, Song XC, Jin CZ, Han FZ. Null mutation of the fascin2 gene by TALEN leading toprogressive hearing loss and retinal degeneration in C57BL/6J mice., 2018, 8(10): 3221–3230.
[14] Tubb B, Mulholland DJ, Vogl W, Lan ZJ, Niederberger C, Cooney A, Bryan J. Testis fascin (FSCN3): a novel paralog of the actin-bundling protein fascin expressed specifically in the elongate spermatid head. Exp Cell Res, 2002, 275(1): 92–109.
[15] Al-Alwan M, Olabi S, Ghebeh H, Barhoush E, Tulbah A, Al-Tweigeri T, Ajarim D, Adra C. Fascin is a key regulator of breast cancer invasion that acts via the modification of metastasis-associated molecules., 2011, 6(11): e27339.
[16] Chen L, Yang SY, Jakoncic J, Zhang JJ, Huang XY. Migrastatin analogues target fascin to block tumour metastasis., 2010, 464(7291): 1062–1066.
[17] Hao LY, Liu Y, Yu XQ, Zhu YR, Zhu YC. Formin homology domains of daam1 bind to fascin and collaboratively promote pseudopodia formation and cell migration in breast cancer., 2021, 54(3): e12994.
[18] Barnawi R, Al-Khaldi S, Bakheet T, Fallatah M, Alaiya A, Ghebeh H, Al-Alwan M. Fascin activates beta-catenin signaling and promotes breast cancer stem cell function mainly through focal adhesion kinase (FAK): relation with disease progression., 2020, 10: 440.
[19] Barnawi R, Al-Khaldi S, Majed Sleiman G, Sarkar A, Al-Dhfyan A, Al-Mohanna F, Ghebeh H, Al-Alwan M. Fascin is critical for the maintenance of breast cancer stem cell pool predominantly via the activation of the Notch self-renewal pathway., 2016, 34(12): 2799–2813.
[20] Huang FK, Han SQ, Xing BW, Huang JY, Liu BQ, Bordeleau F, Reinhart-King CA, Zhang JJ, Huang XY. Targeted inhibition of fascin function blocks tumour invasion and metastatic colonization., 2015, 6: 7465.
[21] Ghebeh H, Al-Khaldi S, Olabi S, Al-Dhfyan A, Al-Mohanna F, Barnawi R, Tulbah A, Al-Tweigeri T, Ajarim D, Al-Alwan M. Fascin is involved in the chemotherapeutic resistance of breast cancer cells predominantly via the PI3K/Akt pathway., 2014, 111(8): 1552–1561.
[22] Xing P, Li JG, Jin F, Zhao TT, Liu Q, Dong HT, Wei XL. Fascin, an actin-bundling protein, promotes breast cancer progression in vitro., 2011, 29(4): 303–310.
[23] Sun JW, He HF, Xiong Y, Lu S, Shen JL, Cheng AN, Chang WC, Hou MF, Lancaster JM, Kim M, Yang SY. Fascin protein is critical for transforming growth factor beta protein-induced invasion and filopodia formation in spindle-shaped tumor cells., 2011, 286(45): 38865–38875.
[24] Wang CQ, Tang CH, Wang Y, Jin LL, Wang Q, Li XN, Hu GN, Huang BF, Zhao YM, Su CM. FSCN1 gene polymorphisms: biomarkers for the development and progression of breast cancer., 2017, 7(1): 15887.
[25] Lee HJ, An HJ, Kim TH, Kim G, Kang H, Heo JH, Kwon AY, Kim S. Fascin expression is inversely correlated with breast cancer metastasis suppressor 1 and predicts a worse survival outcome in node-negative breast cancer patients., 2017, 8(16): 3122–9.
[26] Esnakula AK, Ricks-Santi L, Kwagyan J, Kanaan YM, Dewitty RL, Wilson LL, Gold B, Frederick WAI, Naab TJ. Strong association of fascin expression with triple negative breast cancer and basal-like phenotype in African-American women., 2014, 67(2): 153–160.
[27] Barnawi R, Al-Khaldi S, Majid S, Qattan A, Bakheet T, Fallatah M, Ghebeh H, Alajez NM, Al-Alwan M. Comprehensive transcriptome and pathway analyses revealed central role for fascin in promoting triple-negative breast cancer progression., 2021, 14(12): 1228.
[28] Wang CQ, Tang CH, Chang HT, Li XN, Zhao YM, Su CM, Hu GN, Zhang T, Sun XX, Zeng Y, Du Z, Wang Y, Huang BF. Fascin-1 as a novel diagnostic marker of triple- negative breast cancer., 2016, 5(8): 1983– 1988.
[29] Min KW, Chae SW, Kim DH, Do SI, Kim K, Lee HJ, Sohn JH, Pyo JS, Kim DH, Oh S, Choi SH, Park YL, Park CH. Fascin expression predicts an aggressive clinical course in patients with advanced breast cancer., 2015, 10(1): 121–130.
[30] Rodríguez-Pinilla SM, Sarrió D, Honrado E, Hardisson D, Calero F, Benitez J, Palacios J. Prognostic significance of basal-like phenotype and fascin expression in node- negative invasive breast carcinomas., 2006, 12(5): 1533–1539.
[31] Yoder BJ, Tso E, Skacel M, Pettay J, Tarr S, Budd T, Tubbs RR, Adams JC, Hicks DG. The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course., 2005, 11(1): 186–192.
[32] Liu HL, Zhang Y, Li L, Cao JM, Guo YJ, Wu YY, Gao W. Fascin actin-bundling protein 1 in human cancer: promising biomarker or therapeutic target?, 2021, 20: 240–264.
[33] Duh FM, Latif F, Weng YK, Geil L, Modi W, Stackhouse T, Matsumura F, Duan DR, Linehan WM, Lerman MI, Gnarra JR. CDNA cloning and expression of the human homolog of the sea-urchin fascin and drosophila singed genes which encodes an actin-bundling protein., 1994, 13(8): 821–827.
[34] Sedeh RS, Fedorov AA, Fedorov EV, Ono S, Matsumura F, Almo SC, Bathe M. Structure, evolutionary conservation, and conformational dynamics of Homo sapiens fascin-1, an F-actin crosslinking protein., 2010, 400(3): 589–604.
[35] Yang SY, Huang FK, Huang JY, Chen S, Jakoncic J, Leo-Macias A, Diaz-Avalos R, Chen L, Zhang JJ, Huang XY. Molecular mechanism of fascin function in filopodial formation., 2013, 288(1): 274–284.
[36] Aramaki S, Mayanagi K, Jin MY, Aoyama K, Yasunaga T. Filopodia formation by crosslinking of F-actin with fascin in two different binding manners., 2016, 73(7): 365–374.
[37] Jansen S, Collins A, Yang CS, Rebowski G, Svitkina T, Dominguez R. Mechanism of actin filament bundling by fascin., 2011, 286(34): 30087–30096.
[38] Bros M, Ross XL, Pautz A, Reske-Kunz AB, Ross R. The human fascin gene promoter is highly active in mature dendritic cells due to a stage-specific enhancer., 2003, 171(4): 1825–1834.
[39] Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences., 2019, 51(1): 27–41.
[40] Snyder M, Huang XY, Zhang JJ. Signal transducers and activators of transcription 3 (STAT3) directly regulates cytokine-induced fascin expression and is required for breast cancer cell migration.,2011,286(45): 38886-38893.
[41] Snyder M, Huang J, Huang XY, Zhang JJ. A signal transducer and activator of transcription 3·nuclear factor κB(STAT3·NFκB) complex is necessary for the expression of fascin in metastatic breast cancer cells in response to interleukin (IL)-6 and tumor necrosis factor (TNF)-α., 2014, 289(43): 30082-30089.
[42] Mantaj J, Rahman SM, Bokshi B, Hasan CM, Jackson PJM,Parsons RB, Rahman KM. Crispene E, a cis-clerodane diterpene inhibits STAT3 dimerization in breast cancer cells.,2015,13(13):3882-3886.
[43] Xie Q, Yang ZJ, Huang XM, Zhang ZK, Li JB, Ju JH, Zhang H, Ma JY. Ilamycin C induces apoptosis and inhibits migration and invasion in triple-negative breast cancer by suppressing IL-6/STAT3 pathway.,2019,12(1):60.
[44] Taniguchi K, Karin M. NF-kappa B, inflammation, immunity and cancer: coming of age., 2018, 18(5): 309–324.
[45] Acharya S, Yao J, Li P, Zhang CY, Lowery FJ, Zhang QL, Guo H, Qu JK, Yang F, Wistuba, II, Piwnica-Worms H, Sahin AA, Yu DH. Sphingosine kinase 1 signaling promotes metastasis of triple-negative breast cancer., 2019, 79(16): 4211–4226.
[46] Fang CY, Zhang J, Yang HL, Peng LL, Wang K, Wang YJ, Zhao X, Liu HJ, Dou CH, Shi LH, Zhao CL, Liang SJ, Li DQ, Wang XJ. Leucine aminopeptidase 3 promotes migration and invasion of breast cancer cells through upregulation of fascin and matrix metalloproteinases-2/9 expression., 2019, 120(3): 3611–3620.
[47] Yang HL, Dai G, Wang SS, Zhao Y, Wang XJ, Zhao X, Zhang H, Wei LY, Zhang L, Guo SD, Song WG, Guo L, Fang CY. Inhibition of the proliferation, migration, and invasion of human breast cancer cells by leucine aminopeptidase 3 inhibitors derived from natural marine products., 2020, 31(1): 60–66.
[48] Batlle E, Massague J. Transforming growth factor-β signaling in immunity and cancer., 2019, 50(4): 924–940.
[49] Sun JW, He HF, Pillai S, Xiong Y, Challa S, Xu LY, Chellappan S, Yang SY. GATA3 transcription factor abrogates Smad4 transcription factor-mediated fascin overexpression, invadopodium formation, and breast cancer cell invasion., 2013, 288(52): 36971–36982.
[50] Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer., 2014, 24(1): 26-34.
[51] Wang CQ, Li Y, Huang BF, Zhao YM, Yuan H, Guo DF, Su CM, Hu GN, Wang Q, Long TY, Wang Y, Tang CH, Li XN.EGFR conjunct FSCN1 as a novel therapeutic strategy in triple-negative breast cancer.,2017,7(1):15654.
[52] Jeong BY, Cho KH, Jeong KJ, Park YY, Kim JM, Rha SY, Park CG, Mills GB, Cheong JH, Lee HY. Rab25 augments cancer cell invasiveness through a beta1 integrin/EGFR/ VEGF-A/Snail signaling axis and expression of fascin., 2018, 50(1): e435.
[53] Guan Y, Woo PL, Rubenstein NM, Firestone GL. Transforming growth factor-alpha abrogates the glucocorticoid stimulation of tight junction formation and reverses the steroid-induced down-regulation of fascin in rat mammary epithelial tumor cells by a Ras-dependent pathway., 2002, 273(1): 1–11.
[54] Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities., 2017, 169(6): 985–999.
[55] Lii CK, Chang JW, Chen JJ, Chen HW, Liu KL, Yeh SL, Wang TS, Liu SH, Tsai CH, Li CC. Docosahexaenoic acid inhibits 12-O-tetradecanoylphorbol-13-acetate-induced fascin- 1-dependent breast cancer cell migration by suppressing the PKCδ- and Wnt-1/beta-catenin-mediated pathways., 2016, 7(18): 25162–25179.
[56] Grothey A, Hashizume R, Ji H, Tubb BE, Patrick CW, Yu DH, Mooney EE, Mccrea PD. C-erbB-2/HER-2 upregulates fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines., 2000, 19(42): 4864–4875.
[57] Jabeen A, Sharma A, Gupta I, Kheraldine H, Vranic S, Al Moustafa AE, Al Farsi HF. Elaeagnus angustifolia plant extract inhibits epithelial-mesenchymal transition and induces apoptosis via HER2 inactivation and JNK pathway in HER2-positive breast cancer cells., 2020, 25(18): 4240.
[58] Gkretsi V, Louca M, Stylianou A, Minadakis G, Spyrou GM, Stylianopoulos T. Inhibition of breast cancer cell invasion by Ras suppressor-1 (RSU-1) silencing is reversed by growth differentiation factor-15 (GDF-15)., 2019, 20(1): 163.
[59] Wu ZS, Wang CQ, Xiang R, Liu X, Ye S, Yang XQ, Zhang GH, Xu XC, Zhu T, Wu Q. Loss of miR-133a expression associated with poor survival of breast cancer and restoration of miR-133a expression inhibited breast cancer cell growth and invasion., 2012, 12: 51.
[60] Zhao H, Kang X, Xia XF, Wo LK, Gu XD, Hu YY, Xie XH, Chang H, Lou LH, Shen XN. miR-145 suppresses breast cancer cell migration by targeting FSCN-1 and inhibiting epithelial-mesenchymal transition., 2016, 8(7): 3106–3114.
[61] Wu Y, Yuan MH, Wu HT, Chen WJ, Zhang ML, Ye QQ, Liu J, Zhang GJ. MicroRNA-488 inhibits proliferation and motility of tumor cells via downregulating FSCN1, modulated by Notch3 in breast carcinomas., 2020, 11(10): 912.
[62] Chang CW, Yu JC, Hsieh YH, Yao CC, Chao JI, Chen PM, Hsieh HY, Hsiung CN, Chu HW, Shen CY, Cheng CW. MicroRNA-30a increases tight junction protein expression to suppress the epithelial-mesenchymal transition and metastasis by targeting Slug in breast cancer., 2016, 7(13): 16462–16478.
[63] Wu Q, Yan H, Tao SQ, Wang XN, Mou L, Chen P, Cheng XW, Wu WY, Wu ZS. XIAP 3'-untranslated region as a ceRNA promotes FSCN1 function in inducing the progression of breast cancer by binding endogenous miR-29a-5p., 2017, 8(10): 16784–16800.
[64] Yamakita Y, Ono S, Matsumura F, Yamashiro S. Phosphorylation of human fascin inhibits its actin binding and bundling activities., 1996, 271(21): 12632–12638.
[65] Ono S, Yamakita Y, Yamashiro S, Matsudaira PT, Gnarra JR,Obinata T, Matsumura F. Identification of an actin binding region and a protein kinase C phosphorylation site on human fascin.,1997,272(4):2527–2533.
[66] Zeng FM, Wang XN, Shi HS, Xie JJ, Du ZP, Liao LD, Nie PJ, Xu LY, Li EM. Fascin phosphorylation sites combine to regulate esophageal squamous cancer cell behavior., 2017, 49(5): 943–955.
[67] Villari G, Jayo A, Zanet J, Fitch B, Serrels B, Frame M, Stramer BM, Goult BT, Parsons M. A direct interaction between fascin and microtubules contributes to adhesion dynamics and cell migration., 2015, 128(24): 4601–4614.
[68] Cheng YW, Zeng FM, Li DJ, Wang SH, He JZ, Guo ZC, Nie PJ, Wu ZY, Shi WQ, Wen B, Xu XE, Liao LD, Li ZM, Wu JY, Zhan J, Zhang HQ, Chang ZJ, Zhang K, Xu LY, Li EM. P300/CBP-associated factor (PCAF)-mediated acetylation of fascin at lysine 471 inhibits its actin-bundling activity and tumor metastasis in esophageal cancer., 2021, 41(12): 1398–1416.
[69] Lin SC, Lu S, Mulaj M, Fang B, Keeley T, Wan LX, Hao JH, Muschol M, Sun JW, Yang SY. Monoubiquitination inhibits the actin bundling activity of fascin., 2016, 291(53): 27323–27333.
[70] Gonzalez-Reyes C, Marcial-Medina C, Cervantes-Anaya N, Cortes-Reynosa P, Salazar EP. Migration and invasion induced by linoleic acid are mediated through fascin in MDA-MB-231 breast cancer cells., 2018, 443(1–2): 1–10.
[71] Heinz LS, Muhs S, Schiewek J, Grüb S, Nalaskowski M, Lin YN, Wikman H, Oliveira-Ferrer L, Lange T, Wellbrock J, Konietzny A, Mikhaylova M, Windhorst S. Strong fascin expression promotes metastasis independent of its F-actin bundling activity., 2017, 8(66): 110077–110091.
[72] Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ,Clarke MF. Prospective identification of tumorigenic breast cancer cells., 2003, 100(7): 3983– 3988.
[73] Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu SL, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome.,2007,1(5): 555–567.
[74] Takebe N, Warren RQ, Ivy SP. Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition., 2011, 13(3): 211.
[75] Zhao HY, Yang FQ, Zhao WY, Zhang CJ, Liu JG. Fascin overexpression promotes cholangiocarcinoma RBE Cell proliferation, migration, and invasion., 2016, 15(2): 322–333.
[76] Liang ZG, Wang Y, Shen ZY, Teng XM, Li XJ, Li CW, Wu WJ, Zhou ZH, Wang ZS. Fascin 1 promoted the growth and migration of non-small cell lung cancer cells by activating YAP/TEAD signaling., 2016, 37(8): 10909–10915.
[77] Kang JX, Wang J, Yao Z, Hu YZ, Ma SJ, Fan Q, Gao F, Sun Y, Sun JW. Fascin induces melanoma tumorigenesis and stemness through regulating the Hippo pathway., 2018, 16(1): 37.
[78] Hayashi Y, Osanai M, Lee GH. Fascin-1 expression correlates with repression of E-cadherin expression in hepatocellular carcinoma cells and augments their invasiveness in combination with matrix metalloproteinases.,2011,102(6):1228–1235.
[79] Xu YF, Yu SN, Lu ZH, Liu JP, Chen J. Fascin promotes the motility and invasiveness of pancreatic cancer cells., 2011, 17(40): 4470–4478.
[80] Denkert C, Liedtke C, Tutt A, Von MG. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies., 2017, 389(10087): 2430–2442.
[81] Lamb MC, Tootle TL. Fascin in cell migration: more than an actin bundling protein., 2020, 9(11): 403.
[82] Tampaki EC, Tampakis A, Nonni A, Von Flüe M, Patsouris E, Kontzoglou K, Kouraklis G. Combined fascin-1 and MAP17 expression in breast cancer identifies patients with high risk for disease recurrence., 2019, 23(5): 635–644.
[83] Min KW, Kim DH, Do SI, Chae SW, Kim K, Sohn JH, Pyo JS, Lee HJ, Kim DH, Oh S, Choi SH, Park YL, Park CH, Kim EK, Kwon MJ, Seo J, Moon KM. Negative association between GATA3 and fascin could predict relapse-free and overall survival in patients with breast cancer., 2016, 468(4): 409–416.
[84] Youssef NS, Hakim SA. Association of fascin and matrix metalloproteinase-9 expression with poor prognostic parameters in breast carcinoma of Egyptian women., 2014, 9: 136.
[85] Wang YF, Zhang JJ, Huang XY. Anti-metastasis fascin inhibitors decrease the growth of specific subtypes of cancers., 2020, 12(8): 2287.
[86] Lo RD, Zhou Y, Mucha J, Jones LF, Leahy L, Santocanale C, Krol M, Murphy PV. Synthesis of migrastatin analogues as inhibitors of tumour cell migration: exploring structural change in and on the aacrocyclic ring., 2015, 21(50): 18109–18121.
[87] Riahi N, Kefayat A, Ghasemi A, Asgarshamsi M, Panjehpoor M, Fassihi A. Design, synthesis and molecular docking studies of some tetrahydropyrimidine derivatives as possible fascin inhibitors., 2019, 16(2): e1800339.
[88] Zheng SL, Zhong Q, Xi YL, Mottamal M, Zhang Q, Schroeder RL, Sridhar J, He L, Mcferrin H, Wang GD. Modification and biological evaluation of thiazole derivatives as novel inhibitors of metastatic cancer cell migration and invasion., 2014, 57(15): 6653–6667.
[89] Dinicola S, Pasqualato A, Cucina A, Coluccia P, Ferranti F, Canipari R, Catizone A, Proietti S, D'anselmi F, Ricci G, Palombo A, Bizzarri M. Grape seed extract suppresses MDA-MB231 breast cancer cell migration and invasion., 2014, 53(2): 421–431.
[90] Chen C, Xie BJ, Li ZQ, Chen LN, Chen YX, Zhou JC, Ju SW, Zhou YL, Zhang X, Zhuo WY, Yang JJ, Mao MS, Xu L, Wang LB. Fascin enhances the vulnerability of breast cancer to erastin-induced ferroptosis., 2022, 13(2): 150.
[91] Lin SC, Huang CB, Gunda V, Sun JW, Chellappan SP, Li ZX,Izumi V, Fang B, Koomen J, Singh PK, Hao JH, Yang SY.Fascin controls metastatic colonization and mitochondrial oxidative phosphorylation by remodeling mitochondrial actin filaments.,2019,28(11):2824–2836.e8.
[92] Wu YP, Zhou Y, Gao HY, Wang YJ, Cheng QY, Jian SK, Ding Q, Gu W, Yao YX, Ma J, Wu WJ, Li YY, Tong XH, Song XY, Ma S. LYAR promotes colorectal cancer progression by upregulating FSCN1 expression and fatty acid metabolism., 2021, 2021: 9979707.
[93] Li MH, Gao ZM, Ding HL, Wang ZH, Mu HD, Zhang L, Wei JF, Ma ZS. FSCN1 Promotes glycolysis and epithelial-mesenchymal transition in prostate cancer through a YAP/TAZ signaling pathway., 2022, 2022: 6245647.
[94] Lin SC, Li YZ, Wang DZ, Huang CB, Marino D, Bollt O, Wu CD, Taylor MD, Li W, Denicola GM, Hao JH, Singh PK, Yang SY. Fascin promotes lung cancer growth and metastasis by enhancing glycolysis and PFKFB3 expression., 2021, 518: 230–242.
[95] Pocaterra A, Scattolin G, Romani P, Ament C, Ribback S, Chen X, Evert M, Calvisi DF, Dupont S. Fascin1 empowers YAP mechanotransduction and promotes cholangiocarcinoma development., 2021, 4(1): 763.
[96] Lim B, Park JL, Kim HJ, Park YK, Kim JH, Sohn HA, Noh SM, Song KS, Kim WH, Kim YS, Kim SY. Integrative genomics analysis reveals the multilevel dysregulation and oncogenic characteristics of TEAD4 in gastric cancer., 2014, 35(5): 1020–1027.
[97] Lawson CD, Peel S, Jayo A, Corrigan A, Iyer P, Baxter Dalrymple M, Marsh RJ, Cox S, Van Audenhove I,Gettemans J, Parsons M. Nuclear fascin regulates cancer cell survival.,2022,11.
The role and regulatory mechanism of FSCN1 in breast tumorigenesis and progression
Dong Chang1, Xiangxiang Liu1, Rui Liu2, Jianwei Sun1
FSCN1, an actin-bundling protein,is highly expressed in almost all metastatic tumors and is associated with the poor prognosis. In breast cancer FSCN1 is highly expressed in basal-like and triple negative subgroups. There is significant progress in understanding the role of fascin in breast cancer. Studies on FSCN1 in recent years have revealed that FSCN1 not only promotes tumor migration, invasion, metastic colonization, cancer cell self-renewal and drug resistance, but also regulates glucose and lipid metabolism and mitochondrial remodeling in tumor cells. In this review, we focus on the structure and regulatory mechanism of FSCN1 in breast tumorigenesis and metastasis, and discuss the clinical value of FSCN1 with the aim to provide a direction for further research in this field.
FSCN1; actin bundle; breast cancer; tumorigenesis; tumor progression
2022-11-01;
2022-12-27;
2022-12-28
国家自然科学基金项目(编号:82273460, 32260167),云南省应用基础研究基金(编号:202101AV070002, 2019FY003030),云南省科技计划重大专项(编号:202102AA310055),云南大学研究生科研创新项目(编号:ZC-22223017, KC-22222424)和国家卫生健康委毒品依赖和戒治重点实验室开放课题(编号:2020DAMOP-005)项目资助[Supported by the National Natural Science Foundation of China (Nos. 82273460, 32260167), the Applied Basic Research Foundation of Yunnan Province (No. 202101AV070002, 2019FY003030), the Major Science and Technique Programs in Yunnan Province (No. 202102AA310055), the Graduate Scientific Research Innovation Project of Yunnan University (Nos. ZC-22223017, KC-22222424), and the NHC Key Laboratory of Drug Addiction Medicine (No. 2020DAMPO-005)]
常栋,硕士研究生,研究方向:生物与医药。E-mail: 13648831440@139.com
孙建伟,博士,教授,研究方向:肿瘤发生与转移机制研究。E-mail: jwsun@ynu.edu.cn
10.16288/j.yczz.22-346
(责任编委: 宋质银)
