核受体REV-ERBα整合生物钟与能量代谢
2023-03-03冒姝羽赵昌睿刘畅
冒姝羽,赵昌睿,刘畅,2
综 述
核受体REV-ERBα整合生物钟与能量代谢
冒姝羽1,赵昌睿1,刘畅1,2
1. 中国药科大学生命科学与技术学院,南京 211198 2. 重庆中国药科大学创新研究院,重庆 401135
哺乳动物的各项生理活动以24 h为周期呈现节律性变化。稳定的昼夜节律由生物钟系统所精细调控,而昼夜节律的紊乱会导致代谢性疾病的发生。核受体超家族成员REV-ERBα是哺乳动物生物钟的重要组成部分,参与代谢、炎症、免疫和昼夜节律等多种生理过程的调节,是代谢性疾病、炎症性疾病和癌症的潜在治疗靶点。近年来发现了一系列新的REV-ERBα配体,其中大部分在疾病治疗方面具有潜在的应用价值。本文主要介绍核受体REV-ERBα在能量代谢以及炎症反应中的调节作用,以期为代谢综合征及相关疾病的治疗提供新的策略和参考。
REV-ERBα;代谢;炎症;REV-ERBα配体
REV-ERBα,即核受体亚家族1D组成员1 (nuclear receptor subfamily 1 group D member 1, NR1D1),属于核受体超家族。REV-ERBα主要由N末端配体非依赖性转录激活域1(activation function, AF1)、DNA结合结构域(DNA binding domain, DBD)、铰链区和配体结合结构域(ligand binding domain, LBD)组成,但是与其他核受体不同的是,REV-ERBs的配体结合域缺乏羧基末端激活结构域2(activation function 2, AF2)[1],因此,REV-ERBα不能激活转录,主要作为转录抑制因子发挥作用。REV-ERBα的编码基因位于编码甲状腺激素受体α的原癌基因的反义链[2]。REV-ERBα与NR1D家族的另一成员REV-ERBβ具有高度的同源性以及相似的功能,二者均是哺乳动物分子生物钟系统的核心组成成员。−/−小鼠()表现出以周期缩短为特征的昼夜节律紊乱,然而,−/−小鼠的昼夜节律活动变化则可以忽略不计[3]。因此,与REV-ERBβ相比,REV-ERBα可能在调节昼夜节律方面发挥着更重要的作用[3]。
REV-ERBα普遍存在于多种生物中,在肝脏、心脏、胰腺、脑等多个器官,以及内皮细胞、血管平滑肌细胞、巨噬细胞等多种细胞类型中均有表达。REV-ERBα具有昼夜节律表达模式[3~5],参与调节代谢、免疫、炎症等多个生理过程,其表达节律紊乱会导致炎症性疾病、代谢综合征等多种疾病的发生发展(表1)。REV-ERBα激动剂同时具备抗炎、降糖和改善血脂异常等多种作用,是缓解肥胖、2型糖尿病、脂肪肝、动脉粥样硬化等伴有慢性低度炎症的代谢性疾病的有效方法。
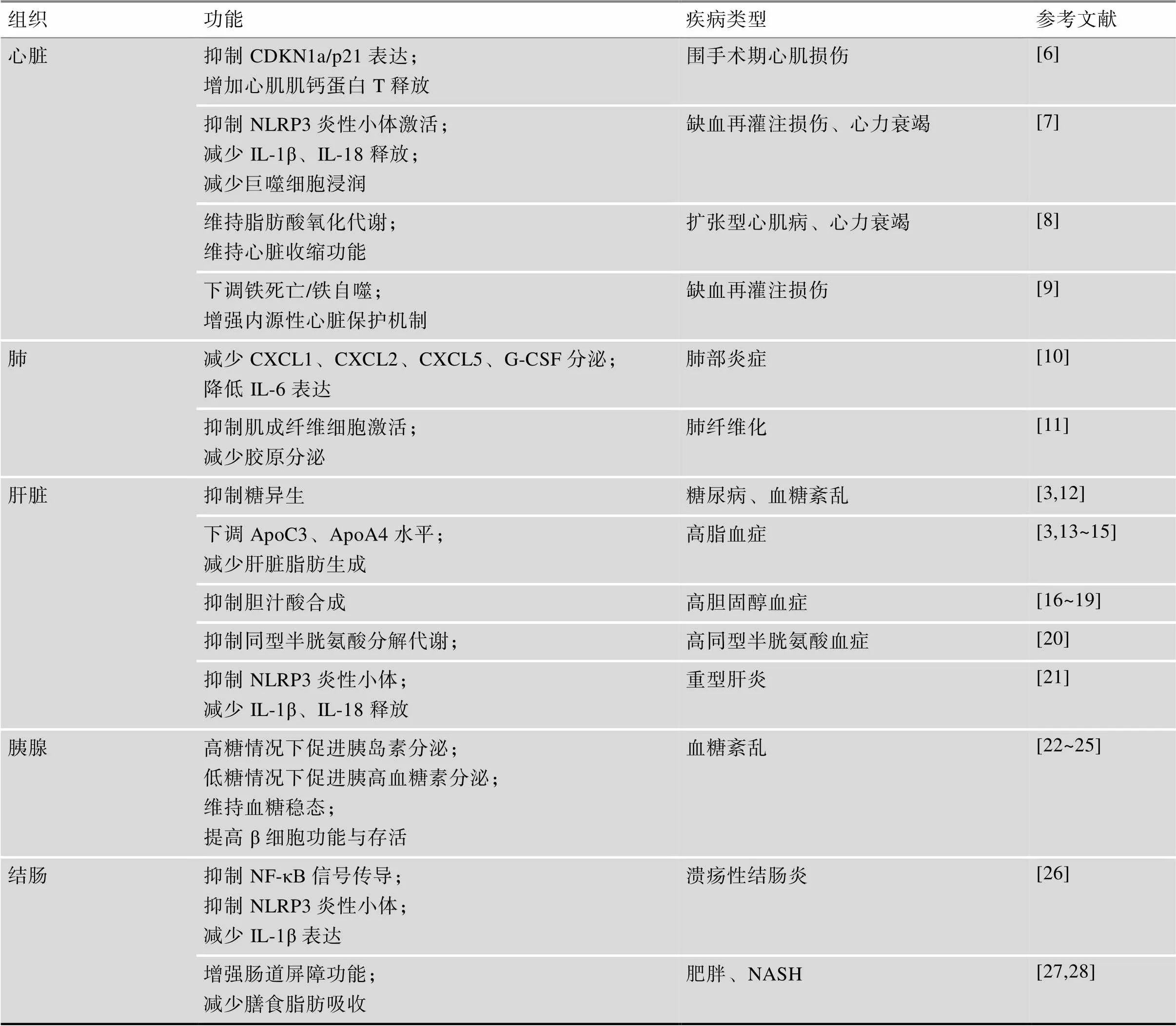
表1 REV-ERBα在机体各组织中的功能
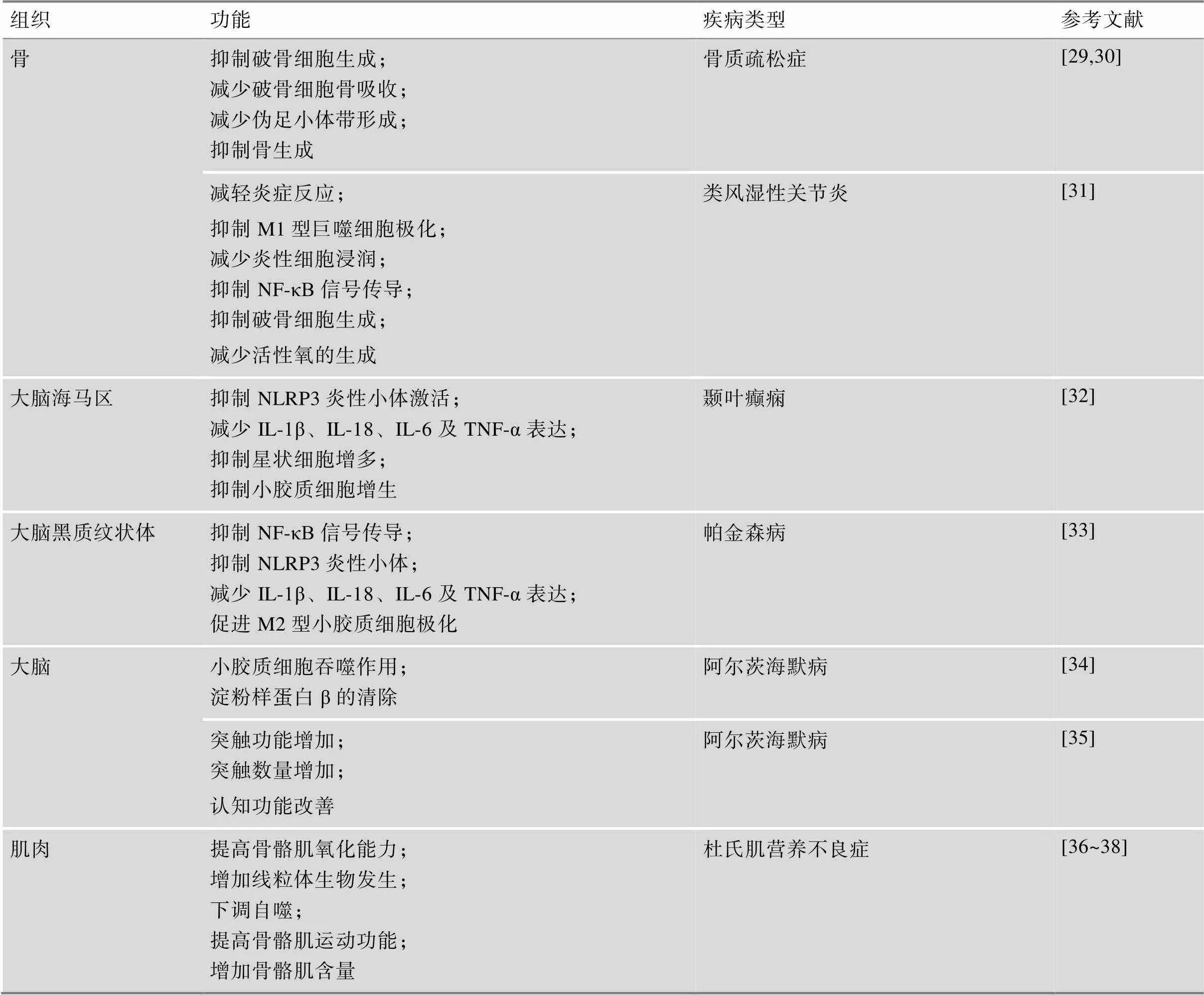
续表
CDKN1a/p21:细胞周期蛋白依赖性激酶抑制剂1a;G-CSF:粒细胞集落刺激因子;NASH:非酒精性脂肪性肝炎。
1 生物钟与REV-ERBα
1.1 生物钟
地球自转产生光照、温度、湿度等环境因素以24 h为周期的昼夜交替变化,而生物钟是地球上的生物体为适应外界环境周期变化而演化的内在自主计时机制[39]。生物钟使得生物体能预见环境的改变,从而调整它们的行为和生理机能(比如摄食和捕食行为)来适应每天的环境变化,使能量利用达到最优状态[40,41]。
哺乳动物体内的生物钟系统包括位于下丘脑的视交叉上核(suprachiasmatic nuclei, SCN)神经元中的中央时钟,以及位于整个人体组织中的一系列外周时钟[42]。SCN接受光线刺激并产生主要的时钟信号,通过体液和神经内分泌通路传递到外周组织中,来协调外周性生物钟[43],两大类生物钟运作同步,维持机体的代谢稳态。
在分子水平上,哺乳动物生物钟系统由转录激活因子昼夜运动输出周期kaput(circadian locomotor output cycles kaput, CLOCK)、神经元PAS结构域蛋白2(neuronal PAS domain protein 2, NPAS2)、芳香烃受体核转运体样蛋白1(brain and muscle ARNT-like 1, BMAL1),转录抑制因子周期节律蛋白家族(period, PER)、隐色素蛋白家族(cryptochrome, CRY),钟控基因蛋白REV-ERBs、维甲酸相关孤儿受体(retinoic acid-related orphan receptors, RORs)以及D-box结合蛋白(D-box binding protein, DBP)等构成。哺乳动物分子生物钟由三条转录–翻译负反馈环路(transcription-translational feedback loop, TTFL)组成[44]。BMAL1和CLOCK/NPAS2以异二聚体形式结合到自身抑制因子基因启动子区域的E-box元件(CACGTG)上,驱动等基因的转录。当细胞内PER和CRY积累到临界浓度时,PER和CRY蛋白异构化,转位至细胞核,与BAML1:CLOCK/NPAS2异二聚体相互作用,抑制其转录活性,从而抑制其自身转录,由此形成分子生物钟第一条负反馈环路。当蛋白质降解导致PER和CRY蛋白水平降低时,PER和CRY从BMAL1:CLOCK/NPAS2复合体中解离出来,开始一个新的转录周期。在第二条负反馈环路中,BMAL1:CLOCK/NPAS2异二聚体驱动REV-ERBs和RORs产生,随后,REV-ERBs和RORs结合作用于等基因启动子中的维甲酸相关孤儿受体反应元件(retinoic acid-related orphan receptor response element, RORE)来抑制和激活靶基因的节律性表达,形成另一条反馈环路。第三条负反馈环路为BMAL1:CLOCK/NPAS2异二聚体驱动产生的DBP与转录因子白细胞介素-3启动子转录激活子(nuclear factor interleukin-3-regulated protein, NFIL3),又名E4启动子结合蛋白4(E4 promoter-binding protein 4, E4BP4),形成异二聚体,通过结合等基因启动子中的D-box元件来激活等的节律性表达[44]。
1.2 核受体REV-ERBα
REV-ERBα是哺乳动物生物钟系统的核心组成部分。REV-ERBα是配体门控转录因子,通过DBD内的两个锌指结构结合靶基因中特定DNA基序发挥直接调控作用。然而,由于其LBD缺乏羧基末端AF2,REV-ERBα主要发挥配体依赖的转录抑制因子作用,在其天然配体亚铁血红素存在的情况下,通过招募辅助抑制因子核受体共抑制因子1(nuclear receptor co-repressor 1, NCOR1)和组蛋白脱乙酰基酶3(histone deacetylase 3,HDAC3)来抑制基因转录[45,46]。REV-ERBα通常可单独结合靶基因启动子上的RORE元件发挥作用(图1A),以同型二聚体形式结合RevDR2(即间隔2 nt的A/GGGTCA二拷贝重复结构域)或两个相邻的RORE抑制基因转录(图1,B和C)。除了直接结合靶基因外,REV-ERBα还通过与肝细胞核因子6(hepatocyte nuclear factor 6, HNF6)等转录因子相互作用间接调节基因转录(图1D)[47]。REV-ERBα的表达受CLOCK、BMAL1的调控,除此之外,过氧化物酶体增殖激活受体α(peroxisome proliferator activated receptor, PPARα)也能通过直接结合REV-ERBα基因上的PPAR反应元件(PPAR response element, PPRE),招募组蛋白标记和辅助因子,驱动REV-ERBα表达[48]。
研究表明,大部分钟控基因及部分糖脂代谢相关节律性表达基因均受到REV-ERBα的直接调控。因此,REV-ERBα在代谢综合征、炎症疾病、心力衰竭和癌症等病理条件中发挥重要作用(表1),可作为治疗相关疾病的药物靶标。近年来发现了一系列以REV-ERBα为靶点的调节剂(如GSK4112、SR9009、SR9011、SR8278),其中大多数在体内具有药理活性,为疾病的治疗提供了新可能。
2 REV-ERBα与能量代谢
在灵长类动物基因组中,约81.7%的基因呈现出昼夜节律性表达,其中很多基因编码在代谢过程中起关键作用的酶[49],昼夜节律失调会导致代谢性疾病的发生。众多研究表明,REV-ERBα在机体能量代谢调节方面发挥着重要作用(图2)。
2.1 REV-ERBα与糖代谢
昼夜节律影响机体葡萄糖稳态,机体内的葡萄糖水平通常会随着昼夜节律变化而周期性振荡。作为分子生物钟TTFL抑制臂的成员之一,REV-ERBα在维持机体葡萄糖稳态方面发挥重要作用。研究表明,与对照组小鼠相比,小鼠具有较高的血糖水平[3,50]。激活REV-ERBα能够下调糖异生限速酶磷酸烯醇丙酮酸羧基激酶1 (phosphoenolpyruvate carboxykinase 1, PCK1)和葡萄糖-6-磷酸酶(glucose-6-phosphatase, G6Pase) mRNA的表达,在体内和体外降低葡萄糖水平[12,51,52]。与此同时,REV-ERBα可维持葡萄糖水平的节律性振荡[53]。膳食铁的摄入通过促进血红素合成来调控REV-ERBα活性,从而改变肝脏糖异生的昼夜节律[54]。
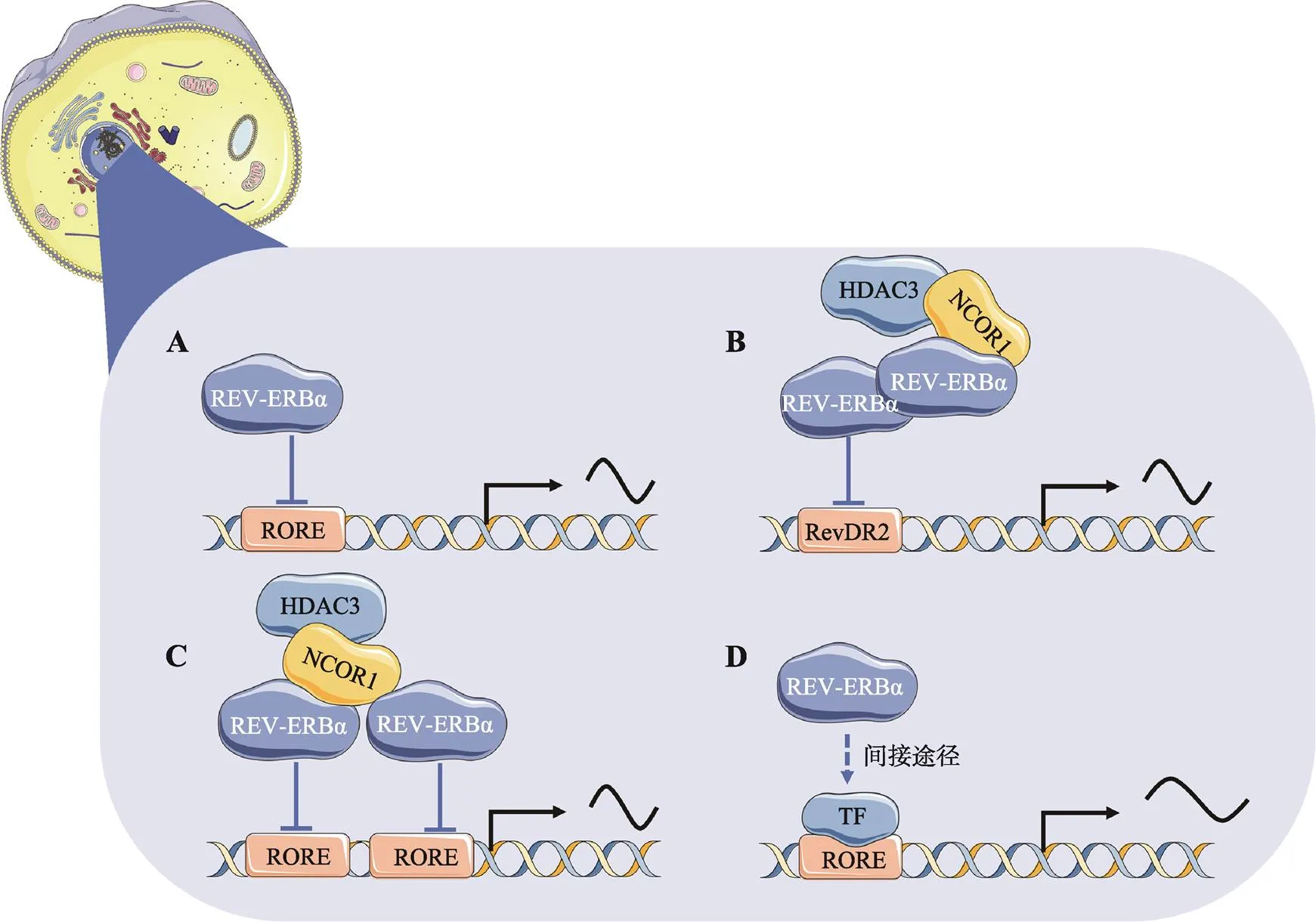
图1 REV-ERBα调控靶基因表达的作用模式
A:REV-ERBα通常作为单体结合在目标基因启动子上的RORE元件发挥调控作用。B:两分子的REV-ERBα形成同源二聚体与RevDR2元件结合,招募共抑制因子(即NCOR1和HDAC3)来调节基因的转录。C:某些情况下,两分子的REV-ERBα可以分别结合两个相邻的RORE元件招募共抑制因子(即NCOR1和HDAC3)来调节基因的转录。D:REV-ERBα通过与某些转录因子相互作用间接调节基因转录。使用Servier Medical Art的素材进行修改绘制,Servier Medical Art使用CC BY-SA 3.0协议(https://creativecommons.org/ licenses/by/3.0/)。
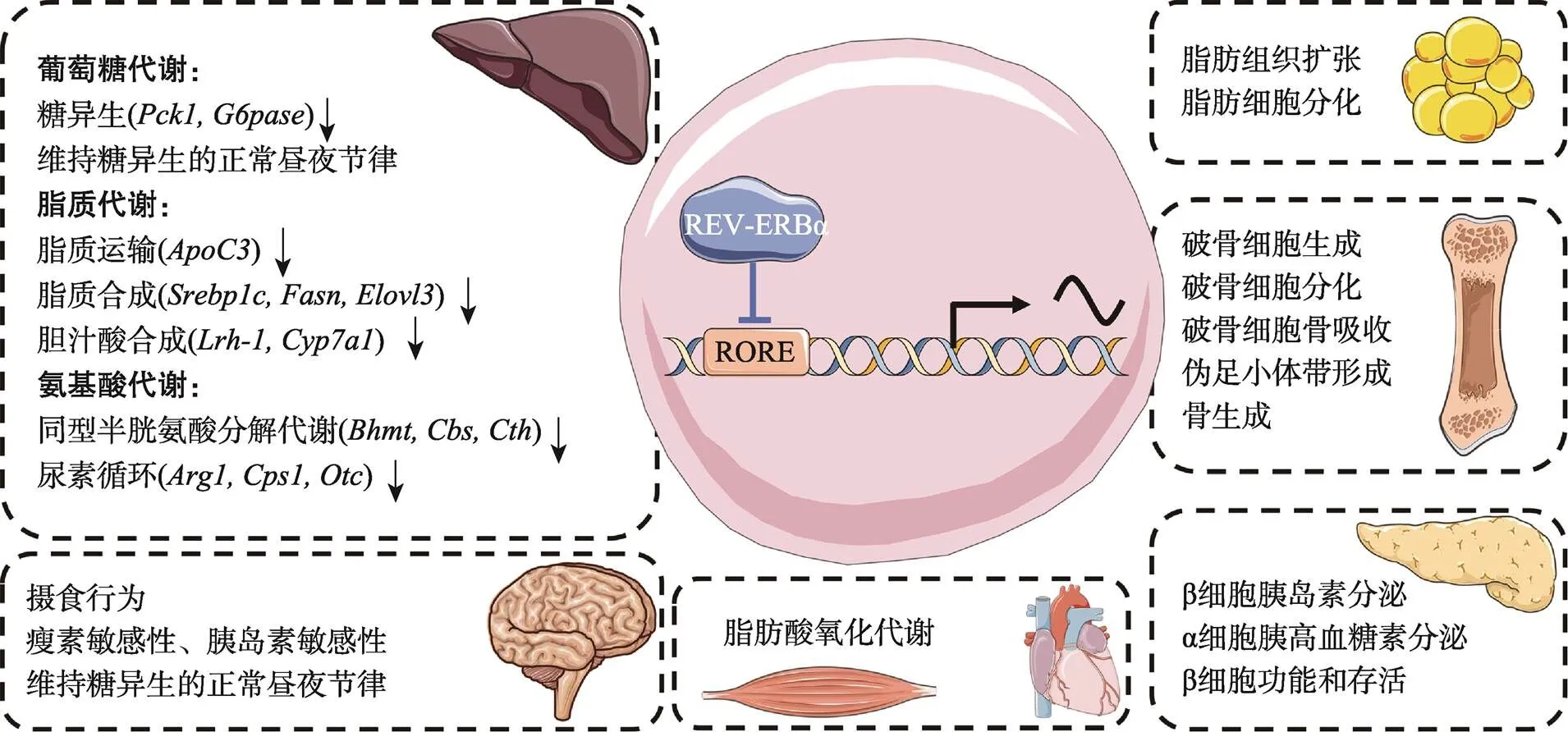
图2 REV-ERBα在机体能量代谢中发挥重要作用
REV-ERBα参与维持机体能量代谢稳态。在糖代谢方面,REV-ERBα直接抑制糖异生,调节胰岛素、胰高血糖素水平,维持机体葡萄糖水平的节律性振荡。在脂代谢方面,REV-ERBα调节肝脏脂质合成、脂质运输、胆汁酸代谢,脂肪细胞分化和脂肪组织的扩张,以及骨骼肌和心脏脂肪酸氧化能力。此外,REV-ERBα在氨基酸代谢和骨代谢方面也发挥着不可或缺的作用。使用Servier Medical Art的素材进行修改绘制,Servier Medical Art使用CC BY-SA 3.0协议(https://creativecommons.org/ licenses/by/3.0/)。
机体葡萄糖水平还受到胰岛素、胰高血糖素等激素的调节。研究表明,REV-ERBα可以调节β细胞中葡萄糖诱导的胰岛素分泌[23,24]。在胰岛α细胞中,REV-ERBα的表达受葡萄糖浓度的调节,并通过AMPK/Nampt/Sirt1途径促进胰高血糖素分泌[24,25]。胰高血糖素水平升高可激活肝脏蛋白激酶A (protein kinase A, PKA)信号,降低肝脏中REV-ERBα的稳定性,进而控制肝脏葡萄糖的生成[52]。敲除REV-ERBα或使用拮抗剂可通过上调自噬提高糖尿病病理情况下β细胞的存活率和活性[22]。
中枢神经系统对外周葡萄糖代谢的时空调控具有重要的意义。REV-ERBα在下丘脑SCN区GABA神经元中高表达,影响肝脏糖异生的昼夜节律[55]。下丘脑SCN区GABA神经元特异性REV-ERBα敲除小鼠表现出觉醒时胰岛素敏感性降低、肝脏葡萄糖生成提高、糖耐量受损的糖代谢表型[56],并且外源性激活SCN区GABA神经元REV-ERBα的同相位表达可改善敲除小鼠觉醒时糖耐量受损。REV- ERBα通过抑制SCN区GABA神经元的过度激活,增强胰岛素介导的肝脏糖异生抑制作用来提高胰岛素敏感性来改善糖代谢表型[56]。
综上所述,REV-ERBα在机体葡萄糖代谢调节中发挥着不可或缺的作用,但机制还需要进一步研究。
2.2 REV-ERBα与脂代谢
在脂代谢方面,Rev-erbα小鼠表现出脂质代谢基因表达紊乱,血脂异常,并伴有血清甘油三酯水平(triglyceride, TG)、血清极低密度脂蛋白、载脂蛋白(apolipoprotein, apo) C3水平升高[3,13,14]。药理激活REV-ERBα可降低小鼠的TG和游离脂肪酸[16,51,57]。而肝脏特异性REV-ERBα敲除小鼠仅在代谢扰动情况下(例如紊乱的进食行为)呈现显著的代谢异常表型,因此,肝脏REVERBα是肝脏能量代谢的状态依赖性调节因子,其真正作用是缓冲代谢扰动的异常反应[58]。心脏特异性REV-ERBα敲除小鼠脂肪酸氧化代谢功能障碍,糖代谢代偿性增强,导致心脏进行性收缩功能障碍,最终引发扩张型心肌病和致死性心衰[8]。此外,REV-ERBα在脂肪组织中的特异性调节作用尚不明确,先前有研究表明,REV-ERBα可促进3T3-L1脂肪细胞分化[59],但这在体内实验中无法得到验证。最新研究表明[60],脂肪组织特异性REV-ERBα敲除仅在肥胖条件下促进白色脂肪组织(white adipose tissue, WAT)的扩张。因此,脂肪组织REV-ERBα主要作用是响应代谢状态的改变,调节WAT的代谢活性和限制组织扩张,这对肥胖相关WAT病理和胰岛素抵抗的发展至关重要。
REV-ERBα可直接下调APOC3水平或通过结合cAMP反应元件结合蛋白H (cAMP-responsive element-binding protein H, CREBH)启动子抑制其转录,从而阻碍CREBH介导的APOA4的合成以及较大脂蛋白的组装[61],预防高脂血症和动脉粥样硬化。
研究表明,超长链脂肪酸延伸酶3 (elongase of very long chain fatty acids, Elovl3)也是REV-ERBα的靶基因之一,REV-ERBα通过下调表达发挥降脂作用[15]。SREBP1是参与脂肪酸合成和胆固醇合成的主要转录因子,REV-ERBα还通过调节内质网中SREBP隔离蛋白胰岛素诱导基因2(insulin induced gene 2, Insig2)的表达间接调节SREBP1的剪切活化,参与胆固醇和脂质代谢[18]。氧-乙酰氨基葡萄糖(O-linked beta-N-acetylglucosamine, O-GlcNAc)修饰是一种特殊的翻译后修饰,可随细胞内葡萄糖、脂肪酸的浓度变化而变化,O-GlcNAc转移酶(O-GlcNAc transferase, OGT)活性可反映细胞的代谢状态[62]。研究表明,REV-ERBα可以与OGT结合,进而调节OGT活性。机体胰岛素水平较低时,胞质REV-ERBα/OGT复合物阻止蛋白激酶B (protein kinase B, AKT)的磷酸化,此时,细胞核中的REV- ERBα同样结合并激活OGT,增强10-11易位酶(ten-of-eleven translocation, TET)的活性,通过表观遗传机制增强了SREBP1c的基础表达,参与肝脏脂肪生成调节[63]。
维持胆固醇稳态对机体健康至关重要,研究表明,REV-ERBα通过直接结合胆固醇合成相关基因调控胆固醇合成代谢[16]。此外,REV-ERBα还参与调控胆汁酸代谢,Rev-erbα小鼠具有胆汁酸合成速率降低,并且胆汁酸分泌功能受损的表型[17,18]。胆固醇7α-羟化酶(cholesterol 7α-hydroxylase, CYP7A1)是催化胆固醇转化为胆汁酸的限速酶。研究表明,REV-ERBα通过E4BP4、小异二聚体伴侣(small heterodimer partner, SHP)或Insig2/Srebp调节Cyp7a1的表达[17,18]。此外,Zhang等[19]的研究表明,REV-ERBα通过抑制Cyp7a1的转录激活因子肝受体同源物1 (liver receptor homolog 1, LRH-1)来下调Cyp7a1表达水平。REV-ERBα拮抗剂可提高野生型和高胆固醇症小鼠肝脏Cyp7a1水平,降低血浆胆固醇水平[19]。因此,靶向REV-ERBα/LRH-1轴可能是治疗胆固醇相关疾病的一种新方法。
2.3 REV-ERBα与氨基酸代谢
氨基酸在肝脏中通过转氨基和脱氨基反应等多种途径代谢,以提供能量并合成葡萄糖、脂肪、非必需氨基酸和其他生物活性分子。机体同型半胱氨酸血水平保持在较低水平对身体健康至关重要。研究结果表明[20],与野生型小鼠相比,−/−小鼠氨基酸代谢相关基因显著变化,血清和肝脏同型半胱氨酸水平降低。REV-ERBα通过直接抑制同型半胱氨酸关键代谢酶基因甜菜碱同型半胱氨酸甲基转移酶(betaine homocysteine methyltransferase, Bhmt)、胱硫醚-β-合成酶(cystathionine β-synthase, Cbs)、胱硫醚γ-裂解酶(cystathionine γ-Lyase, Cth)调节同型半胱氨酸分解代谢,以及抑制CCAAT增强子结合蛋白α(CCAAT/enhancer binding protein α, C/EBPα)的反式激活间接抑制尿素循环基因精氨酸酶1(arginase 1, Arg1)、鸟胺酸氨甲酰基转移酶(ornithine transcarbamylase, Otc)、氨基甲酰磷酸合酶1(carbamoyl phosphate synthase 1, Cps1)转录调节尿素的产生和氨的清除[20]。
2.4 REV-ERBα与骨代谢
昼夜节律的紊乱与骨质疏松症和骨代谢异常有关。激活REV-ERBα表达可抑制核因子κB受体激活因子配体诱导的伪足小体带的形成,并抑制破骨细胞骨吸收,从而改善卵巢切除所致的骨丢失[38]。REV-ERBα通过上调脂肪酸结合蛋白4 (fatty acid-binding protein 4, FABP4)来调节破骨细胞的形成[29]。此外,过表达REV-ERBα可抑制骨间充质干细胞增殖和成骨,而激活Wnt/β-catenin信号传导可部分逆转REV-ERBα的抑制作用[30]。因此,REV-ERBα通过调节破骨细胞和成骨细胞的生成,在维持骨的代谢动态平衡中起着关键作用。
3 REV-ERBα与炎症
慢性低度炎症是肥胖症、2型糖尿病、脂肪肝、心血管疾病等代谢性疾病的主要特征之一[64]。能量过剩引起的代谢性炎症加剧代谢性疾病的发生发展,因此,靶向代谢性炎症可以成为缓解代谢性疾病的新方案。REV-ERBα是公认的炎症调节因子,参与调节NF-κB信号转导、NOD样受体热蛋白结构域相关蛋白3 (NOD-like receptor thermal protein domain associated protein 3, NLRP3)炎症小体复合物的激活、炎症相关基因的转录、巨噬细胞极化以及免疫细胞发育等。
3.1 NLRP3炎症小体的激活与NF-κB信号转导
NLRP3炎症小体的活化包括启动和激活两个步骤。NLRP3炎症小体由病原相关分子模式或损伤相关分子模式等多种因素激活。活化的NLRP3炎症小体可促进caspase-1前体的裂解,产生活化的caspase-1效应蛋白,以及随后促炎症细胞因子IL-1β和IL-18的成熟。NLRP3的表达及其复合体的激活具有昼夜节律性,敲除REV-ERBα会改变巨噬细胞NLRP3的表达模式以及IL-1β和IL-18的产生模式[21]。分子层面上,REV-ERBα是NLRP3基因表达的直接调节因子,通过与启动子特异性结合直接抑制转录,主要在启动阶段灭活NLRP3炎症体。此外,REV-ERBα还抑制了(NF-κB的一个亚单位)的转录,并通过NF-κB途径间接抑制了的转录[26]。类似的,REV-ERBα可通过抑制NF-κB信号转导下调炎症相关基因的表达,如、、、和[65]。除了通过NF-κB信号的间接调节机制外,REV-ERBα还直接下调、[66]、[67]、[68]、和[69]等炎症基因的表达(图3A)。此外,REV-ERBα还能通过NF-κB/NLRP3炎症小体途径抑制巨噬细胞焦亡,减轻氧化应激,发挥血管保护作用[70],或抑制小鼠骨髓源性巨噬细胞和小胶质细胞向促炎表型极化,发挥抗炎和神经保护作用[31,33,71]。
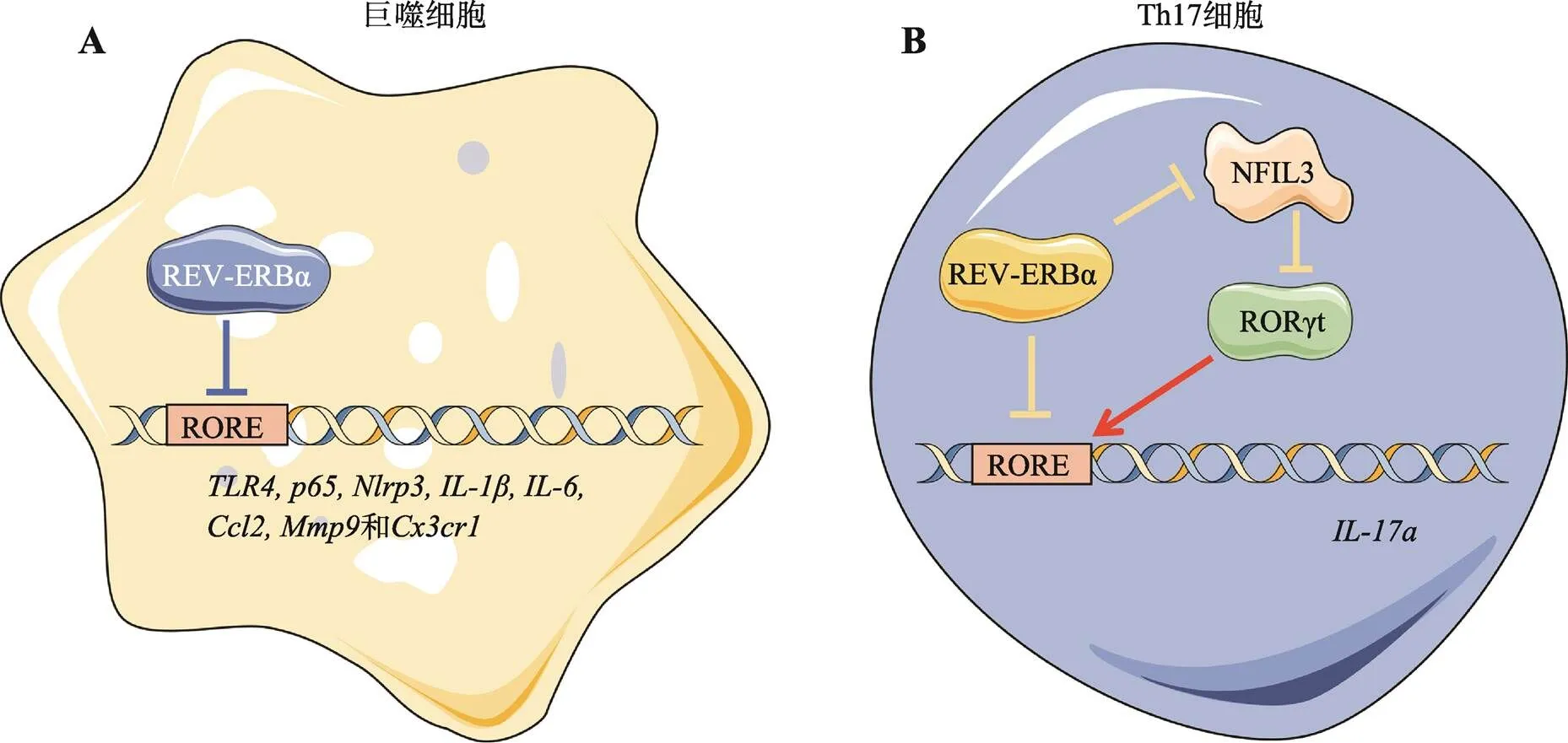
图3 REV-ERBα调控巨噬细胞和Th17细胞的炎症基因的表达
A:REV-ERBα抑制巨噬细胞中多种炎症基因(即、、、、、、和)的表达。B:REV-ERBα在Th17细胞中的调节作用可能与其表达水平有关。当低水平表达时,REV-ERBα通过抑制NFIL3,解除NFIL3对RORγt抑制作用,进而激活RORγt促进Th17细胞的发育;当高水平表达时,REV-ERBα通过与RORγt竞争结合启动子上的RORE元件而负向调节Th17细胞的发育。使用Servier Medical Art的素材进行修改绘制,Servier Medical Art使用CC BY-SA 3.0协议(https://creativecommons.org/ licenses/by/3.0/)。
3.2 免疫细胞发育
3型先天淋巴样细胞(type 3 innate lymphoid cells, ILC3)包括NKp46+和NKp46−两个亚群,在Rev-erbα小鼠中,NKp46+ILC3亚群的发育明显受损,细胞数量减少,RORγt表达减少,IL-22分泌减少[72]。而NKp46−的ILC3亚群发育正常,但IL-17的分泌却增加,可能是因为RORγt不被REV-ERBα拮抗[72]。因为REV-ERBα在调控RORγt方面发挥作用,所以时钟调节因子REV-ERBα是ILC3的发育和功能所必须的[72]。
与先天免疫细胞类似,T细胞和B细胞在血液中表现出强烈的昼夜振荡。辅助性T细胞17(T helper cell 17, Th17)是研究生物钟免疫调节的成熟细胞模型。RORγt是Th17细胞分化所必需的关键转录因子[73]。一项早期研究发现,负调控因子NFIL3通过直接结合和抑制RORγt启动子来抑制Th17细胞的发育,而REV-ERBα通过抑制NFIL3转录激活RORγt进而间接促进Th17细胞分化和IL-17a的产生[74]。Farez等[75]的研究表明,褪黑素通过调节REV-ERBα-NFIL3轴抑制Th17细胞中RORγt和RORα的表达,阻断致病性Th17细胞的分化。Chang等[76]提出,REV-ERBα在Th17细胞分化过程中特异性上调,REV-ERBα在Th17细胞中的调节作用可能与其表达水平有关。在低水平表达时[74],REV-ERBα通过抑制NFIL3促进RORγt的表达,间接促进Th17细胞分化和IL-17的产生;在高表达水平表达时[76],REV-ERBα通过与RORγt直接竞争结合基因的启动子,抑制IL-17a的产生及Th17效应功能(图3B)。综上所述,REV-ERBα是治疗Th17介导的自身免疫性疾病的潜在靶点。
3.3 纤维化
纤维化是指由于炎症导致器官实质细胞坏死,组织内细胞外基质异常增多和过度沉积的病理过程。REV-ERBα激动剂可减轻CCl4诱导的小鼠纤维化,表现为胶原沉积减少和纤维化基因表达减少[77]。成纤维细胞中的REV-ERBα可以改变整合素β1黏着斑的形成,影响肌成纤维细胞的分化,进而抑制肺纤维化的发展,并且REV-ERBα激动剂抑制肺纤维化患者组织中的肌成纤维细胞分化和胶原分泌[11]。因此,靶向REV-ERBα可能是一种治疗纤维化疾病的有效方法。
4 REV-ERBα合成配体
由于早期研究中未能找到内源性配体无法界定其作用,REV-ERBs在发现之初被称为孤儿受体,直至2007年,血红素被鉴定为其内源性配体[45]。作为REV-ERBα典型激动剂,血红素已经在体外研究中被证实对REV-ERB靶基因表达具有抑制作用。此外,随着药物化学的发展,近年来发现了一批新的REV-ERBα配体(图4),其中大多数都具有体内药理活性,为代谢性疾病、炎症性疾病、心力衰竭和癌症等多种疾病的治疗提供了新方向。
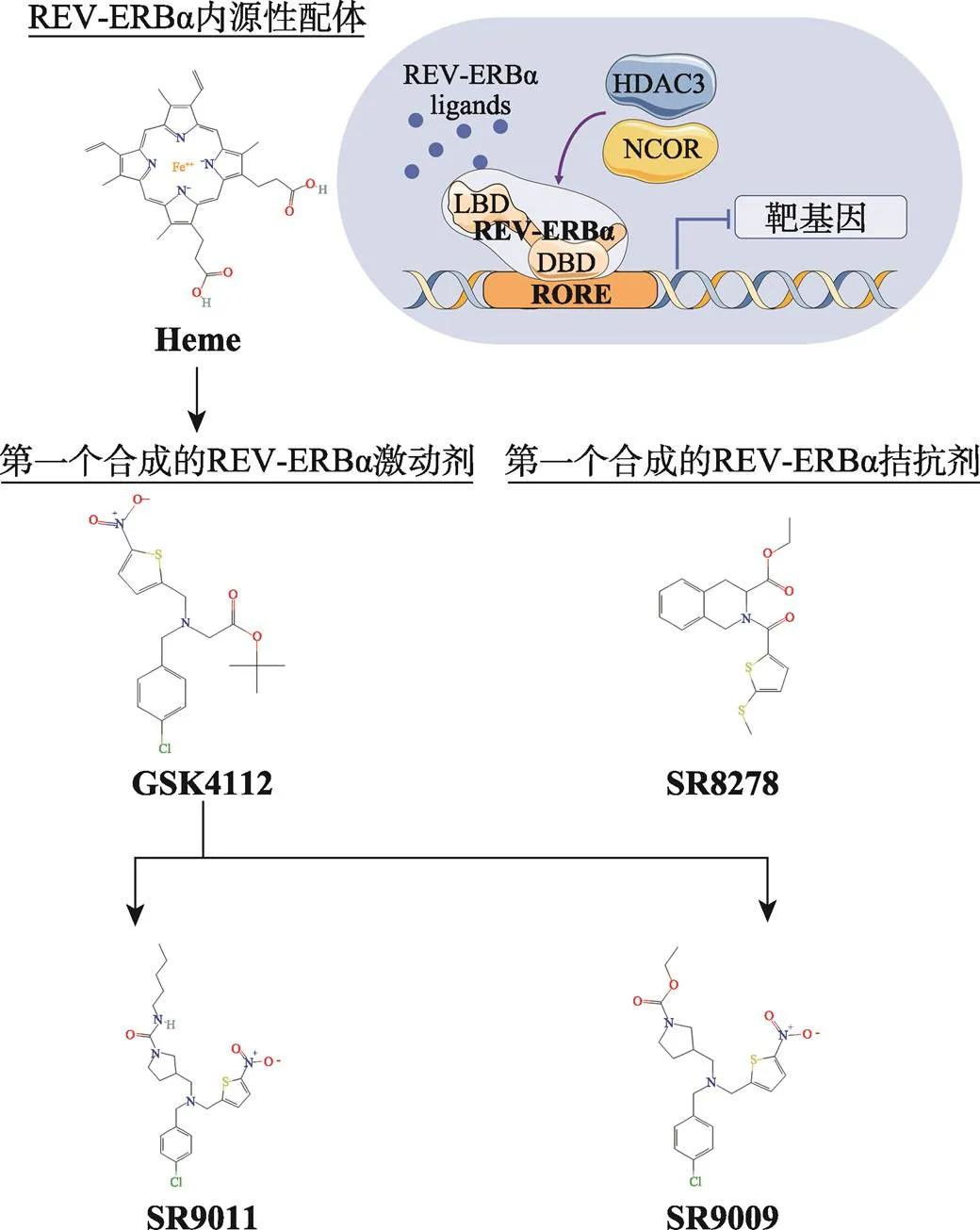
图4 REV-ERBα的生理性配体及代表性的合成配体
血红素是REV-ERBα的内源性配体,但高浓度的血红素具有细胞毒性且对REV-ERBα选择性较差,应用范围有限。随后,研究人员采用FRET技术筛选出第一个合成激动剂GSK4112,通常被用于体外实验。SR9009和SR9011是基于GSK4112的化学结构设计的REV-ERBs激动剂,表现出更好的药代动力学特性,已被广泛应用于体内外研究。SR8278是第一个合成的REV-ERBα拮抗剂,也是迄今为止可用于体内研究的最有效的REV-ERB拮抗剂。使用Servier Medical Art的素材进行修改绘制,Servier Medical Art使用CC BY-SA 3.0协议(https://creativecommons.org/licenses/by/3.0/)。
4.1 REV-ERBα激动剂
4.1.1 GSK4112
GSK4112是第一个合成的非卟啉小分子REV- ERBα激动剂,是通过荧光共振能量转移(fluorescence resonance energy transfer, FRET)技术筛选得到的[78]。体外实验表明[78],GSK4112可抑制以及糖异生基因的表达,减少小鼠原代肝细胞的葡萄糖产生。
遗憾的是,GSK4112药效不强,药代动力学性能不佳,因此通常被用于体外研究。GSK4112具有抗炎作用,是缓解神经炎症以及慢性炎症性肺炎的新策略[79, 80]。此外,GSK4112通过降低糖酵解通量和戊糖磷酸途径来抑制人胃癌细胞的增殖,发挥抗癌作用[81]。
4.1.2 SR9009和SR9011
SR9009和SR9011是基于GSK4112的化学结构设计的REV-ERBs激动剂[51],二者的药效是GSK4112的三倍,并且表现出更好的药代动力学特性。SR9009可减轻野生型小鼠实验性结肠炎,但在REV-ERBα-/-小鼠中无此作用[26],表明SR9009的药理作用是REV-ERBα依赖的。因此,SR9009和SR9011已被广泛用于检测REV-ERBs在体内外对昼夜节律行为和疾病的影响。
REV-ERBα可通过调节骨骼肌线粒体生物发生和线粒体自噬,提高骨骼肌氧化能力[37,38],维持骨骼肌肌浆网钙稳态[36],改善机体运动功能,因此SR9009和SR9011可用于缓解杜氏肌营养不良症等肌病[36]。在干预代谢性疾病方面,SR9009和SR9011可通过增加能量消耗来减少脂肪质量、改善血脂异常和高血糖来缓解小鼠肥胖[51]。另一方面,SR9009激活肠道REV-ERBα可增强小鼠肠道屏障功能,减少膳食脂肪吸收,减轻非酒精性脂肪性肝炎、肥胖等代谢性疾病[27,28]。此外,SR9009和SR9011同样具有心脏保护作用[9]、抗炎作用以及神经保护作用[82,83]。
REV-ERBα参与多种癌症的发生发展。SR9009和SR9011对癌细胞和癌基因诱导的衰老(oncogene- induced senescent, OIS)细胞具有特异性的致死作用,而对正常细胞或组织的活力没有影响[84,85]。因此,REV-ERBα激动剂是高选择性、低毒性、具有广泛治疗窗口的新型广谱抗癌药物,通过抑制癌细胞的增殖与迁移[86]、脂质从头合成[84]、细胞自噬[87]、线粒体代谢[88]以及诱导癌细胞的凋亡[84]来调控癌症的发展。
4.1.3 SR12418
Amir等[73]通过改变SR9009的化学结构合成了一种REV-ERB特异性的合成配体SR12418。SR12418在抑制IL-17等REV-ERBα靶基因方面比SR9009更有效,并且具有更好的药动学特性,可用于治疗实验性自身免疫性脑脊髓炎和结肠炎[73]。
4.2 REV-ERBα拮抗剂
4.2.1 SR8278
SR8278是第一个合成的REV-ERBα拮抗剂[89]。然而,它的药代动力学性质很差,消除半衰期很短,约为0.17 h[90]。SR8278已被广泛用于体内外研究REV-ERBα的功能。SR8278拮抗REV-ERBα可减少糖酵解,增加SGC-7901和BGC-823细胞内乳酸水平。体内研究中,SR8278可增强同型半胱氨酸分解代谢以及尿素的产生,降低小鼠血清和肝脏同型半胱氨酸水平,缓解高同型半胱氨酸血症[20]。SR8278也具有缓解帕金森症情绪障碍[91],减少阿兹海默症淀粉样斑块沉积[34]以及缓解癫痫发作[92]等作用。REV-ERBα是常见血栓性疾病中血小板活化和血栓形成的共同驱动因子,SR8278可减少人和小鼠血小板的聚集和激活,减少血栓形成,改善急性心肌梗死模型中微血栓阻塞和心肌梗死伸展[93]。
尽管近年来越来越多的新拮抗剂被发现,但遗憾的是这些化合物的药物代谢动力学特性都很差,SR8278仍然是迄今为止可用于体内研究的最有效的REV-ERB拮抗剂。
4.2.2 GSK1362
Pariollad等[10]开发了一种新型的基于恶唑的REV-ERBs选择性拮抗剂,命名为GSK1362。基于FRET和荧光素酶报告基因(luciferase reporter gene, luc)分析显示,GSK1362可促进Bmal1转录,并且呈现剂量依赖性。值得注意的是,GSK1362对LXR受体没有激动作用。然而,有研究表明,在骨髓源性巨噬细胞中,GSK1362以REV-ERBα依赖的方式抑制脂多糖诱导的炎性细胞因子IL-6的表达,此现象与REV-ERBα激动剂作用相似,表明GSK1362可能作用于未知的其他靶点。
4.2.3 胆红素
胆红素是血红素在体内分解代谢的有毒终产物,主要在肝脏解毒。Wang等[94]基于Gal4共转染和-luc分析证实胆红素是REV-ERBα的拮抗剂,可诱导已知的REV-ERBα靶基因、、和的表达。胆红素发挥REV-ERBα拮抗作用,保护机体免受高胆红素血症的影响。REV-ERBα拮抗剂为胆红素相关疾病的治疗提供了一种潜在方案。尽管结构上相似,胆红素和血红素对REV-ERBα的作用是相反的,其他结构相似化合物,如SR8278和GSK4112,也有类似的现象。结构相似化合物的不同作用可能是由于REV-ERBα活性对配体结合受体复合物构象变化的高度敏感性,氧化还原条件和少量气体对配体结合的REV-ERBα的轻微修饰会导致配体切换和功能效应的改变[95]。
4.3 其他
4.3.1 GSK2945
GSK2945也是在GSK4112的基础上设计的小分子药物,半衰期为2.0 h[96]。该化合物剂量依赖地抑制U2OS细胞中荧光素酶报告活性,表明对REV-ERBS具有激动性作用[96]。然而,在另一篇报道中GSK2945剂量依赖地拮抗REV-ERBα的活性,GSK2945处理小鼠和人的原代肝细胞可以上调Cyp7a1/CYP7A1的表达水平。体内实验表明,GSK2945可提高野生型和高胆固醇症小鼠肝脏Cyp7a1水平,降低血浆胆固醇水平[19]。因此,靶向REV-ERBα/LRH-1轴可能是治疗胆固醇相关疾病的一种新方法。目前为止,GSK2945是激动剂还是拮抗剂还没有定论,其作用可能具有细胞/组织特异性。
4.3.2 黄连素和葛根素
研究表明,中药单体化合物黄连素是REV- ERBα的激动剂,可抑制和荧光素酶报告基因活性,并以剂量依赖性降低和的表达[97]。黄连素处理可降低骨髓源性巨噬细胞和结肠炎小鼠的炎症反应[97]。葛根素是从葛根中分离出来的,可被用于治疗多种疾病。Chen等[98]基于荧光素酶报告基因、Gal4共转染和靶基因表达分析发现葛根素是REV-ERBα的拮抗剂。葛根素上调肝脏同型半胱氨酸分解代谢基因、和的表达,剂量依赖性缓解蛋氨酸诱导的小鼠高同型半胱氨酸血症,其表现为总同型半胱氨酸和TG水平的降低。黄连素和葛根素在化学结构上与其他合成配体有很大的不同,为REV-ERBα配体提供了新的化学骨架。然而,黄连素和葛根素对REV-ERBα的选择性尚未得到验证。
5 结语与展望
哺乳动物生理和行为的大部分方面受生物钟调控。生物钟与能量代谢关系密切,时钟基因直接或间接参与糖脂代谢调控。生物钟功能的紊乱会导致代谢综合征表型。核受体REV-ERBα作为重要的时钟调节基因在维持机体能量代谢稳态中起着至关重要的作用,是肝脏和WAT等主要代谢器官的代谢状态依赖性调节因子[58,60]。越来越多的证据支持了REV-ERBα在糖脂代谢稳态中的作用,并且REV- ERBα激动剂可增加全身能量消耗,改善血脂异常和高血糖,为代谢性疾病的治疗提供了一种方法。
机体代谢物和代谢过程控制免疫细胞的功能和分化[99]。在肥胖等病理条件下,长期能量过剩导致代谢产物(如游离脂肪酸)激活免疫细胞,导致慢性低度炎症。REV-ERBα是炎症调节因子[21],涉及NLRP3炎症体的激活调控、炎症相关基因的表达、巨噬细胞极化以及免疫细胞的发育等多个过程。越来越多的证据支持REV-ERBα激动剂具有抗炎作用,在治疗代谢性疾病和炎症性疾病方面具有广阔前景。
代谢重编程是癌症的特征之一,使癌细胞能够在营养匮乏、氧气缺乏的肿瘤微环境中快速生长和失控性增殖。REV-ERBα激动剂可通过调控糖脂代谢限速酶基因,减少糖酵解通量和磷酸戊糖途径以及脂质从头合成,抑制癌细胞细胞增殖并诱导癌细胞死亡,从而限制肿瘤的发生发展。由此可见,靶向REV-ERBα是一种很有前途的癌症治疗策略。
虽然REV-ERBα配体已被证明可以在动物临床前研究水平上改善炎症性疾病、代谢紊乱、心脏衰竭、自身免疫性疾病、癌症等病理条件,但是目前在转化为临床试验方面没有取得任何进展。这意味着REV-ERBα配体的药物开发面临着某些挑战。这些挑战包括药物安全问题、较差的生物利用度和药代动力学特性以及人类和啮齿动物之间昼夜节律机制差异。
另一个值得注意的问题是,一些REV-ERB配体具有REV-ERB非依赖的生物效应。肝X受体(liver X receptor, LXR)与REV-ERBs具有相似的生物学功能,参与脂质代谢、糖代谢和炎症的调节,GSK4112类似物SR9009和SR9011共有的叔胺化学结构可激活LXR[96]。为了成功地进行药物开发,使用先进技术在局部分布REV-ERBα药物靶向治疗疾病或许是一种可行的解决方法。
尽管合成配体仅在过去几年才可用于疾病的动物模型,但很明显,改进和优化的配体为睡眠障碍、癌症、免疫性疾病和代谢综合征的治疗提供了新策略,一些改进后的化合物或其类似物在不远的将来很有可能进入临床试验。
[1] Dumas B, Harding HP, Choi HS, Lehmann KA, Chung M, Lazar MA, Moore DD. A new orphan member of the nuclear hormone receptor superfamily closely related to Rev-Erb., 1994, 8(8): 996–1005.
[2] Miyajima N, Horiuchi R, Shibuya Y, Fukushige S, Matsubara K, Toyoshima K, Yamamoto T. Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus., 1989, 57(1): 31–39.
[3] Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β., 2012, 485(7396): 123–127.
[4] Zhao X, Hirota T, Han XM, Cho H, Chong LW, Lamia K, Liu SH, Atkins AR, Banayo E, Liddle C, Yu RT, Yates JR, 3rd, Kay SA, Downes M, Evans RM. Circadian amplitude regulation via FBXW7-targeted REV-ERBα degradation., 2016, 165(7): 1644–1657.
[5] Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism., 2011, 331(6022): 1315–1319.
[6] Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, Potelle C, Berthier A, Gheeraert C, Piveteau C, Deprez R, Eeckhoute J, Duez H, Lacroix D, Deprez B, Jegou B, Koussa M, Edme JL, Lefebvre P, Staels B. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: a single-centre propensity-matched cohort study and a randomised study., 2018, 391(10115): 59–69.
[7] Reitz CJ, Alibhai FJ, Khatua TN, Rasouli M, Bridle BW, Burris TP, Martino TA. SR9009 administered for one day after myocardial ischemia-reperfusion prevents heart failure in mice by targeting the cardiac inflammasome., 2019, 2: 353.
[8] Song SY, Tien CL, Cui H, Basil P, Zhu NX, Gong YY, Li WB, Li H, Fan QY, Min Choi J, Luo WJ, Xue YF, Cao R, Zhou WJ, Ortiz AR, Stork B, Mundra V, Putluri N, York B, Chu MP, Chang J, Yun Jung S, Xie L, Song JP, Zhang LL, Sun Z. Myocardial Rev-erb-mediated diurnal metabolic rhythm and obesity paradox., 2022, 145(6): 448–464.
[9] Huang Q, Tian LQ, Zhao XS, Lei SQ, Zhao B, Qiu Z, Xia ZY. Rev-erbs agonist SR9009 alleviates ischemia- reperfusion injury by heightening endogenous cardioprotection at onset of type-2 diabetes in rats: down- regulating ferritinophagy/ferroptosis signaling., 2022, 154: 113595.
[10] Pariollaud M, Gibbs JE, Hopwood TW, Brown S, Begley N, Vonslow R, Poolman T, Guo BQ, Saer B, Jones DH, Tellam JP, Bresciani S, Tomkinson NC, Wojno-Picon J, Cooper AW, Daniels DA, Trump RP, Grant D, Zuercher W, Willson TM, MacDonald AS, Bolognese B, Podolin PL, Sanchez Y, Loudon AS, Ray DW. Circadian clock component REV-ERBα controls homeostatic regulation of pulmonary inflammation., 2018, 128(6): 2281–2296.
[11] Cunningham PS, Meijer P, Nazgiewicz A, Anderson SG, Borthwick LA, Bagnall J, Kitchen GB, Lodyga M, Begley N, Venkateswaran RV, Shah R, Mercer PF, Durrington HJ, Henderson NC, Piper-Hanley K, Fisher AJ, Chambers RC, Bechtold DA, Gibbs JE, Loudon AS, Rutter MK, Hinz B, Ray DW, Blaikley JF. The circadian clock protein REVERBα inhibits pulmonary fibrosis development., 2020, 117(2): 1139–1147.
[12] Yuan X, Dong D, Li ZJ, Wu BJ. Rev-erbalpha activation down-regulates hepatic Pck1 enzyme to lower plasma glucose in mice., 2019, 141: 310–318.
[13] RaspéE, Duez H, Mansén A, Fontaine C, Fiévet C, Fruchart JC, Vennström B, Staels B. Identification of Rev-erbα as a physiological repressor of apoC-III gene transcription., 2002, 43(12): 2172–2179.
[14] Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang FF, Jager J, Lazar MA. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function., 2012, 26(7): 657–667.
[15] Anzulovich A, Mir A, Brewer M, Ferreyra G, Vinson C, Baler R. Elovl3: a model gene to dissect homeostatic links between the circadian clock and nutritional status., 2006, 47(12): 2690–2700.
[16] Sitaula S, Zhang JS, Ruiz F, Burris TP. Rev-erb regulation of cholesterologenesis., 2017, 131: 68–77.
[17] Duez H, van der Veen JN, Duhem C, Pourcet B, Touvier T, Fontaine C, Derudas B, Bauge E, Havinga R, Bloks VW, Wolters H, van der Sluijs FH, Vennström B, Kuipers F, Staels B. Regulation of bile acid synthesis by the nuclear receptor Rev-erbα., 2008, 135(2): 689–698.
[18] Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Lo Sasso G, Moschetta A, Schibler U. REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis., 2009, 7(9): e1000181.
[19] Zhang TP, Zhao MJ, Lu DY, Wang S, Yu FJ, Guo LX, Wen SJ, Wu BJ. REV-ERBα regulates CYP7A1 through repression of liver receptor homolog-1., 2018, 46(3): 248–258.
[20] Zhang TP, Chen M, Guo LX, Yu FJ, Zhou C, Xu HM, Wu BJ. Reverse erythroblastosis virus alpha antagonism promotes homocysteine catabolism and ammonia clearance., 2019, 70(5): 1770–1784.
[21] Pourcet B, Zecchin M, Ferri L, Beauchamp J, Sitaula S, Billon C, Delhaye S, Vanhoutte J, Mayeuf-Louchart A, Thorel Q, Haas JT, Eeckhoute J, Dombrowicz D, Duhem C, Boulinguiez A, Lancel S, Sebti Y, Burris TP, Staels B, Duez HM. Nuclear receptor subfamily 1 group D member 1 regulates circadian activity of NLRP3 inflammasome to reduce the severity of fulminant hepatitis in mice., 2018, 154(5): 1449–1464.e20.
[22] Brown MR, Laouteouet D, Delobel M, Villard O, Broca C, Bertrand G, Wojtusciszyn A, Dalle S, Ravier MA, Matveyenko AV, Costes S. The nuclear receptor REV-ERBα is implicated in the alteration of β-cell autophagy and survival under diabetogenic conditions., 2022, 13(4): 353.
[23] Vieira E, Marroqui L, Batista TM, Caballero-Garrido E, Carneiro EM, Boschero AC, Nadal A, Quesada I. The clock gene Rev-erbα regulates pancreatic β-cell function: modulation by leptin and high-fat diet., 2012, 153(2): 592–601.
[24] Vieira E, Merino B, Quesada I. Role of the clock gene Rev-erbα in metabolism and in the endocrine pancreas., 2015, 17(Suppl 1): 106–114.
[25] Vieira E, Marroqui L, Figueroa ALC, Merino B, Fernandez-Ruiz R, Nadal A, Burris TP, Gomis R, Quesada I. Involvement of the clock gene Rev-erbα in the regulation of glucagon secretion in pancreatic α-cells., 2013, 8(7): e69939.
[26] Wang S, Lin YK, Yuan X, Li F, Guo LX, Wu BJ. REV-ERBα integrates colon clock with experimental colitis through regulation of NF-kappaB/NLRP3 axis., 2018, 9(1): 4246.
[27] Ni YH, Zhao YF, Ma LY, Wang Z, Ni LY, Hu LT, Fu ZW. Pharmacological activation of REV-ERBα improves nonalcoholic steatohepatitis by regulating intestinal permeability., 2021, 114: 154409.
[28] Yu FJ, Wang ZG, Zhang TP, Chen X, Xu HM, Wang F, Guo LX, Chen M, Liu KS, Wu BJ. Deficiency of intestinal Bmal1 prevents obesity induced by high-fat feeding., 2021, 12(1): 5323.
[29] Song C, Tan P, Zhang Z, Wu W, Dong YH, Zhao LM, Liu HY, Guan HF, Li F. REV-ERB agonism suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss partially via FABP4 upregulation., 2018, 32(6): 3215–3228.
[30] He Y, Lin FW, Chen YQ, Tan Z, Bai D, Zhao Q. Overexpression of the circadian clock gene Rev-erbα affects murine bone mesenchymal stem cell proliferation and osteogenesis., 2015, 24(10): 1194– 1204.
[31] Liu H, Zhu YL, Gao YT, Qi DH, Zhao LM, Zhao LB, Liu CY, Tao TH, Zhou CK, Sun XY, Guo FJ, Xiao J. NR1D1 modulates synovial inflammation and bone destruction in rheumatoid arthritis., 2020, 11(2): 129.
[32] Yue J, He JJ, Wei YJ, Shen KF, Wu KF, Yang XL, Liu SY, Zhang CQ, Yang H. Decreased expression of Rev-Erbα in the epileptic foci of temporal lobe epilepsy and activation of Rev-Erbα have anti-inflammatory and neuroprotective effects in the pilocarpine model., 2020, 17(1): 43.
[33] Kou L, Chi XS, Sun YD, Han C, Wan F, Hu JJ, Yin SJ, Wu JW, Li YN, Zhou QL, Zou WK, Xiong N, Huang JS, Xia Y, Wang T. The circadian clock protein Rev-erbα provides neuroprotection and attenuates neuroinflammation against Parkinson's disease via the microglial NLRP3 inflammasome., 2022, 19(1): 133.
[34] Lee J, Kim DE, Griffin P, Sheehan PW, Kim DH, Musiek ES, Yoon SY. Inhibition of REV-ERBs stimulates microglial amyloid-beta clearance and reduces amyloid plaque deposition in the 5XFAD mouse model of Alzheimer's disease., 2020, 19(2): e13078.
[35] Roby DA, Ruiz F, Kermath BA, Voorhees JR, Niehoff M, Zhang JS, Morley JE, Musiek ES, Farr SA, Burris TP. Pharmacological activation of the nuclear receptor REV-ERB reverses cognitive deficits and reduces amyloid-β burden in a mouse model of Alzheimer's disease., 2019, 14(4): e0215004.
[36] Boulinguiez A, Duhem C, Mayeuf-Louchart A, Pourcet B, Sebti Y, Kondratska K, Montel V, Delhaye S, Thorel Q, Beauchamp J, Hebras A, Gimenez M, Couvelaere M, Zecchin M, Ferri L, Prevarskaya N, Forand A, Gentil C, Ohana J, Piétri-Rouxel F, Bastide B, Staels B, Duez H, Lancel S. NR1D1 controls skeletal muscle calcium homeostasis through myoregulin repression., 2022, 7(17): e153584.
[37] Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MKC, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Nevière R, Burris TP, Schrauwen P, Staels B, Duez H. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy., 2013, 19(8): 1039–1046.
[38] Rovina RL, da Rocha AL, Marafon BB, Pauli JR, de Moura LP, Cintra DE, Ropelle ER, da Silva ASR. One bout of aerobic exercise can enhance the expression of in oxidative skeletal muscle samples., 2021, 12: 626096.
[39] Bass J, Lazar MA. Circadian time signatures of fitness and disease., 2016, 354(6315): 994–999.
[40] Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms., 2020, 21(2): 67–84.
[41] Zeng YZ, Zhang T, Xu Y. Rapid assessment of circadian behavior in mice., 2022, 44(4): 346–357.
曾义准, 张陶, 徐璎. 分析小鼠昼夜节律变化的行为学方法. 遗传, 2022, 44(4): 346–357.
[42] Stenvers DJ, Scheer FAJL, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance., 2019, 15(2): 75–89.
[43] Crnko S, Schutte H, Doevendans PA, Sluijter JPG, van Laake LW. Minimally invasive ways of determining circadian rhythms in humans., 2021, 36(1): 7–20.
[44] Koronowski KB, Sassone-Corsi P. Communicating clocks shape circadian homeostasis., 2021, 371(6530): eabd0951.
[45] Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways., 2007, 318(5857): 1786–1789.
[46] Yin L, Lazar MA. The orphan nuclear receptor Rev-erbα recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene., 2005, 19(6): 1452–1459.
[47] Zhang YX, Fang B, Emmett MJ, Damle M, Sun Z, Feng D, Armour SM, Remsberg JR, Jager J, Soccio RE, Steger DJ, Lazar MA. GENE REGULATION. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock., 2015, 348(6242): 1488–1492.
[48] Griffin P, Sheehan PW, Dimitry JM, Guo C, Kanan MF, Lee J, Zhang JS, Musiek ES. REV-ERBα mediates complement expression and diurnal regulation of microglial synaptic phagocytosis., 2020, 9: e58765.
[49] Millius A, Ueda H. Rhythms: the dark side meets the light., 2018, 359(6381): 1210–1211.
[50] Delezie J, Dumont S, Dardente H, Oudart H, Gréchez- Cassiau A, Klosen P, Teboul M, Delaunay F, Pévet P, Challet E. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism., 2012, 26(8): 3321–3335.
[51] Solt LA, Wang YJ, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists., 2012, 485(7396): 62–68.
[52] Verlande A, Chun SK, Goodson MO, Fortin BM, Bae H, Jang C, Masri S. Glucagon regulates the stability of REV-ERBα to modulate hepatic glucose production in a model of lung cancer-associated cachexia., 2021, 7(26): eabf3885.
[53] Burchett JB, Knudsen-Clark AM, Altman BJ. MYC ran up the clock: the complex interplay between MYC and the molecular circadian clock in cancer., 2021, 22(14): 7761.
[54] Simcox JA, Mitchell TC, Gao Y, Just SF, Cooksey R, Cox J, Ajioka R, Jones D, Lee SH, King D, Huang JY, McClain DA. Dietary iron controls circadian hepatic glucose metabolism through heme synthesis., 2015, 64(4): 1108–1119.
[55] Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus., 2018, 19(8): 453–469.
[56] Ding GL, Li X, Hou XG, Zhou WJ, Gong YY, Liu FQ, He YL, Song J, Wang J, Basil P, Li WB, Qian SC, Saha P, Wang JB, Cui C, Yang TT, Zou KX, Han YH, Amos CI, Xu Y, Chen L, Sun Z. REV-ERB in GABAergic neurons controls diurnal hepatic insulin sensitivity., 2021, 592(7856): 763–767.
[57] Xu YT, Guo JB, Zhang L, Miao GL, Lai PP, Zhang WX, Liu LL, Hou XL, Wang YH, Huang W, Liu G, Gao MM, Xian XD. Targeting apoC3 paradoxically aggravates atherosclerosis in hamsters with severe refractory hypercholesterolemia., 2022, 9: 840358.
[58] Hunter AL, Pelekanou CE, Adamson A, Downton P, Barron NJ, Cornfield T, Poolman TM, Humphreys N, Cunningham PS, Hodson L, Loudon ASI, Iqbal M, Bechtold DA, Ray DW. Nuclear receptor REVERBα is a state-dependent regulator of liver energy metabolism., 2020, 117(41): 25869–25879.
[59] Laitinen S, Fontaine C, Fruchart JC, Staels B. The role of the orphan nuclear receptor Rev-Erbα in adipocyte differentiation and function., 2005, 87(1): 21–25.
[60] Hunter AL, Pelekanou CE, Barron NJ, Northeast RC, Grudzien M, Adamson AD, Downton P, Cornfield T, Cunningham PS, Billaud JN, Hodson L, Loudon AS, Unwin RD, Iqbal M, Ray DW, Bechtold DA. Adipocyte NR1D1 dictates adipose tissue expansion during obesity., 2021, 10: e63324.
[61] Pan XY, Hussain MM. Bmal1 regulates production of larger lipoproteins by modulating cAMP-responsive element-binding protein H and apolipoprotein AIV., 2022, 76(1): 78–93.
[62] Bond MR, Hanover JA. O-GlcNAc cycling: a link between metabolism and chronic disease., 2013, 33: 205–229.
[63] Berthier A, Vinod M, Porez G, Steenackers A, Alexandre J, Yamakawa N, Gheeraert C, Ploton M, Maréchal X, Dubois-Chevalier J, Hovasse A, Schaeffer-Reiss C, Cianférani S, Rolando C, Bray F, Duez H, Eeckhoute J, Lefebvre T, Staels B, Lefebvre P. Combinatorial regulation of hepatic cytoplasmic signaling and nuclear transcriptional events by the OGT/REV-ERBα complex., 2018, 115(47): E11033–E11042.
[64] Samad F, Ruf W. Inflammation, obesity, and thrombosis., 2013, 122(20): 3415–3422.
[65] Griffin P, Dimitry JM, Sheehan PW, Lananna BV, Guo C, Robinette ML, Hayes ME, Cedeno MR, Nadarajah CJ, Ezerskiy LA, Colonna M, Zhang JS, Bauer AQ, Burris TP, Musiek ES. Circadian clock protein Rev-erbα regulates neuroinflammation., 2019, 116(11): 5102–5107.
[66] Zhao WJ, Cui LY, Huang XX, Wang SC, Li DJ, Li LP, Sun Y, Du MR. Activation of Rev-erbα attenuates lipopolysaccharide-induced inflammatory reactions in human endometrial stroma cells via suppressing TLR4-regulated NF-kappaB activation., 2019, 51(9): 908–914.
[67] Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon ASI. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines., 2012, 109(2): 582–587.
[68] Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression., 2014, 192(1): 407–417.
[69] Lam MTY, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, Benner C, Kaikkonen MU, Kim AS, Kosaka M, Lee CY, Watt A, Grossman TR, Rosenfeld MG, Evans RM, Glass CK. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription., 2013, 498(7455): 511–515.
[70] Wu ZN, Liao F, Luo GQ, Qian YX, He XJ, Xu WY, Ding S, Pu J. NR1D1 deletion induces rupture-prone vulnerable plaques by regulating macrophage pyroptosis via the NF-kappaB/NLRP3 inflammasome pathway., 2021: 5217572.
[71] Sitaula S, Billon C, Kamenecka TM, Solt LA, Burris TP. Suppression of atherosclerosis by synthetic REV-ERB agonist., 2015, 460(3): 566–571.
[72] Wang QL, Robinette ML, Billon C, Collins PL, Bando JK, Fachi JL, Sécca C, Porter SI, Saini A, Gilfillan S, Solt LA, Musiek ES, Oltz EM, Burris TP, Colonna M. Circadian rhythm-dependent and circadian rhythm-independent impacts of the molecular clock on type 3 innate lymphoid cells., 2019, 4(40): eaay7501.
[73] Amir M, Chaudhari S, Wang R, Campbell S, Mosure SA, Chopp LB, Lu Q, Shang JS, Pelletier OB, He YJ, Doebelin C, Cameron MD, Kojetin DJ, Kamenecka TM, Solt LA. REV-ERBα regulates TH17 cell development and autoimmunity., 2018, 25(13): 3733–3749.e8.
[74] Yu XF, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, Rothman PB, Takahashi JS, Hooper LV. TH17 cell differentiation is regulated by the circadian clock., 2013, 342(6159): 727–730.
[75] Farez MF, Mascanfroni ID, Méndez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, Balbuena Aguirre ME, Patel B, Ysrraelit MC, Zhu C, Kuchroo VK, Rabinovich GA, Quintana FJ, Correale J. Melatonin contributes to the seasonality of multiple sclerosis relapses., 2015, 162(6): 1338–1352.
[76] Chang C, Loo CS, Zhao X, Solt LA, Liang YQ, Bapat SP, Cho H, Kamenecka TM, Leblanc M, Atkins AR, Yu RT, Downes M, Burris TP, Evans RM, Zheng Y. The nuclear receptor REV-ERBα modulates Th17 cell-mediated autoimmune disease., 2019, 116(37): 18528–18536.
[77] Li T, Eheim AL, Klein S, Uschner FE, Smith AC, Brandon-Warner E, Ghosh S, Bonkovsky HL, Trebicka J, Schrum LW. Novel role of nuclear receptor Rev-erbα in hepatic stellate cell activation: potential therapeutic target for liver injury., 2014, 59(6): 2383–2396.
[78] Grant D, Yin L, Collins JL, Parks DJ, Orband-Miller LA, Wisely GB, Joshi S, Lazar MA, Willson TM, Zuercher WJ. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbalpha., 2010, 5(10): 925–932.
[79] Morioka N, Kodama K, Tomori M, Yoshikawa K, Saeki M, Nakamura Y, Zhang FF, Hisaoka-Nakashima K, Nakata Y. Stimulation of nuclear receptor REV-ERBs suppresses production of pronociceptive molecules in cultured spinal astrocytes and ameliorates mechanical hypersensitivity of inflammatory and neuropathic pain of mice., 2019, 78: 116–130.
[80] Wang QX, Sundar IK, Lucas JH, Muthumalage T, Rahman I. Molecular clock REV-ERBα regulates cigarette smoke-induced pulmonary inflammation and epithelial- mesenchymal transition., 2021, 6(12): e145200.
[81] Tao LL, Yu HY, Liang R, Jia R, Wang JJ, Jiang K, Wang ZG. Rev-erbα inhibits proliferation by reducing glycolytic flux and pentose phosphate pathway in human gastric cancer cells., 2019, 8(10): 57.
[82] Wolff SEC, Wang XL, Jiao H, Sun J, Kalsbeek A, Yi CX, Gao YQ. The effect of Rev-erbα agonist SR9011 on the immune response and cell metabolism of microglia., 2020, 11: 550145.
[83] Tiwari D, Ahuja N, Kumar S, Kalra R, Nanduri R, Gupta S, Khare AK, Bhagyaraj E, Arora R, Gupta P. Nuclear receptor Nr1d1 alleviates asthma by abating GATA3 gene expression and Th2 cell differentiation., 2022, 79(6): 308.
[84] Sulli G, Rommel A, Wang XJ, Kolar MJ, Puca F, Saghatelian A, Plikus MV, Verma IM, Panda S. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence., 2018, 553(7688): 351–355.
[85] Gao L-B, Wang Y-H, Liu Z-H, Sun Y, Cai P, Jing Q. Identification of a small molecule SR9009 that activates NRF2 to counteract cellular senescence., 2021, 20(10): e13483.
[86] Fekry B, Ribas-Latre A, Drunen RV, Santos RB, Shivshankar S, Dai YL, Zhao ZM, Yoo S-H, Chen Z, Sun K, Sladek FM, Younes M, Eckel-Mahan K. Hepatic circadian and differentiation factors control liver susceptibility for fatty liver disease and tumorigenesis., 2022, 36(9): e22482.
[87] Shen WT, Zhang W, Ye WL, Wang HH, Zhang QX, Shen J, Hong QS, Li X, Wen G, Wei T, Zhang J. SR9009 induces a REV-ERB dependent anti-small-cell lung cancer effect through inhibition of autophagy., 2020, 10(10): 4466–4480.
[88] Sun LY, Lyu YY, Zhang HY, Shen Z, Lin GQ, Geng N, Wang YL, Huang L, Feng ZH, Guo X, Lin N, Ding S, Yuan AC, Zhang L, Qian K, Pu J. Nuclear receptor NR1D1 regulates abdominal aortic aneurysm development by targeting the mitochondrial tricarboxylic acid cycle enzyme aconitase-2., 2022: 101161CIRCULATIONAHA121057623.
[89] Kojetin D, Wang YJ, Kamenecka TM, Burris TP. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB., 2011, 6(2): 131–134.
[90] Dong D, Sun H, Wu ZF, Wu BJ, Xue YX, Li ZJ. A validated ultra-performance liquid chromatography- tandem mass spectrometry method to identify the pharmacokinetics of SR8278 in normal and streptozotocin-induced diabetic rats., 2016, 1020: 142–147.
[91] Kim J, Park I, Jang S, Choi M, Kim D, Sun W, Choe Y, Choi JW, Moon C, Park SH, Choe HK, Kim K. Pharmacological rescue with SR8278, a circadian nuclear receptor REV-ERBα antagonist as a therapy for mood disorders in Parkinson's disease., 2022, 19(2): 592–607.
[92] Zhang TP, Yu FJ, Xu HM, Chen M, Chen X, Guo LX, Zhou C, Xu YT, Wang F, Yu JD, Wu BJ. Dysregulation of REV-ERBα impairs GABAergic function and promotes epileptic seizures in preclinical models., 2021, 12(1): 1216.
[93] Shi JF, Tong RY, Zhou M, Gao Y, Zhao YC, Chen YF, Liu WH, Li GX, Lu D, Meng GF, Hu LH, Yuan AC, Lu XY, Pu J. Circadian nuclear receptor Rev-erbα is expressed by platelets and potentiates platelet activation and thrombus formation., 2022, 43(24): 2317–2334.
[94] Wang S, Lin YK, Zhou ZY, Gao L, Yang ZM, Li F, Wu BJ. Circadian clock gene Bmal1 regulates bilirubin detoxification: a potential mechanism of feedback control of hyperbilirubinemia., 2019, 9(18): 5122– 5133.
[95] Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets., 2014, 13(3): 197–216.
[96] Trump RP, Bresciani S, Cooper AWJ, Tellam JP, Wojno J, Blaikley J, Orband-Miller LA, Kashatus JA, Boudjelal M, Dawson HC, Loudon A, Ray D, Grant D, Farrow SN, Willson TM, Tomkinson NCO. Optimized chemical probes for REV-ERBα., 2013, 56(11): 4729–4737.
[97] Zhou ZY, Lin YK, Gao L, Yang ZM, Wang S, Wu BJ. Circadian pharmacological effects of berberine on chronic colitis in mice: Role of the clock component Rev-erbα., 2020, 172: 113773.
[98] Chen M, Zhou C, Xu HM, Zhang TP, Wu BJ. Chronopharmacological targeting of Rev-erbα by puerarin alleviates hyperhomocysteinemia in mice., 2020, 125: 109936.
[99] Artyomov MN, Van den Bossche J. Immunometabolism in the single-cell era., 2020, 32(5): 710–725.
The nuclear receptor REV-ERBα integrates circadian clock and energy metabolism
Shuyu Mao1, Changrui Zhao1, Chang Liu1,2
The physiological processes of mammals show rhythmic changes in a 24-h cycle. Circadian rhythms are under the subtle control of the autonomous circadian clock, and dysregulation of the circadian system can lead to health problems such as metabolic disorders. REV-ERBα, a member of the nuclear receptor superfamily, is an important component of the mammalian circadian clock. REV-ERBα regulates various physiological processes, including the regulation of metabolism, inflammation and immunity as well as the circadian rhythm, making it a potential therapeutic target for metabolic syndrome, inflammatory diseases and cancers. In recent years, an array of new REV-ERBα ligands have been discovered, most of which have potential applications in the treatment of diseases. In this review, we focus on the regulatory role of nuclear receptor REV-ERBα in energy metabolism and inflammation, in order to provide new strategies for the therapy of metabolic syndrome and its related diseases.
REV-ERBα; metabolism; inflammation; REV-ERBα ligands
2022-09-26;
2022-11-09;
2022-11-23
国家自然科学基金(编号:92057112)和国家重点研发计划(编号:2021YFF0702000)资助[Supported by the National Natural Science Foundation of China (No. 92057112) and the National Key Research and Development Program of China (No. 2021YFF0702000)]
冒姝羽,在读硕士研究生,专业方向:细胞生物学。E-mail: 3220030438@stu.cpu.edu.cn
刘畅,博士,教授,研究方向:生物钟与能量代谢。E-mail: changliu@cpu.edu.cn
10.16288/j.yczz.22-310
(责任编委: 孟卓贤)
