Nystose attenuates bone loss and promotes BMSCs differentiation to osteoblasts through BMP and Wnt/β-catenin pathway in ovariectomized mice
2023-01-21QiZhngSijingHuJinjunWuPengSunQunlongZhngYngWngQimingZhoTingHnLupingQinQioynZhng
Qi Zhng,Sijing Hu,Jinjun Wu,Peng Sun,Qunlong Zhng,Yng Wng,Qiming Zho,Ting Hn,Luping Qin,,Qioyn Zhng,
a School of Pharmaceutical Sciences,Zhejiang Chinese Medical University,Hangzhou 310053,China
b Zhejiang Traditional Chinese Medicine&Health Industry Group Co.,Ltd.Hangzhou 310016,China
c School of Pharmacy,Second Military Medical University,Shanghai 200433,China
Keywords:Nystose Bone marrow mesenchymal stem cell Osteoblast Adipocyte BMP pathway Wnt/β-catenin pathway
ABSTRACT Increasing the osteogenic differentiation ability and decreasing the adipogenic differentiation ability of bone marrow mesenchymal stem cells (BMSCs) is a potential strategy for the treatment of osteoporosis(OP).Naturally derived oligosaccharides have shown significant anti-osteoporotic effects.Nystose (NST),an oligosaccharide,was isolated from the roots of Morinda officinalis How.(MO).The aim of the present study was to investigate the effects of NST on bone loss in ovariectomized mice,and explore the underlying mechanism of NST in promoting differentiation of BMSCs to osteoblasts.Administration of NST (40,80 and 160 mg/kg) and the positive control of estradiol valerate (0.2 mg/kg) for 8 weeks significantly prevented bone loss induced by ovariectomy (OVX),increased the bone mass density (BMD),improved the bone microarchitecture and reduced urine calcium and deoxypyridinoline (DPD) in ovariectomized mice,while inhibited the increase of body weight without significantly affecting the uterus weight.Furthermore,we found that NST increased osteogenic differentiation,inhibited adipogenic differentiation of BMSCs in vitro,and upregulated the expression of the key proteins of BMP and Wnt/β-catenin pathways.In addition,Noggin and Dickkopf-related protein-1 (DKK-1)reversed the effect of NST on osteogenic differentiation and expression of the key proteins in BMP and Wnt/β-catenin pathway.The luciferase activities and the molecular docking analysis further supported the mechanism of NST.In conclusion,these results indicating that NST can be clinically used as a potential alternative medicine for the prevention and treatment of postmenopausal osteoporosis.
1.Introduction
Osteoporosis (OP) is a systemic metabolic disease characterized by a reduction in bone mass and deterioration of the bone microarchitecture,eventually leading to the increased risk of fractures.About 50% postmenopausal women worldwide are plagued by OP,and the incidence of fractures in osteoporotic patients is as high as 40% [1,2].OP not only harms the national health and lowers the national happiness but imposes heavy burdens on society and families.Although various medical therapies,including estrogen replacement therapy and the use of bisphosphonates,selective estrogen receptor modulators and calcium preparations,have achieved good therapeutic outcomes,adverse reactions arising from these synthetic drugs have limited their clinical applications [3].Therefore,it is imminent to develop and explore more efficacious and safer anti-osteoporotic medicines to benef it more patients.
Bone remodeling includes osteoblastic bone formation and osteoclastic bone resorption.The excess osteoclastic activity or weaker osteoblastic activity often leads to bone loss,finally resulting in the occurrence of OP,while decreased osteoblastic bone formation is considered as a critical factor of OP [4].Osteoblasts are derived from bone marrow mesenchymal stem cells (BMSCs),which have the potential to differentiate into a variety of functional cells under specific conditions,such as osteoblasts,adipocytes and chondrocytes [5].Accumulating evidence has demonstrated that induction of BMSCs into adipocytes decreases the occurrence of OP,and conversely induction of BMSCs into osteoblasts hinders adipogenesis.Therefore,controlling the differentiation of BMSCs into adipogenic cells or osteoblasts is one of the potential strategies to increase bone formation,and prevent and treat OP [6].
The process of BMSCs differentiation into osteoblasts,including proliferation,differentiation and bone matrix mineralization,is modulated by bone morphogenetic protein (BMP) and Wnt/β-catenin pathway.BMP2,an important member of BMP family,binds to its transmembrane receptor to activate Smad signaling pathway.The activation of Smad1/5/8 and Smad4 increases the expression of osteogenic runt-related gene 2 (Runx2/Cbfa1),and also inhibits the expression of adipogenic peroxisome proliferator-activated receptor-γ(PPAR-γ).Thence,BMP2 directly controls the differentiation of BMSCs into osteoblasts or adipocytes [7].The Wnt family,which consists of large numbers of secreted glycoproteins,is involved in the development,metabolism and maintenance of stem cells,and other critical biological processes as well.Wnt ligands stabilizeβ-catenin and reduce its phosphorylation by binding to the transmembrane Frizzled receptor (FZD) and LRP5/6 coreceptor.Unphosphorylatedβ-catenin translocates into the nucleus and regulates target gene expression of BMSCs differentiation,thus facilitating osteogenic differentiation and inhibiting adipogenic differentiation of MSCs [8-10].
Oligosaccharides,which are carbohydrates with a low polymerization degree,possess diverse biochemical and physiological properties because of their distinctive anomeric configurations,glycosidic linkages,monosaccharide composition and molecular weight [11].Several naturally derived oligosaccharides such as trehalose,Bajijiasu,algal oligosaccharides,fructooligosaccharide fromAchyranthes bidentat,β-1,3-glucanoligosaccharide fromPleurotus sajor-caju,and fructooligosaccharide [12-17],which have been shown to possess significant anti-osteoporotic activitiesin vitroandin vivo.Oligosaccharides fromMorinda officinalisHow.(MO)have been shown to possess anti-depression,anti-Alzheimer’s disease and anti-OP activities [18,19].Our preliminary data demonstrated that oligosaccharides from MO,such as kestose,nystose (NST) and kestopentaose,could promote the differentiation of BMSCs into osteoblasts.NST,being the major constituent of oligosaccharides in MO,is often used as an indicator of quality control of the root of MO in theChinese Pharmacopoeia,requiring that the content of NST in the medicinal material should not be less than 2.0% [20].However,the therapeutic effect of NST on OP and the mechanism of NST in inducing BMSCs differentiation into osteoblasts remains undefined.We therefore hypothesized that NST could prevent bone loss through promoting the differentiation of BMSCs into osteoblasts via the BMP and Wnt/β-catenin pathways.The aim of the present study was to validate the anti-OP effect of NST in animal experiments,and explore the underlying action mechanism of NST in inducing BMSCs differentiation into osteoblasts.
2.Materials and methods
2.1 Reagents and apparatus
Instruments used in this study were high performance liquid chromatography (Agilent 1100 Series,USA);Nuclear magnetic resonance instrument (Bruker 400 Series,Switzerland);Microplate Reader (Thermo Multiskan Go Series,USA);Electrophoresis apparatus (Bio-rad,USA);Chemiluminescence digital imaging system (Bio-rad,USA).All assay kits in this study were obtained from Nanjing Jiancheng Institute of Biological Engineering (Nanjing,China).All the other reagents were of analytical-grade purity,and purchased from Sinopharm Chemical Reagent Co.,Ltd.Deionized water came from a Milli-Q system from Millipore (Milford,MA,USA).
Fetal bovine serum (FBS),α-modified minimal essential medium (α-MEM),phosphate buffered saline (PBS) and penicillin/streptomycin were obtained from Gibco company (USA).Ascorbic acid,β-glycerophosphate and dexamethasone were purchased from Sigma-Aldrich (St.Louis,MO).IBMX and indomethacin were obtained from Solarbio Life Science (Beijing,China).Antibodies against Osterix,BMP2,Dlx-5,COL-I,ALP,OPN,PPAR-γand Adiponectin were purchased from Abcam (Cambridge,MA,USA).Antibodies against Wnt3a were purchased from Boster Biological Technology (Wuhan,China).All the other antibodies used in this study were obtained from Cell Signaling Technology (Beverly,MA,USA).
2.2 Preparation of NST from MO root
MO root was purchased from the medicinal material market in Bozhou of Anhui Province of China.NST was prepared as follows:20 kg MO root was extracted with water,and then separated and purified by silica gel column chromatography.Finally,15 g NST was obtained with the purity >95% .The chemical structure was identified with nuclear magnetic resonance spectroscopy.
2.3 Animal experiment
Sixty ICR female mice weighing 20-25 g were purchased from Shanghai Leigen Biotechnology Co.,Ltd.and were kept in the Experimental Animal Center of the Second Military Medical University (Shanghai,China;Certificate No.SCXK 2017-0022).The mice were fed at 21 °C under a 12 h light/12 h dark cycle with free access to food and water.
2.4 Experimental protocols
Sixty ICR female mice were equally randomized into 6 groups:a sham operation (Sham) group,an ovariectomized model (OVX)group,a positive control group (E2V,0.2 mg/kg),and three different NST dose (40,80 and 160 mg/kg) groups,these dosage of NST in animal experiments were estimated according to the clinical dosage of MO and the content of NST in MO.Sham-operation and OVX were performed after anesthesia induction with intraperitoneal (i.p.)injection of 4% chloral hydrate.After one-week observation,the mice were orally administered with E2V (0.2 mg/kg) or NST (40,80 or 160 mg/kg) for 8 weeks.The Sham group and OVX model group were given the same dose of 0.5% CMC-Na daily.The dosage was adjusted according to the weight change.Finally,all mice were sacrificed,and the femur was collected for micro computed tomography (micro-CT),and urine was collected for determination of the biochemical parameters.
2.5 Uterine weight
The complete uterus was removed,washed with normal saline,dried with absorbent paper,and weighed immediately.
2.6 Micro-CT analysis
The three-dimensional (3D) structure of the mouse femur was reconstructed by using Micview V2.1.2 software.1.0 mm bone tissue 0.16 mm from the growth plate was selected to reconstruct the region of interest (ROI).Bone mineral density (BMD) and morphological parameters,including bone volume to total volume (BV/TV),bone surface to bone volume (BS/BV),trabecular number (Tb.N.),trabecular separation (Tb.Sp.) and trabecular thickness (Tb.Th.),were analyzed by ABA software.
2.7 Determination of biochemical indicators in urine and serum
The levels of calcium (U-Ca) and deoxypyridinoline (DPD) in urine were measured according to the kit manufacturer’s protocol.
2.8 BMSCs culture and identification
BMSCs were isolated from the femur of the 3-week-old SD rat,and cultured in a dish containingα-MEM supplemented with 10% FBS,100 U/mL penicillin,100 mg/mL streptomycinn and 1%L-glutaminea in humidified atmosphere of 5% CO2for 10 days.The medium was changed every 3 days.The subculture cells (passage 2 to 4)were used for subsequent experiments.BMSCs were identified in terms of the morphological character,properties of differentiation into osteoblasts and adipocytes,and expression of CD29 and CD45.
2.9 Flow cytometry analysis
Specific cell membrane markers of BMSCs were detected by flow cytometry.Briefly,BMSCs were digested,and then re-suspended in PBS solution.Then,cells were transferred to a new Eppendorf tube(106cells/tube).The CD29 and CD45 monoclonal antibodies were added into the Eppendorf tube containing 106cells,and then incubated in dark for 30 min at room temperature.The cells in the Eppendorf tube were washed twice with PBS and re-suspended in 400 μL PBS.The expression of CD29 and CD45 was detected by flow cytometry assay.
2.10 Cell proliferation assay
BMSCs were seeded in a 96-well culture plate at a density of 5 × 103cells per well and cultured inα-MEM medium with 10% FBS,treated with NST at dose of 10-10,10-9and 10-8mol/L for 48 h,or treated with Noggin (an inhibitor of the BMP signaling pathway)/DKK-1 (an inhibitor of the Wnt/β-catenin signaling pathway) prior to NST intervention.Cell proliferation was measured by CCK-8 assay.10 μL CCK-8 solution was added to each well and then incubated for appropriate time.The absorbance was determined at 450 nm with a microplate reader.
2.11 ALP staining and activity determination
BMSCs were seeded in a 48-well culture plate at a density of 2.5 × 104cells per well and cultured inα-MEM medium with 10% FBS,and treated with NST at the dose of 10-10,10-9and 10-8mol/L,or treated with Noggin/DKK-1 prior to NST intervention for 7 days to perform ALP staining and determination of ALP activity.The cells were stained with BCIP/NBT assay kit at 37 °C for 30 min.ALP activity was measured according to the manufacturer’s instructions of the assay kit.Briefly,the cells were washed twice with PBS,and then lysed on ice for 30 min.The cell lysate was collected by centrifugation,and mixed with detection buffer and color substrate,and then incubated for appropriate time.The absorbance was determined at 405 nm with a microplate reader.The alkaline phosphatase activity was calculated according to the definition of enzyme activity provided in the instructions of the assay kit.
2.12 Alizarin red staining for analysis of bone mineralized nodule formation
BMSCs were seeded in a 48-well culture plate at a density of 2.5 × 104cells per well and cultured in osteogenic medium (50 µg/mL ascorbic acid,10 mmol/L sodiumβ-glycerophosphate and 10-8mol/L dexamethasone) and treated with NST at dose of 10-10,10-9and 10-8for 21 days.Then,cells were fixed with 4% paraformaldehyde and stained with alizarin red (pH 8.3) at 37 °C for 30 min.Bone nodules were observed under a microscope.To quantify cell mineralization,bone nodules were dissolved with 10% (m/V) cetylpyridinium phosphate chloride,and the absorbance was measured at 570 nm with a microplate reader.
2.13 Analysis of adipocyte differentiation by Oil red staining
BMSCs were seeded in a 48-well culture plate at a density of 2.5 × 104cells per well and cultured in adipogenic medium(0.5 mmol/L IBMX,0.1 mmol/L indomethacin and 1 μmol/L dexamethasone) and treated with NST at dose of 10-10,10-9and 10-8mol/L for 7 days.The adipogenesis of BMSCs were evaluated by qualitative staining of Oil red O (Oil red O stain kits were from Nanjing Jiancheng Institute of Biological Engineering Nanjing,China).The morphology and color of cells were observed and photographed under the microscope.To quantify the activity of adipogenesis,the lipid droplets were dissolved in isopropanol,and the absorbance was measured at 570 nm with a microplate reader.
2.14 Western blot analysis
BMSCs were seeded in a 6-well culture plate at a density of 1 × 105cells per well and cultured inα-MEM medium with 10 % FBS,and then treated with NST at the dose of 10-10,10-9and 10-8mol/L,or treated with Noggin/DKK-1 prior to NST intervention for 7 days.Subsequently,cells were lysed and the protein content was determined using a BCA protein kit.The cell lysates were incubated with a sample buffer of 5 × Loading at 100 °C for 5 min.An equal amount of protein was separated by 10% SDS-PAGE and transferred to the PVDF membrane.The membrane was then blocked with 5% BSA for 2 h and incubated with corresponding primary antibodies overnight at 4 °C,and then washed 3 times with TBST,incubated with the HRP-conjugated secondary antibody for 1 h at room temperature.The protein bands were detected with electro-chemiluminescence (ECL) reagent,and scanned using a chemiluminescence digital imaging system.
2.15 Luciferase reporter gene assay
The transcriptional activity of BMP2 andβ-catenin was determined by luciferase reporter method.293 T cells were seeded in a 96-well culture plate at a density of 5 × 103cells per well overnight,and then transfected with BMP2,β-catenin and PGL-3 using transfection reagent POLO3000.After 8 h incubation,cells were treated with NST at the dose of 10-10,10-9and 10-8mol/L for 48 h.The lysate was collected,and firefly luciferase and renilla luciferase were measured using a multifunctional microplate reader.
2.16 Molecular docking
The structures of BMP2 andβ-catenin were prepared by the Protein Preparation Wizard Workflow provided in the Maestro module of Schrödinger software.As BMP2 does not have a suitable PDB for molecular docking,onlyβ-catenin was verified for molecular docking.The PDB ID ofβ-catenin is 6O9C.The protein was processed through the default pipeline.Subsequently,the Glidedock module of Schrödinger software was used to generate the protein grid of these complexes,and the grid boxes were defined as 12 × 12 × 12 Å,and the spatial region was centered on the original ligand of the complex structures.The 3D structure of the NST compound was constructed by Chem3D Ultra 8.0;stereoisomers and tautomers were generated by LigPrep;and the protonation states of ligands at pH 7.0 ± 2.0 were predicted by Epik.Other parameters were set as default.Ligand docking module in Schrödinger was used to choose the receptor grid file and ligand file with extra-precision(XP) mode,which was generated from ligand preparation and protein preparation.The binding mode was visualized by Pymol [21].
2.17 Statistical analysis
The Graphpad Prism 5.0 statistical software was used to analyze the experimental data.The analysis of ANOVA and Student’st-test were applied to determine significant differences in experimental group data.Data are expressed as the mean ± standard deviation (SD)and the value ofP<0.05 was considered statistically significant.
3.Results
3.1 The chemical structure of NST
1H NMR was used to determine glycosidic bond configuration of NST.Theβ-type glycosidic linkage was less thanδ5.0 andα-type glycosidic linkage was more thanδ5.0 of the anomeric proton chemical-shift value.Only one anomeric proton (residue A)presenting an anomeric signal atδ5.35 was found in the anomeric proton region (δ4.30-6.00) of the1H NMR (Fig.1A).The1H resonance for H2 of residue A was indexed toδ3.44 based on the1H-1H COSY spectrum (Fig.1B).Similarly,the1H resonances of H3,H4 and H5 were assigned.The signal of H6 was assigned in the1H-1H COSY and HSQC spectra (Fig.1C) after C6 signal was identified from the HSQC spectrum.The carbon chemical shifts from C1 to C5 were assigned based on the13C NMR and HSQC spectra.The chemical shift of C6 was indexed toδ60.08 from the13C and DEPT 135 NMR spectra (Fig.1D and 1E). Similarly,the major13C and1H NMR chemical shifts of other residues were assigned using1H-1H COSY,HSQC and DEPT 135 NMR spectra (Table 1).According to the literature [22-25],the sugar residues could be assigned as A,α-DGlcp(1→;B,→1)-β-D-Fruf-(2→;C,→1)-β-D-Fruf-(2→ andD,β-D-Fruf-(2→.
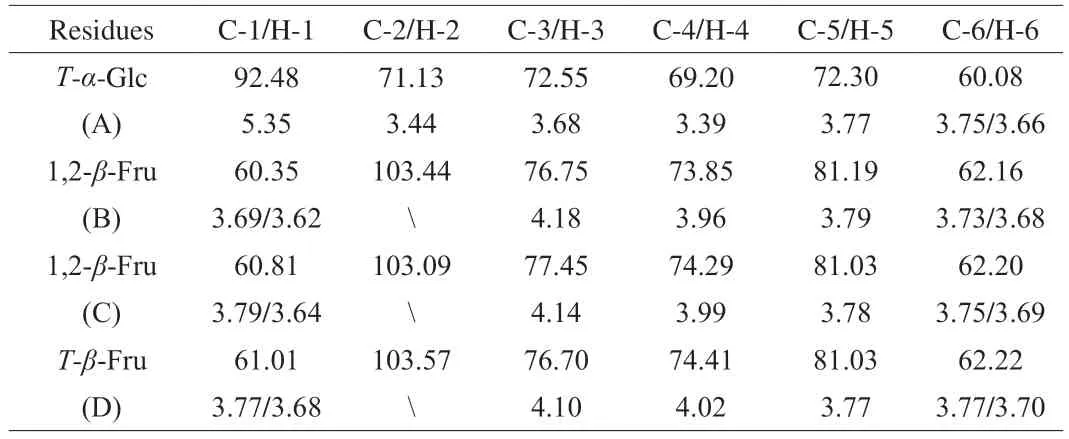
Table 1 The 1H and 13C NMR chemical shifts of the sugar residues of NST.
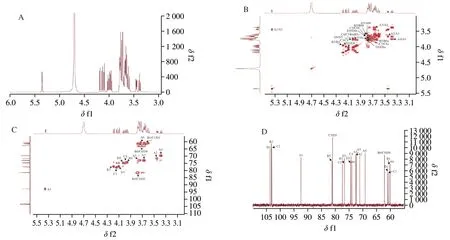
Fig.1 Structure identification of NST.(A) 1H NMR spectrum.(B) 1H-1H COSY NMR spectrum.(C) HSQC NMR spectrum.(D) 13C NMR spectrum.(E) DEPT135 NMR spectrum.(F) HMBC NMR spectrum.(G) Chemical structure of NST.

Fig.1 (Continued)
To determine the main chain structure of NST,the HMBC spectra were recorded.The HMBC spectrum of NST (Fig.1F) revealed an obvious correlation between C2 of B and H1 of A,signifying that C2 of B was connected to the 1-position of A.An intense cross peak between C2 of C and H1a and H1b of B indicated that C2 of C was connected to the 1-position of B.An intense cross peak between C2 of D and H1a and H1b of C indicated that C2 of D was connected to the 1-position of C.According to the aforementioned result,the structure of NST wasβ-D-Fruf-(2→1)-β-D-Fruf-(2→1)-β-D-Fruf-(2→1)-α-DGlcp(Fig.1G),known as nystose,which is in good agreement with the result in the literature [26].
3.2 NST inhibits the increase of body weight but does not affect the uterine weight in OVX mice
Ovariectomy caused estrogen deficiency,leading to the increased body weight and decreased uterine weight.As shown in Fig.2A and 2B,there was no significant difference in body weight of the animals between all groups at the beginning of the experiment.In the 8 weeks of the experiment,the body weight of all mice gradually increased,and the body weight of ovariectomized mice significantly increased compared with the Sham mice at the end of experiment.The treatment of NST and E2V decreased body weight of OVX mice,indicating that NST could inhibit the excessive weight gain caused by ovariectomy.As shown in Fig.2C,the uterus weight of mice in OVX group was significantly reduced when compared with that in Sham group,E2V treatment significantly increased the uterine weight of OVX mice,but NST had no significant effect on their uterine weight,indicating that NST did not stimulate uterine hyperplasia.
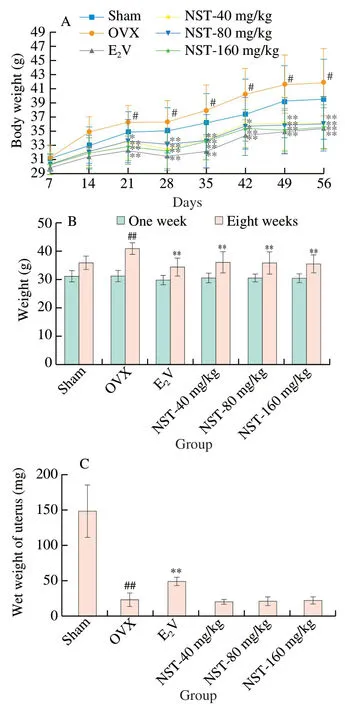
Fig.2 Effects of NST on body weight and uterus weight of OVX model mice.(A) Mouse body weight change during the experiment.(B) Comparison of body weight change in each group at the beginning of the experiment vs.8 weeks after NST treatment.(C) Effects of NST on uterus weight of ovariectomized mice.Data are presented as the mean ± SD.#P <0.05,##P<0.01,compared with Sham group;*P <0.05,**P<0.01,compared with OVX group.
3.3 NST reduces the urine level of calcium and DPD in OVX mice
With the bone loss and the subsequent bone matrix degradation,urine calcium and DPD were significantly elevated in the mice [27].As shown in Fig.3,urine levels of calcium and DPD in OVX group were significantly increased compared with those in Sham group,and treatment with E2V significantly reduced these levels in OVX mice.Similarly,NST at the dose of 40,80,160 mg/kg reduced the urine levels of DPD,and at the dose of 80,160 mg/kg also decreased the urine levels of calcium in OVX mice.
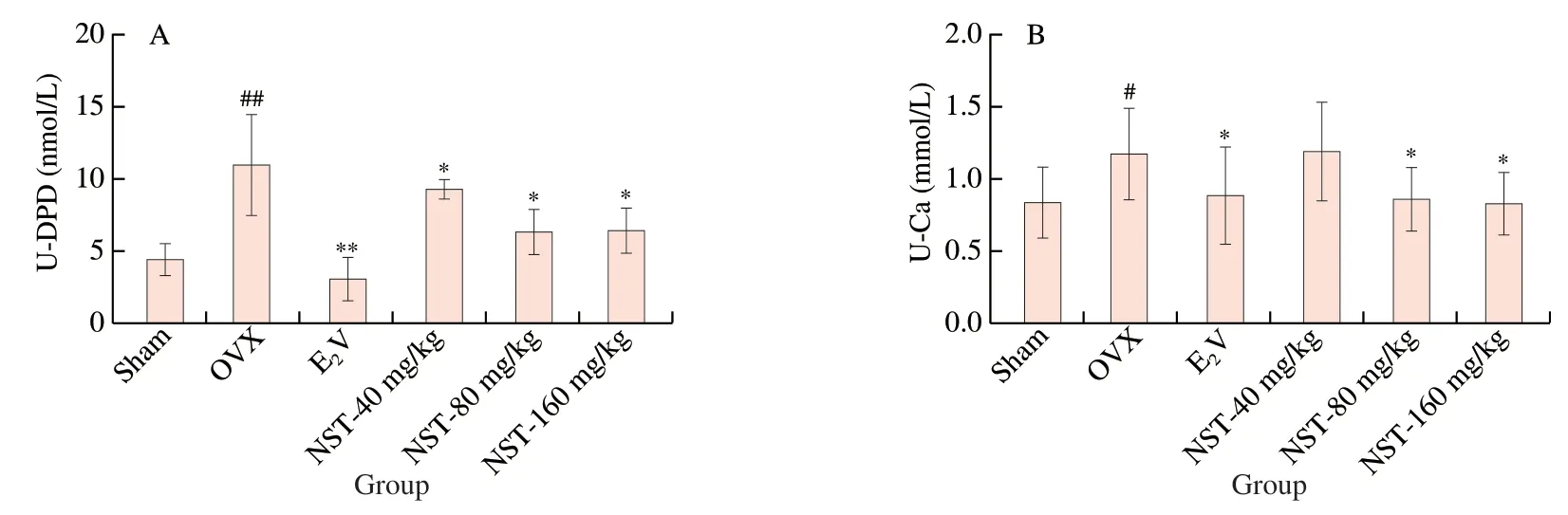
Fig.3 Effects of NST on levels of (A) DPD and (B) Ca in urine of OVX model mice.Data are presented as the mean ± SD.#P <0.05,##P <0.01 compared with Sham group;*P <0.05,**P <0.01 compared with OVX group.
3.4 NST increases BMD and alleviates deterioration of the bone tissue microarchitecture in OVX mice
Ovariectomy induced bone loss as evidenced by decreased BMD and deterioration of the mouse bone tissue microarchitecture.Micro-CT analysis demonstrated that the femoral microarchitecture was intact and the bone trabecula was dense in the mice of Sham group,while the femoral microarchitecture was severely impaired,the bone trabecula was sparse,the number of femoral trabecular bones was significantly reduced,and the trabecular bone space was significantly increased in the mice of OVX group.Administration of E2V and NST at doses of 80 mg/kg and 160 mg/kg for 8 weeks reversed the alteration of the femoral bone microarchitecture in OVX mice as evidenced by the increased number of the bone trabeculae and reduced space between the bone trabeculae in OVX mice (Fig.4A and 4B).In addition,BMD,BV/TV,Tb.N.and Tb.Th.were decreased significantly,and BS/BV and Tb.Sp.were increased significantly in OVX group.Treatment with NST at a dose of 80 mg/kg for 8 weeks significantly increased BMD and BV/TV,Tb.N.and Tb.Th.,and significantly reduced BS/BV and Tb.Sp,showing an inhibitory effect on bone loss in OVX mice (Fig.4C-4H).
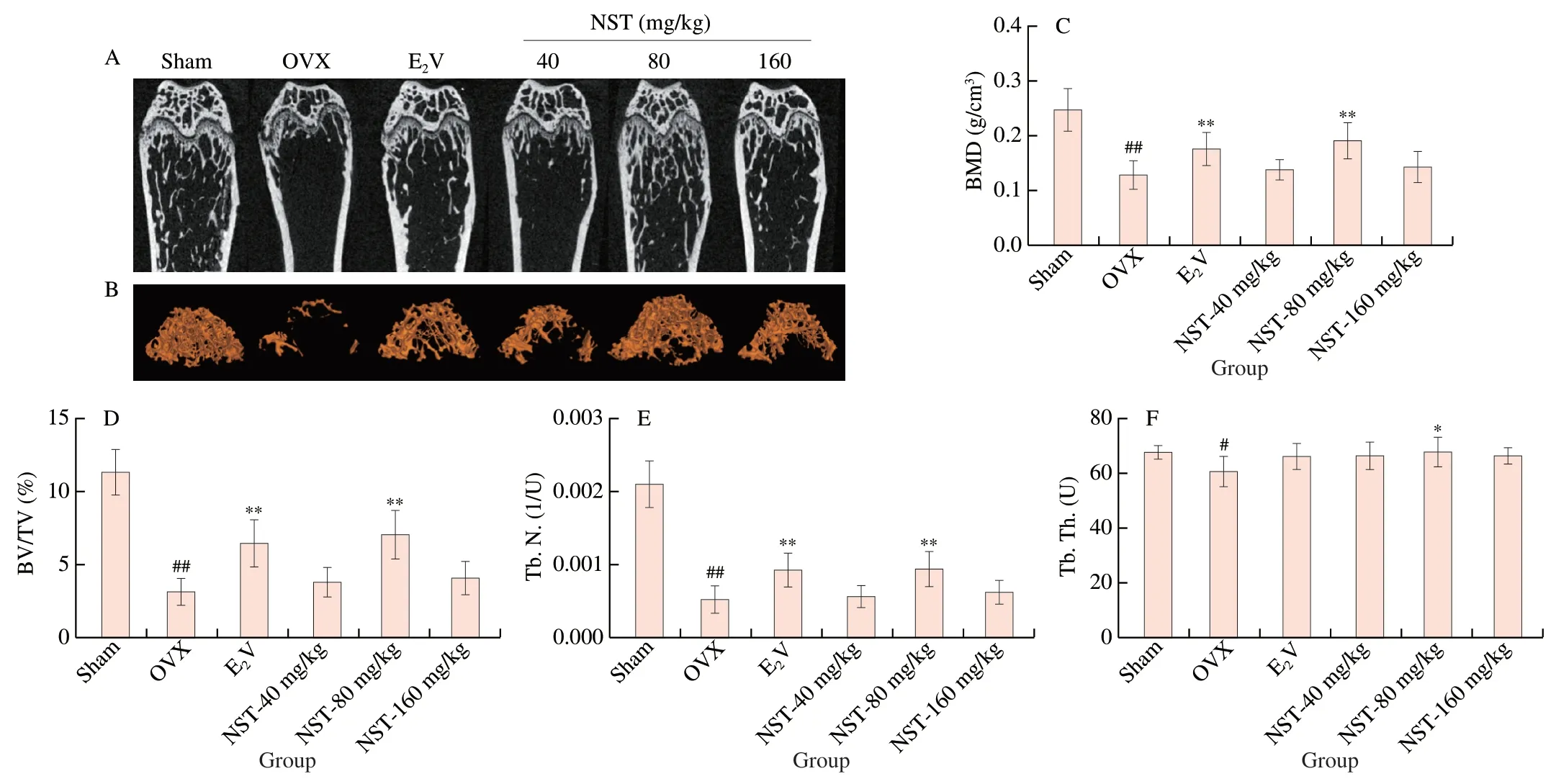
Fig.4 Effect of NST on bone tissue microarchitecture in OVX model mice.Representative images of micro-CT (A) 2D and (B) 3D microarchitecture of femur.Quantification of bone microarchitecture parameters,including (C) BMD,(D) BV/TV,(E) Tb.N.,(F) Tb.Th.,(G) BS/BV and (H) Tb.Sp.Data are presented as the mean ± SD.#P <0.05,## P <0.01 compared with Sham group;*P <0.05,**P <0.01 compared with OVX group.
Fig.4 (Continued)
3.5 NST enhances BMSC proliferation and their differentiation into osteoblasts
Based on their morphological character,expression of alkaline phosphatase,the properties of differentiation into adipogenic and osteoblastic cells,and positive CD29 expression and negative CD45 expression,BMSCs collected from the rat femur were identified as bone marrow mesenchymal stem cells (SI Appendix,Fig.S1).
BMSCs have the potential to differentiate into osteoblasts,which produce certain proteins to form the bone matrix and further mineralize into the bone nodules.As shown in Fig.5,treatment of BMSCs with NST at doses of 10-10,10-9and 10-8mol/L significantly increased BMSCs proliferation and the activity of ALP,significantly increased the expression of bone matrix proteins such as OPN and COL-I,and increased the formation of bone mineral nodules,demonstrating that NST facilitated BMSCs differentiation into osteoblasts and enhanced the bone formation activity.
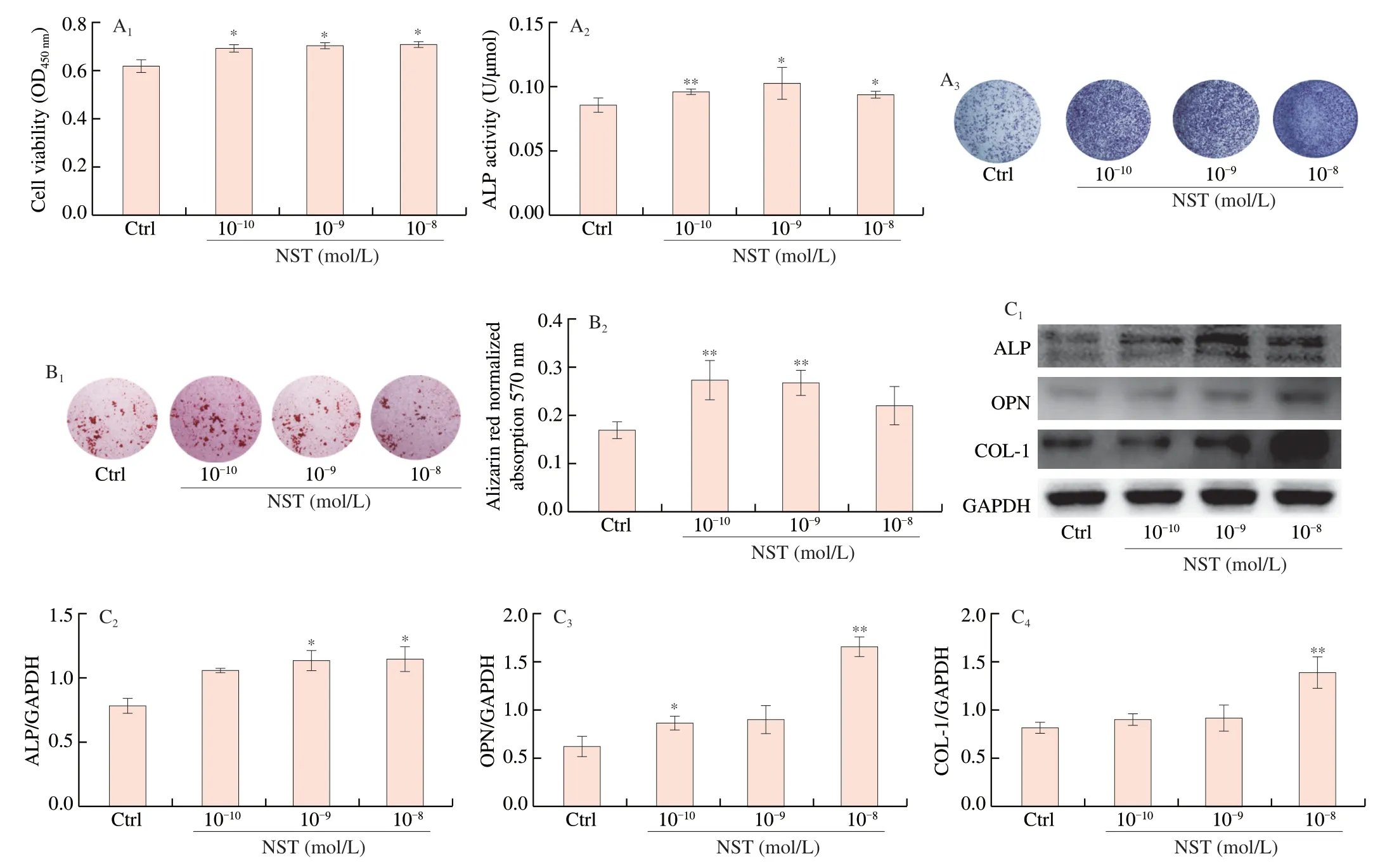
Fig.5 Effects of NST on osteogenic differentiation of BMSCs.(A1) BMSC viability in response to 48 h NST treatment was evaluated by CCK-8 assay;(A2) ALP activity of BMSCs in response to 7 days NST treatment;(A3) Representative images for ALP staining.(B1) Representative images of Alizarin Red staining for matrix mineralization after 21 days NST treatment;(B2) Quantitative Alizarin Red staining assessed by measuring the optical density.(C1)Representative imagines of Western-blot for the expression of ALP,OPN and COL-1 of BMSCs;(C2),(C2-C3) and (C4) quantitative analysis of ALP,OPN and OCL-1,respectively.Data are presented as the mean ± SD.*P <0.05,**P <0.01 compared with Ctrl group.
3.6 NST reduces BMSCs differentiation into adipocytes
The process of BMSCs differentiation into adipocytes is increased in osteoporotic patients,eventually resulting in decreased osteogenesis and bone loss [28].To verify the intervention effects of NST on adipogenesis of BMSCs,Oil Red O staining of adipogenic BMSCs treated with NST for 7 days was performed.As shown in Fig.6,treatment of BMSCs with NST at doses of 10-10,10-9and 10-8mol/L significantly decreased the lipid accumulation in cells,and inhibited the expression of PPAR-γand adiponectin,exhibiting that NST suppressed the adipocytic differentiation of BMSCs.
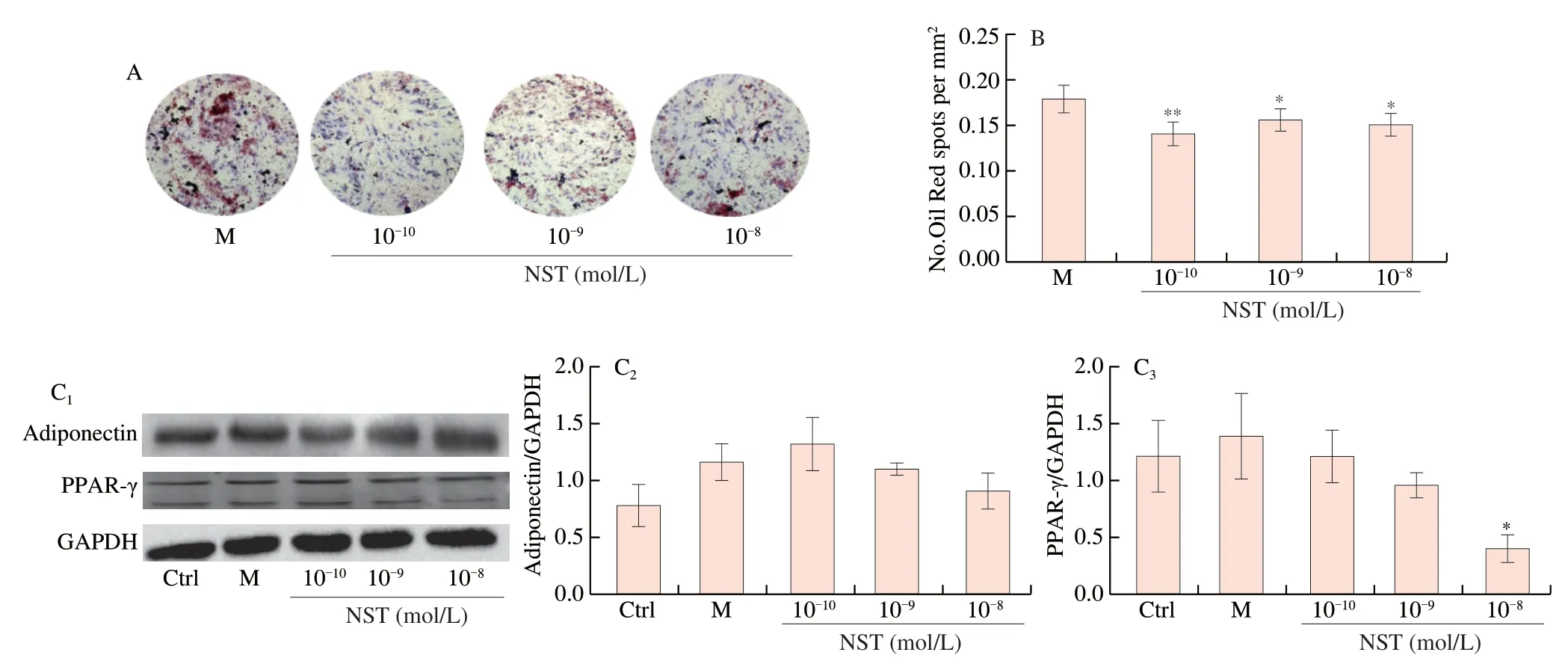
Fig.6 Effects of NST on adipogenic differentiation of BMSCs.(A) Representative images of Oil red O staining for BMSCs treated with NST in adipogenic medium for 7 days.(B) Quantification of Oil red O staining.(C1) Representative imagines of Western-blot for the expression of PPAR-γ and Adiponectin of BMSCs;(C2) and (C3)Quantitative analysis of PPAR-γ and Adiponectin,respectively.Data are presented as the mean ± SD.*P <0.05,**P <0.01 compared with M group.
3.7 NST is involved into the regulation of BMP signaling pathway in BMSCs
Knowing that BMP signaling pathways are involved in the regulation of BMSCs differentiation into osteoblasts [10],we investigated the regulatory effect of NST on BMP signaling pathway.It was found that treatment of BMSCs with NST at doses of 10-10,10-9and 10-8mol/L significantly increased the expression of BMP2,Smad4 and P-smad1/5/8,and enhanced the expression of transcription factors,such as Runx2,Osterix and Dlx5 (Figs.7A and 7B).Further investigation demonstrated that Noggin,the inhibitor of BMP signaling pathway [29],abolished the effect of NST on BMSC proliferation and ALP activity,and counteracted the regulatory effect of NST on BMP signaling pathways(Fig.7C and 7D).These results demonstrated that NST enhanced the differentiation of BMSCs into osteoblasts through BMP signaling pathways.
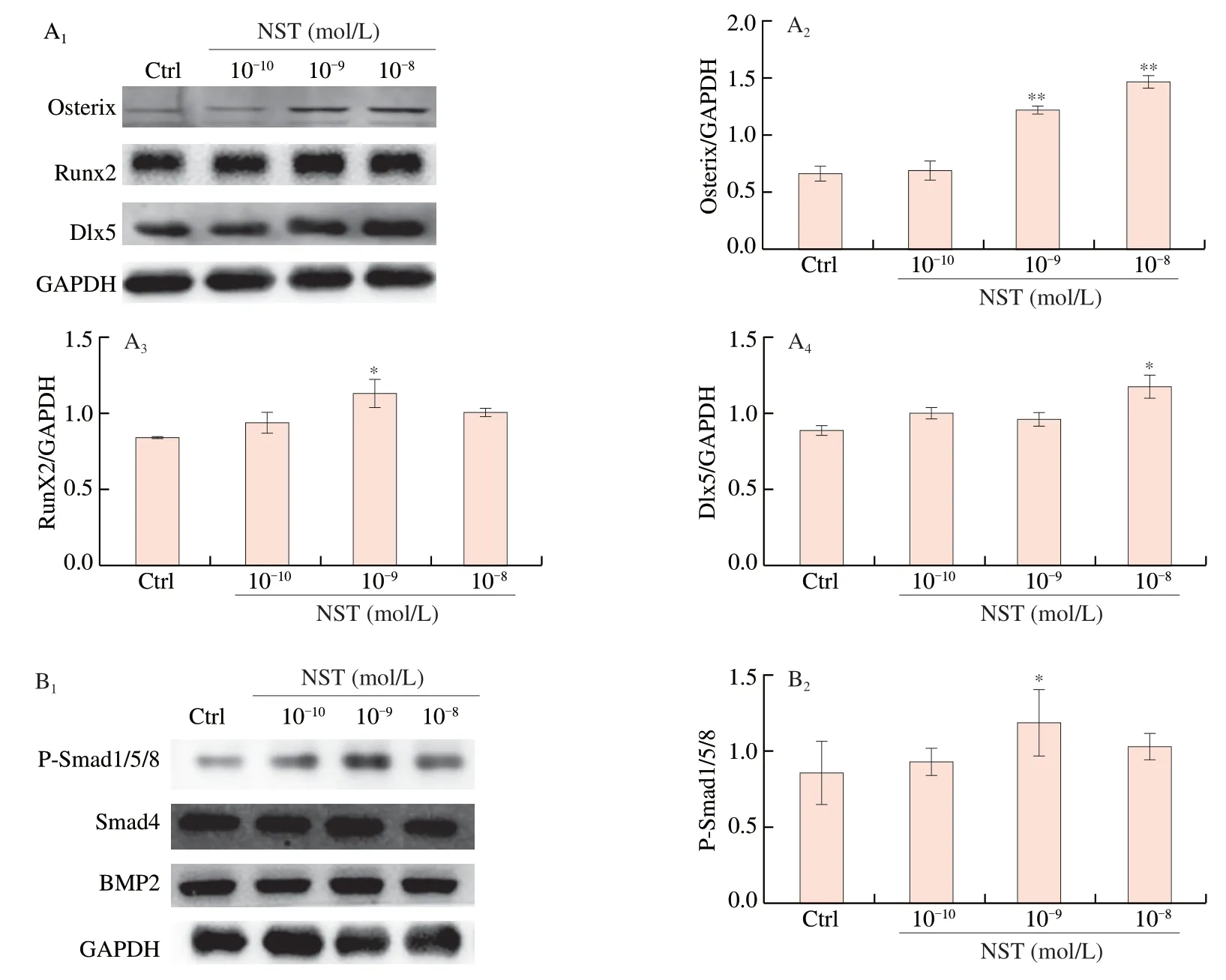
Fig.7 NST activated the BMP signaling pathway in BMSCs.(A1) Representative imagines of Western-blot for the expression of osteogenic related transcription factors of BMSCs and (A2),(A3) and (A4) quantitative analysis of Osterix,Runx2 and Dlx-5,respectively.(B1) Representative imagines of Western-blot for the protein expression of BMP signaling pathway of BMSCs and (B2),(B3) and (B4) quantitative analysis of P-Smad1/5/8,Smad4 and BMP2,respectively.Noggin,an inhibitor of BMP pathway reversed the effects of NST on osteoblast differentiation of BMSCs as evidenced by (C1) proliferation,(C2) ALP activity and (C3) ALP staining.Noggin also reversed the regulatory effects of NST on BMP pathway in BMSCs as showed by (D1) expression of key proteins in representative images of Western-blot and (D2) and (D3) quantitative analysis of P-Smad1/5/8 and Smad4,respectively.
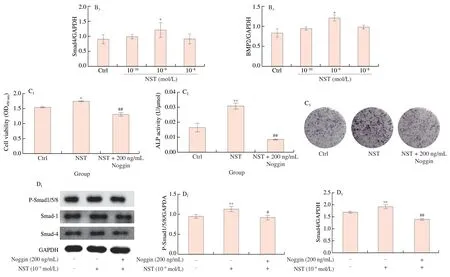
Fig.7 (Continued)
3.8 NST is involved in the modulation of Wnt-β/catenin signaling pathways in BMSCs.
Wnt/β-catenin signaling pathways are also an important regulatory mechanism of BMSCs differentiation into osteoblasts.Treatment of BMSCs with NST significantly increased the phosphorylation ofβ-catenin and the expression of LRP5 and wnt3a (Fig.8A).In addition,DKK-1,the inhibitor of Wnt-β/catenin signaling pathway [30],reversed the effect of NST on BMSC proliferation,ALP activity and activation on Wnt-β/catenin signaling pathways (Fig.8B and 8C).All these results suggest that NST enhanced the differentiation of BMSCs into osteoblasts also by activating Wnt-β/catenin signaling pathways.
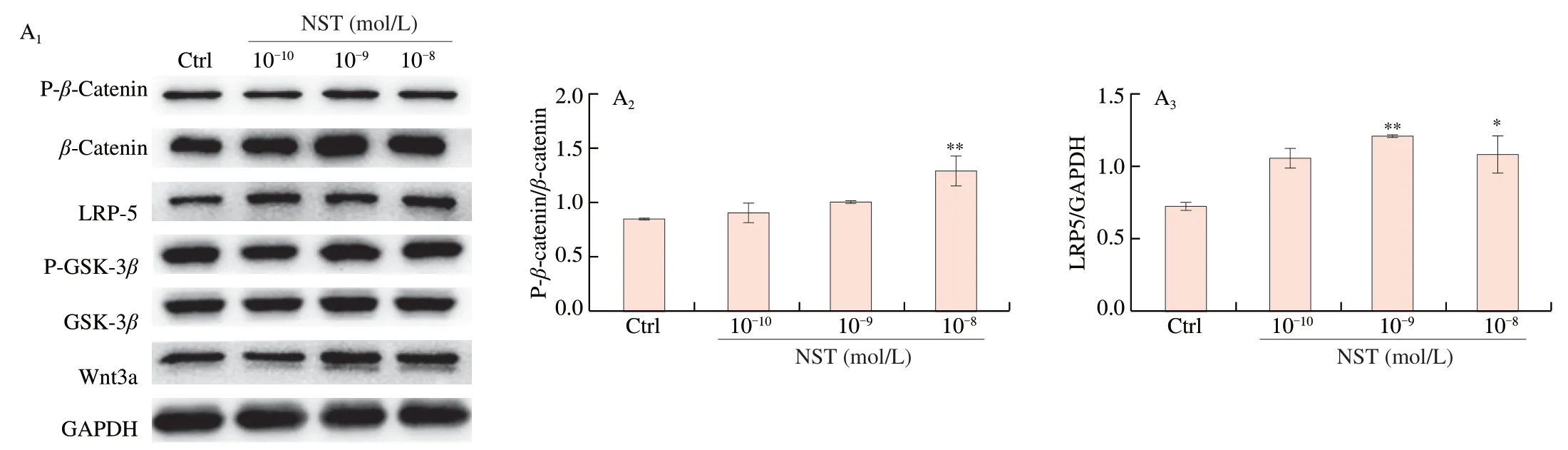
Fig.8 NST activated the Wnt/β-catenin signaling pathway in BMSCs.(A1) Representative imagines of Western-blot for the expression of key proteins of Wnt/β-catenin pathway in BMSCs and (A2),(A3),(A4) and (A5) quantitative analysis of P-β-catenin/β-catenin,LRP-5,P-GSK-3β/GSK-3β and Wnt3a,respectively.DKK-1,an inhibitor of Wnt/β-catenin pathway reversed the effects of NST on osteoblast differentiation of BMSCs as evidenced by (B1) proliferation,(B2) ALP activity and (B3) ALP staining.DKK-1 also reversed the regulatory effects of NST on Wnt/β-catenin pathway in BMSCs as showed by (C1) expression of key proteins in representative images of Western-blot and (C2),(C3) and (C4) quantitative analysis of LRP-5,P-β-catenin/β-catenin and P-GSK-3β/GSK-3β,respectively.Data are presented as the mean ± SD.#P <0.05,##P <0.01,compared with NST group;*P <0.05,**P <0.01,compared with Ctrl group.
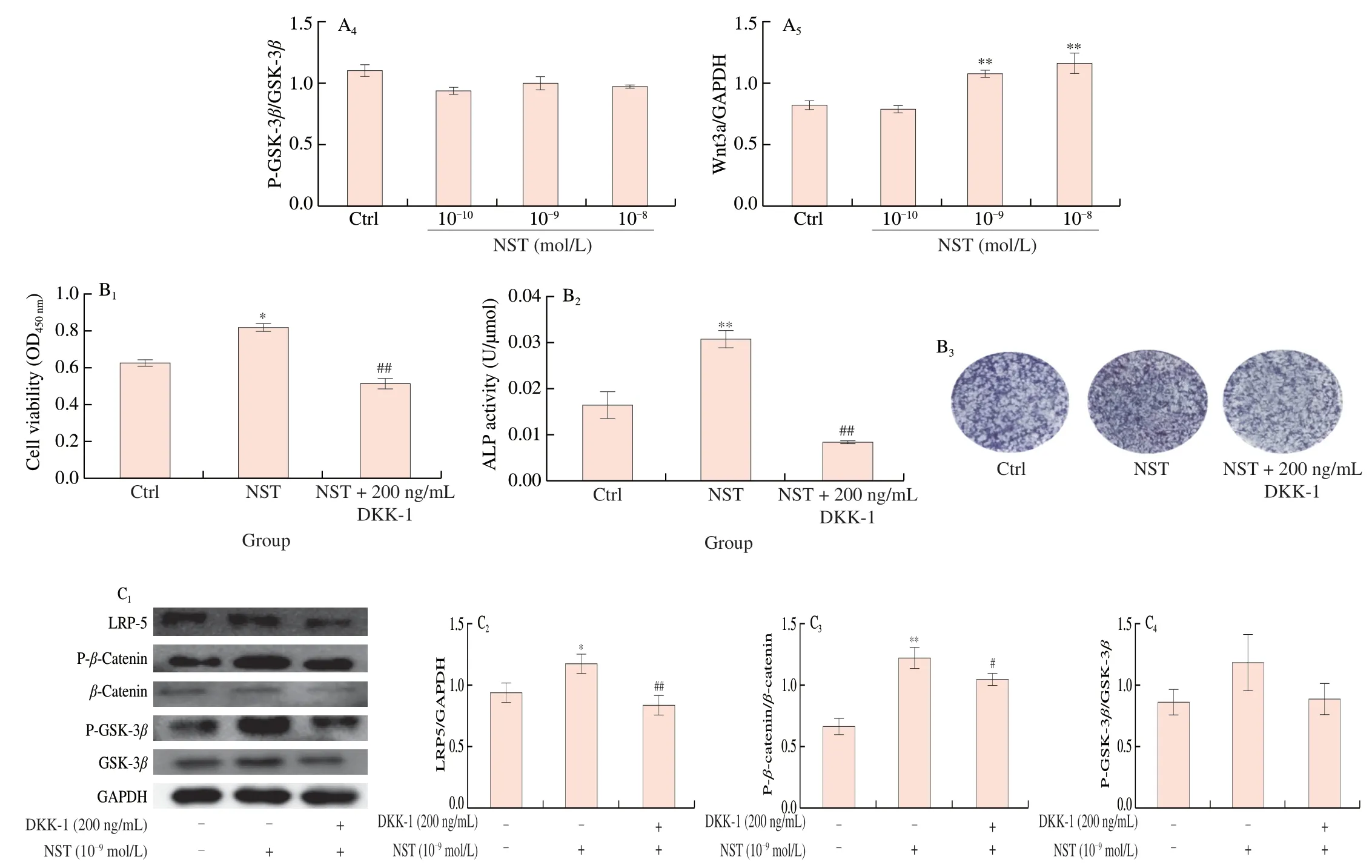
Fig.8 (Continued)
3.9 Effects of NST on the activity of BMP2 and β-catenin luciferase and their molecular docking study
To substantiate the above findings,we detected the effect of NST on the activity of BMP2 andβ-catenin luciferase,and performed molecular docking analysis of NST withβ-catenin crystal structures(PDB ID:6O9C) by adopting the Glidedock module from the Schrödinger suite [21].As shown in Fig.9A and 9B,NST at a dose of 10-8mol/L significantly increased the activity of BMP2,and NST at a dose of 10-9and 10-8mol/L enhanced the activity ofβ-catenin luciferase in cells.The docking estimation was performed by the docking score,knowing that a lower docking score value implies a better binding affinity between the protein and the ligand.The docking score for NST withβ-catenin was -10.843.The binding mode of NST is provided in Fig.9C and 9D.The whole molecule of NST fit well in the active site ofβ-catenin surrounded by GLU A: 229,LEU A:230,VAL A: 231,LYS B: 111,VAL B: 113,LEU B: 107,ILF B: 27,LYS B: 26,PRO B: 25,THR B: 24,TRP A: 244,and seven hydrogen bonds were formed with GLU A: 229,LEU A: 230,LYS B: 111,THR B: 24,PRO B: 25,LYS B: 26 and ILF 27.Therefore,the results of Luciferase reporter gene assay and docking energies may further explain the regulatory effect of NST on BMP and Wnt/β-catenin pathways [31,32].
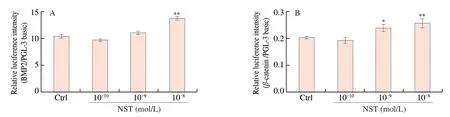
Fig.9 Effects of NST on the activity of BMP2 and β-catenin luciferase and their molecular docking studies.(A) and (B) The activation of BMP2 and β-catenin by NST detected by luciferase reporter gene assay,respectively.(C) and (D) Predicted binding mode of NST with β-catenin by molecular docking.Data are presented as the mean ± SD.*P <0.05,**P <0.01 compared with Ctrl group.
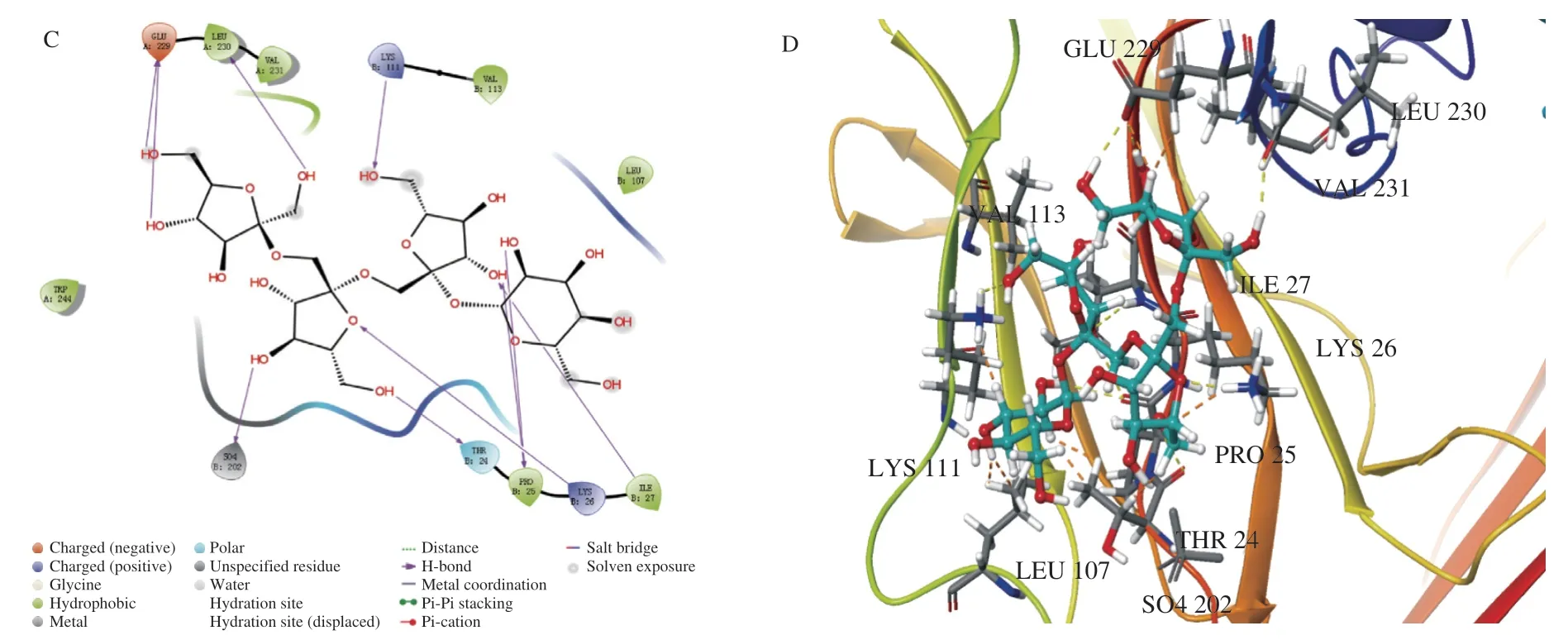
Fig.9 (Continued)
4.Discussion
The present study investigated the anti-osteoporotic effect of NST and its underlying mechanism inducing differentiation of BMSCs into osteoblasts.The results showed that NST prevented the bone loss of ovariectomized mice,enhanced the osteogenic differentiation of BMSCs,while suppressing adipogenic differentiation of BMSCs through regulating BMP and Wnt/β-catenin pathway,and inhibiting expression of adiponectin and PPAR-γ.
Owing to the estrogen deficiency,postmenopausal women often present with bone metabolic disorders,body weight gain and uterine atrophy.Estrogen replacement therapy can relieve OP of postmenopausal women and attenuate weight gain,but may increase the risk of endometrial hyperplasia or endometrial carcinoma [27].Ovariectomy caused the decrease in secretion of estrogen of mice,and finally led to the body weight gain and reduction of uteri weight of mice.It was found in the present study that treatment with NST significantly attenuated the body weight gain but showed no significant effect on uterus weight in OVX mice,suggesting that NST may have the potential to attenuate body weight gain but had no hyperplasic effect on the uterus in the treatment of OP in postmenopausal women.NST belongs to fructo-oligosaccharides (FOS) in chemical structure,whereas FOS,composed of 1-kestose,nystose and 1-beta-fructofuranosyl nystose,have scientifically proven to be health benefits,such as promoting role in absorption of calcium and minerals,reducing levels of lipids and cholesterol,and improving intestinal microbiota,which help to regulate the lipid metabolism and then reduce body weight.Moreover,FOS are often used as substitutes of sugar and fat in food products.Hence,the inhibitory effects of NST on weight gain of OVX mice may be attribute to the above-mentioned biological activities [33,34].Certainly,whether NST can decrease the body weight gain under normal or obesity condition should be further investigated.
With the increase of age,postmenopausal women are prone to OP,which is characterized by decreased BMD and deterioration of the bone tissue microarchitecture,accompanied with the increased urine level of calcium and collagen degradation products such as DPD and CTX-1 [35,36].The present study showed that treatment with NST enhanced the BMD and improved the bone tissue microarchitecture of OVX mice,and reduced the urine level of calcium and DPD.Consistent with our study,Tanabe et al.[17]reported that fructooligosaccharide,which consists of 1-kestose,nystose and 1F-β-fructofuranosyl-nystose,reduced bone resorption by modifying gut microbiota in SAMP6 mice.Zhang et al.[15]also reported that fructooligosaccharide fromAchyranthes bidentatasignificantly increased the relative fluorescence intensity of bone mass in a zebrafifish model of glucocorticoid-induced osteoporosis.
BMSCs have the potential to differentiate into osteoblasts and adipocytes,and the increased differentiation of BMSCs into adipocytes and the decreased differentiation into osteoblasts may reduce bone formation and the risk of OP occurrence [37,38].The present study found that NST could increase the osteogenic differentiation of BMSCs as evidenced by increased the proliferation,ALP activity and levels of OPN,COL-I.These increased indicators are known to be involved in the differentiation and mineralization of osteoblasts derived from BMSCs,and inhibit adipogenic differentiation as assayed by the fat deposition,exhibiting that NST induced the differentiation of BMSCs into osteoblasts,and inhibited differentiation of BMSCs into adipocytes.In consistent with our findings,Yodthong et al.[16]reported thatβ-1,3-Glucanoligosaccharide fromPleurotus sajor-caju(Fr.) Sing enhanced the proliferation,differentiation and mineralization of MC3T3-E1 Cells through mediation of BMP-2/Runx2/MAPK/Wnt/β-Catenin signaling pathway.
It was reported [10]that BMP pathway participated the regulation of BMSCs differentiation into osteoblasts,and the activation of BMP induced bone and cartilage formationin vivo.BMP activation upregulated the expression of Smad protein,and subsequent expression of Runx2 and Osterix proteins in BMSCs improved the activity of ALP,and differentiation and mineralization of osteoblasts.In addition,Dlx5,a transcription factor,stimulated the expression of Runx2,and then increased the differentiation and development of osteoblasts [39,40].The present study showed that NST increased the expression and luciferase activity of BMP2,Smad4 and P-smad1/5/8 in BMP signaling pathway,and also enhanced the expression of Runx2,Osterix and Dlx5,indicating that NST induced the differentiation of BMSCs into osteoblasts through regulating BMP signaling pathways.
Studies [41]showed that Wnt/β-catenin signaling pathway played an important role in the recruitment and differentiation of preosteoblasts.When Wnt ligand bound to receptor Frizzled (Fzd) and low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6),Wnt/β-catenin signal was activated,and phosphorylation of GSK-3βled to stability and nuclear translocation ofβ-catenin,thus inhibiting the phosphorylation ofβ-catenin.In addition,signal proteinβ-catenin accumulated in cytoplasm and entered the nucleus to interact with transcription factor TCF/LEF to activate the transcription of target genes and regulate the proliferation and differentiation of osteoblasts.The present study showed that NST promoted the expression of the key proteins in Wnt/β-catenin pathway and had a good activation and affinity withβ-catenin,further confirming that NST promoted BMSCs differentiation into osteoblasts through regulating Wnt/β-catenin pathway.
Aging and estrogen deficiency are key causes for OP by altering the elaborate balance between osteogenic differentiation and adipogenic differentiation in BMSCs [42].Adipogenic differentiation in BMSCs is modulated by adiponectin and PPAR-γ,and in old ages,the increased expression of PPAR-γpromotes adipogenesis and suppresses osteogenesis [43].Adiponectin,one of the most important adipocytokines secreted by adipocytes,enhances the differentiation in adipocytes [44].PPAR-γmodulates the expression of genes involved in adipocyte differentiation of BMSCs,and the upregulation of PPAR-γpromotes adipogenesis in BMSCs [45].Our results demonstrated an opposing effect of NST on osteoblast versus adipocyte differentiation,and an inhibitory effect of NST on expression of PPAR-γand adiponectin.These results may explain the inhibitory effect of NST on body weight gain and its protective effect on bone loss in OVX mice.
The content of saccharides in MO nearly reaches 50%,and is mostly composed of oligosaccharides such as inulin-type hexasaccharide,bajijiasu,kestose,NST and kestopentaose [46,47].Bajijiasu has been reported to be able to reduce osteoclast formation and bone resorption through interfering with RANKL-induced NFκB and NFATc1 activation [13].Here,we showed that NST enhanced the differentiation of BMSCs into osteoblasts through modulating BMP and Wnt-β/catenin signaling pathways,and inhibiting the differentiation of BMSCs into adipocyte.In addition,oligosaccharides fromM.officinalishave been approved as a prescribed Chinese medicine by the Chinese Food and Drug Administration (CFDA) for the treatment of depression.They have also been histopathologically confirmed as having no organ toxicity in the kidneys,pituitary gland,or thyroid glands [48,49].Collectively,our study,together with other studies,have demonstrated that the oligosaccharides in MO,especially NST,possess a better safety,and have the potential to be useful for the treatment of OP.
5.Conclusion
Oligosaccharides of MO possess diverse pharmacological effects including anti-osteoporosis,anti-depression and anti-senile dementia.NST is the major component of the oligosaccharides from MO,which is used as the quality control indicator of MO byChinese Pharmacopoeia.Our investigation revealed that NST could improve the bone tissue microarchitecture and reduce bone loss in OVX mice,and induce BMSCs to differentiate into osteoblasts through regulating BMP and Wnt-β/catenin signaling pathways,while inhibit BMSCs to differentiate into adipocyte by suppressing the expression of PPAR-γand adiponectin (Fig.10).Therefore,this study provides new insights into the potential use of NST as a bone formation-acceleration agent for the treatment of OP.
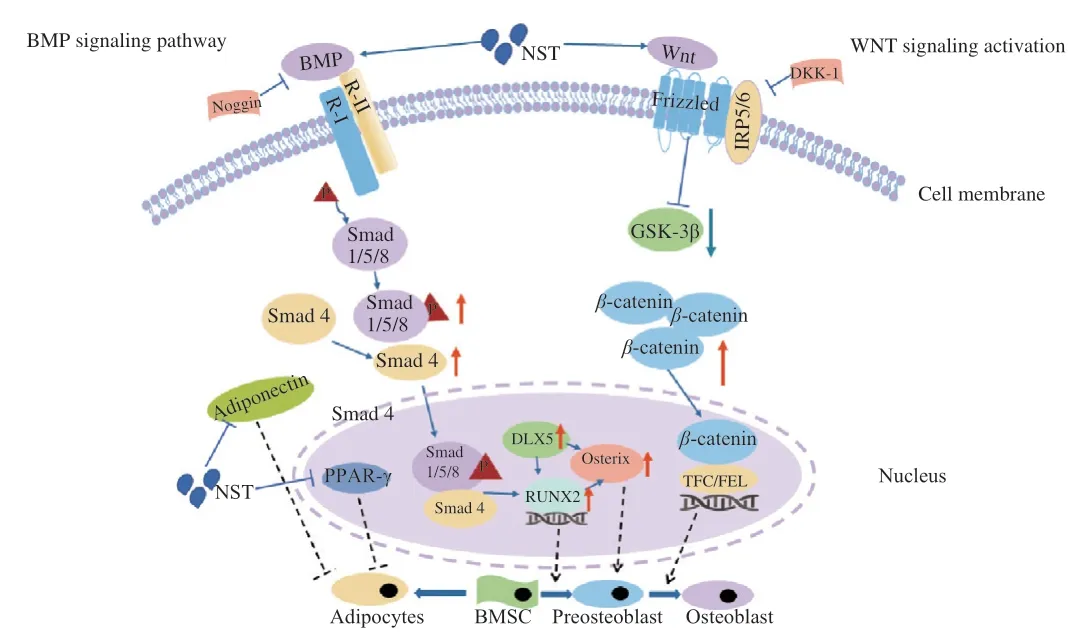
Fig.10 A schematic diagram illustrating the promoting effects of NST on osteoblast differentiation of BMSCs via activation BMP and Wnt/β-catenin pathway.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgments
We appreciate the technical support from the Public Platform of Medical Research Center,Academy of Chinese Medical Science,Zhejiang Chinese Medical University.The project was sponsored by the National Natural Science Foundation of China (81973534,U1505226).The funding body did not have any influence on the design of the study and collection,analysis,and interpretation of data and in writing the manuscript.
Ethics approval and consent to participate
All animal protocols were approved by the Experimental Animal Ethic Committee of the Second Military Medical University(Certificate No.SCXK 2017-0022) and the Bioethic Committee of Zhejiang Chinese Medical University.(Certificate No.SCXK 2017-0005)
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.1016/j.fshw.2022.07.066.
杂志排行
食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil
