Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil
2023-01-21LinaTaoPanpanWangTingZhangMengzhenDingLijieLiuNingpingTaoXichangWangJianZhong
Lina Tao,Panpan Wang,Ting Zhang,Mengzhen Ding,Lijie Liu,Ningping Tao,Xichang Wang,Jian Zhong*
National R&D Branch Center for Freshwater Aquatic Products Processing Technology (Shanghai),Integrated Scientif ic Research Base on Comprehensive Utilization Technology for By-Products of Aquatic Product Processing,Ministry of Agriculture and Rural Affairs of the People's Republic of China,Shanghai Engineering Research Center of Aquatic-Product Processing and Preservation,College of Food Science&Technology,Shanghai Ocean University,Shanghai 201306,China
Keywords:Ionotropic gelation Millimeter-sized spherical capsule Monoaxial dispersion electrospraying Multicore Specif ic and sustained release
ABSTRACT Specific and sustained release of nutrients from capsules to the gastrointestinal tract has attracted many attentions in the field of food and drug delivery.In this work,we reported a monoaxial dispersion electrospraying-ionotropic gelation technique to prepare multicore millimeter-sized spherical capsules for specif ic and sustained release of f ish oil.The spherical capsules had diameters from 2.05 mm to 0.35 mm with the increased applied voltages.The capsules consisted of uniform (at applied voltages of ≤ 10 kV) or nonuniform (at applied voltages of >10 kV) multicores.The obtained capsules had reasonable loading ratios(9.7% −6.3%) due to the multicore structure.In addition,the obtained capsules had specific and sustained release behaviors of f ish oil into the small intestinal phase of in vitro gastrointestinal tract and small intestinal tract models.The simple monoaxial dispersion electrospraying-ionotropic gelatin technique does not involve complicated preparation formulations and polymer modification,which makes the technique has a potential application prospect for the f ish oil preparations and the encapsulation of functional active substances in the field of food and drug industries.
1.Introduction
Fish oils are important functional active substances for human being [1].It also can be used as carriers for fat-soluble functional active substances.However,the wide applications of fish oils in food and drug industries have been restrained due to their low stability to environments (e.g.oxygen,light,and temperature),fishy taste/smell,and poor water solubility [2-4].
Research and development of encapsulation techniques has become one of the hot spots in food and pharmaceutical industry due to their huge advantages for active substances [5-8]: i) covering of unpleasant taste or smell,ii) reduction of negative effects from environmental factors such as oxygen and heat,iii) controllablein vitroandin vivoretention and release,and iv) conversion of liquid dosage forms to solid dosage forms.The mainstream encapsulation techniques in the academic and industry fields are those techniques for producing centimeter-sized capsules and micro/nano capsules such as spray-dried nanocapsules [9]and electrosprayed nanocapsules [10].The centimeter-sized capsules are difficult to be swallowed for some special people such as children,elderly,and patients with diseases in the mouth and/or throat.With the same shell layer materials,in theoretically,micro/nano capsules have lower stability for active substances than millimeter-sized and centimeter-sized capsules due to their nanoscale shell thicknesses.Furthermore,there is concern from consumers,regulatory agencies,and food industry about potential toxicity associated with nano-sized products [11].In addition,specific and sustained delivery behavior is an important requirement for active substance delivery in human organs [3,12,13].Therefore,it is meaningful to develop millimeter-sized particles with specific and sustained release behaviors for some food and drug active substances.
Some preparation techniques of millimeter-sized capsules have been explored over the past two decades [14-16].These methods were mainly based on injection and made significant advances in the field of capsule preparation.However,they cannot be applied to encapsulate active substances due to their disadvantages such as hightemperature treatment [14],use of organic solvents [15],and complex photopolymerization and manual microinjection [16].
By collecting electrically charged jet of polymer solution,electrohydrodynamic processes (electrospinning and electrospraying)can be applied to produce fibers or particles at millimeter,micron,submicron and nanoscale [17-21].They are simple,cost-effective(only about 4 000 US dollars for one machine) and flexible methods.Electrospinning has been widely explored in air filtration [17,22,23],fast detection [24],oil-water separation [3,25],actuators [26,27],tissue engineering [28-31],drug delivery [13,32,33],and food engineering [34,35],while electrospraying has been widely explored in tissue engineering [36,37],drug delivery [38,39],and food engineering [40,41].
Until now,two different electrospraying-based strategies have been developed to prepare millimeter-sized spheres: (1) twostep fabrication method (electrospraying+pipette injection) for encapsulating micrometer-sized samples such as probiotics [42],which is complex;(2) coaxially electrospraying method using a coaxial needle for encapsulating fish oil/nutrients [43].In this method,fish oil/nutrients and alginate sodium solutions entered the inner hole and the outer hole,respectively,of the coaxial needle.Electrically charged jet of fish oil/nutrients-encapsulated alginate solutions were formed on the needle and solidified into the CaCl2solution.These resulted coaxial electrosprayed particles generally have controllable burst release behaviors of fish oil/nutrient at the particular time point and have no sustained release ability due to their monocore-shell structures.Considering that the sustained release behavior of functional bioactive substances has a significant application prospect [44],it is important to explore the fabrication method of millimeter-sized capsules with sustained release behaviors of fish oil.
Herein,we report an monoaxial dispersion electrosprayingionotropic gelation technique using a single hole needle to prepare multicore millimeter-sized spherical capsules for specific and sustained release of fish oil in the small intestinal phase.Firstly,fish oilloaded alginate-stabilized dispersion is prepared and used to prepare millimeter-sized spherical capsules using monoaxial dispersion electrospraying-ionotropic gelation technique.Then,the fish oil loading ratios of the millimeter-sized spherical capsules are measured.Finally,fish oil sustained release behaviors of the millimeter-sized capsules in thein vitrodigestion models are determined.The results will prove that the monoaxial dispersion electrospraying-ionotropic gelatin technique is simple and has outstanding application prospect for food research and development.
2.Materials and methods
2.1 Materials
Fish oil was purchased from Xi’an LvTeng Biological Technology Co.,Ltd.(Shaanxi Province,China).Lipase from porcine pancreas was purchased from Lantuo Biotechnology Co.,Ltd.(Shanghai,China).Porcine bile extract was purchased from Shanghai Macklin Biochemical Co.,Ltd.(Shanghai,China).Sodium alginate,pepsin from porcine gastric mucosa and other common chemicals were bought from Sinopharm Chemical Reagent Co.Ltd.(Shanghai,China).
2.2 Preparation and characterization of fish oil-loaded alginate-stabilized dispersion
The preparation and characterization of fish oil-loaded alginatestabilized dispersion was performed referring to previous works [45-47].Briefly,2.0% sodium alginate solution was prepared and stood for one hour to degas air bubbles.Then,2 mL of fish oil (food grade,DHA+EPA ≥ 70%) was mixed with 6 mL of 2.0% sodium alginate solution.The mixtures were mechanically sheared using a 10 mm head ULTRA-TURRAX®homogenizer (T 10 basic,IKA,Guangzhou,China).The applied homogenizing speed was 8 000 r/min.The applied homogenizing time was 60 s.The air bubbles were not seen by naked eyes and ordinary inverted optical microscope (MS600F,Shanghai Minz Precision Instruments Co.Ltd.,China),and therefore,the dispersions were not stood to degas air bubble.The obtained dispersion was observed by a digital camera (EOS700D,Canon,Japan),an ordinary inverted optical microscope,and a confocal laser scanning microscope (CLSM) (TCS SP8,Leica,Wetzlar,Germany).For optical microscopy observation,the sample was prepared by adding 5 μL dispersion onto glass microslide.For CLSM observation,the dispersion was mixed with 320 μL 0.1% Nile Red(Sangon Biotech Co.,Ltd.,Shanghai,China) prior to homogenization under dark conditions,and then the sample was prepared by adding 5 μL dispersion onto glass microslide.The CLSM observation was performed with an oil objective of 63×,a 552 nm laser for excitation,a scanning frequency of 200 Hz,and a scanning density of 1 024×1 024.
2.3 Monoaxial dispersion electrospraying-ionotropic gelation process
The process was performed by a custom-designed electrospraying instrument [48].Fish oil-loaded alginate stabilized dispersion and CaCl2solution (25 mmol/L) were applied as an electrically charged jet solution and collecting solution,respectively.A high voltage power supply (Tianjin Dongwen High Voltage Power Supply Co.,Ltd.,Tianjin,China) was applied to produce different operational voltages(0,5,10,15,and 20 kV).The spherical capsules were prepared with a stainless needle of 20# (inner diameter: 0.60 mm and outer diameter:0.91 mm),a jet solution feeding rate of 0.03 mL/min,a distance between the needle and the collecting solution of 10 cm.After 15–60 min,the obtained spherical capsules were moved into 25 mmol/L fresh CaCl2solution and stored into the CaCl2solution.
2.4 Capsule observation
The capsules were transferred to 35 mm Petri dishes and the surrounding water was removed by filter paper until no obvious water was observed on the surface of capsules by naked eyes [47].Then,the obtained spherical capsule shapes were photographed by a digital camera,an upright optical microscope (ML8000,Shanghai Minz,China),an ordinary inverted fluorescence microscope (MS600F,Shanghai Minz,China),a CLSM,and a scanning electron microscope(SEM) (S-3400N,Hitachi,Tokyo,Japan).For optical microscopy observation,MeizsMcs 6.0 software from the commercial optical microscopy company was applied to measure the maximum and minimum diameters (n=50) of the capsules,which was to understand the capsule shapes.For CLSM and ordinary fluorescence observation,320 μL of 0.1% Nile Red was added to the alginate/oil mixtures prior to homogenization and the Nile Red-loaded dispersion was used to prepare millimeter-sized capsules.The capsules were immediately and directly detected by CLSM or ordinary fluorescence.3D reconstructed CLSM images of the capsules were obtained using XYZ scanning mode with a slice number of 30 for the whole capsule height and reconstructed using Leica Application Suite X software.
2.5 Water content measurements
Water contents of the capsules were measured by a hightemperature drying method.Briefly,the capsules in CaCl2solutions were took out and the surrounding water was removed by filter paper until no obvious water was observed on the surface of capsules by naked eyes.Then,the capsules were put into a clean constant-weight bottle (m0) and the weight of the bottle with capsule was recorded (m1).Subsequently,the capsules were dried at 100 °C in an electrothermal constant temperature drying oven (Model 202,Beijing Ounisi Technology Development Co.Ltd.,China).After two hours,the capsules were weighed every one hour until the weight change was less than 3% .The final weight (m2) was used to calculate the water content (Wc) according to the following equation:

2.6 Loading ratio measurements
The oil contents inside the capsules were measured by the Rose-Gottlieb method with some modification [49].The capsules were transferred to 35 mm Petri dishes and the surrounding water was removed by filter paper.After 12 h,exactly 1 g (m3) of dried capsules were transferred into liposuction bottle.Then,1.25 mL of 25% (V/V)ammonia solution was added and the mixture was incubated at 60 °C water bath for 5 min.The mixture was vigorously vortexed for 2 min and 10 mL of ethanol was added into the bottle.Then,the mixture was cooled by tap water.Subsequently,25 mL of diethyl ether was added and the bottle was immediately sealed and shook for 30 s.After that,25 mL of petroleum ether was added and the bottle was immediately sealed and shook for 30 s.After 30 min,the ether layer volume (V1(mL)) was recorded.Then,a part of the ether layer(V2(mL)) was transferred to a constant weight (m4) flask and concentrated at 60 °C using a rotary evaporator (RE52AA,Shanghai Yarong Biochemistry Instrument Factory,Shanghai,China).Then,the flask was further dried at (102 ± 2) °C for 2 h.The weight (m5) of the flask was recorded.Finally,the loading ratios (LR) of the spherical capsules were calculated according to the following equation:
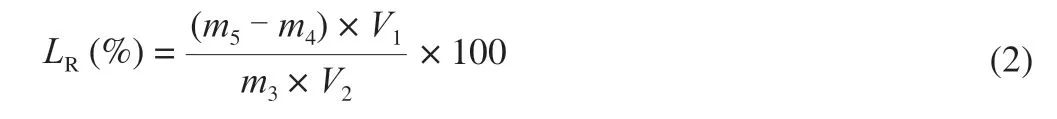
2.7 In vitro digestion measurements
Two simulated gastrointestinal tract digestion models were applied to simulate thein vitrodigestion of the capsule samples [50,51]:(1) gastrointestinal tract model: gastric and intestinal phases;(2) small intestinal tract model: small intestinal phase.
In the model (1),2 g of NaCl and 7 mL of HCl (37%,V/V) were added into 1 L of water.Then,3.2 g of pepsin from porcine gastric mucosa was dissolved into the solution.The solution pH was adjusted to 1.2 to obtain pepsin solution.Exactly 1 g of dried capsules was added into 15 mL of pepsin solution.The pH was adjusted to 2.0.The mixture was incubated at 37°C for 2 h in an incubator shaker(THZ-C,Haocheng Experimental Equipment Manufacturing Co.Ltd.,Taicang,China) with continuous agitation at 100 r/min.During the gastric incubation period,the pH was maintained to 2.0.After the gastric incubation,the pH was adjusted to 7.0.Immediately,porcine bile extract solution (3.5 mL,54 mg/mL) and salt solution (1.5 mL,10 mmol/L CaCl2and 150 mmol/L NaCl) were added into the solution.The pH was adjusted to 7.0 again.2.5 mL of lipase phosphate buffer (75 mg/mL) was added to the solution.The solution was moved into a water bath with controlled temperature (37 °C) with continuous agitation at 100 r/min for small intestinal phase incubation.Subsequently,the pH of the solution was monitored and maintained to pH 7.0 using 0.5 mol/L NaOH every 20 min in this intestinal phase.The adding amount of NaOH was applied to neutralize free fatty acids(FFA) released from the capsules by lipid digestion.
In the model (2),exactly 1 g of dried capsules was added into 15 mL water.The solution was incubated at 37 °C and the pH was adjusted to 7.0.Then,like model (1),porcine bile extract solution and salt solution were added,the pH was adjusted to 7.0 again,lipase phosphate buffer was added.The solution was moved into a water bath with controlled temperature (37 °C) with continuous agitation at 100 r/min for small intestinal phase incubation.Subsequently,the pH of the solution was monitored and maintained to pH 7.0 every 20 min.The adding amount of 0.5 mol/L NaOH was applied to neutralize FFA released from the capsules by lipid digestion.
FFA could be produced by the triglyceride if they were all digested (assuming 2 FFAs are produced per triacylglycerol molecule)by lipase.Therefore,the percentage of FFAs released from the capsules could be calculated according to the following equation:
whereVNaOHis the cumulative volume of NaOH,cNaOHis the molarity of NaOH,Mlipidis the molecular mass (868 g/mol) of fish oil [52],mcapsulesis the total mass of dried capsules,andLRis the fish oil loading ratios of capsules.
2.8 Observation of the capsules in different phosphate buffer saline (PBS)
The capsule used in this experiment was prepared at below conditions: 2.0% sodium alginate solution,an applied voltage of 10 kV,a needle of 20#,a feeding rate of 30 μL/min,and a receiving distance of 10 cm.Fifty obtained capsules were added to 10 mL of two types of PBS at room temperature: (1) 10 mmol/L NaH2PO4,pH 2.0,adjusted with phosphoric acid;and (2) 8.4 mmol/L Na2HPO4,1.6 mmol/L NaH2PO4,pH 7.0.Then,at the designated time points(0.5,1,and 2 h),the capsules were observed by an upright optical microscope.MeizsMcs 6.0 software from the commercial optical microscopy company was applied to measure the maximum and minimum diameters (n=15) of the capsules,which was applied to calculate the diameter change by dividing the measured values by the average value of the untreated capsules.
2.9 Statistical analysis
The data are expressed as mean value ± standard deviation of three parallel experiments.Statistical comparisons were performed using one-way ANOVA test.TheP<0.05 was considered significant.
3.Results and discussion
3.1 Preparation and characterization of fish oil-loaded alginate-stabilized dispersion
Homogenization method was applied to prepare fish oil-loaded alginate-stabilized dispersion at an alginate sodium concentration of 2.0% .The dispersion was milk-white (Fig.1A).As shown in Fig.1B and 1C,the dispersion consisted of fish oil droplets with micrometer size.The sizes were similar to fish oil dispersion stabilized by 1% of alginate in a previous work [53].
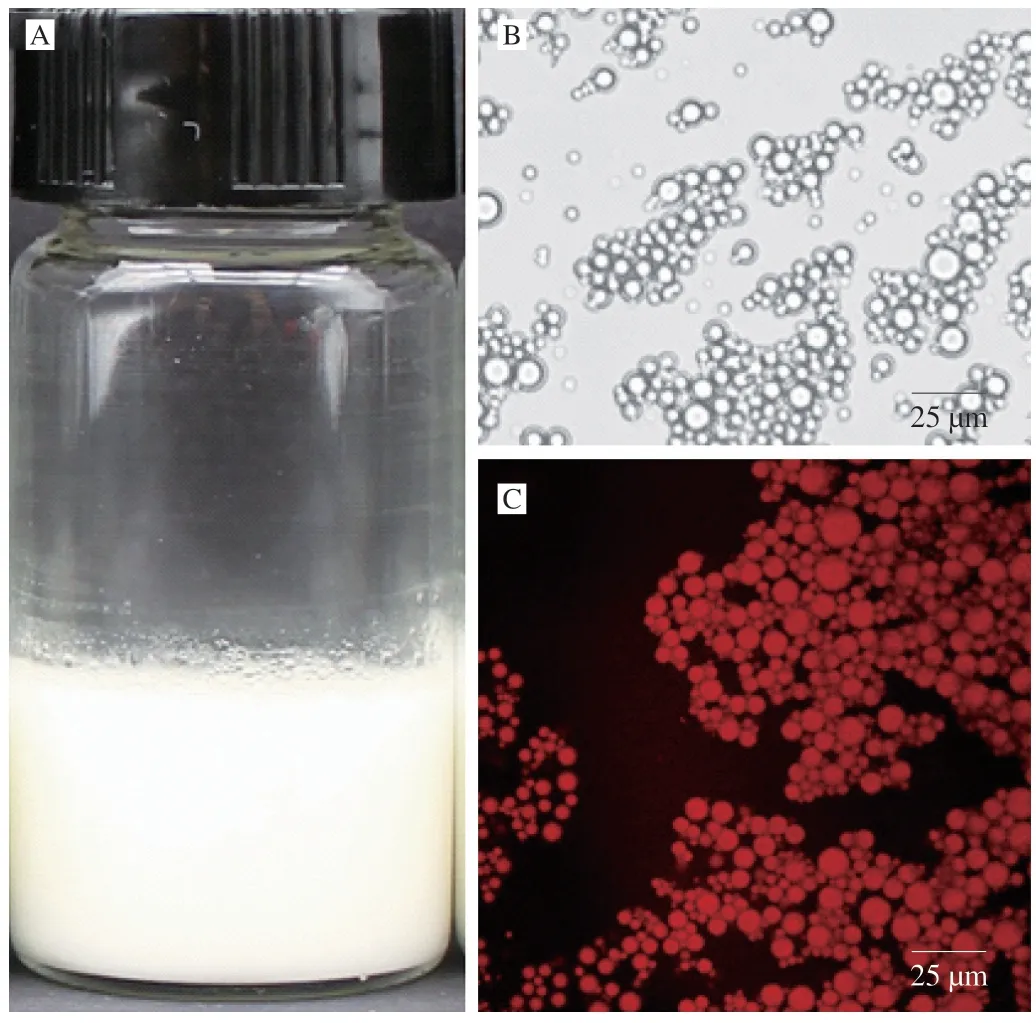
Fig.1 Digital camera (A),optical microscopy (B),and CLSM (C) images of fish oil-loaded alginate-stabilized dispersion.Nile Red was applied for CLSM observation of fish oil.Scale bars in optical microscopy and CLSM images indicate 25 μm.
3.2 Preparation and structural characterization of millimeter-sized capsules
Using a customed-designed electrospraying instrument with a single hole needle [48],spherical millimeter-sized spherical capsules were successfully fabricated by a monoaxial dispersion electrospraying-ionotropic gelation technique in this study.It is well known that the water solvent in the fish oil-loaded alginatestabilized dispersion was evaporated between single hole needle and the collecting solution in a typical electrospinning/electrospraying process [54].The obtained capsules were collected in CaCl2solutions.Therefore,the stabilization mechanism of the capsule shell layers could be described as ionotropic gelation [55].
During the electrospraying process,some oils were floated on the surface of CaCl2collecting solution.After moving into fresh CaCl2solution,no fishy odor was smelled by our noses on these capsules,which implied the fish oil molecules were compactly encapsulated by the capsules.As shown in the digital camera images in Fig.2 and diameter statistical analyses in Fig.3,spherical capsule diameters decreased with the increase of the applied voltages.The average maximum diameters of spherical capsules were from 2.05 mm to 0.35 mm.It is well known that increased voltage will decrease the electrospun nanofiber diameter in part by the increased electrostatic forces [56].Therefore,in part by the increased electrostatic forces,the increased voltage might decrease the diameter of spherical capsules.The optical microscopy images in Fig.2 showed there were many light spots in the capsules,which were more obviously at higher voltages.A previous work used fluorescence microscopy to demonstrate that fish oil was lighter than alginate in the optical microscopy images [43].Therefore,fish oil might be distributed into the capsules with a multicore way according to the optical microscopy images in this work (Fig.2).
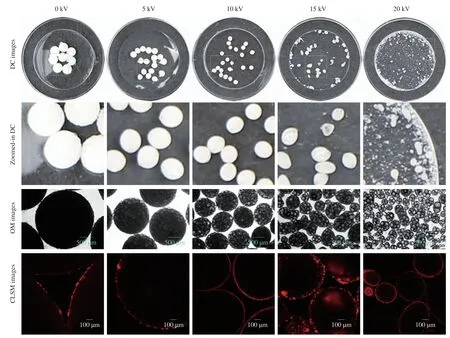
Fig.2 Digital camera (DC),optical microscopy (OM),and CLSM images of fish oil-loaded millimeter-sized spherical capsules prepared at different alginate preparation voltages (0,5,10,15,and 20 kV).The light circle (the central part of 35 mm Petri dish) diameters in digital camera images were 15 mm.Zoomed-in digital camera images were from the corresponding sky-blue squares in digital camera images.Green arrows indicate capsules with large fish oil masses.Nile Red was used for CLSM observation of fish oil.Scale bars in optical microscopy and CLSM images indicate 500 and 100 μm,respectively.CLSM could only observe dyes in the crust layer (near the surface) of the capsules because visible laser could not transmit in the capsule cores [71].
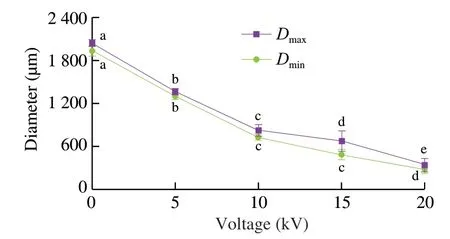
Fig.3 Maximum and minimum diameters of fish oil-loaded millimeter-sized capsules prepared at different voltages (0,5,10,15,and 20 kV).Values with different letters indicate significance at P ≤ 0.05.Same as Fig.7 and 9.
In order to further analyze the structure,CLSM,SEM,3D reconstructed CLSM and ordinary fluorescence images were applied to analyze the obtained capsules.Previous work suggested visible light cannot transmit through opaque fluids [57],human body [58],and semiconductor layers [59].Therefore,we could reasonably predict that visible laser could not transmit in the multicore capsules due to the presence of polymer in the core.CLSM could only observe dyes in the crust layer (near the surface) of the multicore capsules(Fig.2),which confirmed our prediction.If the fish oil (dye) was only located on out layer of the capsules,light spots might not appear in the optical microscopy images (Fig.2).Therefore,the phenomena that only dyes in the crust layer were observed in CLSM images was due to the multicore structural characteristics of the capsules and the technical characteristics (visible laser could not transmit in the multicore capsules) of CLSM.SEM image (Fig.4) showed that there were many hemisphere-like humps on the surface of capsules.It was reasonable to assume the capsules were formed by the ionotropic gelation of many dispersion droplets in the CaCl2solution.Therefore,the capsule surface was consisted of hemisphere-like humps and was not smooth.3D reconstructed CLSM images of the capsule crust layer(Fig.5A) demonstrated that fish oil was relatively uniformly and noncontinuously distributed in the crust layer of the capsules.
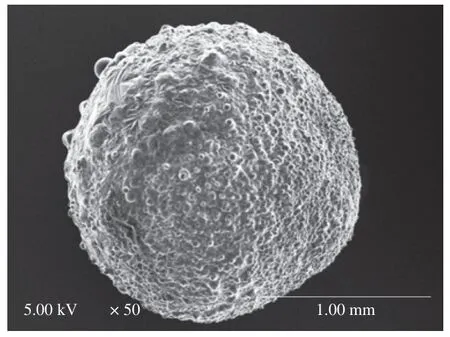
Fig.4 SEM image of fish oil-loaded millimeter-sized capsule prepared at below conditions: 2% sodium alginate solution,an applied voltage of 5 kV,a needle of 20#,a feeding rate of 30 μL/min,and a receiving distance of 10 cm.
Some large fish oil masses (white arrows in Fig.5A) implied that some large fish oil masses were also occasionally present in the capsules.The digital camera images also demonstrated the presence of large fish oil masses in the capsules prepared at applied voltages of 15 and 20 kV (green arrows in Fig.2).Though SEM and 3D reconstructed CLSM images of the capsules prepared at other voltages were not provided in Figs.4 and 5,we could reasonably assume that all the SEM and 3D reconstructed CLSM images of the capsules prepared at different voltages were similar to Figs.4 and 5 except the capsule sizes according to digital camera images,optical microscopy images,and CLSM images in Fig.2.Furthermore,ordinary fluorescence image of the capsule (Fig.5B) demonstrated fish oil was also present in the core of the capsule.Therefore,these results confirmed that fish oil might be distributed into the capsules with a multicore way.
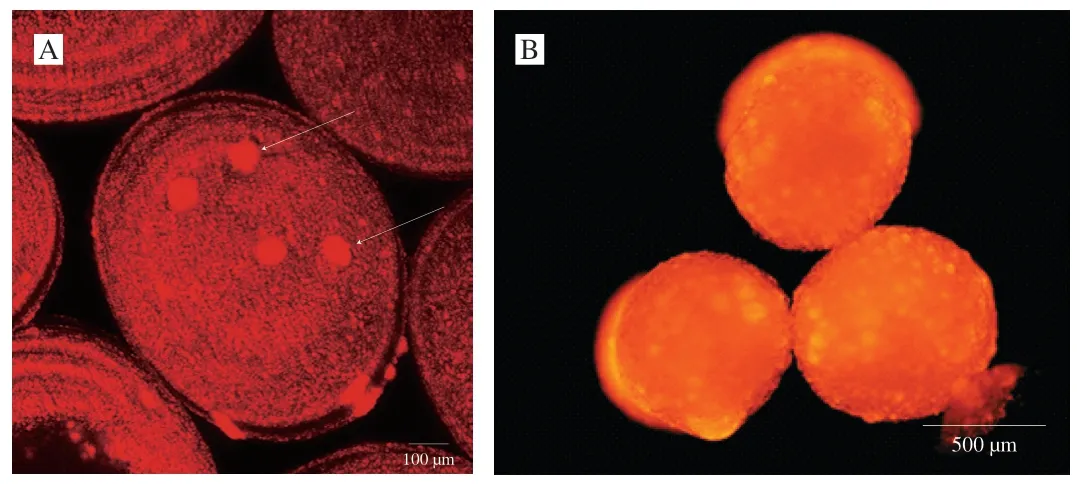
Fig.5 Fluorescence microscopy images of fish oil-loaded millimeter-sized capsules prepared at below conditions: 2% sodium alginate solution,an applied voltage of 10 kV,a needle of 20#,a feeding rate of 30 μL/min,and a receiving distance of 10 cm.Nile Red was used for the observation of fish oil.(A) 3D reconstructed CLSM image of the crust layer (near the surface) of fish oil-loaded millimeter-sized capsule.Scale bar indicates 100 μm.White arrows indicate large fish oil masses in the crust layer (near the surface) of the capsule.(B) Ordinary fluorescence image of fish oil-loaded millimeter-sized capsule.Scale bar indicate 500 μm.
Water contents of fish oil-loaded millimeter-sized capsules prepared at different voltages (0,5,10,15,and 20 kV) were measured,as shown in Figs.6 and 7.All the white capsules became yellow after water evaporation due to high-temperature (100 °C)treatment for 2 h (Fig.6).The water evaporation made the alginate became transparent.A previous work demonstrated the color of wheat gluten/chlorophyll film became from green to yellow during oxidation process of oil [60]and heating could significantly induce color formation in oil [61].Therefore,the color change of fish oil-loaded millimeter-sized capsules might be caused by fish oil color change(became yellow) after oxidation and the alginate change (became transparent) after heating.The water contents of the capsules were 54% -60% (Fig.7).Therefore,the continuous phase of the capsules consisted of water and hydrophilic alginate calcium.Previous works suggested alginate is a good oil encapsulation agent and can be applied to prepare four types capsules [62]: irregular shaped capsules with sizes of 0.5 μm–3.0 mm,polynuclear capsules with sizes of 200 μm–5.0 mm,oil core shell capsules with sizes of 200 μm–3.5 mm,and emulsion core shell capsules with sizes of 200 μm–5.0 mm.Our millimeter-sized capsule is a kind of polynuclear capsule.Compared with previous polynuclear capsule prepared by an emulsion extrusionionotropic gelation technique [63],our capsules were prepared by an dispersion electrospraying-ionotropic gelation technique,which made the preparation process easy to control the capsule formation speed and the capsule size.

Fig.6 Digital camera images of fish oil-loaded millimeter-sized capsules prepared at different voltages (0,5,10,15,and 20 kV) before and after hightemperature (100 °C) treatment.The capsules were put in clean constant-weight 50-mm-diameter bottles.
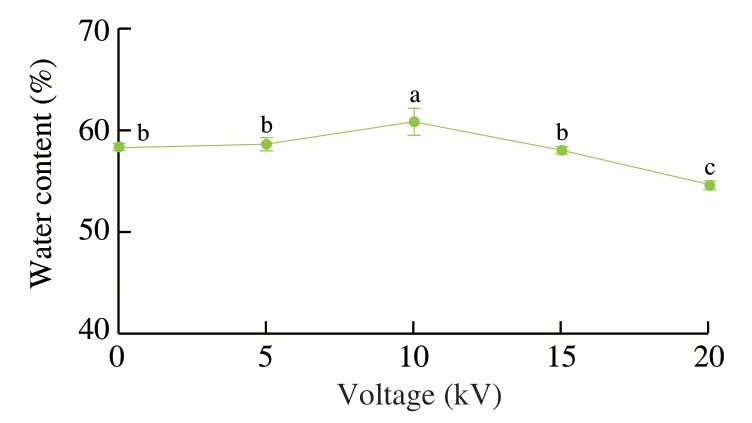
Fig.7 Water contents of fish oil-loaded millimeter-sized capsules prepared at different voltages (0,5,10,15,and 20 kV).
According to above analyses,in this work,fish oil-loaded alginate-stabilized dispersions were used to prepare fish oil-loaded millimeter-sized uniform and nonuniform multicore spherical capsules by a monoaxial dispersion electrospraying-ionotropic gelation technique (Fig.8).The obtained capsules were in a typical multicore structure: the non-continuous core consisted of fish oil and the continuous wall layer consisted of water and alginate calcium.The multicore structures in this work were significantly different to the electrosprayed millimeter-sized capsules in a previous work [43]:(1) The preparation methods were different.Coaxial (coaxial syringe needle and two syringe pumps) electrospraying method was applied in the previous work and monoaxial (monoaxial syringe needle and one syringe pump) electrospraying method was applied in this work.(2) The preparation solutions were different.Fish oil and sodium alginate solution were used as core and outer,respectively,injection solutions in the previous work.Alginate-stabilized fish oil dispersion was used as the injection solution in this work.(3) The capsule structures were different.Monocore capsules were obtained in the previous work and multicore capsules were obtained in this work.
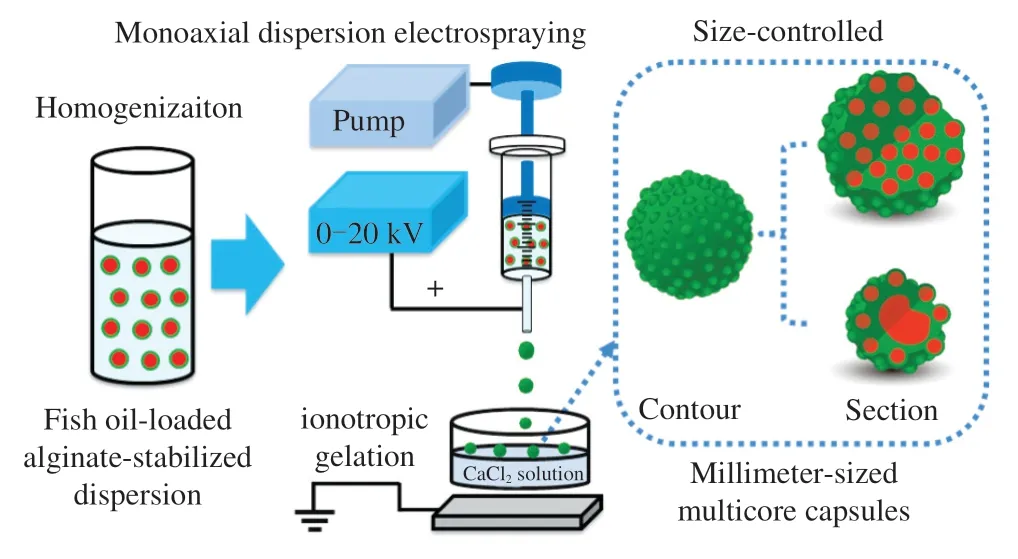
Fig.8 Schematics of monoaxial dispersion electrospraying-ionotropic gelation technique for the fabrication of fish oil-loaded millimeter-sized uniform and nonuniform multicore spherical capsules.Large uniform multicore capsules and small nonuniform multicore capsules are produced at low and high voltages,respectively.
The multicore capsules could be classified into relatively uniform(≤ 10 kV) and nonuniform (>10 kV) multicore capsules.The relatively uniformity and nonuniformity were classified according to fish oil distribution in the capsule,as shown by the light spots (fish oil) in the optical microscopy images.Moreover,these results also suggested that the fish oil core sizes were comparable (micrometer level) to the sizes of alginate gel-stabilized fish oil droplets (Fig.1).According to the above analyses,the preparation of fish oil-loaded millimeter-sized capsules could be proposed as shown in Fig.8.Considering the large fish oil mass was randomly distributed into the obtained capsules,the reason for the nonuniform multicore might be the difference on the voltage.At higher voltage in an electrospinning process,the polymer jet becomes highly unstable and may produce nanofibers with a larger variation [64].Therefore,in our electrospraying process,the dispersion jet was stable at lower voltages and produced the capsules with uniform multicores,whereas the dispersion jet becomes highly unstable at higher voltages and produced the capsules with nonuniform multicores.
3.3 Fish oil loading ratios of the millimeter-sized capsules
Fish oil loading ratio is one of important parameters of multicore capsules.The fish oil contents in the spherical capsules prepared at different applied voltages (0,5,10,15,and 20 kV) were measured by the Rose-Gottlieb method with some modification [49].As shown in Fig.9,the loading ratio of the capsules prepared at 0 kV was (9.7 ± 1.7)% (n=3).The applied voltages (5–20 kV) slightly decreased the loading ratios to 6.3% –7.1% and they had no obvious differences in the loading ratios.Some fish oil substances might not be encapsulated in the capsules during the electrospraying process.These millimeter-sized spherical capsules had higher loading ratios than whey protein aerogel as blended with cellulose crystalline particles ((2.61 ± 0.53)%) by freeze drying method [65]and silicone oil-loaded polyacrylonitrile uniform microcapsules(0.08-0.92 g/g polymer) by solvent evaporation method [66].They were similar to the optimized fish oil spray-dried inulin microcapsules (1.5% -7.5%) [67].Considering that fish oil was distributed into alginate matrix with uniform (≤ 10 kV) or nonuniform (>10 kV) structures (Fig.8) with a multicore structure,the measured loading ratios were reasonable.Compared with the capsules prepared at 0 kV,the electrospayed capsules had lower loading ratios (Fig.9) and less diameters (Figs.2 and 3).Therefore,the capsule sizes could be controlled after the electrospraying technique.
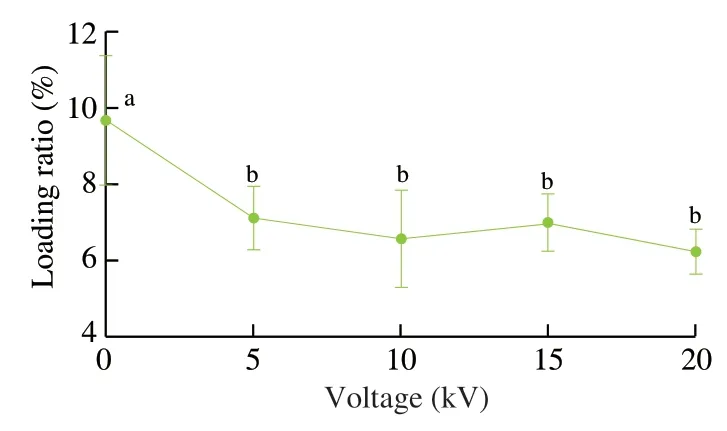
Fig.9 Fish oil loading ratio of millimeter-sized capsules with different applied voltages (0,5,10,15,and 20 kV).
3.4 Fish oil sustained release behaviors of the millimetersized spherical capsules in the in vitro digestion models
In order to simulate the digestion behaviors of millimeter-sized spherical capsules,the spherical capsules prepared at different applied voltages (0,5,10,15,and 20 kV) were put in the gastrointestinal and small intestinal tract models.We did not simulate oral phase because drinking with the millimeter-sized capsules only lasts a few seconds in the oral though they may taste gritty or sandy.The percentage of released FFAs from the capsules in the gastrointestinal (Fig.10A)and small intestinal (Fig.10B) tract models could be thought as the percentage of released fish oil from the capsules.No lipase is present in human stomach,and therefore,no lipase was present in the gastric phase of the gastro-intestinal tract model [50,51].The lipid digestion did not occur and FFAs were not released in the gastric phase.Therefore,FFA release was calculated not in the gastric phase but in the small intestinal phase of the gastro-intestinal tract model (Fig.10A),which was similar to previous works [51,52].
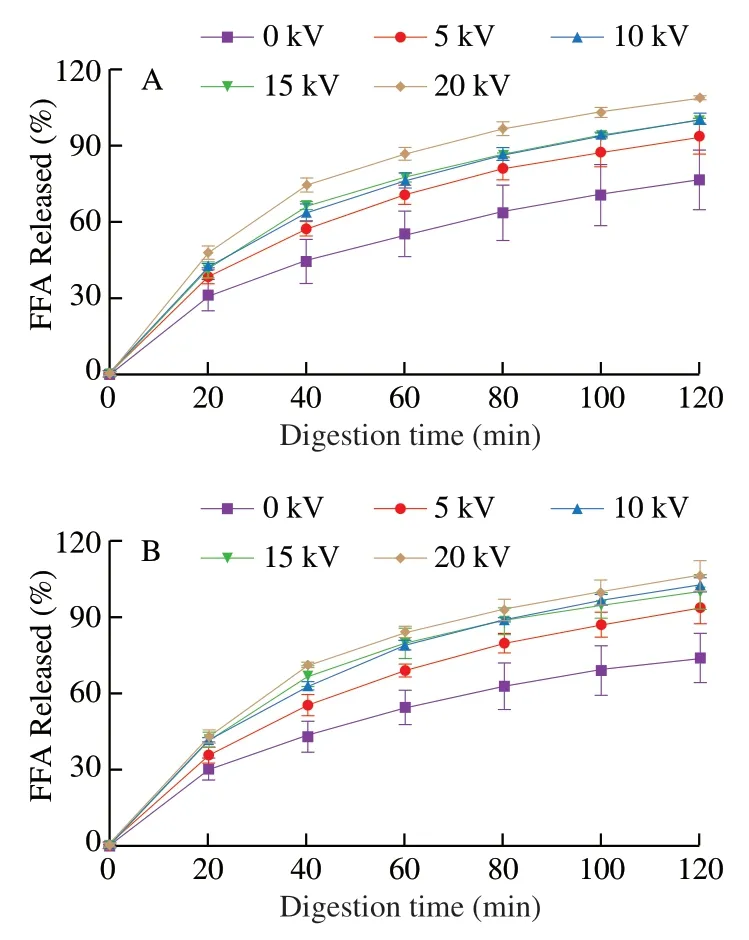
Fig.10 Calculated FFA release from the fish oil-loaded millimeter-sized capsules during in vitro digestion in the small intestinal phase of the gastrointestinal tract model (A) and the small intestinal model (B).The capsules were prepared under different applied voltages (0,5,10,15,and 20 kV).
The results showed fish oil was sustainedly released from the capsules without obvious burst release and the released amounts increased with the increase of applied voltages (Fig.10).The capsule size significantly decreased with the increased applied voltages.Therefore,the surface-to-volume ratio significantly increased with the increased applied voltages,which might be the main reason for the increased fish oil release with the increased applied voltages.In addition,the multicores were nonuniform at higher applied voltages (Fig.2).Nonuniform multicore capsules might have faster release than uniform multicore capsules.The large core in nonuniform multicore capsules may release more fish oil when fish oil was released from the large core,which might be the minor reason for the increased fish oil release with the increased applied voltages.Compared with the uniform multicore capsules prepared at lower voltages,the nonuniform capsules prepared at higher voltages had some potential benefits such as relatively fast release and smaller size for easier swallow.Therefore,the choice of applied voltages would be dependent on the research requirement.
The capsules in PBS at pH 2.0 and pH 7.0 at room temperature were observed,as shown in Fig.11.The results showed the capsules shrank at pH 2.0.A previous work showed insulin-loaded alginate beads shrank and only release about 20% -30% insulin at low pH(0.1 mol/L HCl) for two hours [68].The capsule significantly swelled(about 130%) at pH 7.0 (Fig.11).Therefore,fish oil was mainly released from the capsules after expansion in the small intestinal phases (Fig.10).Moreover,the release percentages in the small intestinal phase of gastrointestinal tract model showed no obvious differences to that in the small intestinal tract model (Fig.10),which also demonstrated that fish oil was not mainly released in the gastric phase.The specific release behavior of fish oil to the small intestines was consistent with previous electrosprayed fish oil/alginate coreshell capsules [43].
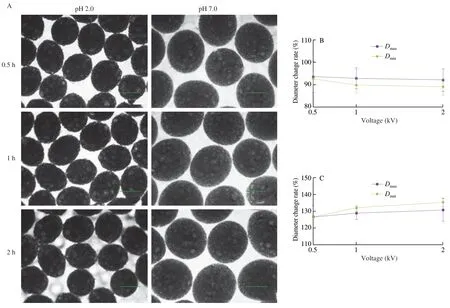
Fig.11 Observation of the capsules in PBS at pH 2.0 and pH 7.0 at room temperature.The capsule used in this experiment was prepared at below conditions:2% sodium alginate solution,an applied voltage of 10 kV,a needle of 20#,a feeding rate of 30 μL/min,and a receiving distance of 10 cm.(A) Optical microscopy images of the capsules at different incubation times.Diameter change rates of the capsules at different incubation times in PBS at pH 2.0 (B) and pH 7.0 (C).
According to thein vitrodigestion behaviors,we could reasonably conclude the obtained millimeter-sized spherical capsules could be applied for specific and sustained release of fish oil in the small intestines of human being.Fish oil is rich in omega-3 polyunsaturated fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA),which have been suggested to be beneficial in autoimmune,inflammatory and cardiovascular disorders [69,70].Therefore,the development and application of millimeter-sized multicore capsules might have good potential in the field of functional foods.Considering fish oil is also a good carrier for water insoluble nutrients/drugs such asβ-carotene [43],the obtained capsules could be also applied for specific and sustained release of water insoluble nutrients/drugs in the small intestines.
4.Conclusions
To sum up,a facile monoaxial dispersion electrosprayingionotropic gelation approach was explored for fabricating millimetersized fish oil-loaded multicore alginate capsules using fish oil-loaded alginate-stabilized dispersion.The diameters of spherical capsules could be controlled from 0.35 mm to 2.05 mm by adjusting the applied voltages.The millimeter-sized multicore spherical capsules could be classified into two types: (1) uniform multicore capsules at applied voltages of ≤ 10 kV;(2) nonuniform multicore capsules at applied voltages of >10 kV.The millimeter-sized capsules had reasonable fish oil loading ratios and fish oil could be specifically and sustainedly released in the small intestinal phase ofin vitrogastrointestinal and small intestinal tract models.Moreover,the sustained release behaviors could be controlled by the applied voltages.This technique also converted liquid fish oil to solid capsules,which were convenient for food processing and storage.In addition,alginate is an FDAapproved food additive.Therefore,with oral administration,the obtained millimeter-sized spherical capsules by monoaxial dispersion electrospraying-ionotropic gelation technique are particularly suitable for specifically and sustainedly delivering fish oil and fat-soluble functional active substances to small intestines.They have significant and broad application potentials for the encapsulation of active substances in food and drug industries.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgements
This research has been supported by research grants from the National Key R&D Program (2019YFD0902003).
杂志排行
食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Structural characteristics,anticoagulant and antithrombotic mechanism of a novel polysaccharide from Rosa Chinensis Flos
