Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
2023-01-21MjKozrskiAnitKlusLovnGrinsvnDrgiJkovljviNinTooroviWnAlQrImWnMohtrJovnVunuk
Mj Kozrski,Anit Klus,Lo vn Grinsvn,Drgi Jkovljvi,Nin Toorovi,Wn A Al Qr Im Wn-Mohtr,Jovn Vunuk
a Institute of Food Technology and Biochemistry,Faculty of Agriculture,University of Belgrade,Belgrade 11080,Serbia
b Plant Research International,Wageningen University and Research Centre,Wageningen 6700 AA,The Netherlands
c Institute of Chemistry,Technology and Metallurgy,University of Belgrade,Belgrade 11000,Serbia
d Functional Omics and Bioprocess Development Laboratory,Institute of Biological Sciences,Faculty of Science,Universiti Malaya,Kuala Lumpur 50603,Malaysia
e Institute of General and Physical Chemistry,University of Belgrade,Belgrade 11158,Serbia
Keywords:Mushrooms β-Glucan Polyphenols Immunity boosters Superfood Natural cosmetics
ABSTRACT Mushrooms are experiencing a kind of renaissance as a part of the contemporary human diet.These valuable organisms are more than food,they f it in perfectly as a novel market group known as nutra-mycoceuticals.Immune-balancing mushroom dietary fibers and secondary metabolites such as polyphenols are the main focus of the healthcare industry.Wellness and cosmetic companies are increasingly using mushroom extracts rich in these ingredients.This review considers the basic molecular immunomodulatory mechanisms of action of the most commonly used mushroom dietary fibers,β-glucans.The literature data on their bioavailability,metabolic transformations,preclinical and human clinical research,and safety are discussed.Immunomodulatory mechanisms of polyphenol ingredients are also considered.These molecules present great potential in the design of the new immunity balancer formulations according to their widespread structural diversity.Finally,we draw attention to the perspectives of modern trends in mushroom nutraceutical and cosmeceutical formulations to strengthen and balance immunity.
1.Introduction
Over the last decade,the abundance and diversity of mushrooms marketed as functional food and medicine around the world have steadily grown [1-5].From the primary/nutritional aspect of food,mushrooms are placed between meat and vegetables.According to available data,the content of proteins,fats,carbohydrates and dietary fiber in the most popular edible mushrooms,such asAgaricusbisporus,Lentinulaedodes,Pleurotusspp.andVolvariellavolvacea,which are commercially grown worldwide,are in the range of 16% -31%,2% -6%,51% -73% and 8% -12%,respectively,depending on their dry weight [3].Low in calories,without cholesterol,mushrooms contain ergosterol and essential polyunsaturated fatty acids (PUFAs) such as linoleic,oleic and linolenic in their lipid prof ile,usually as the main ingredients [3,6,7].For example,it has been found that at least 80% of total fatty acids are unsaturated inA.bisporus[3].Moreover,they contain all the essential amino acids needed for human metabolism and fit perfectly into the trend of vegetarian/vegan nutrition [8].Mushrooms are a good source of B-complex vitamins and are rich in carotenoids,lycopene,tocopherols and vitamin C [1-3,9].Likewise,mushrooms are a rich source of ergosterol [7,10].The transformation of ergosterol into ergocalciferol/vitamin D2takes place during the development of fruiting bodies exposed to sun’s UV light and thermal reaction [7,11].However,its formation can be additionally stimulatedinvitroby exposing caps or gills which are fresh,dried,ground,and suspended in ethanol to UV light [7].Moreover,due to their low caloric value and low glycemic index,high-value proteins and fibers,as well as the high potassium:sodium ratio,mushrooms are suitable for the diet of diabetics and patients with hypertension [12].With a growing population of vegetarians,vegans and lactose intolerant,this non-animal source of nutrients is an excellent choice.They are even marketed as new non-animal meat.
The secondary function of food,which is becoming increasingly popular,is the ability to modulate physiological systems -immune,endocrine,nervous,circulatory and digestive in addition to proven,beneficial nutritional effects [13].Among the scientific community,mushrooms are declared nature’s pharmaceutical factories [1,2,14,15].They are rich in a vast array of active constituents and metabolites(classified as non-nutrients) such as dietary fibers and secondary metabolites,as well as polyphenols,nitrogen-containing compounds e.g.,ergothioneine,terpenoids,sterols,lovastatin,and others that can improve cellular longevity and lifespan.They are present in small quantities and have only a limited caloric value [2,14].The bioactivity of these compounds includes not only health protection but also the treatment of health problems caused by various forms of homeostasis disorders,such as immunodeficiency,inflammation,and oxidative stress which plays a crucial role in the development of age-related diseases including arthritis,diabetes,dementia,cancer,atherosclerosis,vascular diseases,obesity,osteoporosis,and metabolic syndromes [1,16].Consequently,mushrooms are more than food,they fit perfectly as a novel market group which is known as nutraceuticals/mycoceuticals.
Medicinal mushrooms such asGanodermalucidum(lingzhi or reishi),Agaricusblazei(sun mushroom or himematsutake),Lentinulaedodes(shiitake),andGrifolafrondosa(maitake or huishu-hua) are usually consumed in China,Japan,Thailand,and Korea as immune response modifiers for prevention of cancer,or as nutritional support during chemotherapy,and against chronic inflammatory conditions such as hepatitis [17].Immune-balancing mushroom dietary fibers,primarilyβ-glucans,and secondary metabolites such as polyphenols are a top focus of the health industry [18-21].Wellness and cosmetic companies are increasingly using mushroom extracts rich in these ingredients.Interest is growing in Western countries,and an increasing number of preclinical studies of mushroom polysaccharides and polyphenols indicate pronounced anticancer and regenerative properties [18,22].Besides,they can prevent autoimmunity as well as transplant rejection via the activation of cytoprotective and antioxidant enzymes [23].Recently,there have been indicators these mushroom compounds might be beneficial in lessening the severity of coronavirus infections via suppression of inflammatory cytokine storm and inhibition of viral protease [24,25].In recent decades,fungal immunomodulatory proteins (FIPs) have been widely studied for their pharmaceutical utilizations [26].Ling-Zhi 8 (LZ-8) was the first FIP discovered inG.lucidum[27].Till now,more than 38 types of FIPs have been identified with their anti-allergic,anti-inflammatory,and anti-cancer bioactivity.Research has shown that some FIPs can retain their bioactivity upon oral administration,e.g.,some vital domain,responsible for their bioactivity,reaches the intestinal tract intact [26].
Finally,it is important to mention that mushrooms attract attention as a rich source of chitin and its derivate-chitosan,which are not only known as dietary fibers but also as one of the vital innovations in designing biopolymer-based products that are gaining momentum in diverse biomedicine arenas [28].The advantages of using chitin and chitosan are linked to their renewability,biodegradability,and nontoxicity [28].Thus they find application in a large variety of fields,for example as constituents of artificial bones and skin or for drug delivery in the biomedical field and the detection and removal of organic and inorganic pollutants for environmental remediation [28].
Hereby we draw attention to the perspectives of contemporary trends in mushroom nutraceutical and cosmeceutical formulations to strengthen and balance immunity.We consider the basic molecular immunomodulatory mechanisms of action of the most commonly used mushroom dietary fibers,β-glucans.The literature data on their bioavailability,metabolic transformations,preclinical and human clinical research,and safety are discussed.Immunomodulatory mechanisms of mushroom polyphenol ingredients are also considered.These molecules present great potential in the design of the new immunity balancer formulations according to their widespread structural diversity.Finally,we tried to give a clear answer whether small molecules such as polyphenols are more bioavailable and efficient than macromolecules such asβ-glucans after oral application.
2.Mushroom nutraceuticals as a health and wellness trend
Mushroom nutraceuticals are a refined or partially refined extract or dried biomass derived from either mycelium or fruiting body,with a potential therapeutic application,Fig.1a.Mushroom formulations available on the market include: (1) artificially cultivated fruiting body powders and hot water or alcohol extracts obtained from these fruiting bodies,(2) dried and pulverized preparations made by a combination of substrate,mycelium and primordial form,(3)biomass or extracts derived from mycelium which is harvested from a submerged liquid culture grown in a fermentation tank or bioreactor,(4) naturally grown,dried mushroom fruiting bodies in the form of powders,capsules or tablets,and (5) spores and their extracts [29].

Fig.1 Photos by Jovana Vunduk: (a) Mushroom nutraceuticals and cosmeceuticals,a1-year 2022,Belgrade,Serbia and a2-year 2019,New York,USA;(b) Cultivation of medicinal mushroom G.lucidum,b1-fruiting bodies grown on wood logs and b2-antler form grown on sawdust-year 2019,Fuzhou,China.
Commercial nutraceutical and cosmeceutical formulations found on the market may not only consist of extract from one mushroom but can also be combinations of extracts from different mushroom species,(Table 1).Also,mushroom formulations may be found along with plant ingredients and extracts.
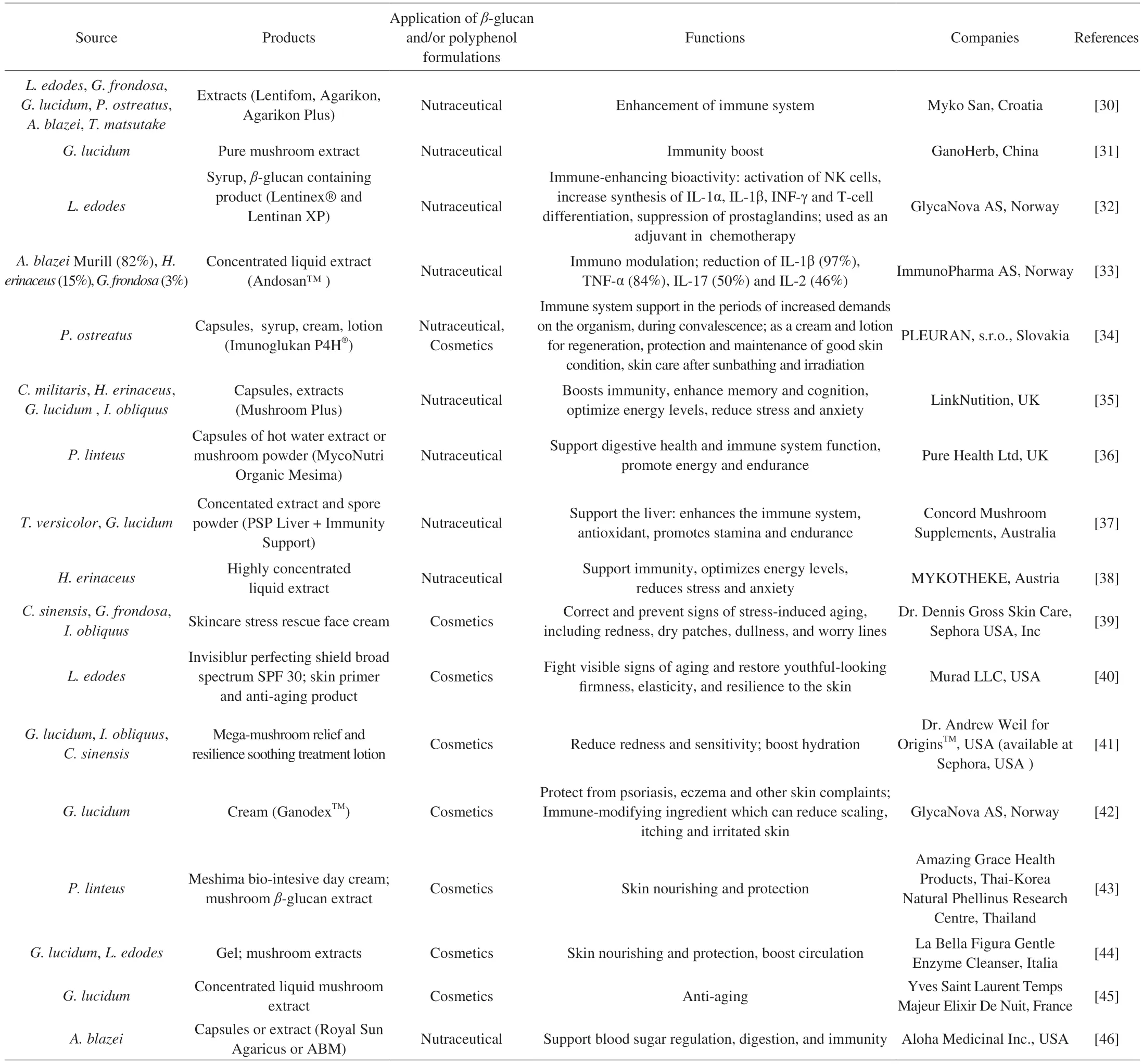
Table 1 Some of the nutraceutical and cosmeceutical formulations rich in β-glucans and/or polyphenols available on the world market
In the modern global market,mushroom nutraceuticals designed to balance and support immunity are among the most sought after [47].This fact goes in line with the global market projections by which the mushroom nutraceuticals market is estimated to reach over $90 billion by 2027,growing at a compound annual growth rate (CAGR) of 7.6% during 2021-2027 [48].The largest and most popular market is in the Asia-Pacific countries,while Europe is the fastest-growing market.It should be noted that,despite the growing popularity,mushroom nutraceutical therapies are still mostly unrecognized in Western countries,especially in conventional medical institutions.The use of these nutraceuticals is becoming significant within the complementary and integrative medicine (CIM) communities of Europe and the USA.There is great interest from cancer patients in CIM methods;studies have shown that 20% -50% of these patients use some form of CIM therapy as an addition to conventional treatments [22].Given the growing demand for mushroom nutraceuticals,today many companies deal with the development and marketing of these substances [16,49].It should also be noted that the ingredients of mushrooms are finding their application in the nutricosmetic/mycocosmetic industry,and many potential products are likely to be formulated [50].G.lucidumhas been used in anti-aging skin care products since the 1980 s,starting with the Japanese brand Menard.In the early 2000 s,mushrooms hit the Western market for skincare applications [50].Advances in fields such as nanotechnology are also driving innovations in mushroom nutraceutical science and products [51].
3.Mushroom β-glucan and polyphenol formulations as natural immune system boosters and balancers
Immune-supportive,anti-inflammatory and antitumor activities are among the predominant effects of mushroom nutraceuticals in the modulation of physiological systems [47,52].Mushrooms contain diverse immunomodulatory compounds such as polysaccharides,polyphenols,terpenoids,and proteins [26,27,52].However,in the marketplace,these beneficial properties are most often attributed to the mushroom dietary fiber formulations,primarily consisting ofβ-glucans since their structure,structure-immunomodulating activity relationship,preclinical and clinical studies are among the most investigated [19].Exceptional interest in their immunomodulatory potential arose due to structural diversity in relation to another important food source,plantβ-glucans;e.g.β-glucans from cereals,at variance,have no side-chain branching,nor 1→6 linkages [19,53-58].Mushroomβ-glucans are primarily based onβ-(1→3) bonds in a backbone with shortβ-(1→6) branches or rarely without branches,Table 2 (contrary to long-branched chainsβ-(1→6) present in yeast).The triple helix structure determines the immunomodulatory and anticancer properties of mushroomβ-glucans [20,53,56].In general,mushroomβ-glucans whose molecular weight is greater than 104Da are more active [58].Besidesβ-glucans,immunomodulating properties of diverse mushroom homopolysaccharides e.g.β-mannans and heteropolysaccharides such as fucogalactans,fucomannogalactans,glucogalactomannans,xylomannans,and others are reported [58].In their last review,Yin et al.[58]pointed out that because of the complexity,the complete structure of these polysaccharides was difficult to determine,and the structure-activity relationship could not be completely established.Also,most of the reported studies of immunomodulatory activities have been performedinvitroorin vivoon animal models,which cannot fully reflect the actual role of these polysaccharides in the human body [58].
In the last few years,there has been a growing interest in immunesupporting formulations based on mushroom polyphenols due to their widespread structural diversity [59,60].The PubMed search with “polyphenols and immune system” as keywords,resulted in 2225 publications (on 06 April 2022) [61];this underlines the actual great interest of researchers in the scientific bases of polyphenols’immunomodulating properties.To be able to compete with the very popular herbal polyphenolic immunomodulatory formulations on the market,rapid genomics,proteomic,metabolomic,metagenomics,and transcriptomic research on mushroom polyphenols are underway [62].Currently,intensive research on mushroom polyphenols aims to complete the characterization and quantification of sets of their biologically active secondary metabolites (Table 2).
3.1 Mushroom β-glucan formulations-nature of immunomodulation
Mushroom nutraceutical formulations ofβ-glucans are commercially available as purified or partially purified extracts in the form of capsules,pills,and powders (Table 1).The available content ofβ-glucans in commercial formulations depends on mushroom species as well as a part of the mushroom from which extraction is performed,e.g.,stalks or caps.As an example,there is an investigation by Sari et al.[63]on the enzymatic determination ofβ-glucan contents in 39 species of commercially cultivated and wild-growing mushrooms.Theβ-glucan contents of nine culinary mushrooms,includingAgaricusbisporus(white and brown button mushroom),Lentinulaedodes,Cantharellus cibariusand five differentPleurotusspecies,showed a range of 8-27 g/100 g dry mass (dm).In these species,percentages ofβ-glucans to all glucans were in the range of 71-96.Among wild species,the highest content ofβ-glucans was observed inBoletus edulisstalk(58 g/100 g dm) and fruiting bodies ofTrametes versicolor,not dividable in cap and stipe parts (61 g/100 g dm) [63].It is important to mention that the quantification ofβ-glucans is still quite difficult to standardize because of their diversity.The most commonly used methods for the detection ofβ-glucans from mushrooms are:(1) enzymatic method or McCleary method (Megazyme kit),(2)enzyme-linked immunosorbent assay (ELISA) method,(3) fluorimetric method with aniline blue,and (4) colorimetric method with Congo red [64].An ELISA method has been developed by Mizuno et al.[65]to determine high molecular weightβ-glucans,with grifolan and lentinan as prototypes of branchedβ-(1→3)(1→6)-glucan with a triple helix for immune reactions to form specific antibodies.Besides,it is important to emphasize that extraction ofβ-glucans is a difficult job and requires special attention to produce the consistent raw material.The nature of the extraction procedure has a profound effect on the molecular weight ofβ-glucan,which in turn affects its functional behavior.The extraction methodologies are based on the solubility ofβ-glucan in hot water and alkaline solutions [55,57,64].Separation of the dissolved proteins by isoelectric precipitation and/or enzymatic deproteinization of the bound proteins and precipitation of theβ-glucan by ammonium sulfate,2-propanol,ethanol,or freezethawing are general ways [64,66].Additional purification of particularβ-glucan bioactive fractions is commonly achieved by different chromatography techniques [67,68].
The most commonly reported and applied in nutraceutical formulationsβ-(1→3)(1→6)-glucans are: grifolan from maitakeβ-glycanD-fraction (G.frondosa),lentinan (L.edodes),pleuran(P.ostreatus) and krestin (T.versicolor) [20,56,69-79].Their molecular weight varies from 100 to 1 000 kDa,e.g.,lentinan has an average of about 500 kDa with a branching level of 0.40 (2/5) [20].Some studies related to mushroomβ-glucans are shown in Table 2.

Table 2 Some research on β-glucans and polyphenols in mushrooms
An important use of mushroomβ-glucans is in the prevention of immune disorders and in maintaining a good quality of life,especially in immunodeficient and immunodepressed conditions [23,80].They act primarily as adaptogens and immunostimulators: they stimulate and enhance antitumor activities in patients with different types of cancer as well as in patients on chemotherapy or radiotherapy[23,80].Many studies have shown thatβ-glucan treatment leads to the induction of apoptotic death of cancer cells [81,82].Apoptosis sometimes called “a guardian angel” or “cell policeman”,is carried out by a multistage chain of reactions and arises in cancer and other life-threatening cells [81].The ability ofβ-glucans to stimulate apoptotic pathways or the proteins involved in apoptosis prompts a new domain in cancer therapy [81].Also,β-glucans stimulate protective activities against fungal,bacterial and viral infections,e.g.in chronic blood-borne viral infections such as hepatitis B,C,and D,human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS),and herpes simplex virus (HSV) [80].
3.1.1 Intestinal uptake
Unlikeα-glucans,β-glucans administered orally are not digested by human enzymes and are only to a lesser extent fermented in the lower gastrointestinal tract (GI) by the human microbiome (gut microbiota) that produces active carbohydrate enzymes [83].
In the small intestine,despite their apparent high molecular weight,β-glucans are captured by the M cells and intestinal epithelial cells (IECs) in Peyer’s Patches,and/or subsets of CXCR3+macrophages or CD103+dendritic cells (DCs),which stimulates mucosal and systemic immunity [53,56](Fig.2).It was observed that the ileum,rather than the jejunum,is the main absorption site for glucans due to the abundance of M cells in this intestinal segment [84].Mushroomβ-glucans can recognize pattern recognition receptors (PRRs),including the dectin-1 receptor which belongs to the class of C-type lectin-like receptors and tolllike receptors (TLRs),to enter DCs and macrophages [56].This observation has been confirmed previously by Rice et al.[85]with fluorescently labeled and orally administered solubleβ-glucans in rats.They demonstrated that the first step inβ-glucan uptake is its internalization by IECs and/or M cells in Peyer’s patches.Uptake by these cells appeared to be independent of PRRs,whereas uptake by follicle-associated epithelium resulted in increased expression of dectin-1 as well as TLR2 on DCs and macrophages,suggesting that these two receptors contribute to the uptake ofβ-glucans.Studies using syntheticβ-glucans revealed that binding to dectin-1 requires a configuration consisting of aβ-(1→3) linkage [86].The previous structural analysis provided by Adams et al.[87]demonstrated that glucan backbone chain length andβ-(1→6) sidechain branching strongly influenced dectin-1 binding affinity.Also,several other PPRs have been shown to bind structurally differentβ-glucans (Fig.2b).For example,lactosylceramide receptor (LacCer),scavenger receptor,and complement receptor 3 (CR3) were reported to bind glucans [56].It should be noted thatβ-glucan receptors are not limited to the binding of singleβ-glucans,and vice versa,β-glucans are not limited to single receptors [56].Moreover,Rice et al.[85]showed that uptake ofβ-glucans was accompanied by an increase in systemic IL-6 and IL-12 levels.Multiple reports are pointing out thatβ-glucans result in up-regulated expression of pro-inflammatory cytokines and other mediators,most likely derived from macrophages and DCs [56](Fig.2).Exposure toβ-glucans induces DCs and macrophages to migrate via the bloodstream to the bone marrow or via the lymph system to mesenteric or more distant lymph nodes [56].
3.1.2 Basic molecular mechanisms of immunomodulation
There are three major pathways in the integrated transcriptional response ofβ-glucans [53,56]: (1) modulation of the signaling for nuclear factor kappa B (NF-κB) via NF-κB essential modulator(NEMO) complex and,in parallel,via activation of mitogen-activated protein kinase (MAPK) and initialization of AP-1 expression after protein kinase C-delta (PKCδ) activation;NF-κB is a crucial transcription factor that participates in a number of physiological and pathological conditions including immune response,apoptosis,carcinogenesis and inflammatory processes [81,82,88];(2) by triggering of phospholipase C-gamma (PLCγ),and subsequently calcineurin,which modulates nuclear factor of activated T-cells (NFAT);(3) controlling the signaling pathway in which reactive oxygen species(ROS) are generated,by modulating monocyte NADPH oxidase,which initialize activation of NLR family pyrin domain containing 3(NLRP3) protein and secretion of IL-1β (Fig.2b).
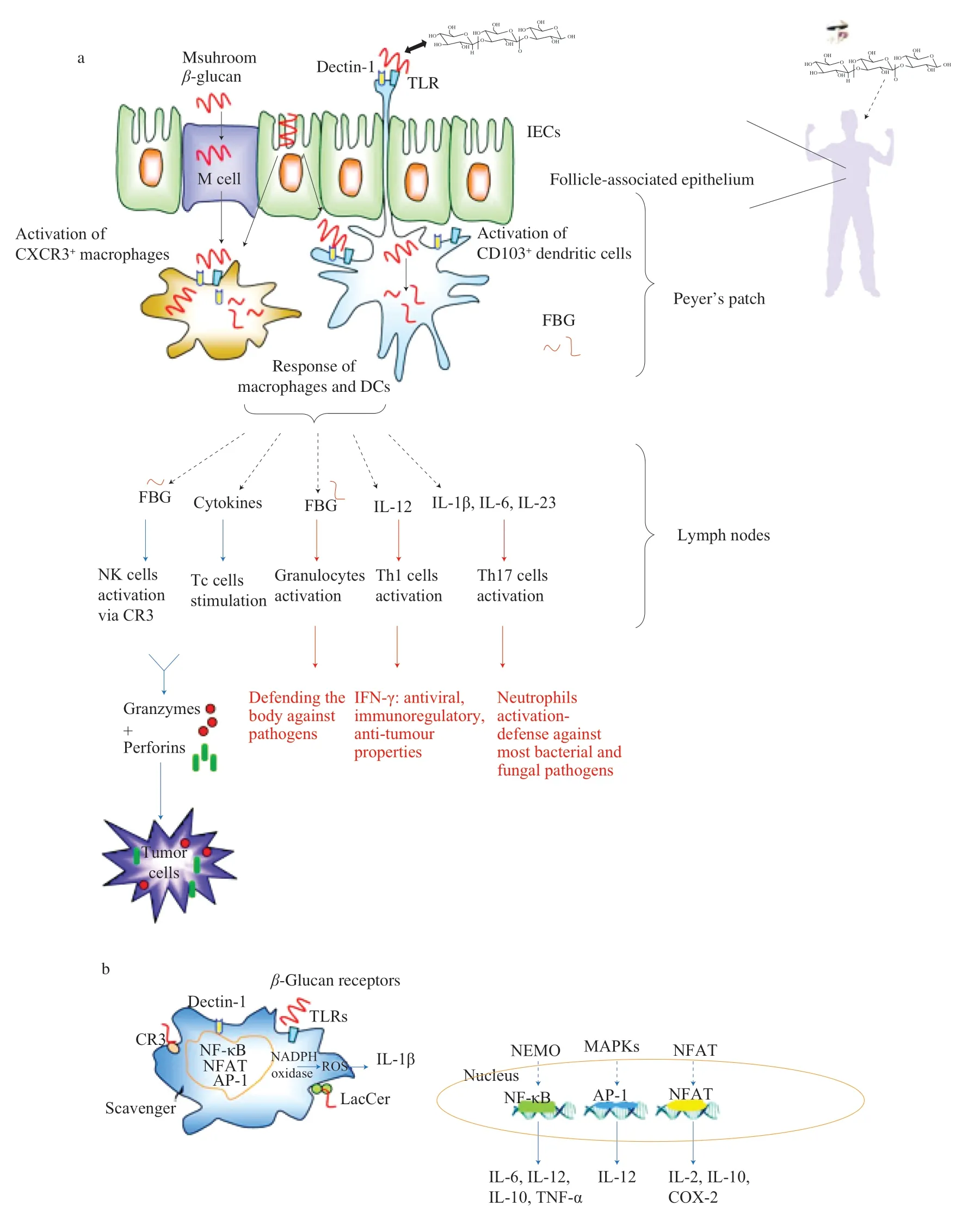
Fig.2 (a) Intestinal uptake and immune cells activation of mushroom β-glucans;(b) β-glucans bind to innate immune cells via pattern recognition receptors PPRs which generally leads to signaling through conserved pathways and leads to cell activation.
Mushroom glucans engulfed by macrophages and DCs undergo fragmentation.Fragmented glucans (FBGs) are released from the cells and activate natural killer (NK) cells and granulocytes [53](Fig.2a).Activated NK cells release perforins and granzymes,which make pores and disintegrate the DNA of tumor cells,respectively [53,56].Activated granulocytes protect the body from pathogens.Moreover,activated macrophages and DCs can release cytokines and interferons which activate cytotoxic T lymphocytes (Tc) and helper T lymphocytes (Th) and further provide an immunomodulatory response in the body’s defense against pathogens [2,53](Fig.2a).
It has been suggested that dietary administration ofβ-glucans is as effective as parenteral administration to potentiate systemic immunity and protect against pathogens [53].Detection of plasmaβ-glucan was reported in rats after oral administration of solubleβ-(1→3)(1→6)-glucan for 14 days [89].After two weeks of consecutive oral administration (5-6 mg/day),each rat absorbed approximately 30 ng.In another study,orally administered fluorescently labeledβ-glucan particles were detected 3 days after ingestion in spleen and lymph node macrophages in mice,as well as 4 days after ingestion in bone marrow macrophages,indicating migration of intestinal immune cells after they were exposed toβ-glucans.Glucan aggregates were found to be concentrated at the edges of the cytoplasm near the cell membrane and appeared to be the same size as the starting material [56,90].Xie et al.[84]observed that translocation of model glucan microparticles in rats to various organs of the reticuloendothelial system (RES) appears at 12 h and augments at 24 h with about 1.3% and 2.3% distribution of the particles to the four RES organs,liver,lung,spleen and kidney,in total.A recently published study by Zheng et al.[91]reported after intravenous administration of 32 mg/kg of fluorescenty labeled lentinan to rats that its circulating plasma levels decreased with apparent biphasic elimination.Tissue distribution showed that most of the administered lentinan was concentrated in the liver,followed by the kidney,spleen,heart,stomach,and intestine.The slow degradation of lentinan in the liver,recovered up to 7 days,was confirmed by liver perfusion methods and liver microsome assays,which indicated that CYP450 enzymes,mainly CYP2D6 and CYP2C9,and epoxide hydrolase are involved in the metabolic degradation of this polysaccharide in the liver [91].
Other studies using a cultured macrophage cell line examined the fate of labeled glucan added to the cells.These experiments showed that phagocytosed glucan was slowly degraded within cells and that soluble biologically highly-active fragments of glucan were begun to release after 4 days into the surrounding cells.Complete macrophage degradation of glucan required approximately 13 days [92].Fragmented and solubleβ-glucans bind to the receptors such as complement receptor 3 (CR3) present on innate immune cells [56].It is important to mention that smaller fragments might not bind/stimulate the same receptors as full-lengthβ-glucans.For example,fragmented/solubleβ-glucans with a backbone length below seven glucose units cannot bind to dectin-1 and cannot initiate clustering of dectin-1 in immunological synapses [56].Consequently,they are unable to activate the dectin-1 receptor [56].
Attention should also be paid to the synergistic effect of the extracts.Mushroomβ-glucan formulations are often based on a mixture of extracts obtained from different species.As an example,in the study on human macrophages,nine commercially available preparations from three mushroom species,G.lucidum,L.edodes,andG.frondosa,were analysed forβ-andα-glucan contents and three extracts were selected based on the highestβ-glucan contents.These extracts led to a dose-dependent increase in cytokine expression in both non-LPS and LPS stimulated macrophages,with a synergistic effect on the expression of IL-1α,IL-6 and tumor necrosis factoralpha (TNF-α) and an antagonistic effect on the expression of IL-10.A combined mushroom formula had EC50values lower than 100 μg/mL and even lower in TNF-α expression from LPS treated macrophages compared to the individual extracts,suggesting a potential synergistic effect of the mushroom formulas [93].
3.1.3 Selected oral clinical trials on the mushroom derived β-glucans in the supporting of the immune system
As therapy with mushroomβ-glucans has achieved success in preclinical animal models,investigations have been made to determine their therapeutic efficacy in human patients.These results mostly cover trials in cancer therapy,improvements in immune reactions,and lowering cholesterol.Among theβ-glucans obtained from mushrooms,lentinan and krestin are of special importance.In Japan and China,lentinan and krestin have been licensed as successful drugs since the 1980s [22].
Many clinical oral,intravenous or intramuscular studies have been conducted on the complementary use ofβ-glucans,e.g.,in conjunction with chemotherapy [22,92,94].Oral clinical researches are among the most sought after for the investigation of nutraceutical formulation efficacy.Verified results indicate relevant health benefits ofβ-glucans such as the overall disease-free survival of gastrointestinal cancer patients.An example is an investigation on krestin and its efficacy in the therapy against 21 gastric cancer patients which was implemented by Akagi and Baba [95].Patients were randomly assigned to receive 300 mg tegafur/uracil (UFT) alone or 3 g krestin together with 300 mg UFT daily for at least one year after surgery and immunological parameters were monitored and measured.Overall survival was markedly improved in the krestin group,with a 3-year overall survival of 62.2% compared with 12.5% in the krestin untreated group.This result was related to the observed reduction in CD57+T-cells,known to indicate a poor prognosis in patients with advanced gastric cancer.Moreover,a meta-analysis involving 1 094 patients with curatively resected colorectal cancer treated with krestin,showed an overall survival risk ratio of 0.71.The disease-free survival risk ratio was 0.72 [22].Another oral clinical trial on the safety and effectiveness of superfine dispersed lentinan (SDL) formulation in patients with advanced colorectal cancer was reported by Hazama et al.[96].Adverse events that were or were suspected to have been related to SDL were observed in 4 (5%) out of the 80 patients.All of these symptoms were grade 2 and disappeared or remitted within the study period.Regarding the onset of adverse events associated with chemotherapy,12 adverse events were observed in only 9 (14%) out of 64 patients who received chemotherapy [96].Among the 48 patients assessed for quality of life (QOL),the patients with low QOL scores before SDL treatment(n=23) reported a significant improvement in their QOL scores after 12 weeks of SDL administration.Moreover,the rates of lentinanbinding peripheral blood CD14+monocytes in the QOL-improved group were significantly higher than those in the QOL-not-improved group (P<0.05).SDL was confirmed as safe and effective for suppressing the adverse effects of chemotherapy as well as improving QOL.The binding ability of peripheral blood monocytes to lentinan appears to be a promising predictor of QOL improvement after SDL administration [96].In addition to studies on oral therapies,a more recent human clinical intramuscular injection study by Wang et al.[97]showed that lentinan based chemoimmunotherapy seems to be a promising strategy for antitumor activity via enhancing the proliferation of cytotoxic T cells (CD3+CD8+) and CD3+CD56+NKT cells,followed by the elevation of proinflammatory chemokines/cytokines (IFN-γ,TNF-α) and proinflammatory IL-12,which would then lead to a shift in the Th1/Th2 balance towards Th1.
One of the first studies on the therapeutic use of glucan for asthma involved anA.blazei-derivedβ-glucan in the treatment of a bronchitic patient with asthma-like symptoms [98].After oral supplementation for 60 days,the attenuation of bronchitic symptoms was observed accompanied by changes in Th2-dependent IL-10 and Th1-dependent IFN-γ production [98].Moreover,Jesenak et al.[99]conducted a double-blind,placebo-controlled,randomized,multicenter trial involving 175 children,for 6 months,with more than five respiratory infections to assess the effect of immunoglucan syrup formulation(P4H®) consisting of 10 mg pleuran and 10 mg vitamin C per mL.A higher proportion of the treated group compared to the placebo group did not suffer from any respiratory infection throughout the treatment.Furthermore,in the treated group,there were significant reductions in the frequency of flu and flu-like disease and the number of lower respiratory tract infections,as well as a statistically significant modulation of humoral and cellular immunity.The concentration of IgG and IgM increased during the treatment period and remained heightened throughout the study.Furthermore,IgA increased more in the treated group than in the placebo one.During the study,pleuran was also associated with an increase in NK cells and prevented the decline in CD8+T cytotoxic lymphocytes [99].Another randomized,double-blind,placebo-controlled study of the prevalence of atopy in a group of children with recurrent respiratory tract infections and the potential anti-allergic effect of pleuran (Imunoglukan P4H®)showed a significant reduction of peripheral blood eosinophilia and stabilization of the levels of total IgE in serum.Pleuran showed a potential anti-allergic effect,suggesting the use of mushroomβ-glucans as a substituent for corticosteroids [100].This effect was more evident in atopic subjects.
The latest most promising clinical trials in cancer research involve the use ofβ-glucans together with monoclonal antibodies.The advantages of combined therapy with monoclonal antibodies andβ-glucans offer possibility of an easier and more efficient elicitation of antibody response [92,101].In human studies,the tested and suggested daily dose ofβ-glucans remains in the range of 100–500 mg for stimulation of the immune system.For comparison,the lethal dose of 50(LD50) for lentinan,in mice and rats was found to be over 2 500 mg [92].
3.1.4 Safety profile of mushroom β-glucans
Mycoceutical formulations based onβ-glucans are generally considered safe.Consumption of concentratedβ-glucan was not associated with any obvious signs of toxicity [56,102].Adverse events and consequences of treatment with mushroom polysaccharidebased nutraceuticals were poorly reported and appeared to be rare.Gastrointestinal symptoms were the most common problem,which may be due to high levels of dietary fiber and prebiotics known to cause mild stomach upset such as bloating,abdominal pain,diarrhea,and constipation [22].Most major polysaccharides/β-glucans from medicinal mushrooms are known to have undergone extensive preclinical toxicological studies.For example,a polysaccharideprotein complex (PSP) isolated fromC.versicolorunderwent a wide range of preclinical toxicological tests,which demonstrated no effect on ovarian follicle development,steroidogenesis,oocyte quality,pregnancy,or embryonic development and showed no evidence of genotoxicity [16].Klaus et al.[103]confirmed the lack of toxicity of up to 10 mg/mL of the commercial water polysaccharide extract obtained from an industrial strain ofG.lucidum(Fujian,China).Furthermore,this extract stimulated theinvitroproliferation of HTR-8/SVneo cells,which are essential for normal placentation,the establishment of pregnancy,and maintaining fetal growth in humans.Moreover,according to the European Food Safety Authority(EFSA),the safety of Lentinex® as a novel food ingredient has been established in the specific proposed conditions of use and proposed intake levels: 2.5 mL Lentinex® containing 1mg lentinan/mL corresponding to 41.7 mg/kg body weight for a person of 60 kg [16].
3.1.5 Possibility of prebiotic action and cholesterol reduction of mushroom β-glucan formulations
Apart from immunomodulatory and antitumor effects,many other biological activities of mushroomβ-glucans have been reported,including cholesterol reduction and postprandial glucose metabolic activities [53].For example,dietary fibers can be used as a suitable therapy for the treatment of patients with dyslipidemia,as these are natural molecules that do not cause any significant side effects [104].These functionalities ofβ-glucans have been mainly attributed to their ability to form a viscous layer in the small intestine which hinders the resorption of cholesterol and bile acids [104,105].This leads to the excretion of bile acids in the feces and stimulates its neosynthesis from cholesterol in the liver [105].Increased bile acids activate the utilization of circulating cholesterol,thus reducing its level in the blood [104].β-Glucans may be more effective as they have a different mechanism of action than statins;while statins block the action of the liver enzyme responsible for cholesterol production,β-glucans promote physiologically based rebalancing of cholesterol levels [104].
Besides,β-glucans can reduce unwanted inflammation of the colon after microbiota-mediated fermentation into short-chain fatty acids [56].These fatty acids can function as bioactive compounds and exert a beneficial effect on specific intestinal bacteria associated with anti-inflammatory effects (Fig.3).Cosola et al.[106]reported a decrease in urinaryp-cresyl sulfate levels and an increase in shortchain fatty acid levels in feces after two months of oral administration of barleyβ-glucans in healthy volunteers,suggesting a saccharolytic shift in gut microbiota metabolism.Also,this nutritional treatment significantly reduced low-density lipoprotein (LDL) cholesterol and total cholesterol,as expected [106].A recent study of the modulation of human intestinal microbiota in a clinical trial by consumption of aβ-D-glucan-enriched extract obtained fromL.edodeswas provided by Morales et al.[107].A mixture,corresponding to 3.5 g/day of glucans,incorporated in three different commercial food creams,was given to hypercholesterolemic subjects,in a controlled,randomized,double-blind trial.After the eight-week intervention,no changes in lipid-or cholesterol-related parameters were reported in either subject receivingβ-glucans or placebo;no differences were also observed in the concentrations of inflammatory cytokines (IL-1β,IL-6,and TNF-α) or oxidized LDL,at the end of the intervention in the two groups.On the other hand,in the same trial,the mushroom mixture modulated the microbiota differently from the placebo,with cholesterol and dietary fiber intake being the most associated factors of the observed microbiota variations [107].
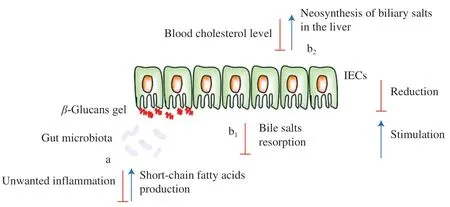
Fig.3 Possible effects of dietary intake of β-glucans on (a) inflammation of the colon and (b) cholesterol balance.
3.1.6 Cosmeceutical applications
β-Glucans are known to enter the stratum corneum and epidermis,penetrating deep into the dermis [108,109].They do not enter the cell directly but penetrate the skin through the intercellular space [108].β-Glucans can occur in many conformations and are typically extracted in the form of aggregate particles >1 μm which can be visible under a light microscope.Such large particles may not enter the skin and the effects ofβ-glucans were thought to be limited to the skin’s surface.In aninvitropenetration study,oat glucans consisting ofβ-(1→3,1→4) linkages in a backbone were subjected to submicron filtration to produce an aggregate-free,low-turbidity solution without particles visible under a light microscope [108].It was shown that purifiedβ-glucans penetrate the skin through the intercellular space [108].Such a process may be facilitated by a diffusion gradient and by lipid and phospholipid interactions [97].Therefore,it has been suggested thatβ-glucans form a thin film above the stratum corneum and epidermis to promote hydration and radioprotection.Within the dermis,they can boost collagen synthesis through direct interaction with macrophages to induce the production of IL-1 which indirectly promotes the production of procollagen by fibroblasts [108],(Fig.4).Moreover,a clinical study on 27 subjects performed to evaluate the effects ofβ-glucans on facial fine lines and wrinkles indicated a significant reduction of wrinkle depth and height,and overall roughness [108].
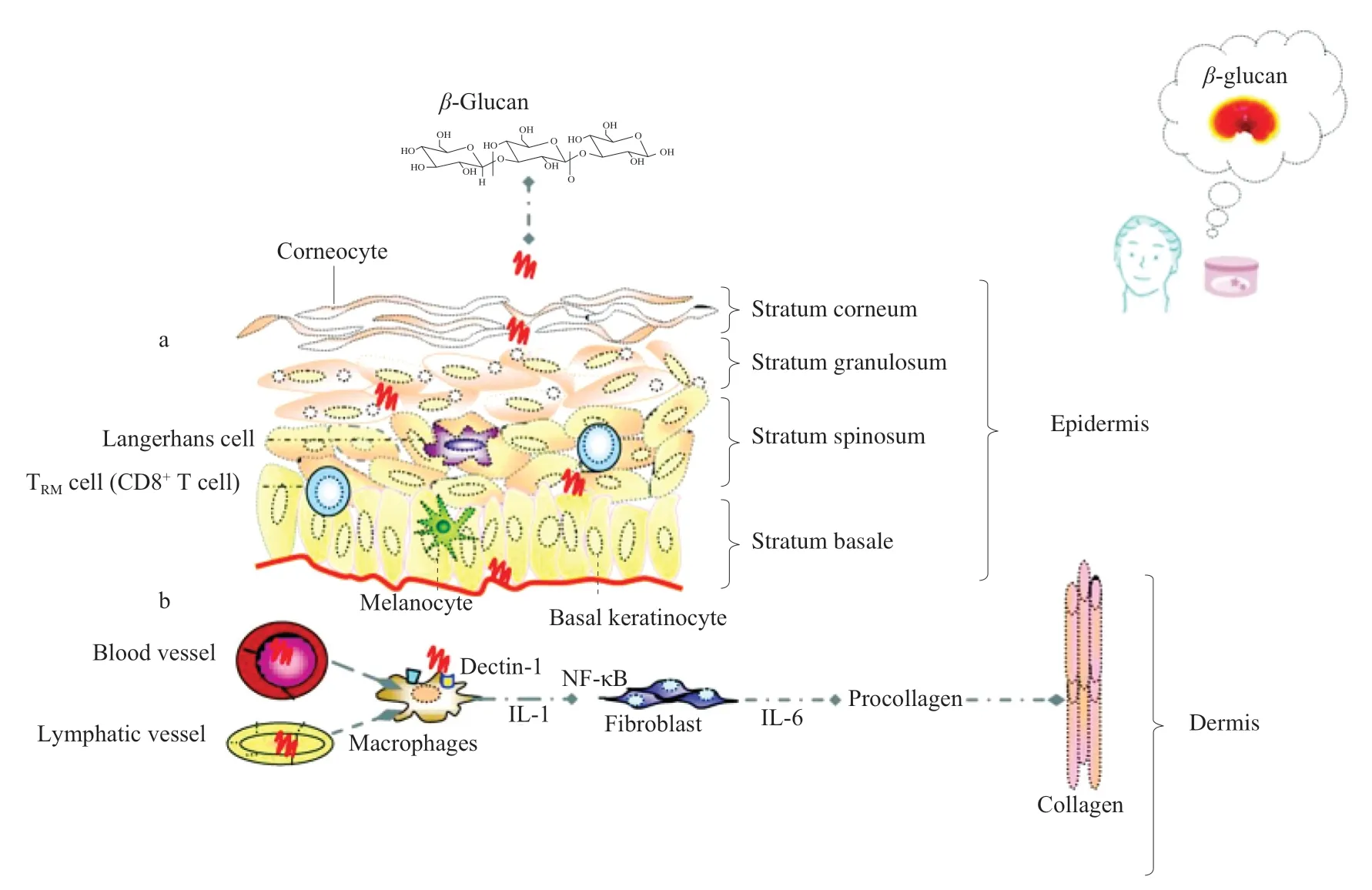
Fig.4 Skin structure and cells (a) above the stratum corneum and epidermis in the intercellular space β-glucans form a thin film that stimulates hydration;(b) within the dermis β-glucans are able to boost collagen synthesis through direct interaction with macrophages as well as through indirect,cytokine mediated interaction with fibroblasts.TRM represent CD8+ tissue-resident memory T cell.
The ability ofβ-glucans to enter the stratum corneum and epidermis,and to penetrate deep into the dermis offers a cosmetic alternative to other more invasive treatments aimed at reducing fine lines and wrinkles in the elderly population [108].Increased demand forβ-glucans extracted from mushrooms in nutricosmetic products has also occurred due to their pH neutrality [67,109,110].Trend analysis shows that earnings from cosmetics based onβ-glucans derived from mushrooms on the market are expected to reach about $20 million by 2026 with a CAGR of 8.1% over the forecast period [111].
3.2 Mushroom polyphenol formulations-nature of immunomodulation
Mushroom formulations rich in polyphenols are commercially available as extracts in capsule,pill or powder form.In the last few years,they have become more and more available in cosmetic formulations in the form of lotions and creams [50,112,113].Mushroom polyphenols are non-energy secondary metabolites,meaning they are not required for growth and reproduction [114-122].Mushrooms synthesize these compounds for protection against radiation,mechanical damage and microbial infection [18,123].Accordingly polyphenols content in mushrooms depends on the locality and environmental conditions of growth [114-122].Besides they possess the ability to absorb polyphenols from the substrate on which they grow or from neighboring plants [124].Depending on the extraction efficiency and type of extractant/solvent applied,different concentrations and profiles of polyphenols were reported in mushrooms and their commercial formulations [114-122,125].For example,Kim et al.[122]reported that phenolic compounds concentration analyzed in dimethylsulfoxide (DMSO) was greater in medicinal mushrooms than in edible mushrooms.The total average concentration of phenolic compounds was found to be 174 and 477 μg/g in edible and medicinal mushrooms,respectively.S.crispa,a medicinal mushroom,contained the highest total concentration of phenolic compounds (i.e.,764 μg/g),whileA.bisporuswas the edible species with the most phenolics (i.e.,543 μg/g).The mushroom species that contained the least number of phenolic compounds wereL.edodesandPleurotuseryngii,which had only three phenolic compounds each,whileSparassis crispacontained 15 among the 30 phenolic compounds [122].In another study by Janjusevic et al.[114],concentration of polyphenols inT.versicolorwater,ethanol and methanol extract quantified by the spectrophotometric method were reported to be 142.17,71.55 and 64.76 mg of gallic acid equivalents(GAE)/g dry weight (dw) of the extracts,respectively.Among polyphenolic compounds detected by high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS),p-hydroxybenzoic acid was observed in the highest amount in allT.versicolorextracts (ethanol-65 μg/g,methanol-184 μg/g and water-41.00 μg/g) [114].A variety of polyphenols have been found in mushrooms,[1,18,21,112-122].Although they are classified according to different characteristics,the structure of all polyphenols is based on an aromatic ring and at least one hydroxyl group [1,18,126].Different structural elements may be bound to this ring and to each other.The main phenolic compounds found in mushrooms are phenolic acids and can be divided into two major groups-hydroxybenzoic acids (HBA) and hydroxycinnamic acids (HCA) [1,126].HBA and HCA compounds are derived biosynthetically from the shikimate-derived phenylpropanoid pathway whichL-phenylalanine andL-tyrosine are the crucial building blocks.They are devoid of any nitrogen-based functional group in their basic structural expression [1,126].HBA derivatives in mushrooms commonly occur in bound form and are typically a component of a complex structure such as lignins and hydrolyzable tannins [127,128].They can also be found linked to sugars or organic acids.The prevalent HBA derivatives found in mushrooms are reported to bep-hydroxybenzoic,protocatechuic,gallic,gentisic,homogentisic,vanillic,5-sulphosalicylic,syringic,veratric,vanillin [1,127,128](Table 2).HCA derivatives are mainly present in bound form,linked to cell-wall structural components,such as cellulose,lignin,and proteins,as well as with organic acids,such as tartaric or quinic acids (i.e.,chlorogenic acids),through ester bonds.The majority of identified HCA derivatives in mushrooms arep-coumaric,o-coumaric,caffeic,ferulic,sinapic,3-o-caffeoylquinic,4-o-caffeoylquinic,5-o-caffeoylquinic (Table 2).The presence of ellagic and tannic acids is also observed [1,127,128].
Intensive research has been conducted over the last decade to determine the presence of flavonoids in mushrooms,another large group of phenolic compounds.Flavonoids (flavonols,flavones,flavanones,isoflavones,flavonols,and anthocyanins) are all structurally derived from the parent substance flavone consisting of two benzene rings (A and B) combined with a pyran one (C) [126].In nature,they are most often found in the form of glycosylate or esterificate conjugates.Scientists have reported various observations and conclusions regarding the metabolic entry point for flavonoid biosynthesis in mushrooms and the presence or absence of the enzymes chalcone synthase (CHS) and chalcone isomerase (CHI),as well as other enzymes involved in further biosynthesis steps [62,124,129].According to some research,the reported presence of flavonoids in mushrooms may be due to the ability of these organisms to absorb many nutrients and compounds from the substrate on which they grow or from neighboring plants by spreading their hyphae or forming mycorrhizae [124].Contrary to this observation,Mohanta [62]in his latest report on a genome-wide study across the genome sequences of the fungal kingdom shows the presence of all gene/protein sequences associated with the flavonoid biosynthesis,e.g.chalcone-flavanone isomerase (CHFI) inL.edodes.However,he pointed out that the presence of genes associated with the flavonoid biosynthesis pathway is not universal in fungi.Shao et al.[129]in the investigation of secondary metabolites in sixSanghuangporus sanghuangstrains,which are used for large-scale industrial production,confirmed the presence of genes involved in flavonoid biosynthesis.Seven core genes were found: phenylalanine ammonia-lyase (PAL),chalcone isomerase 1(CHI1),flavonoid-3’,5-hydroxylase (F3’5’H),F3’H-flavonoid-3’-hydroxylase (F3’H),flavanone-3-hydroxylase 1(F3H1),flavanone-3-hydroxylase 2 (F3H2),and flavonol synthase(FLS).No gene encoding CHS,the first enzyme specific for the flavonoid synthesis pathway in plants,has been found in theS.sanghuanggenome,suggesting a different catalytic mechanism for naringenin chalcone production in this fungus.Moreover,it was revealed thatS.sanghuangmay produce more flavonoids in the mycelial stages than in the fruiting body stages [129].The presence of flavonoids in mushrooms has been confirmed by using liquid chromatography-mass spectrometry (LC-MS),high-performance liquid chromatography-mass spectrometry (HPLC-MS),and ultrahigh performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS) [1,114,119-122,127,130,140].
3.2.1 Intestinal uptake
To confirm the biological activities of polyphenols in mushroom formulations after oral consumption,it is necessary to pay attention to their bioavailability.Many different factors affect bioavailability,such as liberation from matrix/extract formulation,expression of appropriate transporters that drive the transport of these compounds into the enterocytes and other cells,and presence of adequate metabolic enzymes [131].From a nutraceutical perspective,bioavailability is the rate and extent to which a bioactive compound is absorbed and made available at the site of action [131].After oral intake,dietary polyphenols are recognized by the human body as xenobiotics and only a small amount is hydrolyzed in the active compounds and absorbed in the small intestine [132].
It is known that polyphenols are absorbed in different rates through the GI tract.Certain chemical characteristics,for example,molecular weight,lipophilicity,stereochemistry,and the presence of a hydrogen-bonding group,affect the transport and permeability of the polyphenols into the cytosol enterocytes from the gut lumen.For example,gallic acid is easily absorbed.On the other hand,numerous phenolic compounds are absorbed at a rate of 0.3% –43% and the content of metabolites circulating in the plasma may be low [133].Similarly,the use of quercetin as a potential component for health promotion is inadequately effective due to reduced bioavailability as a result of low absorption rate,low water solubility,and increased instability in alkaline and neutral media including various organs,such as the small intestine,colon and kidney [133].Some phenolic components require structural modification for their absorption.The number of sugar molecules seems to play an effective role in phenol absorption.If they contain glucose,galactose or xylose,they will be absorbed through the small intestine by cytosolicβ-glucosidase/lactase phlorizin hydrolase [131].Otherwise,phenols that have rhamnose in their molecule cannot be absorbed.They are broken down by rhamnosidases produced by the colon microflora.Phenolic compounds are believed to be absorbed by a passive diffusion mechanism or by carriers present in the intestine,such asP-glycoprotein and sodium-dependent glucose cotransporters(SGLT1).These transporters are expressed on the cell membrane and transport phenols/drugs inside the cell [133].For example,aglycones pass through the epithelial cell membrane via passive diffusion,whereas on the other hand glycosides,esters and polymers cannot cross the membrane by passive diffusion [133].
Once adsorbed phenolic compounds are metabolized by deconjugation and reconjugation reactions in the liver;they are hydrolyzed to their free aglycones and then conjugated by methylation,sulfation,glucuronidation,or a combination of these reactions.The final compounds enter the circulation from the liver to other organs or through the blood to the urine.During this transit through various tissues and organs,they can perform their biological activities.There are often significant differences between the activities of the metabolic form of phenols and their form in the mushroom nutraceutical matrix [133].As an example,dihydoferulic acid,a reduced metabolite of the ferulic acid,expresses anti-inflammatory activity,opposite to metabolites derived after sulfation and glucuronidation,dihydroferulic acid-4-O-sulfate and dihydroferulic acid-4-O-glucuronide [134].Improving the bioavailability of mycoceutical polyphenols is essential to improving their bioefficacy.Several approaches to improving their bioaccessibility and bioavailability have been assessed nowadays.These include technological and chemical modifications of molecules to improve their solubility or site of absorption,the design of colloidal systems (micelles and vesicles),and the use of nanosystems/nanoparticles [131].
3.2.2 Interference between mushroom polyphenols and gut microbiota
When it comes to phenols,it is necessary to pay attention to their connection with gut microbiota (GM).Polyphenols that are not hydrolyzed and/or adsorbed in the small intestine accumulate in the lumen of the colon where they can be hydrolyzed by GM enzymatic activities into various metabolites before further absorption [132].Large interindividual variations in metabolites may be associated,at least in part,with differences in GM composition,and thus in the way polyphenols are catabolized.Accordingly,GM plays a key role in the maturation,development and function of the innate and adaptive host immune system,leading to the regulation of intestinal tissue homeostasis [132].Dietary polyphenols have a prebiotic-like effect,contributing to maintaining intestinal health and reducing inflammation levels by stimulating the growth of beneficial bacteria and inhibiting the development of pathogenic microbes.Among the important immunological parameters are macrophages which promote tolerance toward commensal flora,while allowing reactivity to pathogens.The imbalance of immune defense and tolerance between intestinal microbiota and macrophages can trigger dysbiosis and promote tumorigenesis [132].
3.2.3 Basic molecular mechanisms of immunomodulation
Numerousinvitroandin vivotrials include polyphenols that can modulate immune cell regulation,cytokine synthesis,and gene expression,in both innate and adaptive immune responses [18,21,59](Fig.5).
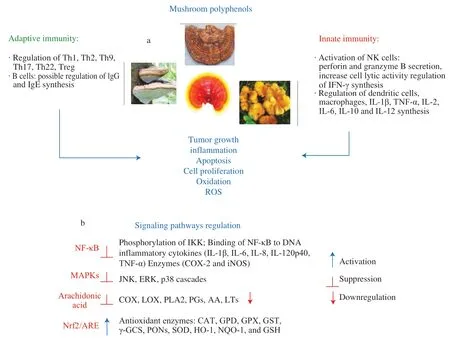
Fig.5 Possible immunomodulatory activities of polyphenols on (a) innate and adaptive immune systems;(b) signaling pathways regulation.
Mushroom formulations rich in polyphenols may influence multiple immunomodulatory processes involved in the inflammatory response and carcinogenesis.They have been reported to play a useful role in the prevention and process of chronic inflammatory diseases,e.g.bowel disease,allergic asthma and rhinitis,atopic eczema or dermatitis [1,21].Moreover,the concept of combined therapy of anti-cancer drugs with natural polyphenol formulations has become a very promising approach by reducing the dose-related toxicity and resistance to the drugs [132].The immune system is also responsible for self-tolerance,by which host tissues are protected from immunological action.Polyphenols have been shown to play a beneficial role in some common autoimmune diseases e.g.rheumatoid arthritis and multiple sclerosis by regulating signaling pathways,suppressing inflammation and limiting demyelination [21].It is important to note that the main difficulty in elucidating the immunomodulatory effects of polyphenols on health is the large number of phenolic compounds found in food and nutraceutical formulations,which give different biological activities [135].
When it comes to the effect on immune cells,there are different pathways in the integrated immunomodulatory polyphenol response [21,59,136,137](Fig.5b),such as: (1) nuclear NF-κB signaling pathway to regulate the accumulation and degradation of kappa inhibitorB(IκB) protein,phosphorylation of NF-κB,hyperphosphorylation of IκB kinase complex (IKK),and processing of NF-κB precursors,leading to suppression of various inflammatory cytokines expression,e.g.IL-1β,-6,-8,-12p40,TNF-α and enzymes such as cyclooxygenase-2/prostaglandin synthase-2 (COX-2) and inducible nitric oxide synthase (iNOS);(2) MAPKs play a key role in a number of fundamental cellular processes such as cell growth,proliferation,death and differentiation,and also regulate gene transcription and transcription factor activities involved in inflammation.Polyphenols can regulate the activity of different groups of MAPKs expressed in mammals i.e.extracellular signalregulated kinase (ERK)1/2,c-Jun amino-terminal kinases (JNK1/2/3),p38-MAPK (α,β,δ,γ) and ERK5;(3) arachidonic acid (AA)signaling pathway by the reduction of the release of inflammatory mediators: AA,prostaglandins (PGs),and leukotrienes (LTs).Their action is mainly due to their ability to inhibit cellular enzymes,such as phospholipase A2 (PLA2) responsible for AA release,the cyclooxygenase family (COX-1,-2,-3) and lipoxygenase (LOX)which metabolizes AA and produce PGs and thromboxane A2(TXA2),and hydroxyeicosatetraenoic acids and LTs;(4) nuclear factor (erythroid-derived 2)-like 2/antioxidant response element (Nrf2/ARE) signaling pathway.Nrf2/ARE is involved in the activation of genes that encode antioxidant enzymes such as superoxide dismutase(SOD),heme oxygenase-1 (HO-1),glutathione S-transferases(GST),and catalase (CAT) leading to the inhibition of ROS production [21,59,136,137].
Dietary polyphenols affect macrophages,dendritic cells,different types of Th cells,Th1/Th2 balance,and NK cells.As an example,Zhang et al.[138]in the study ofInonotus sanghuangmushroom known for its medicinal value,rich in rutin,quercetin,quercitrin,isorhamnetin,and chlorogenic acid,have been shown suppression of the interaction between RAW264.7 macrophages and 3T3-L1 adipocytes,attenuations of chronic inflammation in adipose tissue and improvement of obesity-related insulin resistance and complication.It was observed thatI.sanghuangcan be mediated through inhibition of ERK,p38-MAPK,and signal transducer and activator of transcription 3 (STAT3) factor.Moreover,it has been suggested that it may improve insulin resistance and metabolic syndrome in this way [138].In recent years,there has been a growing emphasis on the importance of the immune response against cancer and the search for therapies that can interact with the immune system to eliminate tumor cells [18].In this context,mycoceutical formulations of polyphenols may emerge as adjuvant therapies.Kang et al.[139]conducted a placebocontrolled,double-blind,and parallel clinical trial of 79 healthy men randomized to the oral intake of 1.5 g/day ofCordyceps militarisethanol extract (two capsules per dose,twice daily) or placebo for 4 weeks.After the attempt,the mushroom group showed increased levels of IFN-γ and IL-2 compared to baseline and placebo.However,IL-12 and TNF-α did not differ after the experiment.In the same research results fromex vivoassay of the peripheral blood mononuclear cells (PBMCs) cytotoxicity in the K-562 cell line showed increased NK-cells activity and lymphocyte proliferation compared to baseline in the treated group.Accordingly,C.militarisincreased NK cell activity and lymphocyte proliferation and partially increased Th1 cytokine secretion.Ethanol extract of this mushroom can be expected to be useful for immunocompromised patients with malignancies,chemotherapy or radiation therapy,viral infection such as AIDS,influenza or chronic viral hepatitis,or bacterial infection,long-term steroid therapy,or chronic dysfunctions of organ systems,such as congestive heart failure,chronic kidney disease,chronic obstructive pulmonary disease or diabetes mellitus,as well as for those whose immunity is naturally reduced [139].In the study of polyphenol-rich medicinal mushroom preparations Agarikon and Agarikon Plus,on colorectal (HCT-116,SW620) cell linesinvitroand colorectal mice model (CT26.WT)in vivo,a significant antiproliferative and proapoptotic effect was observed [125].In the same study,the effect on the human fibroblast cell line (WI-38) was proliferative,emphasizing the specificity towards tumor cell lines.The antitumor effects of the tested formulations,with or without 5-fluorouracil,were shown to be based on improved polarization of M1 macrophages,inhibition of M2 and tumor-associated macrophage (TAM) polarization,effects on T helper cell -Th1/Th2/Th17 cytokine profiles,direct inhibition of CT26.WT tumor growth,inhibition of vascular endothelial growth factors (VEGF) and metalloproteinases 2 and 9 (MMP-2 and MMP-9)modulation.In addition,the administration of Agarikon and Agarikon Plus did not show a genotoxic effect [125].
As already mentioned,mushrooms are a rich source of HCAs [113,140].HCAs have the potential to regulate inflammatory skin disorders,as well as atopic dermatitis and photocarcinogenesis.In addition to corticosteroids,they are applied as alternative natural additives in cosmeceutical formulations [50,113].Taofiq et al.[113]summarized some of the most interesting anti-inflammatory studies,bothin vivoandinvitro,regarding HCAs and derivatives such as ferulic acid,p-coumaric acid,caffeic acid and its phenethyl,butyl and octyl esters,as well as rosmarinic and chlorogenic acid.The anti-inflammatory mechanism is attributed to the ability of these compounds to inhibit the NF-κB pathway,block the activation of the AP-1 transcription factor,suppress the secretion of PGE2,and expression of IL-1β,IL-6,TNF-α,COX-1 and COX-2,iNOS,vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1).One of the clinical studies on HCAs was provided by Lee et al.[141].They evaluated the potential of rosmarinic acid-based emulsions in twenty individuals between the ages of 5 and 28 years to reduce the severity of atopic dermatitis.The cream was applied twice a day,for eight weeks,and then the condition of the skin was assessed.Significant improvement of the disease from erythema and dryness to other common symptoms of atopic dermatitis has been observed,thus showing the therapeutic benefits of rosmarinic acid as an alternative against the disease.HBAs and HCAs are also reported as effective in the modulation of the Nrf2 pathway.In the review of Zhou et al.[137],some antioxidant studies were reported,bothin vivoandinvitro,on gallic,ferulic and caffeic acid.The potential of signaling pathway modulation was attributed to the ability of these acids to increase Nrf2 nuclear translocation and target protein expression,including HO-1,glutamate-cysteine ligase (GCLC),and glutathione (GSH).Moreover,a novel possible approach to modulatating the immune system and preventing autoimmunity or transplant rejection is the activation of cytoprotective and antioxidant enzymes such as HO-1.HO-1 is a key protein in the cell stress response and its upregulation is a common event during pro-inflammatory conditions [142].After being expressed by the regulator proteins,HO-1 affects downstream elements such as heme,bilirubin and carbon monoxide (CO) [142].It has also been suggested that polyphenols may modulate B cell function [21].In aninvitrostudy on U266 myeloma cells,polyphenolic compounds with gallate groups were observed to reduce IgE production dose-and timedependent manner,without mediated apoptosis or cell death [143].Another study showed that polyphenols inhibited the proliferation of CD19+cells and reduced IgG production by the PBMC cells [144].However,this is poorly described among polyphenols in mushroom formulations,and further research is required.
3.2.4 Interactions with cellular receptors
It is important to mention that each type of polyphenol can target and bind to one or more proteins and/or receptors on immune cells and thus triggers intracellular signaling pathways that ultimately regulate the host immune response [126].Manyinvitrostudies have shown that proteins (amino acids) can bind to polyphenols and provide a systems view of a wide variety of biochemical processes affected by these compounds,from central metabolism to signaling events[145,146].This binding is mainly non-covalent and may subsequently be stabilized by hydrogen (H) bonding [146].Non-covalent binding includes hydrophobic and van der Waals interactions,ionic interactions,and H bridge/bonding,and it is always reversible [146].These interactions can influence protein structure leading to a possible change in the protein folding and its functionality,which triggers or inhibits intracellular signaling pathways [146].For example,Lacroix et al.[145]in a databases study of the polyphenol-protein interactome reported 61 unique polyphenols that can interact with nineteen subclasses of the TNF receptors.Besides it was reported that kinases are also major targets of polyphenols because they have the potential to bind to ATP-binding sites thereby modulating the action of MAPK,phosphoinositide 3-kinase (PI3K),Akt/protein kinase B(Akt/PKB),tyrosine kinases,and protein kinase C (PKC) pathways.Inhibiting or stimulating these pathways influences phosphorylation events and modulation of gene expression [145].The fact that various polyphenols interact with cyclin-dependent kinases (CDKs)highlights their potential control of cell cycle events,including cell proliferation and cancer development [145].Major proteins involved in diabetes,such as AMP-activated protein kinase (AMPK),peroxisome proliferator-activated receptors (PPARs),dipeptidyl peptidase 4 (DDP-4),and others have been reported to interact with polyphenols,suggesting a potential therapeutic application for natural bioactive in the modulation of metabolic diseases [145].Furthermore,mushroom polyphenols can inhibit PI3K which are involved in the regulation of cellular functions such as growth and survival,aging and malignant transformation [126].PI3K itself possesses oncogenic activity and also forms complexes with some viral or cellular oncoproteins (src,ras,rac,alb,T-antigen),whose transformational activities are realized only in presence of PI3K [126].Moreover,polyphenols can target receptors that are expressed on immune cells and allow the transmission of stimuli to activate processes within the cellin vivo[126,145].It has been observed that compounds with gallate groups present in mushrooms [1,126,127]can target different cellular receptors e.g.67 kDa laminin receptor (67LR) and zeta chain-associated 70 kDa protein (ZAP-70).67LR is expressed by neutrophils,monocytes/macrophages,mast cells and T cells and regulates the adhesion and inflammatory processes of these cells.These compounds can inhibit the activity of ZAP-70 by inhibiting the T cell-induced pathway mediated by CD3 in leukemic cells.Another example is bacalein,flavones,which targets receptors on T and B cells such as TLR 4,T cell receptor (TCR) αβ,and IgM-(sIgM-)B-cell receptor [126].Janjusevic et al.[114]reported that baicalein was among the most prevalent flavonoids detected in ethanol,methanol and water extracts ofT.versicolor.Accordingly,baicalein can regulate innate and adaptive immune systems by regulating these immune receptors.
3.2.5 Safety profile and interference with drug metabolism
The potential interference of mushroom formulations rich polyphenols with drug metabolism is one of the important facts that should be taken into account when using them [145,147].Polyphenols can induce certain enzymes that metabolize drugs and thus affect the metabolism of important therapeutic agents [147].Polyphenols are substrates of enzymes such as cytochrome P450 monooxygenases (CYPs) and phase II conjugation enzymes,as well as of transporters involved in the deactivation and excretion of many drugs and xenobiotics [145,147].Accordingly,they share the same metabolic pathways with many therapeutic drugs.Numerous studies have demonstrated inhibition of various CYPs and drug transporters by polyphenols which have been described to interact and inhibit CYPs with mechanisms similar to those of single-target drugs [145].Among mushroom formulations,the data for CYPs inhibition was reported forA.blazeiextract [148].Invitroherb interaction studies with this extract,demonstrated a very low inhibition of CYP metabolism,less than that for green tea,making clinically relevant adverse effects unlikely [148].It is important to mention that effects of some metabolites,in a modulating of the immune system,can vary depending of the patient genetic predisposition and medical condition.Accordingly,side effects of polyphenol formulations may differ from person to person or with disease type,and consequently cannot be easy to document [149].
Likewise,one of the essential aspects of polyphenols supplementation is its potential prooxidative effect [150].Besides immunomodulating properties and being well-known antioxidants,polyphenols may possess prooxidative capacity based on their concentration,structure and cellular redox context which may include increased levels of oxidant scavenging proteins or decreased levels of oxidized proteins and lipids [128,133,150].In recent years,the potential toxicity of some polyphenols,such as catechins,to the DNA of mouse spleen cells has been reported.In this regard,cytotoxicity studies have shown that polyphenols may play a positive role in the safe concentration range.In addition,compounds with gallate groups were found to have higher potential toxicity than compounds without gallate groups.However,the concentration and structure of polyphenols are not the only determinants,and their prooxidative properties may be also related to synergistic effects and exposure time [133].
4.Synergistic effect of mushroom β-glucans and polyphenols
Many studies have found that polyphenols can interact with macromolecules like carbohydrates,i.e.β-glucans [146,151,152].It has been shown that these interactions can affect the bioaccessibility of polyphenols in a food matrix as well as in nutraceutical formulations [146,153].Therefore,β-glucans have an impact on polyphenols’ immunomodulating activities if they were applied together in commercial formulations.The main types of interactions involved in the complexation mechanism are non-covalent bond formation and hydrophobic interactions [146,153].β-Glucans have many OH groups in their structure,which gives them the possibility of noncovalent interactions (H bonds and van der Waals interactions) [153].H bonds are formed between OH groups ofβ-glucans and polyphenols as well as between OH groups of polyphenols and oxygen (O) atoms of the glucan glycosidic linkages [146,153].When H bonds are created first,the distance between polyphenol and dietary fiber molecules can become shorter which than allows van der Waals interactions [153].The structural and conformational organization ofβ-glucans could also play an important role in these interactions [153].They can be linear molecules or branched molecules with cavities/hydrophobic pockets and with various pore sizes [153].Accordingly,polyphenols can bind to the surface of these molecules or can penetrate into these pores as suggested for interactions between quercetin andβ-glucan [151,153].For polyphenols,it seems that the presence of OH,CH3and galloyl groups,sugar molecules on the aglycon,the flexibility and number of phenolic rings,and the molecular size and spatial configuration all affect polyphenols/β-glucan interactions [146].Furthermore,their hydrophilic or hydrophobic character is important [146].
All these interactions can change polyphenols’ bioaccessibility from nutraceutical formulation and bioavailability,affecting the actual amount that can be absorbed in the intestine and detected in plasma.Unabsorbed polyphenols can be carried byβ-glucans,reach the colon,where they might be released by the action of bacteria and exhibit potential beneficial effects [146].Besides,in a colon,their catabolites/metabolites might be absorbed.Accordingly,there are several different effects arising from the transport of polyphenols to the colon: (1) the release of polyphenols from their associated molecules/β-glucans can enhance their bioaccessibility in the colon,(2) polyphenols andβ-glucans can positively affect the growth of colon microflora,(3) microorganisms of the digestive tract can metabolize released polyphenolic compounds,(4) metabolites can exhibit various positive effects,and (5) polyphenols and their metabolites,in general,can create a positive bioactive environment in the colon [146,152,153].Reported activities of the polyphenol metabolites are anti-inflammatory activities,detoxification processes and phytoestrogenic activities [146].Most of the positive role relates to the protection against the risk of development of colorectal cancer [146].β-Glucans might be a mechanism for controlling the amount of bioaccessible polyphenols in the upper or lower parts of the digestive tract.
5.Conclusions and future directions
This review describes the most used mushroom ingredients,β-glucans and polyphenols,and their nutraceutical and cosmeceutical formulations in the world market which are used to boost and balance immunity.Their mechanisms of action,as well as bioavailability,metabolic transformations,preclinical and human clinical research,and safety,are highlighted.In recent years wellness and cosmetic companies are showing an increase interest in using these ingredients.
A promising application is observed on mushroomβ-glucans.They affect the immune system,and many of their clinical effects may be based on this.Oral applications are focusing on cancer treatment,as mushroom extracts rich inβ-glucans showed anti-tumor effects.The latest clinical trials in cancer research involve the use ofβ-glucans together with monoclonal antibodies.Studies on oral therapies for asthma and respiratory tract infections among adults and children revealed their application as bronchodilator medications.The ability ofβ-glucans to enter the stratum corneum and epidermis,and to penetrate deep into the dermis offers a cosmetic alternative to other more invasive treatments aimed at reducing fine lines and wrinkles in the elderly population.
Widespread structural diversity of polyphenols was observed in mushrooms and their commercial formulations.Different observations and conclusions were reported by the scientists in relation to the metabolic entry point to flavonoid biosynthesis in mushrooms and the existence or absence of enzymes involved in their biosynthesis steps.Mushroom polyphenols may influence multiple immunomodulatory processes involved in the inflammatory response and carcinogenesis.They can be useful for immunocompromised patients with malignancies,viral or bacterial infection and chronic dysfunctions of organ systems as well as for those whose immunity is naturally reduced.Moreover,a novel possible approach of polyphenols to modulate the immune system and prevent autoimmunity or transplant rejection is the activation of cytoprotective and antioxidant enzymes such as HO-1.Cosmeceutical formulations rich in mushroom polyphenols have the potential to regulate inflammatory skin disorders,as well as eczema or atopic dermatitis and photocarcinogenesis.In addition to corticosteroids,they are applied as alternative natural additives in cosmeceutical formulations.Concerning the dietary application of mushroom polyphenols,it is necessary to mention that after oral consumption they are recognized by the human body as xenobiotics and often a small amount is hydrolyzed into active compounds and absorbed in the intestine.Likewise,there are significant differences between the activities of the metabolic form of phenols and their form in the mushroom nutraceutical matrix.Accordingly,the modern focus is on improving and increasing their bioavailability by designing colloidal systems and using nanosystems.One of the disadvantages in the commercialization of polyphenol-based formulations is quantitative and qualitative variations in the polyphenol content in mushroom species.As secondary metabolites with a protective role against radiation,mechanical damage,and microbial infection,their content in mushrooms depends on the locality and environmental conditions of growth.Besides,mushrooms possess the ability to absorb polyphenols from the substrate on which they grow.Using extracts or preparations that are based on mycelia grown under controlled conditions may be the solution for the reduction of the natural variability in polyphenol composition.
β-Glucans might be beneficial for controlling the number of bioaccessible polyphenols in the upper or lower parts of the digestive tract.Polyphenols can interact withβ-glucans by complexation mechanism which includes non-covalent bond formation and hydrophobic interactions.Unabsorbed polyphenols from the small intestine,carried byβ-glucans,can reach the colon,where they might be released and metabolized by the action of bacteria and show potential beneficial effects as well as subsequent modulation of postprandial lipid metabolism and anti-inflammatory activities.
Considering the presence of mushroom extracts rich inβ-glucans and polyphenols in the nutraceutical and cosmeceutical sector to strengthen and balance immunity,all this integrated information regarding complete aspects of functionality will reduce or fill the gap in their application among complementary and integrative medicine and conventional medicine.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgment
This review was the result of research within the “Agreement on the implementation and financing of scientific research work in 2022 between the Faculty of Agriculture in Belgrade and the Ministry of Education,Science and Technological Development of the Republic of Serbia”,contract record number: 451-03-68/2022-14/200116,451-03-68/2022-14/200051 and supported by the Science Fund of the Republic of Serbia,#Grant No: 7748088,“Composite clays as advanced materials in animal nutrition and biomedicine-AniNutBiomedCLAYs”.
杂志排行
食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil
- Structural characteristics,anticoagulant and antithrombotic mechanism of a novel polysaccharide from Rosa Chinensis Flos
