Biological factors controlling starch digestibility in human digestive system
2023-01-21ChngLiYimingHuSongnnLiXurYiShuioShoWnwnYuEnpngLi
Chng Li,Yiming Hu,Songnn Li,Xur Yi,Shuio Sho,Wnwn Yu,Enpng Li
a School of Health Science and Engineering,University of Shanghai for Science and Technology,Shanghai 200093,China
b Joint International Research Laboratory of Agriculture and Agri-Product Safety,the Ministry of Education of China,Institutes of Agricultural Science and Technology Development of Yangzhou University,Yangzhou 225009,China
c Department of Pathology,Zhongshan Hospital,Fudan University,Shanghai 200031,China
d Department of Food Science&Engineering,Jinan University,Guangzhou 510632,China
e Jiangsu Key Laboratory of Crop Genetics and Physiology / State Key Laboratory of Hybrid Rice,College of Agriculture,Yangzhou University,Yangzhou 225009,China
Keywords:Starch digestion Human gastrointestinal tract Biological factors Gastric emptying rate Gut microbiota
ABSTRACT Starch digestion rate and location in the gastrointestinal tract (GIT) are critical for human health.This review aims to present a comprehensive summary on our current understanding of physiological,biochemical,anatomical and geometrical factors of human digestive system that are related to in vivo starch digestibility.It is shown that all digestive compartments including mouth,stomach,small intestine,and large intestine play critical roles in regulating the overall starch digestion process.A proper investigation of starch digestion pattern should thus be based on the consideration of all these compartments.Main biological factors are summarized as oral mastication and salivation,gastric emptying and motility,small intestinal enzymes and motility,large intestinal resistant starch (RS)-microbiota interactions and gut-brain feedback control,as well as glucose adsorption and hormonal feedback control.However,connections among these biological factors in determining starch digestive behaviors remain elusive.This is due to the inherent complexity of human GIT anatomy,motility and biochemical conditions,as well as ethical,financial and technical issues in conducting clinical studies.Much technological and scientific efforts from both clinical studies and in vitro simulation models are required for a better understanding of in vivo starch digestion behaviors.
1.Introduction
Starch is the main macronutrient in our daily foods,which supplies 50% of our daily energy.Its digestion and absorption in our multi-compartmental gastrointestinal tract (GIT) are initiated from mouth and continuously through the stomach,small and large intestine [1].The starch digestion rate and location in the GIT have critical impacts on the postprandial metabolism and human health [2].If the starch portion passes the small intestine and enters the colon,it could be fermented by the inhabited gut microbiota into short-chain fatty acids (SCFAs),which are the energy source for the colonocytes and beneficial for a healthy gut [3].This starch portion is commonly referred as resistant starch (RS).On the other hand,the starch portion digested rapidly in the duodenum could be referred as rapidly digestible starch (RDS).It contributes to the large fluctuation of postprandial blood glucose level and should be avoided by patients with obesity and type 2 diabetes [2].Compared to the RDS,the starch portion that is digested and absorbed slowly through the duodenum to ileum is normally referred as slowly digestible starch (SDS) [4].SDS can provide more sustainable energy supply and has a relatively moderate influence on the postprandial glycemic response compared to the RDS.In this respect,SDS is an ideal starch portion for the type 2 diabetic patients [5].
In vivobiological factors related to the starch digestibility still remain elusive.This is mainly because that conducting human clinical trials is ethically and technically challenging [1].In vivostudies are also relying on expensive instruments such as nuclear magnetic resonance and ultrasonic scanner.Invasive techniques such as gastric barostat and intraluminal manometry are sometimes needed.Therefore,there are still many “unknowns” that are determining starch digestion from the GIT.It is noted that some starch digestion patterns have been learnt fromin vitrodigestion models [6-12],which are efficient,cheap and without ethical restrictions compared to human clinical studies [1,10].However,there are significant differences between thesein vitromodels with actual human conditions,in terms of GIT morphologies,biochemical conditions,and hormonal controls [1].These factors are playing critical roles in determining the starch digestion rate and location.Thus,reliable predictions of the extent and rate of starch digestion in human are still extremely difficult and often deficient byin vitrodigestion models.
This review aims to present a systematic summarization on the current understanding of human GIT tract and biological factors controlling the starch digestion property.GIT compartments including mouth,stomach,small and large intestine are discussed.It could serve as the basis for a better understanding of bothin vivoandin vitrostarch digestive characteristics,and help the development of new generation of foods with better manipulation of the starch digestion property.
2.Oral processing
After food ingestion,oral processing is the first step involving a mechanical process that breaks foods into particles,which are simultaneously hydrated by the saliva into food bolus.Although it is a rapid process,the oral processing step has an important role on the overall starch digestion process [13-15],mainly through two complementary steps,including mastication and salivation.
2.1 Mastication
The mastication phase maintains until the food particle size,water uptake and amount of saliva are sufficient to develop the food bolus with desirable rheological properties to be swallowed [13].The main function of mastication is to reduce the size of solid foods through mechanical cutting,crushing,grinding,compressing and shearing food into fragments (Table 1).Whereas,this process is negligible for liquid foods.Based on the textural characteristics of ingested solid foods,the residual fragments are normally with a particle size around 0.8–3.0 mm when the food bolus is formed;this size is rather similar among different subjects [16]and frequently referred as swallowing threshold to trigger the swallowing action [14].Mastication is thus able to increase the effective surface area of solid food particles for the access of digestive enzymes.It has been tested bothin vivoandin vitrothat larger particle sizes of cereal grains have lower starch digestibility and glycemic responses compared with milled fractions [17].The lack of mastication efficiency causes larger food particles remained in the bolus and increases the intactness of food architectures such as plant cell walls and protein matrices,which influence largely the bio-accessibility of starch and other macronutrients [17].For example,in elderly populations,the swallowable bolus always has a higher proportion of large food particles and a lower degree of food structural breakdown [18].
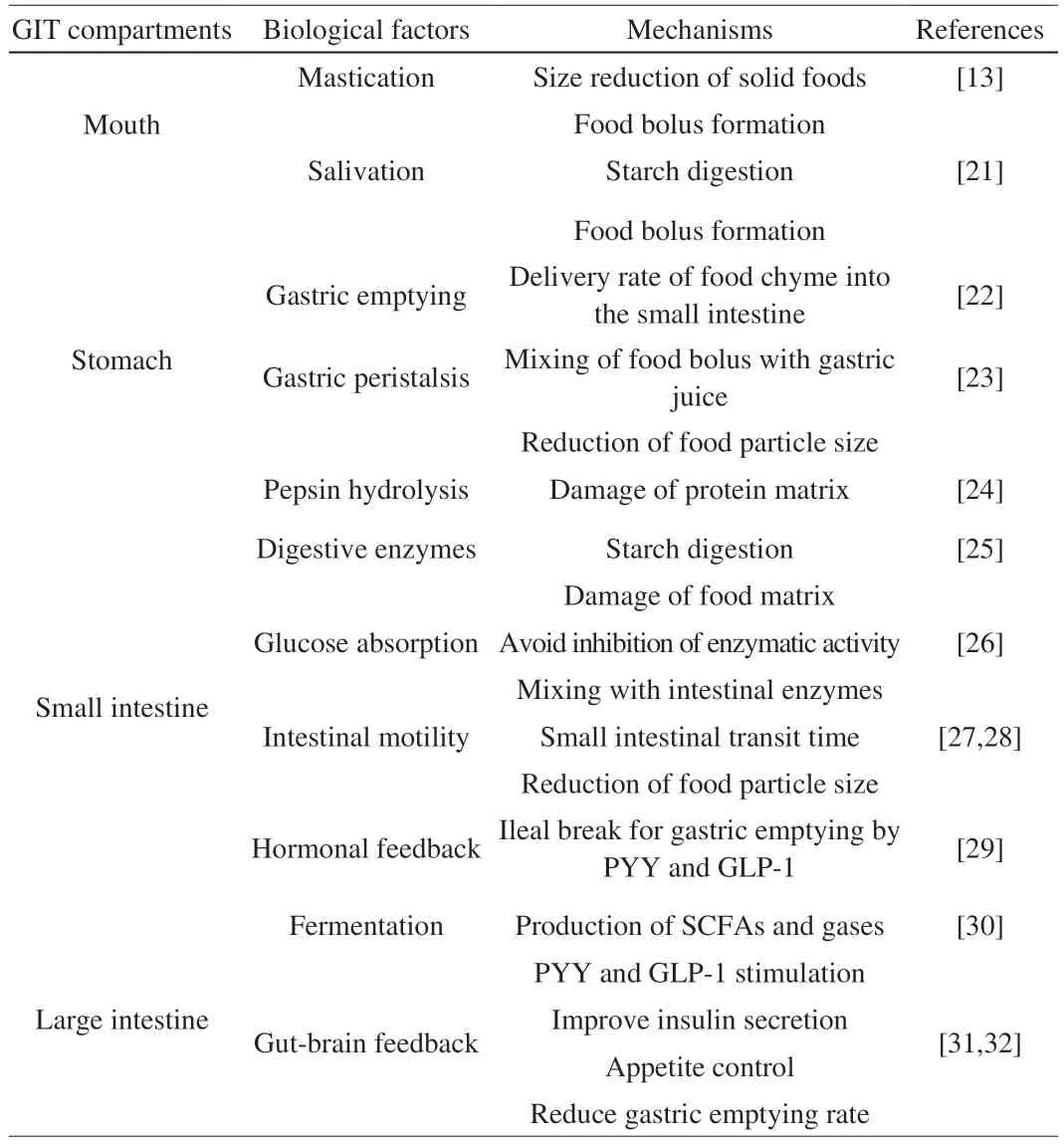
Table 1 Biological factors affecting the in vivo starch digestibility and the possible mechanisms.
Mastication characteristics including the number of cycles,masticatory frequency,muscular forces and velocities,are varied significantly among individuals [19].It could at least partially explain the significant variance of starch digestibility observed for the same food among different individuals [20].
2.2 Salivation
Salivation is a simultaneous process with mastication during oral processing.The saliva is produced by salivary glands,which contains 99.5% of water with various electrolytes including sodium and potassium,and 0.3% of proteins [33].The composition and flow rate of saliva are modulated by many intrinsic and extrinsic physical,chemical,psychological,and physiological factors,e.g.,the food texture and stimuli such as acid.The volume of saliva incorporated during chewing could vary from 0.4 to 1.7 mL prior to swallowing [34]with flow rates in the range of 0.3-0.5 mL/min [35].
The main enzymes related to starch digestion during salivation is salivaα-amylase,which breaks down starch into maltose,maltotriose,andα-limit dextrins by cutting theα-1,4 glycosidic linkages(Table 1).It has been shown that the amylase in mouth substantially lower starch viscosity in seconds [21].It is known that viscosity is a critical factor in determining the gastric emptying rate of starchy bolus [36,37].The salivaryα-amylase can also frequently survive from the strong acidic conditions in stomach and still show some activity in the small intestine [38].
3.Gastric processing
After oral processing,ingested food is further mixed with gastric juices containing acid,pepsin,lipase and salts,with a pH value around 2 [25].The main body of stomach is anatomically divided into four sections,including the fundus,body,antrum and pylorus.The fundus and body mainly act as the reservoir for the ingested food bolus,while the antrum is functioning as a grinder and mixer contributing to the reduction of solid food particles to pass the pyloric sphincter into the intestine.Food particles are thus degraded both physically and chemically by the peristalsis of stomach walls and the acidic/enzymatic reactions,respectively.
3.1 Gastric emptying rate
Gastric emptying rate is a critical determinant of thein vivostarch digestibility and postprandial glycaemia (Table 1).Lower gastric emptying rate would result in a slower delivery of food into the small intestine [22],where most of the starch is digested and absorbed.However,a slower gastric emptying may increase the degree of food damage by acidic/enzymatic hydrolysis and peristaltic contractions [39],and once these foods are released into the small intestine a faster degradation by intestinal enzymes could be reached.In addition,depending on the specific pH condition of the stomach,salivaryα-amylase may still be present for up to 15-30 min into gastric digestion and even reach to the small intestine without being inactive [40].Hence,there is still some sort of starch digestion that may occur in the stomach due to the presence of salivaryα-amylase.
The relationship between gastric emptying rate and postprandial blood glucose levels (that is related to the digestion and absorption rate of starch molecules) is bidirectional.Gastric emptying rate can contribute to~35% of the peak variance of postprandial glycaemia,while the gastric emptying rate itself is regulated by the acute changes of the glycaemia [41].It has been reported that even minor differences from the gastric emptying rate can cause a significant variance on the postprandial glycaemia [42].On the other side,the gastric emptying rate is slowed down reacting to the hyperglycaemia and accelerated in order to mitigate the hypoglycaemia [43,44].
3.2 Gastric motility
Gastric motility is another biological factor determining starch digestibility,as it can change the pressure,specific flow profile and force encountered by the starch-contained food bolus (Table 1).The contractions move the food bolus towards the pylorus with a propagation velocity of~2.5 mm/s and forces ranging from 0.2 to 1.89 N depending on the fed or fasting state of stomach [45].The chyme is then propelled back to the stomach main body through the retropulsion,which causes the drastic mixing and emulsifying of the food particles by the gastric juices.The food bolus is finally ground into particles with a diameter less than 2 mm by the contractions.These small food particles then enter the small intestine in a pulsatile fashion against the pyloric resistance [29].
3.3 Pepsin hydrolysis
As commonly observed in the literature,protein component within the food system could act as physical barriers for both thein vivoandin vitrodigestion of starch granules [17].In addition,protein could act as amylase inhibitor during starch digestion process [46].However,the property of such protein matrix/amylase inhibitor could be greatly modified by the pepsin in the gastric juice [24](Table 1).Pepsin prefers to cleave bonds adjacent to hydrophobic and aromatic amino acids [47],and its diffusion into food bolus is influenced by many food particle-related properties,including its density,porosity and surface-to-volume ratio [23].For example,Somaratne et al.[48]has reported that the egg white gel with more compact and microstructurally homogeneous structure had a much lower diffusion coefficient towards the fluorescein isothiocyanate (FITC) labelled pepsin (FITC-pepsin) compared to the egg white gel with more heterogenous and loose protein matrix.Another study has reported that the diffusion coefficient of FITC-pepsin was decreased 2.4 times in dairy gels,which had an increase of casein concentration from 32.5 to 130.0 g/kg [49].It was further rationalized by the large increase of gel density and corresponding decrease in the average gel pore size.This is reasonable as the more compact gel matrix provide more steric hindrance towards the pepsin diffusion.
3.4 Lipase hydrolysis
The hydrolysis of lipid by the action of lipase in the stomach may have critical roles in determining starch digestibility in human GIT,as it could affect the availability of amylose-lipid complex to the intestinal digestion and absorption.Amylose-lipid complex is known as a form of RS,due to its hindrance to the access of starch digestive enzymes [50].The transformation of amylose conformation into a helical structure in the amylose-lipid complex also changes the torsion angles of amylose glycosidic bonds and their binding affinity to the amylolytic enzymes [51].Furthermore,if the starch-lipid-protein ternary complexes presented,they are more resistant to the enzymatic digestion than the corresponding starch-lipid binary complexes,possibly due to their higher degree of steric hindrance and greater structural order [52].
4.Small intestinal digestion
Small intestine is the main location for starch digestion and absorption.Small intestine can be divided into duodenum,jejunum,and ileum.Most starch is digested in the duodenum (receiving the digestive secretions from pancreas and liver),then absorbed at the jejunum and ileum.Starch digestive enzymes include pancreatic enzymes and other enzymes anchored on the inner walls of small intestine.Furthermore,bicarbonate is secreted from the pancreas into the duodenum in order to adjust the chyme pH value to around 6 for the optimal activity of starch digestive enzymes.The food chyme is transported in the small intestine through segmentation and peristaltic muscle contractions,which aid the mixing and forward movement of the food chyme [36].
4.1 Starch digestive enzymes
Pancreaticα-amylase and four small intestine mucosalα-glucosidase subunits are employed in the small intestine to generate glycemic glucose from starchy foods [53](Fig.1).Pancreaticα-amylase is secreted by pancreas into the lumen and hydrolyzes starch by an endo mechanism at innerα-1,4 glucosidic linkages but bypasses theα-1,6 glucosidic linkages,which produces linear glucans and branched dextrin (Fig.1).The mucosalα-glucosidases include two types of enzyme complexes,which are maltase-glucoamylase(MGAM) and sucrase-isomaltase (SI).MGAM and SI are anchored on the membrane of villus cells through the N-terminals of maltase and isomaltase [54].They can hydrolyze the degraded products from pancreaticα-amylase to absorbable glucose (Fig.1).In vivo,SI accounts for 80% of neutral mucosal maltase activity,all neutral sucrose activity,and almost all isomaltase activity [55].MGAM accounts for all mucosal neutral glucoamylase exoenzyme activity for amylose and amylopectin substrates,1% of isomaltase activity,and 20% of neutral maltase activity [55].The spectrum of MGAM and SI activities,with complementary substrate specificities,is thus indispensable for small intestinal digestion of starches.
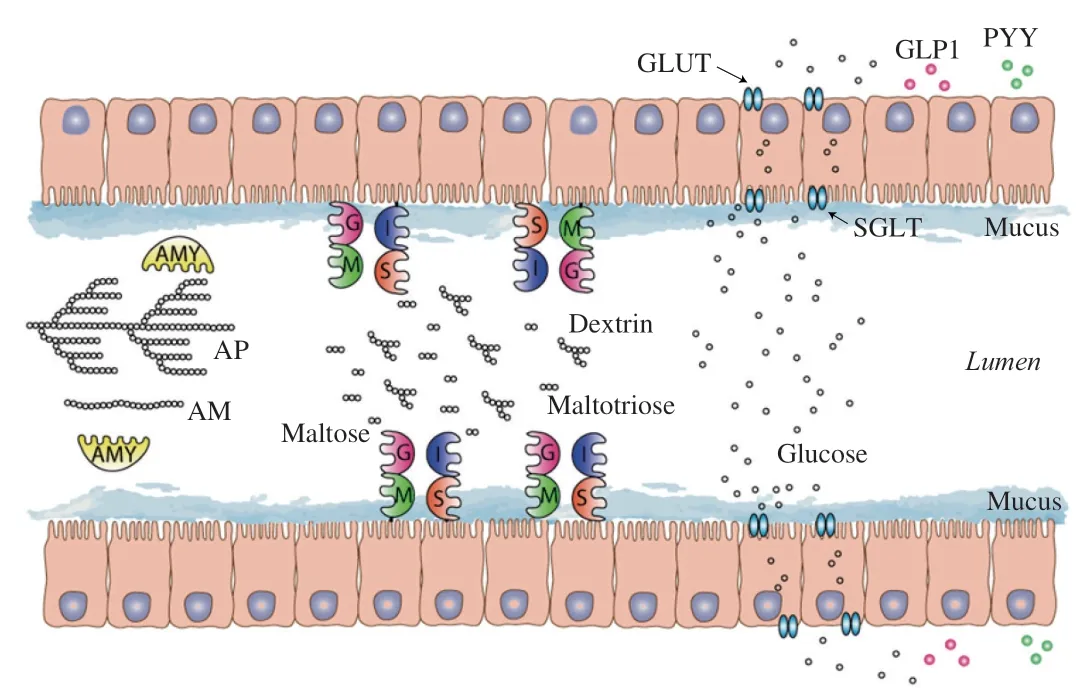
Fig.1 A schematic representation of starch digestion and absorption process in human small intestine.It starts with pancreatic α-amylase,moving on to the digestion of α-limit dextrin,maltose and maltotriose by the combination of four mucosal α-glucosidases.The produced glucose is then absorbed by glucose transporter into blood.If the starch were digested in the ileum,it could activate the secretion of GLP-1 and PYY.AM is amylose;AP is amylopectin;AMY is α-amylase;G is glucoamylase;M is maltase;S is sucrase;I is isomaltase;GLUT and SGLT are glucose transporters.
4.2 Glucose absorption
Proper gut glucose absorption rate is important for maintaining a normal glucose homeostasis and avoiding the activity inhibition of starch digestive enzymes by the hyperglycemia [26](Table 1).The absorption process occurs via two types of mechanisms through the epithelial cells,namely,indirect active co-transport and facilitated diffusion [56].There are two families of glucose transporters,glucose transporters (GLUTs) and sodium-glucose transport proteins(SGLTs) [56](Fig.1).The GLUTs are Na+independent and use the diffusion gradient of glucose (and other sugars,e.g.,fructose) to cross plasma membranes.Fourteen members of GLUTs have been identified in humans,which are expressed at different locations and for different substrates,e.g.,GLUT2 is the major glucose transporter in hepatocytes and intestine while GLUT5 is primarily responsible for fructose absorption into the epithelial cells of intestine [56].GLUT4 is another important glucose transporter,which is highly expressed in adipose tissues and skeletal muscles and is notoriously known as the “insulin-responsive glucose transporter” [56].The SGLTs are primarily expressed in the intestine and kidney cortex,which are Na+dependent and use the Na+electrochemical gradient provided by the Na+-K+ATPase pump to transport glucose into cells against its concentration gradient [57].There are six SGLT proteins identified in humans,with SGLT1 primarily expressed in the intestine [56].Gut glucose moves out of the intestinal cells into the blood stream via facilitated diffusion (Fig.1).
Once in the bloodstream,the glucose travels to the liver via the portal vein.The liver may form glucose into glycogen (1/3 of total glycogen formed in different tissues) that can be used to maintain normal blood glucose levels,burn it for energy or release it to the bloodstream for use in other parts of the body.Circulating glucose is taken up by cells (regulated by insulin) and through a series of metabolic reactions (glycolysis,conversion of pyruvate to acetyl CoA,citric acid cycle and electron transport chain) in mitochondrion is broken down to carbon dioxide and water,and releases energy in a form the body can use (oxidation process).In the muscle tissues,the glucose can be synthesized into glycogen and fuels muscle activity.Blood glucose level is very tightly controlled by two hormones,insulin and glucagon,perhaps the most of any nutrient entering the body.
4.3 Small intestinal motility and transit time
Gut motility,a key physiologic parameter governing the digestion and absorption of nutrients including starch molecules (Table 1),is affected by diet [58],gut microbes [59],the enteric nervous system(ENS,local system of nerves at the gut wall) [60],and host genetics [61].For example,dietary changes could introduce modifications on the microbiota,altering the motility of duodenum and jejunum in both animal models and human subjects [62].It was partially rationalized by that the fermentation of unabsorbed carbohydrates by the colonic microbiota produced methane,which can directly inhibit the gut motor activity [63].In small intestine,muscle contractions~12 times/min,compared to~3 times/min in stomach.Slow peristalsis is found in large intestine,which sweeps food along the GIT.The peristalsis could break chyme into smaller pieces and mix the chyme with digestive juices by forward and backward movements.ENS could also partly control the blood flow,water and electrolyte transport and acid secretion.At present,detailed understanding of the complex and dynamic interrelationships between these factors are limited,particularly in the global context of diverse cultural traditions concerning foods,their methods of preparation,and the varied human gut microbiota that have evolved under these dietary conditions.
4.4 Hormonal feedback regulation of gastric emptying
In the small intestine,nutrients interact with the mucosa and induce the release of hormones,such as GLP-1,cholecystokinin and PYY,which could modulate the gastric emptying through feedback inhibition [29](Table 1 and Fig.1).It has been shown that the release rate of the incretin hormones GLP-1 and gastric inhibitory polypeptide(GIP) from L cells (in the distal small intestine and colon) and K cells (in the duodenum and jejunum),respectively,are dependent on the rate of entry of nutrients such as starch molecules into the small intestine [43,44,64,65].Preloads of a small amount of protein or fat before the ingestion of a meal can interact with the small intestine to induce the release of GLP-1 and GIP before the main nutrient load enters the small intestine [66].These hormones could slow down the gastric emptying rate while promote insulin secretion.Besides,normal gastric emptying relies on the coordination of the contractile activity of the proximal and distal stomach,pylorus and the upper small intestine through complex interplay between the extrinsic and enteric nervous systems and neurohumoral pathways [29].
5.Large intestinal fermentation
The large intestine (cecum,colon,rectum,and anal canal),which is characterized by slow flow rate and neutral to mildly acidic pH,harbors by far the largest microbial community (gut microbiota).By comparison,the small intestine provides a more challenging environment for microbial colonizers given the fairly short transit times (3-5 h) and high bile concentrations [67,68].The dominant bacterial phyla in the large intesine of healthy human are Firmicutes,Bacteroidetes,and Actinobacteria,with Proteobacteria and Verrucomicrobia also presented in lower numbers.However,most of the current available information on the composition of gut microbiota derives from fecal samples collected from the lumen of distal large intestine [69,70].Aspects of microbial communities in the whole large intestine might thus be obscured by fecal sampling.
Only short glycan substrates can penetrate bacterial cell walls.Thus,the digestion of dietary polysaccharides such as RS requires export of sugar-cleaving enzymes into the intestinal lumen.These secreted enzymes include proteins that remain attached to the outer membrane or cell wall.There are two types of enzymes that cleave glycosidic bonds between carbohydrates: glycoside hydrolases(GHs) [71],which cleave bonds by the insertion of a water molecule(hydrolysis),and polysaccharide lyases (PLs),which cleave complex carbohydrates by an elimination mechanism [72].Enzymes from the GH13 family are the most representative ones that are associated with the breakdown of starch [71].
5.1 RS acting as the prebiotics
RS could potentially function as the prebiotics [30].Prebiotics are defined as non-digestible food ingredients that beneficially support the growth and/or activity of healthy-promoting bacteria that colonize the GIT.Under the anaerobic conditions of the large intestine,undigested starches (RS) are fermented mainly to SCFAs and gases,such as hydrogen,carbon dioxide,methane and hydrogen sulphide (Table 1).It has been reported that an increase ofBifidobacteria[73]andBacteroides[74]from the consumption of RS from serveral studies.An enrichment of phylotypes related toRuminococcus bromii[75]and bacteria related toEubacterium rectale[76]was also observed by consuming the RS.The alteration of gut microbiota could further change the SCFAs production [77].Acetate,propionate,and butyrate(found in roughly 60:25:15 ratio in the colon) account for 80% of the SCFAs produced by the gut microbiota [78].The calorific value per mole of RS (approximately 8 kJ/g) is considerably less than for a fully digestible carbohydrate (approximately 15 kJ/g),which depends on the extent of fermentation and of SCFA absorption [79,80].The chemical structure of RS determines its accessibility by different groups of colonic bacteria [81].In a recent study,it showed that resistant starch type 3 (RS3) with larger molecular size and fewer chains of degree of polymerization (DP) 36-100 promoted the relative abundance of some classes of gut bacteria,while other classes were promoted by RS3 with smaller molecular size and more chains of DP 36-100 [82].Furthermore,crystallinity polymorph and differeneces in physical surface of RS3 has also been shown to affect the microbiota [82].
5.2 Gut-brain feedback
SCFAs have multiple effects on the host.Butyrate is mainly consumed by the colonic epithelium (colonocytes) as energy substrates,while propionate and acetate become available systemically [83]and can be transported to the liver through the portal vein and used as the substrates for lipogenesis and gluconeogenesis in the liver.SCFAs are able to bind to the G-protein-coupled receptors (GPCRs)in the gut and activate different hormones (Table 1).For example,they can modulate the secretion of the hormone GLP-1 and PYY by enteroendocrine L-cells in the distal small intestine and colon [31].Both hormones can improve the insulin secretion and reduce the gastric emptying rate [32].This is supported by significant reductions in food intake following the peripheral administration of physiological levels of PYY in both rodents and human subjects [32].Another study has also demonstrated that the mice lacking SCFAs receptorsffar2orffar3exhibited reduced SCFAs-triggered GLP-1 secretion and a corresponding impairment of glucose tolerance [31].Other influences reported in literature include anticancer effects (especially for butyrate),anti-inflammatory properties [84]and changes in gut motility [85]and energy expenditure [86].
6.General comments and future perspectives
Understanding thein vivostarch digestion process is important in terms of developing starchy foods with low glycemic index values.Although substantial knowledge has been learnt,it is still challenging to fully understand all the factors controlling thein vivostarch digestibility.This is due to the complexity of human GIT,such as the anatomy,peristaltic movements,hormonal/nervous feedback,local immune system,and biochemical conditions.The following are few recommendations for the future work in order to better understand the biological factors related to thein vivostarch digestibility.
1) The collection and storage ofin vivodigesta samples for further characterizations are still challenging.As mentioned,fecal samples are commonly obtained to investigate the interaction of resistant starch with gut microbiota.However,this may not reflect the realistic situation in human’s colon.In addition,it is almost impossible to extract starch digesta from the small intestine of healthy individuals.Healthy ileostomy participants have been recruited for the investigation of the starch bio-accessibility in wheat endosperm [2].Whereas,the disadvantage is that it may not reflect the situation in a healthy individual in terms of investigating its starch digestion pattern.The feedback regulation of starch digestibility through the gut microbiota-brain axis is also lack in these ileostomates.
2) More advanced and economic techniques are needed to conduct futurein vivostudies.Currently,many clinical studies have to rely on some advanced and expensive instruments,including ultrasonic scanner,scintiscanner and nuclear magnetic resonance [23].Sometimes,invasive procedures including aspiration from the stomach or small intestine have to be applied [87,88].This kind of study is however not always technically and financially feasible.
3) The human GIT should be taken as a whole in order to fully understand the overallin vivodigestion behavior of starch.As summarized,each digestion step in the GIT is critical while considerably complex in determining the overall digestion rate of starchy foods.Connections among these compartments towards controlling thein vivostarch digestibility as a whole should be further explored.The interpretation of starch digestion results based on only a single or few digestion compartments should always be careful.
4) In addition toin vivobiological factors,starch digestibility is also regulated by the food properties ingested [36,89].Food properties could include moisture content,cooking/preparation procedure,particle size,texture and sensory attributes.Factors from both sides should thus be considered in order to fully understand the nature ofin vivostarch digestibility.
7.Conclusion
The current review presented a holistic view on biological factors within human digestive tract related to the regulation of starch digestion.It is shown that each digestive compartment plays a critical role in regulating the starch digestion process.This mainly include oral mastication and salivation,gastric emptying and motility,small intestinal digestive enzymes and motility,large intestinal RS-gut microbiota interactions and gut-brain feedback control,as well as glucose adsorptions and hormonal feedback control.Although valuable scientific insights have been learnt from the literature,it is still challenging to fully understand the wholein vivostarch digestion process,due to the ethical,financial and technical issues.More efforts are needed in the future from both human trials andin vitrosimulation models with a considerable collaboration between food scientist and biologist.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgement
Dr.Cheng Li would like to thank the financial support from National Natural Science Foundation of China (32001646),Natural Science Foundation of Jiangsu Province (BK20190906),Jiangsu Yangzhou Key Research and Development Program(SSF2018000008),Jiangsu Provincial Entrepreneurial and Innovation Phd Program,and Yangzhou Lvyangjinfeng Talent Program.
杂志排行
食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil
- Structural characteristics,anticoagulant and antithrombotic mechanism of a novel polysaccharide from Rosa Chinensis Flos
