Effect of fructooligosaccharides on the colonization of Lactobacillus rhamnosus AS 1.2466T in the gut of mice
2023-01-21ZhihuNiuMeijunZouTingtingBeiZhngDongyoLiMioshuWngChenLiHongtoTin
Zhihu Niu,Meijun Zou,Tingting Bei,N Zhng,Dongyo Li,Mioshu Wng,Chen Li,d,*,Hongto Tin,d,e,*
a College of Food Science and Technology,Hebei Agricultural University,Baoding 071000,China;
b College of Biochemistry and Environmental Engineering,Baoding University,Baoding 071000,China;
c New Hope Tensun (Hebei) Dairy Co.,Ltd,Baoding 071000,China;
d Hebei Technology Innovation Center of Probiotic Functional Dairy Product,Baoding 071000,China;
e National Engineering Research Center for Agriculture in Northern Mountainous Areas,Baoding 071000,China
Keywords:Intestinal microbiota Synbiotics Fructooligosaccharides Lactobacillus rhamnosus
ABSTRACT Lactobacillus rhamnosus and fructooligosaccharides (FOS) have been widely studied so far.However,the effects of L.rhamnosus on the intestinal microecological environment at the species level and the effect of different proportions of FOS on L.rhamnosus colonization in different parts of mice intestine are still unclear.The study results indicated that the specif ic bands of enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR) in the L.rhamnosus (LR) group significantly increased at 7 days.Although the number of bands was similar to the natural recovery (NR) group,the brightness of few bands significantly enhanced in the later stage of recovery.Besides,Southern-blot maps showed strong signals,indicating that the ERIC-PCR f ingerprint could accurately ref lect the changes in the mouse gut microbiota diversity.Further,the high-throughput results confirmed that the Lactobacillus and Akkermansia had different changes at different periods,but all of them showed an upward trend,while the Klebsiella were inhibited,thereby maintaining the intestinal microecology balance.Moreover,FOS exerted a positive effect on L.rhamnosus colonization in the gut.
1.Introduction
The gut is the largest host defense organ of the human body,containing a microbial ecosystem with a large number of microbes,approximately 1−5.5 kg microbes in an adult [1].Gut microbes play a vital role in the gut [2],and thus,a slight imbalance could lead to various diseases [3,4].Therefore,maintaining the intestinal microecological balance has attracted the researchers’ attention to develop healthy foods.Accumulating studies on intestinal microbes have reported that oral pro-or prebiotics could prevent immunemediated destruction by increasing the beneficial symbiotic groups in the gut [5].In recent studies,Lactobacillus rhamnosus,a probiotic,has been shown to have the potential to influence the immune response and prevent and treat allergic inflammation [6],it is more resistant to bile and gastric acid and has a stronger ability to colonize and stably exist in the dairy products for a long time in the human body [7].Currently,more than 40 countries are producingL.rhamnosusrelated products,L.rhamnosushas become one of the most widely documented and widely used probiotics in the world.AlthoughL.rhamnosuscan regulate the gut microbiota structure and enhance the body’s immunity and other probiotic functions [8],its specif ic impact on the gut microbiota structure is still unclear.
Prebiotic is a non-viable food ingredient selectively metabolized by beneficial intestinal bacteria.They can cleave into numerous indispensable substances by microbial enzymes to maintain the metabolic and functional activities of the intestinal mucosa.Prebiotics supplementation could promote the growth of genetic intestinal microbes or probiotics [9].Probiotic substances selectively stimulate microorganisms in the host’s intestinal ecosystem,thereby eliminating the need to compete with bacteria.The stimulation of prebiotics on the intestinal microflora affects the level of intestinal short-chain fatty acids (SCFA),which brings health benefits to the host [10].In addition,probiotics can lower the pH of the intestine and maintain the osmotic pressure of water in the intestine [11].Therefore,an indepth study of prebiotics is of great significance to develop healthy foods.Prebiotics are of many types,including oligosaccharides,polyols,protein hydrolysates,and natural plant extracts.At present,the functional oligosaccharides are mostly studied and applied.Inulin intake can stimulate the growth ofBifidobacteriumadolescentis[12],the intake of FOS,and significantly increaseBifidobacteriaabundance [13].
As early as 1960,researchers noticed that synbiotics (a combination of pro-and prebiotics) could be used in the nutrition field to interfere with the human intestinal microbes [14].However,the lack of prominent technologies at the time retained synbiotics unnoticed.With the emergence of new technologies,such as highthroughput sequencing,synbiotics and their effect on improving dietinduced obesity and other metabolic disorders have been conducted.Thiennimitr et al.[15]have shown that long-term consumption ofLactobacillus paracaseiHII01,xylooligosaccharide (XOS)and synbiotics can equally improvement in insulin sensitivity and dyslipidemia in obese and insulin resistant rats.Currently,various synbiotic products are available on the market,but the effect of proand prebiotics on the gut microbiota and the optimal compatibility of synbiotics have become obstacles to the development of related products.
Intestinal colonization of probiotics plays a beneficial rolein vivo[16].In this study,the prebiotic fructooligosaccharide (FOS)with a better proliferation effect ofL.rhamnosuswas obtained throughin vitroscreening.Based on the previous research [17],enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR),Southern-blot,and high-throughput methods were employed to analyze further the effect ofL.rhamnosuson the typical gut microbiota of mice and explore the effect of FOSin vivoonL.rhamnosus.The results of the study show thatL.rhamnosusAS 1.2466Tcan accelerate the relative abundance ofLactobacillusandAkkermansia,while inhibiting the proliferation ofKlebsiella,thereby regulating the gut microbiota.FOS could change the microecological environment in the gut [18].The combination of 2 × 109L.rhamnosusAS 1.2466Tand 3.99 g/kg bw FOS can prolong the colonization time ofL.rhamnosusAS 1.2466Tin the intestine and increase the colonization time ofL.rhamnosusAS 1.2466Tnumber.The aim of this study was to study the effect of FOS on the colonization ofL.rhamnosusAS 1.2466Tin the gut of mice and the effect ofL.rhamnosuson gut microbiota imbalance in mice.This study emphasizes the matching of probiotics and suitable prebiotics,which can not only improve the theoretical research level of synbiotics,but also provides a scientific basis for the further product development,promotion and application of synbiotics,and its theoretical contribution will promote the development of precision nutrition technology.
2.Materials and methods
2.1 Probiotics and prebiotics
L.rhamnosusAS 1.2466Twere obtained from the China General Microorganisms Collection,andEscherichia coliDH5α (pMG36e dsred2) was procured from the preserved stock of Laboratory of Food Fermentation and Brewing Innovation,Hebei Agricultural University.The FOS,oligo-isomaltose (IMO),XOS,and inulin were obtained from Baolingbao Biotech Co.,Ltd.(Hebei Province,China).Plasmid pMG36e-dsred2 used in this study was constructed in Laboratory of Food Fermentation and Brewing Innovation,Hebei Agricultural University.
2.2 Animals,diets,and sample preparation
We studied the effect ofL.rhamnosusAS 1.2466Ton gut microbiota imbalance in mice caused by ampicillin sodium [17].All animal procedures were done following an approved protocol from the Animal Welfare and Ethical Committee of Hebei Agricultural University,China (certificate No.2020030).24 male BALB/c mice(4 weeks old;Beijing Weitong Lihua Experimental Animal Technology Co.,Ltd.,Beijing,China) were housed in a temperaturecontrolled room ((26 ± 1) °C and relative humidity of 40% –70%)with 12 h light/12 h dark cycles,6 mice per cage.All mice had free access to drinking water.One week after the adaptation to the environment,gavage mice with a syringe at 10 a.m.,once a day.The mice were were given 125 mg/mL ampicillin sodium (twice a day,4 days) by gavage to induce gut microbiota imbalance in the mice.The mice were randomly divided into two groups: the natural recovery(NR) group (n=12),intragastrically administered with the same amount of sterile normal saline,and theL.rhamnosus(LR) group(n=12),intragastrically administered with 109CFU/mLL.rhamnosusAS 1.2466Tsuspension,these supplements are given by gavage at a volume of 0.1 mL/10 g bw once a day for 14 consecutive days.In order to determine the amount of supplements by gavage,this experiment uses forced gavage.The mice were sacrificed by cervical dislocation on the 0,7,and 14 days of gavage,and the cecum contents were taken aseptically.
2.3 ERIC-PCR and southern-blot
PCR amplification was performed according to the primers designed by Versalovic et al.[19].ERIC-PCR fingerprint can distinguish fragments based on DNA mobility,with different sequence fragments in the same band.Southern-blot was performed on the amplified product to reduce the error estimation of the map difference.
2.4 Fecal genome extraction and 16S rRNA gene analysis
Feces (0.1 g) was mixed with 5 mL of sterile phosphate-buffered saline.The bacterial components were separated by differential centrifugation according to the method reported by Apajalahti et al.[20].Later,it was verified by 1% agarose gel electrophoresis,and the extracted DNA was frozen at −20 °C for further use.16S rDNA gene PCR amplification and sequencing were performed according to the method reported by Li et al.[17].As reported earlier,the default settings of Illumina processing in QIIME were used to analyze the original FASTQ files.UPARSE was used to cluster non-repetitive sequences based on 97% similarity by operating taxon units (OTU),and UCHIME was used to identify and discard the chimeric sequences.Mothur was used for alpha diversity analysis.Chao estimated the total number of OUTs in the sample,reflecting the species richness and represented the community species composition and species abundance information through a heatmap.The analysis method was built into the R language vegan package rda.Welch’st-test uses thet-distribution theory to infer the probability of a difference,was employed to compare the significance difference level between 2 averages.
2.5 Effects of four prebiotics on the growth of recombinant L.rhamnosus AS 1.2466T in vitro
The colonization ofL.rhamnosusAS 1.2466Tin the gut was observed by constructing a red fluorescent protein-labeledL.rhamnosusAS 1.2466T,as reported by Li et al.[21].Four types of probiotics were contained in the basal medium at different proportions.The added amount of XOS and IMO were 0.4%,0.8%,1.2%,1.6%,2.0%,while the added amount of FOS was 1.2%,1.6%,2.0%,2.4%,2.8%,and the added amount of inulin was 0.2%,0.4%,0.6%,0.8%,1.0% .After determining the growth curve of the recombinantL.rhamnosusAS 1.2466T,after being incubated at 37 °C for 16 h,the strain enters the end of logarithmic phase,and then the strains were inoculated in a liquid basal medium containing erythromycin (1 μg/mL) and a medium supplemented with different amounts of prebiotics at 1% inoculum.Later,it was placed in an incubator at 37 °C.Then,the samples were taken after 12,14,and 16 h,and the optical rotation density was measured at 600 nm (TU-1810 Ultraviolet Spectrophotometer,Prose General Instrument Co.,Ltd.,Beijing,China).The medium containing the corresponding prebiotics without inoculated strains was used as a reference.The effects of different prebiotics on the growth of recombinantL.rhamnosusAS 1.2466Tin vitrowere compared by spectrophotometry,and the prebiotics with faster growth rate effects on the tested strains was screened out for subsequent analyses.
2.6 Preparation of L.rhamnosus AS 1.2466T suspension
The activatedL.rhamnosusAS 1.2466Twas implanted in a liquid bacterial growth medium (MRS) containing 1 μg/mL erythromycin at a rate of 1%,cultured at 37 °C for 16−18 h,and centrifuged at 10 000 r/min for 5 min to collect the bacteria (Hettich UNIVERSAL 320 high-speed refrigerated centrifuge,Hettich,Germany).Then,the bacterial sludge was washed 2−3 times with PBS,resuspended in PBS,and the bacterial concentration was adjusted to 1010CFU/mL.
2.7 In vivo persistence of synbiotics
The dose groups were set up as 5,10,and 30 times,and the low FOS (FOS-L),medium FOS (FOS-M),and high FOS (FOS-H)groups,respectively,according to the recommended dose of human body weight and dose ratio [22],the recommended dose of oligofructose is 8.3 g/day for adults.Prepare FOS mother liquor with a concentration of 400 g/L with sterilized distilled water for use.84 male BALB/c mice were randomly divided into 4 groups,each group contained 21 mice,and with a 0.2 mL/10 g bw supplement orally once a day at 10 a.m.for 14 consecutive days.FOS-L,FOS-M and FOS-H groups were gavaged 0.016,0.033 and 0.100 mL/10 g bw FOS mother liquid and 2 × 109L.rhamnosusAS 1.2466T,sterilized PBS was used to make up to 0.2 mL/10 g bw,control (CON)group,2 × 109L.rhamnosusAS 1.2466Tand PBS were orally administered,and the other groups were intragastrically administered with the corresponding dose of FOS and the same concentration ofL.rhamnosusAS 1.2466T,the solutions are all prepared with sterile distilled water.Three mice were sacrificed each time on 7,14,15,20,25,and 31 days.After body surface disinfection,2 cm of the gut was taken aseptically,rinsed with sterilized PBS according to 100 μL/cm intestinal segments,and the washing liquid was collected and mixed.Then,100 μL of each wash liquid was serially diluted with sterile PBS buffer.After mixing the diluted washing solution,100 μL was taken and spread on the MRS solid plate containing erythromycin(5 μg/mL).Later,the washing solution was applied 3 times,and the plates were coated,incubated at 37 °C for 24 h,and counted.
3.Results and discussion
3.1 Effect of L.rhamnosus AS 1.2466T on the gut microbiota of mice
After gavage of mice withL.rhamnosusAS 1.2466Tfor 7 days,the ERIC-PCR specific bands of the natural recovery 7 days (NR7)group showed more scattered patterns,and the brightness was lower,while theL.rhamnosus7 days (LR7) group had more specific bands,concentrated between 2 000 bp and 200 bp,and the brightness was higher than the NR7 group (Fig.1A).After 14 days of intragastric administration,no significant difference was observed in the number of specific bands between the natural recovery 14 days (NR14) andL.rhamnosus14 days (LR14) groups.The band sizes were concentrated between 2 000 bp and 500 bp,but the brightness of some bands in the LR14 group was significantly higher than the NR14 group,especially at 1 000 bp and 500 bp (Fig.1B).The results showed that after 14 days,L.rhamnosusAS 1.2466Tsignificantly affected the recovery of the types and abundance of the intestinal microbiota in mice.Although the ERIC-PCR can reflect the structural characteristics of the microbiota to a certain extent,it cannot distinguish the fragments with the same mobility and different base sequences.Southern-blot maps were used to reduce the error rate of estimation of the gut microbiota diversity through fingerprints and analyze the similarities and differences between the microbiota in different environments.The ERIC-PCR products of the NR group samples were used as the hybridization probes.The ERIC patterns at 7 and 14 days exhibited strong signals after hybridization with the respective probes,indicating that the ERIC-PCR fingerprint could accurately reflect the changes in the mouse gut microbiota diversity.
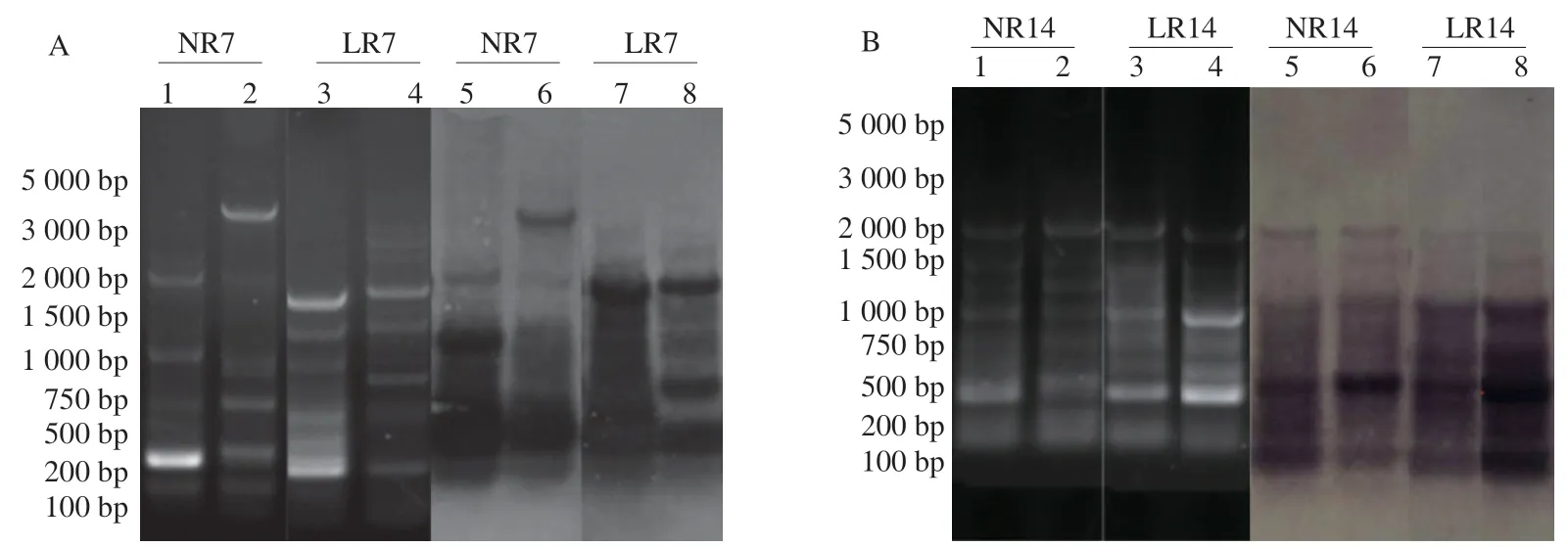
Fig.1 Agarose gel fingerprints of the ERIC-PCR amplicons from fecal samples of the natural recovery group and L.rhamnosus group.(A) The bacterial genome ERIC-PCR of the cecum contents after gavage for 7 days;(B) ERIC-PCR of the cecal contents of bacterial genome after 14 days gavage.1,2: Natural recovery group;3,4: L.rhamnosus group,the Southern-blot gavage for 7 days;5,6: Natural recovery group;7,8: L.rhamnosus group.
In a previous study,the imbalance of gut microbiota was adjusted through different intervention methods [17].The results showed that the abundance of probiotics (such asLactobacillus) was increased 7 days after gavage ofL.rhamnosusAS 1.2466T,while opportunistic pathogens (such asKlebsiella) were inhibited.Herein,the effect ofL.rhamnosusAS 1.2466Ton the gut microbiota was analyzed at the species level based on the previous stage.
Compared with NR7,the abundance of LR7 gut microbiota was significantly restored,and the degree of recovery was close to NR14.Statistically,the abundance of LR14 gut microbiota was not significantly higher than NR14,but the number was still higher than NR14 (Fig.2A).The typical probiotic strains (Lactobacillus,Blautia,andAkkermansia) and opportunistic pathogens (Klebsiella) used in this study were selected from the top 15 abundant intestinal strains.Fig.2B depicts the relative abundance of strains in the community.Therefore,combining the analysis with Fig.2A is necessitated to analyze the actual situation more accurately.Upon natural recovery,the relative abundance ofLactobacillusincreased from 0 to 0.026 7(NR14).When intragastrically administered withL.rhamnosusAS 1.2466T,Lactobacillusrapidly multiplied to a relative abundance of 0.036 5 on the 7th day,which increased by 36.7% than NR14.However,the relative abundance decreased to 0.008 in the late recovery period.The relative abundance changes inBlautiawere similar toLactobacillus,while contradictory to the natural recovery.The supplementation ofL.rhamnosusAS 1.2466Tinhibited the proliferation ofBlautia,whileAkkermansiawas recovered under the two recovery methods.The difference was that after the intervention ofL.rhamnosusAS 1.2466T,theAkkermansiaabundance in the LR14 group reached 0.207 5,which was much higher than the NR14 group(0.058 2),and its proliferation mainly occurred in the late recovery period.UnlikeLactobacillusandAkkermansia,the relative abundance of opportunistic pathogensKlebsiellawas high (0.290 6) in the early stage,butKlebsiellawas inhibited (close to 0) when the gut microbiota was restored,and the inhibition rate was accelerated after the gavage ofL.rhamnosusAS 1.2466T.
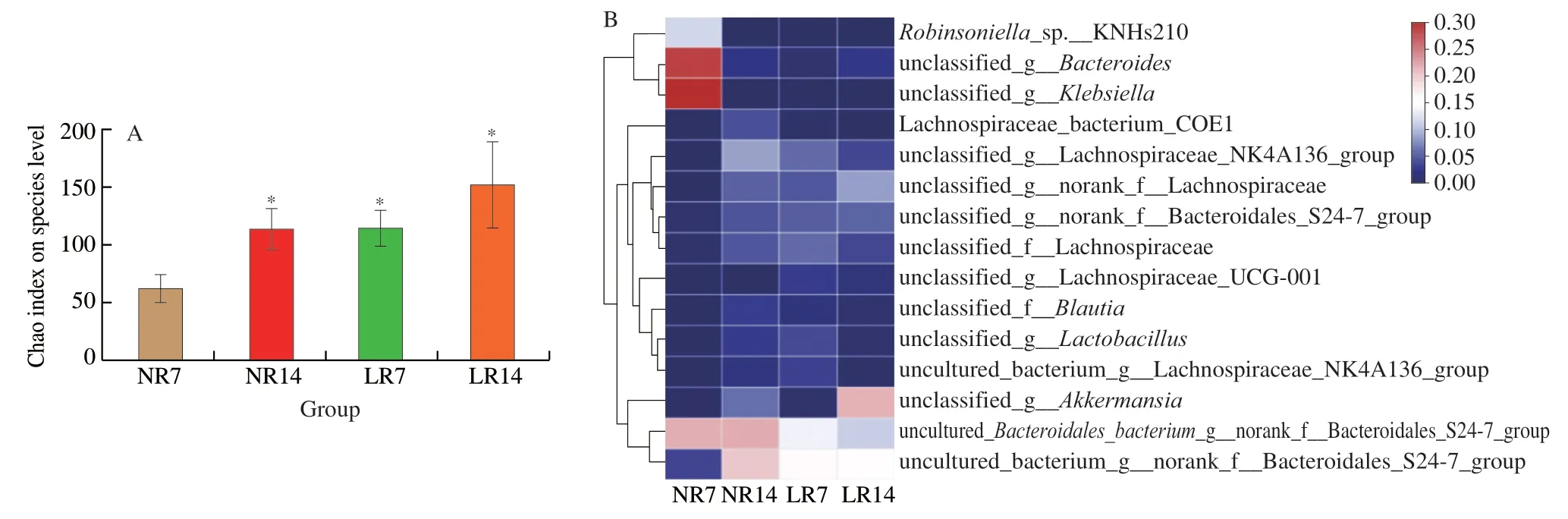
Fig.2 High-throughput sequencing results.(A) Analysis of the difference in Chao index between NR7,NR14,LR7,and LR14 group.Error bars denote SD.(B) Heatmap of NR7,NR14,LR7,and LR14 group.P ≤ 0.05 is marked as *.
3.2 Effects of four prebiotics on the growth of recombinant L.rhamnosus AS 1.2466T in vitro
The activatedL.rhamnosusAS 1.2466Twas inoculated into a medium supplemented with four prebiotics and a basal medium at 1% of the inoculum and cultured at 37 °C.Then,the samples were taken at 12,14,and 16 h to measure the optical density at 600 nm.The uninoculated medium containing the corresponding prebiotics was used as a reference.L.rhamnosusAS 1.2466Thas the highest optical density value at 14 h,so compare the optical density values at 16 h to evaluate their influence on the growth of recombinantL.rhamnosusAS 1.2466Tin vitro.
Four percent of inulin increased the OD600nmofL.rhamnosusAS 1.2466Tto 0.54.Inulin exhibited a poor growth-promoting effect on probiotics (Fig.3B),while IMO exhibited no positive effect on bacterial growth (Fig.3A).Similarly,2.4% FOS and 1.2% XOS increased the probiotics optical density to 0.86 and 0.88 at 600 nm and showed significant growth-promoting effects onL.rhamnosusAS 1.2466T(Figs.3C and 3D).As depicted in Fig.3E,the 4 prebiotics had the most appropriate amount of addition in thein vitroproliferation ofL.rhamnosusAS 1.2466Tscreening test.The results show that in addition to IMO,each prebiotic has an appropriate amount added to promote the growth ofL.rhamnosusAS 1.2466T,andL.rhamnosusAS 1.2466Tand prebiotics are selective,XOS and FOS have better growth-promoting effects onL.rhamnosusAS 1.2466T.However,in the dairy industry alone,the market share of FOS is 21 times that of xylooligosaccharides [23].And we found through market research that the market price of XOS in China is 3−5 times the market price of FOS.Therefore,in the next experiment,we choose FOS as the research object to explore the effect of different FOS doses on the colonization ofL.rhamnosusAS 1.2466Tin the gut of mice.
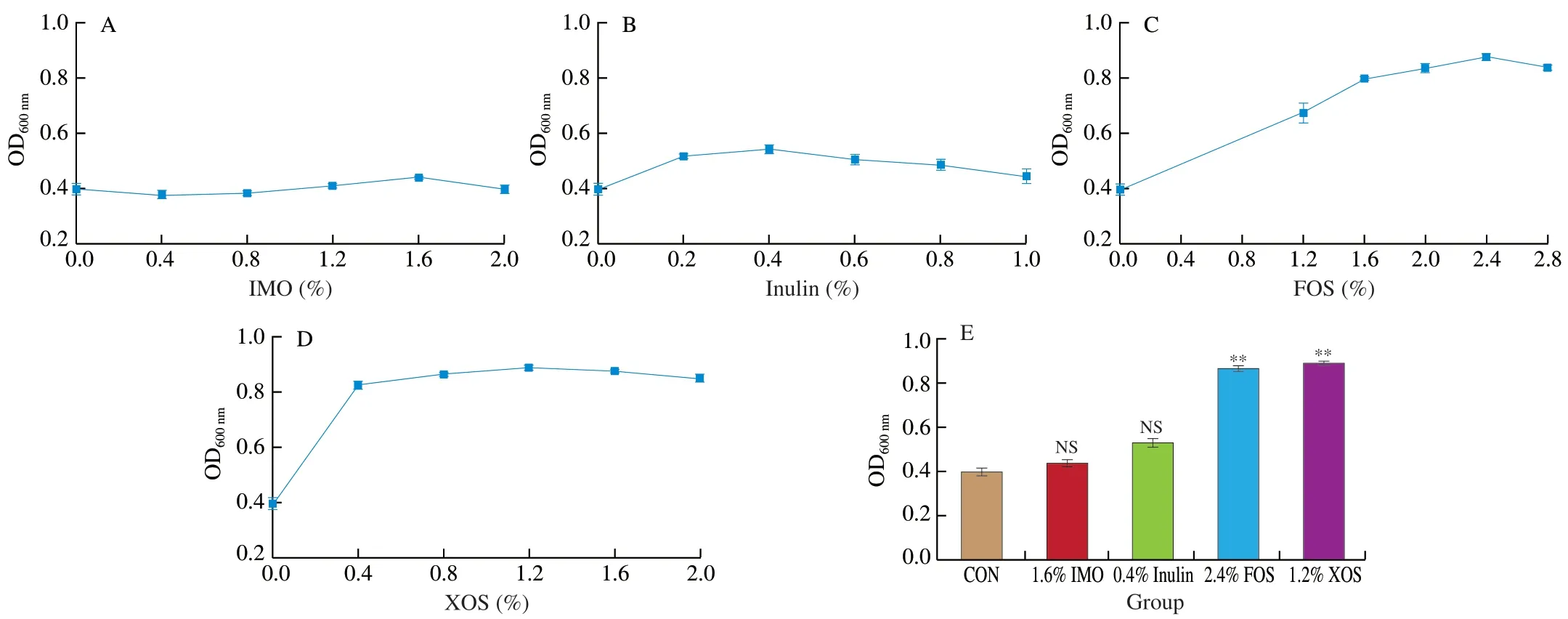
Fig.3 Effects of four prebiotics on the growth of recombinant L.rhamnosus AS 1.2466T in vitro.(A) Effect of different inulin concentrations on L.rhamnosus AS 1.2466T;(B) Effect of different isomalto-oligosaccharides concentrations on L.rhamnosus AS 1.2466T;(C) Effect of different xylooligosaccharides concentrations on L.rhamnosus AS 1.2466T;(D) Effect of different fructooligosaccharide concentrations on L.rhamnosus AS 1.2466T.P <0.05 is marked as *,P <0.1 is marked as **.
3.3 Effects of different doses of FOS on L.rhamnosus AS 1.2466T in the gut of mice
The mice were supplemented with different doses of FOS andL.rhamnosusAS 1.2466Tby gavage.During the feeding period,the colonization number ofL.rhamnosusAS 1.2466Tin various parts of the gut gradually increased,but different FOS doses did not affect the colonization number ofL.rhamnosusAS 1.2466T.After the gavage was stopped,the colonization ofL.rhamnosusAS 1.2466Tin the gut gradually decreased.As depicted in Fig.4A,after 25 days,the number ofL.rhamnosusAS 1.2466Tdecreased faster,while on the 31st day after the gavage was stopped,the number ofL.rhamnosusAS 1.2466Tin the gut reduced by two orders of magnitude compared with the 25th day.During this period,the intervention of FOS did not change the trend ofL.rhamnosusAS 1.2466T,but it increased the number ofL.rhamnosusAS 1.2466Tcolonization.On day 31,the colonization number ofL.rhamnosusAS 1.2466Tin the H-FOS group in the ileum was 4.34 (lg(CFU/mL)),which was an order of magnitude higher than the control and L-FOS groups (Fig.4B).However,FOS did not affect the number ofL.rhamnosusAS 1.2466Tin the duodenum and jejunum,and no significant difference between the M-FOS and H-FOS groups was observed.Since the microecological environment of different parts of the gut were quite different,the colonization ofL.rhamnosusAS 1.2466Tin different parts was significantly different.On the 31st day (the 16th day when the probiotics were stopped),the colonization ofL.rhamnosusAS 1.2466Tin the colon and ileum was an order of magnitude higher than the duodenum(Fig.4C).Interestingly,the colonization number ofL.rhamnosusAS 1.2466Tin the present study was two orders of magnitude higher than the previous experiment (Fig.4D),indicating that increasing the supplementation time ofL.rhamnosusAS 1.2466Tcould improve the colonization ability ofL.rhamnosusAS 1.2466Tin the colon and ileum of mice.
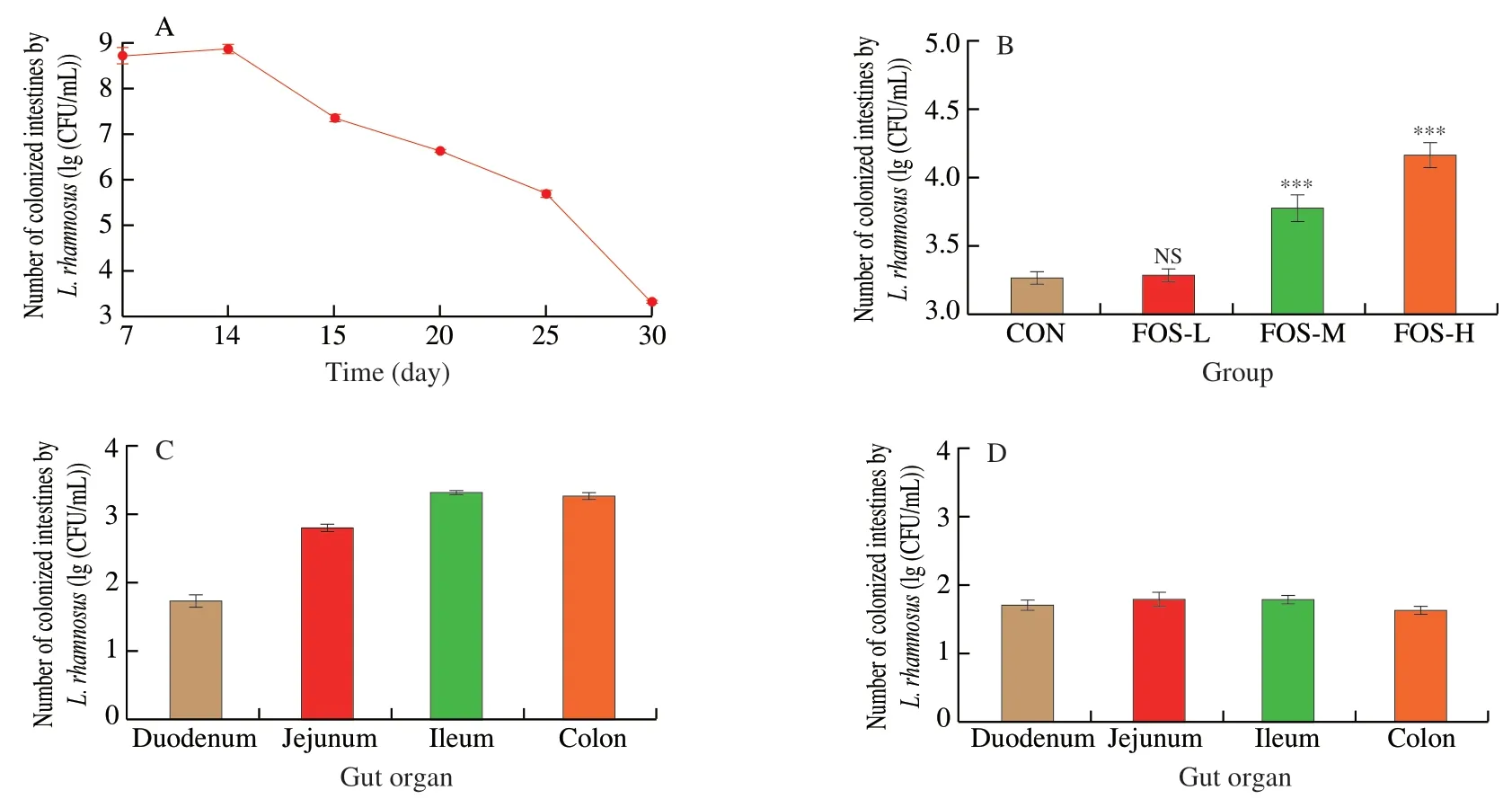
Fig.4 Colonization of L.rhamnosus in the gut of mice after the intervention of different doses of fructooligosaccharide.(A) The colonization of L.rhamnosus AS 1.2466T at different times after intragastric administration of L.rhamnosus AS 1.2466T;(B) The effect of different doses of FOS on the colonization of L.rhamnosus AS 1.2466T in the colon on the 31st day of intragastric administration;(C) The colonization of L.rhamnosus AS 1.2466T in different parts of the gut on the 31st day after intragastric administration;(D) The colonization of L.rhamnosus AS 1.2466T in different parts of the gut after 15 days with only one L.rhamnosus AS 1.2466T gavage.
4.Discussion
In recent years,probiotics have attracted the attention of most researchers due to their inhibitory effect on opportunistic pathogens.It also plays a vital role in maintaining the microecological environment in the gut [24].The intervention ofLactobacillus coryniformisCECT5711 can reduce the pro-oxidant and pro-inflammatory state of blood vessels in obese mice [25].Similarly,Lactobacillus plantarumHAC01 can alleviate the negative effects of obesity by regulating the gut microbiota structure [26].As a recognized probiotic,L.rhamnosushas good resistance to stress and adjuvant therapeutic effect on many gastrointestinal-related diseases [8].Zhao et al.[27]proposed that the study of intestinal bacteria should be accurate to the species level to achieve the purpose of precise nutrition.Although our previous research has established thatL.rhamnosusAS 1.2466Tcould regulate the gut microbiota,this study further aimed to analyze the changes in some gut microbiota at the species level.L.rhamnosusAS 1.2466Tcan inhibitKlebsiellaand accelerate the recovery ofLactobacillus[28],which can regulate the intestinal environment,andAkkermansia[29],which can regulate the body’s immunity,but it cannot restore the relative abundance ofBlautia[30],which maintains intestinal homeostasis.The effects of different recovery periods on the strains were different,which were complicated by the intestinal microecological environment.The gut microbiota had a lower diversity in the early stage of recovery of the gut microbiota,andL.rhamnosusAS 1.2466Thad more lactobacilli to regulate the gut microbiota.When the intestinal bacteria were recovered to a certain level,Akkermansiaproliferated faster to make the intestinal bacteria healthier,indicating that the supplement of a single probiotic bacterium could regulate the gut microbiota by affecting the overall proportion of the intestinal bacteria rather than a single strain.
In the food industry,synbiotics have potential application [31].However,the highly individualized gut microbiota makes the supplement of pro-and prebiotics fully consider the characteristics of the host’s gut microbiota [32].Thus,in the future development of probiotic functional products,accurate supplementation of pro-and prebiotics will be an important research direction.Meanwhile,some researchers have proved that different probiotic strains have different preferences for prebiotics due to the differences in the pro-and prebiotics characteristics [33],which is consistent with the present study results.
The incidence of inflammatory bowel disease (IBD) has shown a rapid increase over the past decades,specifically in the populations of Western Europe and North America [34].The emerging diseases,such as colitis and colon cancer [35],have necessitated the regulation of the intestinal micro-ecological environment through diet [36].Oral probiotic preparations are one of the important intervention methods.After probiotics are colonized in the body,they can inhibit the reproduction of conditional pathogenic bacteria by forming biofilms and producing a variety of metabolites [37,38].In the previous studies,when mice were administered withL.rhamnosusAS 1.2466Tby gavage for one day,the intestinal bacteria ofL.rhamnosusAS 1.2466Twere less than 100 CFU/mL after 15 days,and the colonization effect of the strain in the ileum was significant [16].On the contrary,in this experiment,when the FOS dose was higher than 1.33 g/kg bw,the colonization number ofL.rhamnosusAS 1.2466Tin the colon remained high after the gastric administration was stopped just after 16 days.This result indicates that FOS consumption should be limited for some time.It has an important impact on the micro-ecological environment in the colon,allowingL.rhamnosusAS 1.2466Tto colonize easily.This phenomenon helps the synbiotics to maintain the health of the colon and prevent diseases.In this study,it was found that the ratio of pro-to prebiotics should be paid attention to during synbiotics use.An appropriate amount of prebiotics will significantly increase the number of probiotics colonized in the gut and prolong the colonization time.An increase in the number of probiotics in the intestinal tract allows it to better regulate the intestinal microecological environment.Similarly,an extension in the colonization time also prolongs the action time of the probiotics,making the effect of the probiotics of synbiotics more significant.
5.Conclusion
In conclusion,thein vivoandin vitroexperiments showed good compatibility betweenL.rhamnosusAS 1.2466Temulsion and FOS than other prebiotics.The combination of FOS andL.rhamnosusAS 1.2466Tcould extend the colonization time ofL.rhamnosusAS 1.2466Tin the intestine of mice to 31 days and significantly increase the colonization number ofL.rhamnosusAS 1.2466Tin the ileum to 4.34 (lg(CFU/mL)).Notably,the combination of an appropriate amount of FOS andL.rhamnosusAS 1.2466Tcould mediate better colonization ofL.rhamnosusAS 1.2466Tin the gut.This study revealed the potential application of synbiotics.
Ethics statements
All the animal procedures were performed according to the approved protocol of the Animal Welfare and Ethical Committee of Agricultural University of Hebei,China,and the ethical guidelines of the European Community guidelines (Directive 2010/63/EU).
Conflict of interest
The authors declare to have no conflict of interest towards the publication of this manuscript.
Acknowledgements
This research was supported by the Science and Technology Research Projects of Colleges and Universities in Hebei province(ZD2021059),the Key Research and Development Program of Hebei Province (19227134D),the Natural Science Foundation of Hebei province (C2016204129,C2017204094),and the Food Processing Discipline Group of Hebei Agricultural University (Grant No.2021-08).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.1016/j.fshw.2022.07.063.
杂志排行
食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil
