Effects of resistant starch III on the serum lipids levels and gut microbiota of Kunming mice under high-fat diet
2023-01-21XuhuiChenZhirongWangDiWangJianquanKan
Xuhui Chen,Zhirong Wang,Di Wang,Jianquan Kan,*
a College of Food Science,Southwest University,Chongqing 400715,China
b Laboratory of Quality&Safety Risk Assessment for Agro-products on Storage and Preservation (Chongqing),Ministry of Agriculture and Rural Affairs of the People’s Republic of China,Chongqing 400715,China
Keywords:Novelose 330 Hypolipidemic effect Cecal environment Gut microbiota change
ABSTRACT Resistant starch III (RS3),as a prebiotic,provides health benefits.This study aimed to investigate the role of RS3 in lowering serum lipids and regulating gut microbiota by administering Novelose 330 to Kunming(KM) mice.The results demonstrated that RS3 intervention significantly decreased body weight,food intake,levels of serum total cholesterol (TC),triglyceride (TG),low-density lipoprotein cholesterol (LDL-C),and liver fat.RS3 could remarkably improve the quality of the entire cecum,quality of the cecal wall,and wall surface area of mice;enhance the moisture content;and reduce the pH value.Moreover,the decrease in the liver TC content and the increase in the fecal TC content were related to RS3 intervention.The concentrations of total short-chain fatty acids (SCFAs) in the colon and individual levels of acetate,propionate,and butyrate increased with RS3 supplementation.An Illumina-based sequencing approach showed that RS3 notably increased the Bacteroides/Firmicutes ratio in the mice fed a high-fat diet.At the genus level,the relative abundance of Bacteroides,Ruminococcus,and Bifidobacterium and the number of main SCFA producers increased in the mice fed an RS3 diet.These findings provided insights into specif ic gut microbiota shifts to the hypolipidemic effect of RS3.
1.Introduction
The prevalence of obesity accompanied by hyperlipidemia has increased among almost all age and socioeconomic groups with the alterations in lifestyle and diet structure in recent years [1].The long-term intake of a high-fat and a high-energy diet can lead to fat accumulation and high lipid levels in the body [2].In particular,hyperlipidemia is the main risk factor for chronic metabolic diseases such as diabetes,atherosclerosis,and cardiovascular disease [1].To combat this epidemic,controlling and lowering the serum lipid levels are important measures to prevent and treat these diseases.Several published studies have proved that dietary interventions,including starch-derived functional compositions,could control and ameliorate obesity in a safe,accessible,and low-cost manner [3].Resistant starch(RS) has received considerable attention because of its health benef its in hyperlipidemia and related metabolic syndrome [4,5].
RS is considered as a prebiotic that has natural enzymatic resistance and can successfully escape the digestion of the small intestine [6].RS has been classified into f ive types according to the starch source and enzymolysis resistance (RS1–RS5) [7].In detail,RS1,RS2,RS3,RS4,and RS5 are defined as physically entrapped,ungelatinized,retrograded,chemically modified,and amylose-lipid complexed starches,respectively [8].Unfortunately,RS1 and RS2 encounter significant losses during processing (mainly milling) and heating compared with RS3.In addition,RS3 is mainly formed during production and food processing and is the major component of RS in human diet;it still maintains anti-enzymatic stability after heating treatment [9].Hence,RS3 can be widely used as a functional food with low calorie and high nutrition and,therefore,has the most potential commercial application.
An increasing number of studies elucidated that abundant microorganisms are present in the intestine of adult animals for a long-term coexistence and dependence,forming a highly complex intestinal microecosystem [10].Zhang et al.[11]suggested that the gut microbiota was a necessary medium for translating food intake signals and transmitting the nutrition to the body.Studies supported the beneficial effects of RS3 in promoting the growth of beneficial microbiota and inhibiting harmful microbial populations that produced a range of potentially pathogenic microbial species [12].Interestingly,the gut microbiota via RS3 administration can undergo complex interactions in regulating lipid metabolism between the gastrointestinal tract and its host during fat storage and maturation,efficiently controlling body weight and changing the serum lipid levels [13,14].In addition,evidence revealed that RS with different crystallinity types and physical surfaces affected microbiota competition and utilization [15].However,previous studies focused more on the prebiotic effects of RS3in vitro[16].The research on intestinal microbiotain vivois more complicated than thatin vitro.The research on the relationship between the structural properties and the hypolipidemic effect of RS3in vivois limited to date.
Therefore,this study was performed to investigate the effects of RS3 (Novelose 330) on serum lipid profile,intestinal environment(cecal weight,surface area,cecal content moisture,and pH),variation in the gut microbiota of KM male mice fed a high-fat diet (HFD),the relationship between structural features and physiological function of RS3in vivo.The aim of this study was to provide the experimental basis by which Novelose 330 with fine structural features of starch could shift the gut microflora of KM mice to regulate the serum lipid levels and lipid metabolism.
2.Materials and methods
2.1 Materials
Novelose 330 (containing 41% RS3 obtained from high-amylose maize starch) was supplied by the National Starch and Chemical Company (USA).Casein was obtained from Zhengzhou TianTong Shipinpeiliao Co.(China).Soybean oil was acquired from Chongqing Grain Group Co.,Ltd.(China).Acetic acid and crotonic acid (standard,purity >99.7%) were obtained from Johnson Matthey (London,UK).Propionic acid,butyric acid,and isobutyric acid (standard,purity >99%) were purchased from Tokyo Chemical Industry Co.,Ltd.(Japan).Other chemicals were of reagent grade.
2.2 Animals and diets
Forty-eight healthy male KM mice weighing (20 ± 2) g (4 weeks old and SPF grade) were purchased from the Institute of Chongqing Traditional Chinese Medicine (permitted by SCXK 2012-0006[Chongqing]).They were housed under controlled environmental conditions ((24 ± 2) °C;normal day/night cycle (08:00 on;20:00 off);and humidity of (50 ± 10)% .They were acclimated by feeding an AIN-93G diet (Table 1) for 1 week and allowed free access to food and water.Subsequently,48 mice were randomly divided into three groups with eight mice in each group,using a randomized design based on weight.They were assigned to the following three dietary groups (n=16 per group,two mice in the same group housed per cage): negative control (NC) group fed a normal AIN-93G diet,blank control (BC) group fed an HFD with additional 100 g/kg lard and 10 g/kg cholesterol,and RS3 group fed an HFD with excess 15 g/kg RS3 (36.585 g/kg Novelose 330).The mice were given the aforementioned dietary regimen for 4 weeks.The composition of the experimental diets is shown in Table 1.All experimental procedures were approved by the Animal Care and Use Committee of Southwest University (Permit SYXK 2009-0002) and strictly conducted following the International Guiding Principles for Biomedical Research Involving Animals.
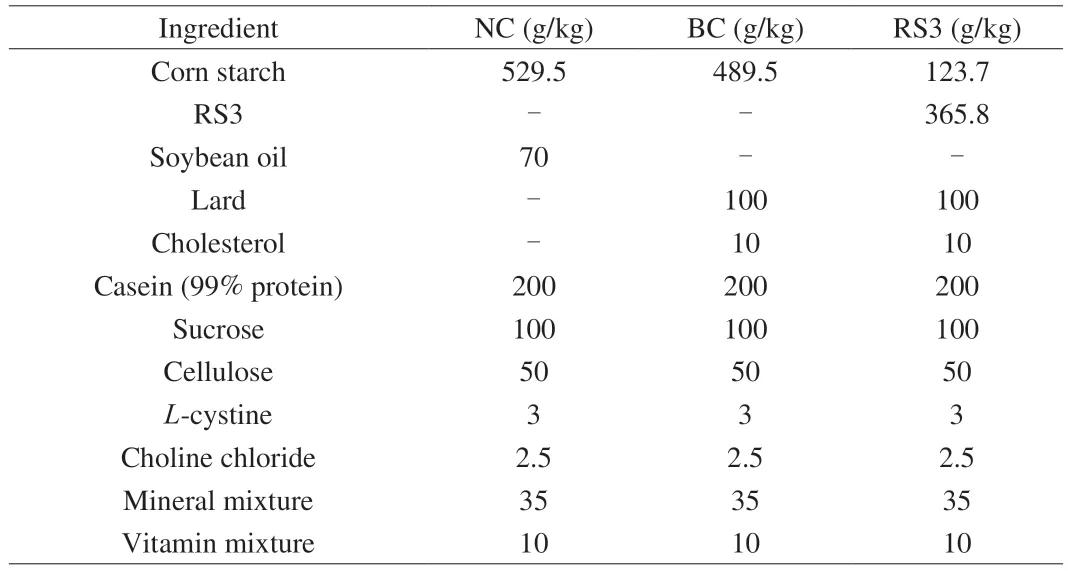
Table 1 Composition of experimental diets.
2.3 Sample collection
After treatment,the mice were weighed,fasted overnight,and lightly anesthetized [17].After decapitation,blood was collected from the eye sockets of mice and transferred into heparinized tubes.The serum samples were obtained through centrifugation at 1 500 ×gfor 15 min at 4 °C and stored in 1.5 mL centrifuge tubes at–80 °C until analysis [18].The liver was removed,washed,dried,weighed,and then stored at–80 °C until analysis.The cecum of each mouse was removed from the abdomen and weighed together with the contents.Approximately 0.2 g of fresh cecal contents of each mouse were placed in test tubes,and their pH was determined.An appropriate amount of fresh cecal contents was placed in weighing bottles to determine their water content.Finally,the cecum of each mouse was flushed,dried,weighed,and then stored at 4 °C for determining the cecum surface area.The colon contents were collected and freeze-dried.Up to 0.2 g of colon contents of each mouse were placed in 2 mL microcentrifuge tubes and then stored at–20 °C for short-chain fatty acids (SCFAs) determination;the other colon contents were stored at–80 °C for DNA extraction to determine the gut microbiota.
2.4 Serum lipid analysis
The levels of total cholesterol (TC),triglyceride (TG),lowdensity lipoprotein cholesterol (LDL-C),and high-density lipoprotein cholesterol (HDL-C) in the serum were analyzed using assay kits(Jiancheng Bioengineering Institute,Nanjing,China).The atherogenic index (AI) and anti-atherogenic index (AAI) were calculated using the following equation:

Total lipid content in the liver was extracted according to the method proposed by Folch et al.[19]and subsequently measured gravimetrically [20].
2.5 Liver histopathological analysis
The liver tissue of the mice was washed with cold saline and then fixed in 10% formaldehyde overnight.Subsequently,the specimens were subjected to standard procedures,including dehydrating in graded ethanol,embedding in paraffin,staining with H&E dye,and examining microscopically.
2.6 Analysis of intestinal environment factors
The pH value and water content of fresh cecal contents were determined as described by Shen et al.[21].The cecal surface area was measured as described by Loeschke et al.[22]with some modifications.The cecum was spread and delineated on scale papers to obtain the profiles of cecum walls.The profiles were copied to new papers and then dried to constant weights (m1,accurate to 0.001 g).Meanwhile,the per unit area (1 cm2) weights (m2,accurate to 0.001 g)for each paper were measured to calculate the cecal surface area as the following equation:
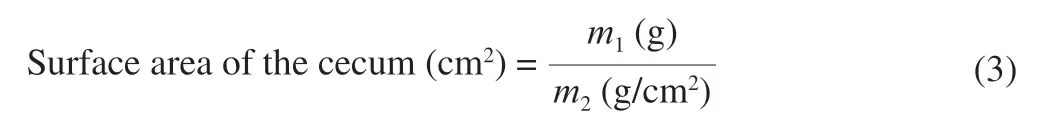
The SCFAs concentrations of colon contents were analyzed using a 7890A gas chromatograph (Agilent Technologies,CA,USA)equipped with a DB-WAX capillary column (122-7032,30 m ×0.25 mm,0.25 μm,Agilent Technologies) as previously described with slight modification [23].The heating procedures were as follows: the initial column temperature (90 °C) was maintained for 0.5 min,increased to 150 °C at 5 °C/min,and then held for 3.0 min.The detector temperature was 230 °C,the hydrogen flow rate was 40 mL/min,the airflow rate was 400 mL/min,and the nitrogen flow rate was 30 mL/min.
2.7 Determination of fecal and liver tissue cholesterol levels
The sample processing methods were performed as described by Kim et al.[24].Subsequently,the feces of treated mice were dried to constant weight and ground to a uniform powder;0.1 g of feces was accurately weighed and extracted using methanol-chloroform (2:1,V/V) mixture for 1 h at 45 °C.The contents of fecal cholesterol were determined using theo-phthalaldehyde (OPA) method [25].
The liver tissues (1 cm3) were ground uniformly,placed in 50 mL centrifuge tubes,mixed with 10 mL of methanol-chloroform (2:1,V/V)in a water bath extract at 45 °C,and freeze centrifuged (1 400 ×g,20 min,4 °C).Subsequently,1.2 mL of physiological saline was added to the collected chloroform liquid and separated using freeze centrifugation (1 400 ×g,10 min,4 °C).These steps were repeated to collect the chloroform layer,which was blow-dried in the fume hood.Then,160 µL of isopropanol and Triton-100 (9:1,V/V) were added for resolution.Next,240 μL of ultrapure water was added and evenly blended.The obtained solution was the total fat of the liver tissue.
2.8 DNA extraction and barcoded pyrosequencing
2.8.1 DNA extraction
The genomic DNA of colon microbiota from NC,BC,and RS3 groups (n=3) was extracted from pooled colon contents using a TIANamp Stool DNA Kit following the manufacturer’s specifications.The DNA was quantified using a NanoDrop 2000 spectrophotometer(Thermo Scientific,MA,USA) at OD260nm,where the concentration and purity were assessed using theA260nm/A280nmratio.Universal primers 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 533R(5’-TTACCGCGGCTGCTGGCAC-3’) were used to amplify the hypervariable V3–V4 regions by polymerase chain reaction (PCR)of the 16S rDNA gene.The purified PCR products were pooled in equimolar ratios,and pyrosequencing was performed on an Illumina HiSeq platform (Novogene bio Mdt InfoTech Ltd.,Beijing,China)following the manufacturer’s protocols.Paired-end sequencing was employed to construct a small fragment library.Raw sequence data were deposited into the NCBI sequence read archive database (https://www.ncbi.nlm.nih.gov/sra) under Accession no.SRP071820.
2.8.2 Bioinformatics and statistical analysis
Raw pyrosequencing reads were merged and filtered according to the unique barcode using FLASH software [26]and QIIME software (Version,1.7.0,http://qiime.org/) [27].Chimera sequences were identified and removed using a UCHIME algorithm to collect the high-quality clean tags [28].Reads with low-quality scores and short lengths and those that did not contain exact matches with the primer sequence were removed using PRINSEQ (Version 0.20.4,http://prinseq.sourceforge.net/) [13].The obtained tags were clustered into operational taxonomic units (OTUs) using UPARSE software based on 97% sequence identity [29].These OTUs were analyzed by a Greengenes database using PyNAST software and annotated to taxonomic information (family and genus) [30].α-Diversity andβ-diversity were analyzed using QIIME software and R software.The taxonomy of all high-quality sequences at the phylum and genus levels was selected to recalculate the proportion with the R software package (http://cran.r-project.org/) [31].
2.9 Statistical analyses
The results were expressed as the “mean ± standard deviation” of the indicated number of experiments.The data were analyzed by One-Way analysis of variance with SPSS 20.0 software (IBM).The figures were drawn using GraphPad Prism (version 7.0).P<0.05 indicated statistically significant differences.
3.Results
3.1 Morphological analysis
The crystallographic structures of the RS3 sample (Novelose 330) are presented in Fig.1A.A strong peak in the diffractogram of RS3 was observed at 2θat 21.8° and weak peaks at 5.423°,14.951°,16.776°,19.726°,21.837°,22.104°,and 23.887°,which presented a B-type intermediate diffraction pattern,suggesting a high degree of crystallinity of RS3.As shown in Fig.1B,the RS3 particles had smooth surfaces with uneven fold structures.Also,starch granules were regular in shape with different sizes.
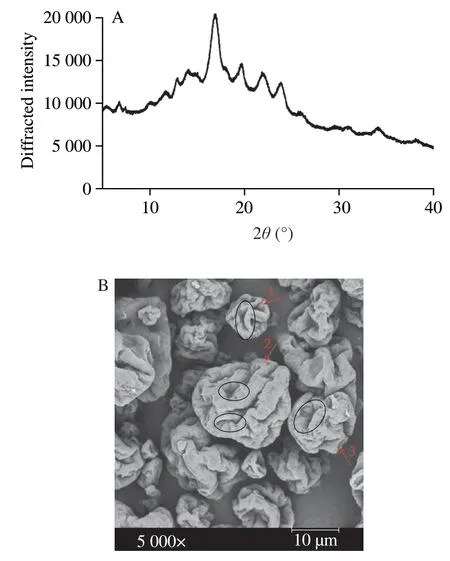
Fig.1 X-ray diffraction pattern (A) and scanning electron micrograph (B)of RS3.The red arrows and numbers represent regular starch structure with different granule sizes.While the black circles represent a smooth surface with fold structures.
3.2 Effects on body weight and food intake
The effects of RS3 administration on the body weight and food intake of mice are presented in Fig.2 and Table 2.The body weight gain of mice was lower in the RS3 group than in the BC group(P<0.01),but higher than that in the NC group (P<0.05).Given that an increased food intake corresponded to increased growth [32],the food intake was significantly lower in the RS3 group than in the BC group (P<0.05) and was significantly higher than that in the NC group (P<0.05).
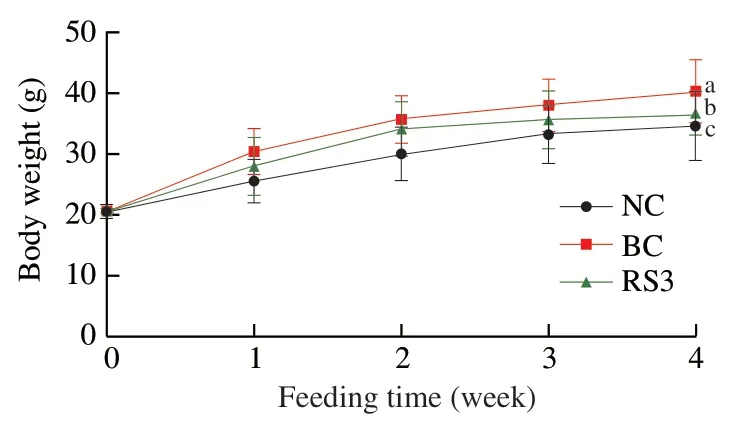
Fig.2 Effect of different feed types on body weight in mice.Significant differences are indicated with different letters (a,b and c) (P <0.05).
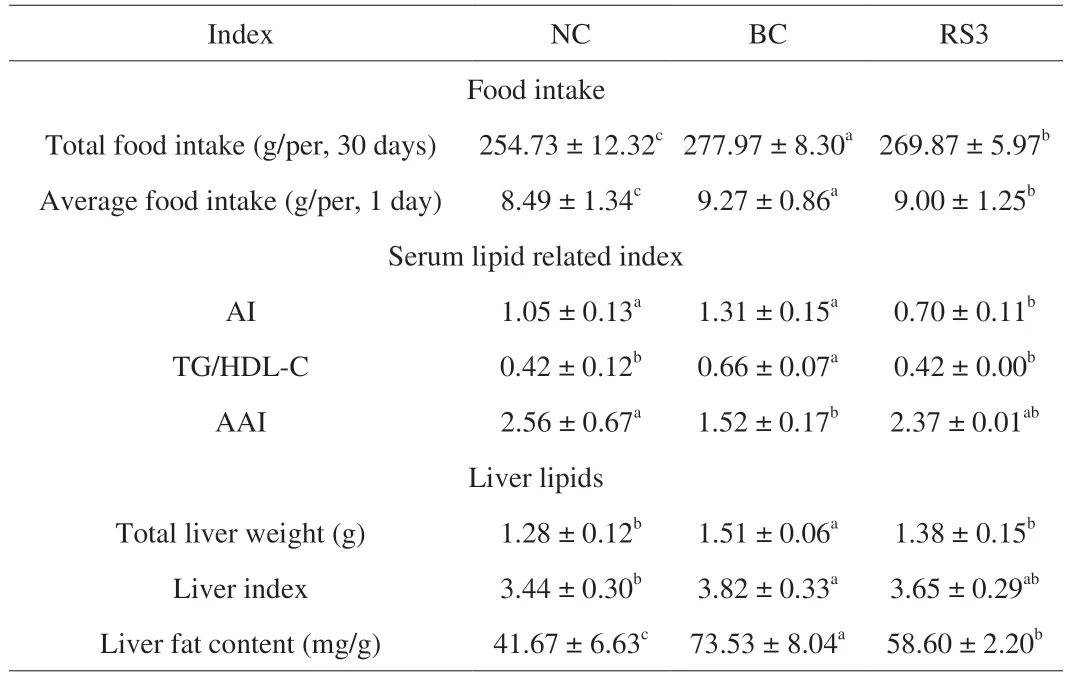
Table 2 Effect of different feed types on the food intake,atherogenic index,and liver lipids of mice.
3.3 Serum lipid and liver lipid concentrations
The effects of different feed types on serum lipid levels in mice are shown in Fig.3.The serum levels of TC,TG,and LDL-C were significantly higher in the BC group than in the NC group (P<0.05).Compared with the BC group,the mice in the RS3 group showed significantly lower levels of TC and TG (P<0.05) but higher HDL-C levels (P<0.05).The risk of atherosclerosis and coronary heart disease was reflected by the AI value.The AI value in the RS3 group was lower than that in the BC group (P<0.05) (Table 2).In contrast,the AAI value in the RS3 group was significantly higher compared with that in the BC group (P<0.05) and showed no significant difference compared with that in the NC group.
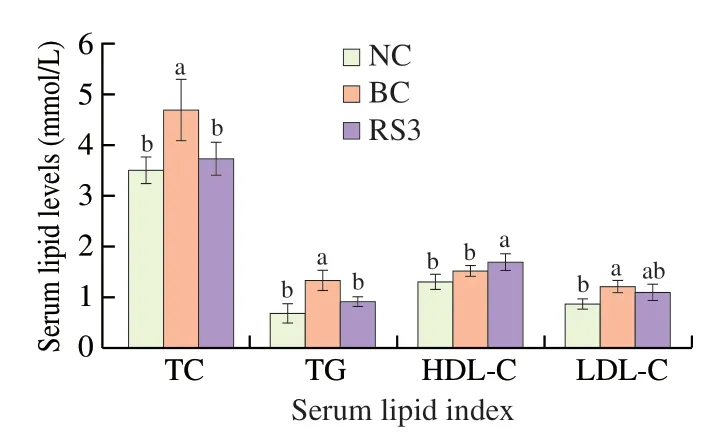
Fig.3 Effect of different feed types on serum lipids in mice.a,b,and c mean values of the same index in the three groups with unlike letters are significantly different (P <0.05).TC,total cholesterol;TG,triglyceride;HDL-C,highdensity lipoprotein cholesterol;LDL-C,low-density lipoprotein cholesterol.
As shown in Table 2,the total liver weight,liver index,and liver fat content of mice were significantly higher in the BC group than in the NC group (P<0.05).Compared with the BC group,the total liver weight and liver fat content in the RS3 group significantly decreased(P<0.05),while the liver index was not noticeably reduced.
3.4 Cecal indexes and cholesterol contents
The cecal weight,surface area,and contents were determined(Table 3) as a measure of indigestible residues (starch,dietary fiber,and so on) and fermentative activity [33].No significant difference was observed in these indexes between the NC and BC groups except the surface area of the cecal wall (P<0.05).Compared with the BC group,the RS3 group showed significantly increased cecal weight,cecal wall weight,surface area,and water content of the cecal contents (P<0.05) and significantly lower pH value of the cecal contents (P<0.05).In addition,the liver and fecal TC were significantly higher in the BC group than in the NC group (fecal TC,P<0.05).The liver TC was significantly lower in the RS3 group than in the BC group (P<0.05),while the fecal TC showed the opposite results.
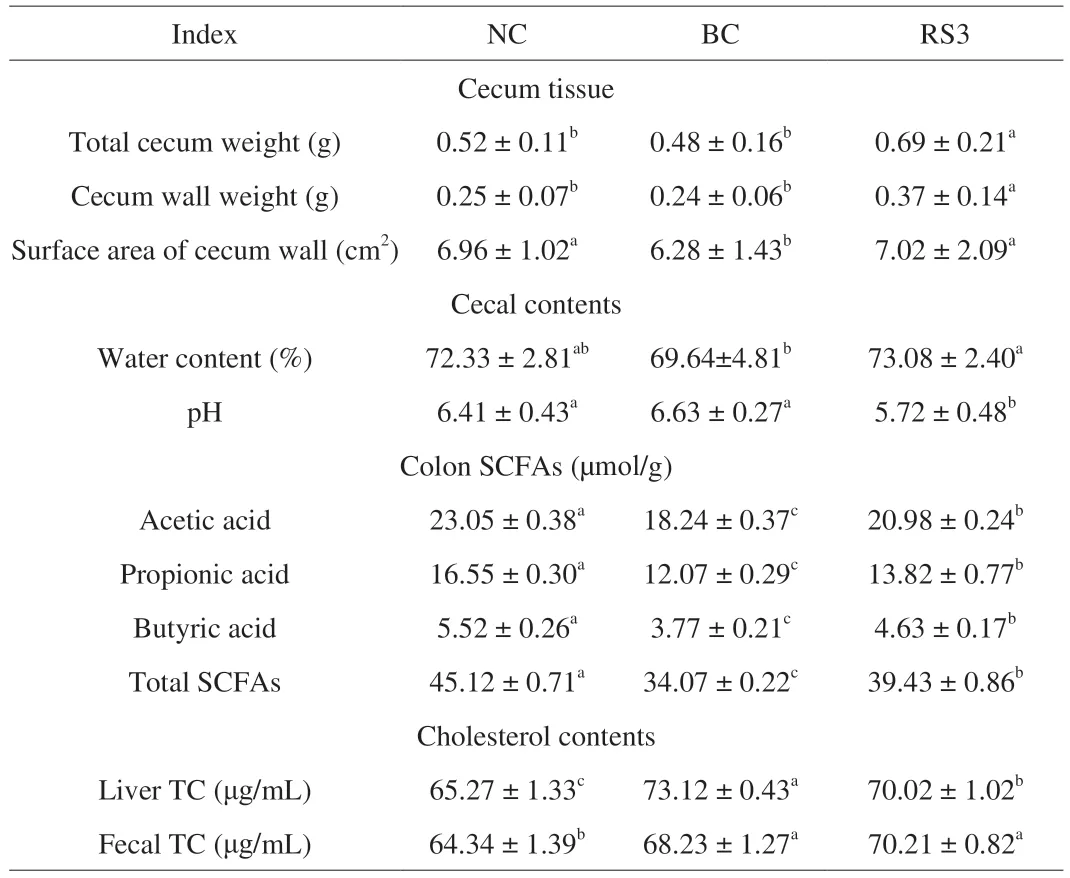
Table 3 Effect of different feed types on the intestinal environment factors and cholesterol contents.
3.5 Liver histopathological changes
As shown in Fig.4,the histopathological analysis of liver tissues showed that the cells were neatly arranged,and the nuclei were round and obvious in the NC group (Fig.4A).However,the liver morphology of mice in the BC group exhibited a disordered cellular structure with cytoplasmic vacuolation and accumulation of lipid droplets (arrows in Fig.4B).Comparatively,the accumulation of liver lipid droplets was relatively less,and liver damage was relieved in the RS3 group (Fig.4C).
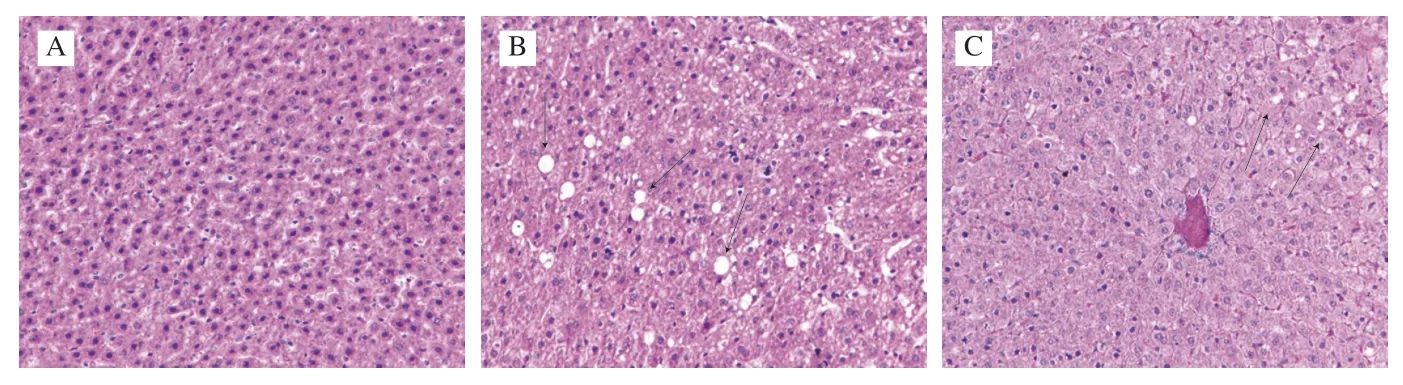
Fig.4 Liver histopathological analysis of mice in different groups (magnification of 400×).NC group (A);BC group (B);RS3 group (C);Black arrows indicate vacuolation of cytoplasm and accumulation of lipid droplets.
3.6 SCFAs in the colon contents
As shown in Table 3,the generation of SCFAs in the frozen colon contents was determined.The levels of acetate,propionate,butyrate,and total SCFAs were significantly lower in the BC group than in the NC group (P<0.05).After the RS3 interventions,the concentrations of total SCFAs and individual levels of acetate,propionate,and butyrate were significantly higher than those in the BC and NC groups(P<0.05).
3.7 Gut microbiota
The intestinal tract is considered a “superorganism” containing abundant gut microorganisms that form a dynamic and diverse microbial community.As shown in Fig.5A,α-diversity indexes of gut microbiota,including the observed species,Chao1,Ace,and Shannon,were significantly lower in the BC group than in the NC and RS3 groups (P<0.05),indicating that community biodiversity was reduced by the HFD.However,the Simpson and Coverage indexes in the three groups showed no significant difference.
The top five bacterial phyla were identified in colon content samples (Fig.5B).Almost all sequences (accounting for >95% of the reads) belonged to the following five phyla: Bacteroidetes,Firmicutes,Verrucomicrobia,Proteobacteria,and Actinobacteria.Bacteroidetes and Firmicutes had the major proportion of reads,but the ratio of Bacteroidetes and Firmicutes (B/F) changed with different diet interventions.The relative abundance of Bacteroidetes (45.68%)was higher than that of Firmicutes (33.53%) in the NC group.However,the relative abundance of Bacteroidetes in the BC group was 40.93%,which was lower than that of Firmicutes (43.28%).The RS3 intervention altered the proportion of Bacteroidetes (46.13%)and Firmicutes (40.44%).As shown in Fig.5C,the ratio of B/F in mice fed normal diet,HFD,and RS3 diet was 1.36,0.95,and 1.14,respectively.
At the genus level,the top 10 most abundant bacterial genera were ranked to exhibit an intuitionistic relative abundance in gut microbiota(Fig.5D).The results showed that some dominant genera were dramatically altered,showing significant differences in each group of mice fed different diets.The most notable changes at the genus level were observed in the three groups includingBacteroides(relative abundance of (10.94 ± 1.30)%,(7.95 ± 0.82)%,and (8.53 ± 1.12)% for NC,BC,and RS3 groups,respectively),Ruminococcus(relative abundance of (0.55 ± 0.05)%,(0.48 ± 0.60)%,and (4.61 ± 0.20)% for NC,BC,and RS3 groups,respectively),Lactobacillus(relative abundance of (1.55 ± 0.20)%,(6.85 ± 0.50)%,and (6.57 ± 0.34)% for NC,BC,and RS3 groups,respectively),andBifidobacterium(relative abundance of (1.09 ± 0.20)%,(3.32 ± 0.25)%,and (4.61 ± 0.32)% for NC,BC,and RS3 groups,respectively).
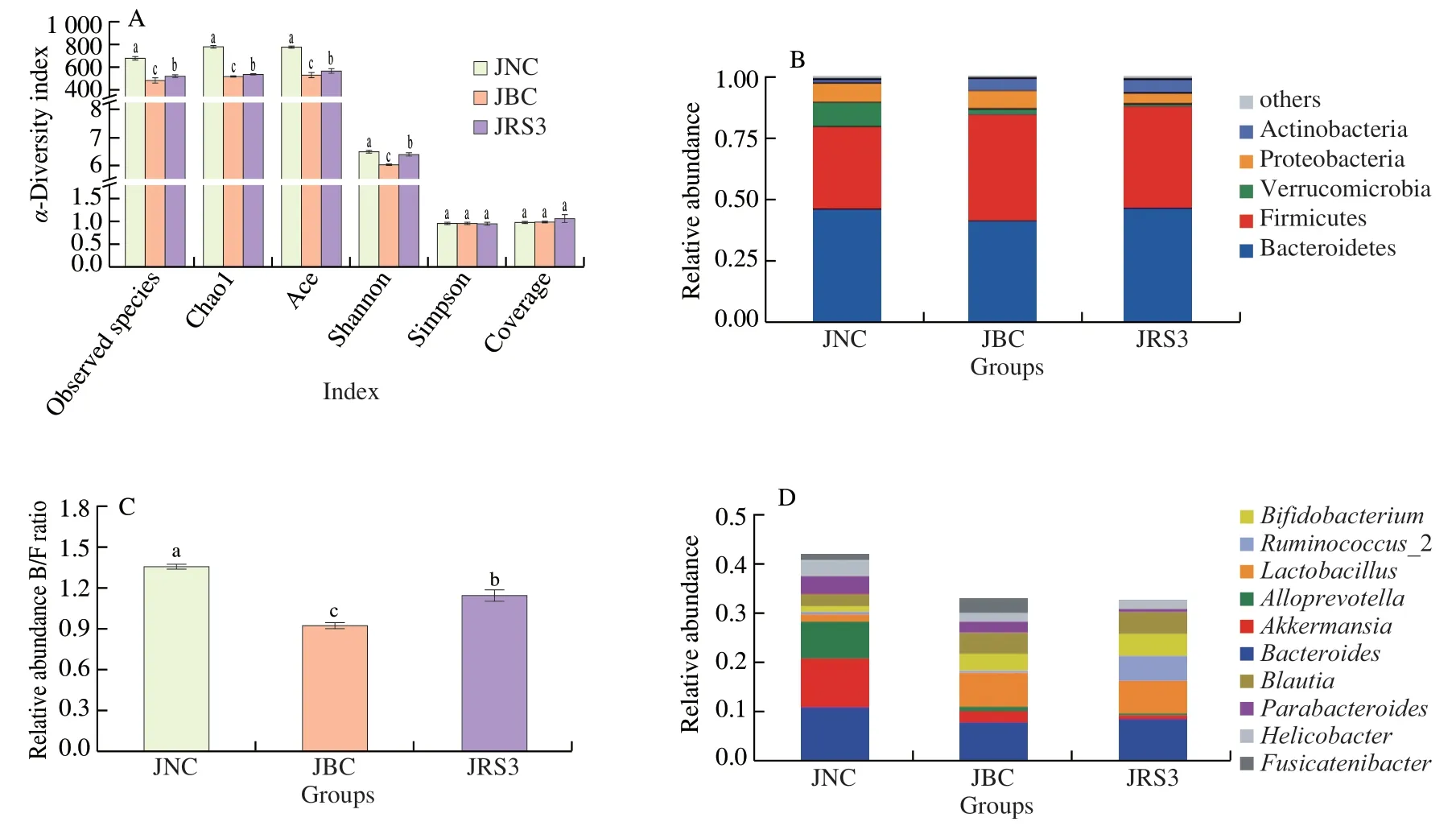
Fig.5 Gut microbiota composition in mice colon for the three groups.(A) α-Diversity indexes of gut microbiota including observed species,Chao1,Ace,Shannon,Simpson and Coverage of each group;(B) Bacterial community abundance at the phylum taxa level of each group;(C) Relative abundance ratio of Bacteroides/Firmicutes (B/F);(D) Bacterial community abundance at the genus taxa level of each group.a,b and c indicate that the difference of α-diversity indexes is significant (P <0.05).
Moreover,the study focused on the enteric bacteria (Fig.6)(the main producers of SCFAs),including Lachnospiraceae_NK4A136_group,Blautia,Bifidobacterium,Ruminococcus_2,Ruminofilibacter,andRoseburia,among which Lachnospiraceae,Roseburia,andBifidobacteriumwere the main butyrate-producing bacteria [34,35].Interestingly,the result revealed that the abundance of all these bacteria was markedly increased by RS3 intervention(P<0.05).Taken together,compared with the HFD diet,the RS3 diet could significantly promote the growth and reproduction of SCFA producers.
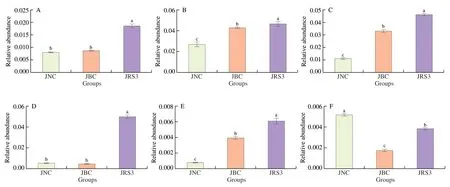
Fig.6 The relative abundance of short chain fatty acids-producing bacteria.(A) Lachnospiraceae_NK4A136_group,(B) Blautia,(C) Bifidobacterium,(D)Ruminococcus_2,(E) Ruminofilibacter,(F) Roseburia.
4.Discussion
Excessive consumption of high-calorie food is the leading cause of obesity,which has become a worldwide epidemic with an increasing risk of hyperlipidemia and related complications.Functional RS as a dietary food can exert potential effects on obesity.The interventional effect of Novelose 330 on serum lipid indexes,intestinal environment,and gut microbiota was investigated in HFDfed rats.The data demonstrated that that RS3 could reduce weight gain (Fig.2) and serum lipid levels (Fig.3),decrease the fat content in the liver (Fig.4),ameliorate the cecal environment (Fig.3),alter the gut microbiota (Fig.5),and increase the abundance of SCFAproducing-bacteria (Fig.6) in KM mice fed an HFD.
Many kinds of RS3 are available on the market,which have different contents of amylose,crystal type,and molecular structure,affecting consumption and utilization.Owing to the health benefits of RS3,studies have claimed that the shifts of gut microbiota are mainly impacted by the utilization of RS3 with different fine structures and crystallinity types [36].Previous studies suggested that RS3 with B-type crystallinity had a longer-chain and more proportional ordered double-helix configuration,compared with A-type and C-type crystallinity,which made it more resistant to starch decomposition and digestion,impacting the bacterial fermentation pattern [37,38].RS3 used in this study was Novelose 330,which was derived from high-amylose maize starch;RS3 already lost the granular structure during modification [39].The structural properties of RS3 exhibited B-type crystallinity and a smooth physical surface with a fold structure,which possessed high enzymatic resistance (Fig.1).A study reported that Novelose 330 was mainly degraded and fermented in the upper part of the large bowel [40].Hence,the physical accessibility and generation of fermentation products for Novelose 330 substrates occurred in the cecum and proximal colon of KM mice in the HFD model,resulting in the change in the cecal environment,favoring the production of SCFAs and gut microbiota,as well as ultimately affecting the lipid metabolism to varying degrees.
The findings of this study were based on the animal model unlike other studies,and RS3 with starch had a fine structure.The body weight and food intake tests indicated that,compared with control HFD (BC group),Novelose 330 inhibited the increase in body weight and feed intake.Other studies demonstrated that reduced body weight was associated with the fermentation of RS in animal models,such as Sprague-Dawley rats [41],Goto-Kakizaki rats [42],and C57BL/6J mice [43].Likewise,the present study found that RS3 effectively reduced the body weight of KM mice (Fig.1).Further,the RS3 diet lowered daily and monthly food intake in HFD mice (Table 2).A possible reason was that the sources of RS3 could induce satiety and impact energy consumption,thereby causing a decrease in food intake.
In the last 20 years,evidence from clinical researches proved that increased LDL-C and reduced TC,TG,HDL-C levels were potential indicators of hyperlipidemia,leading to coronary heart disease and atherosclerosis [44].Hence,lowering serum lipid profile and levels of relevant markers is deemed an important measure to prevent and cure such diseases [45].The liver is known as a crucial organ to examine lipid and cholesterol metabolism.RS3 could regulate lipid metabolism by significantly reducing the levels of TC,TG,and LDL-C (P<0.05) (Fig.3)and relieving liver damage (Fig.4) and related hyperlipidemia syndrome(Table 2) in HFD mice.Meanwhile,the decrease in AI and the increase in AAI were in line with the changes in serum lipid levels (Table 2),which might improve cholesterol profile,accommodate the levels of blood lipids in HFD mice,and lessen the incidence of atherosclerosis.
The fermentation process of Novelose 330 occurred in the upper part of the large bowel as mentioned earlier and produced SCFAs,such as acetate,propionate,and butyrate,which were important substrates for maintaining intestinal epithelium and gut health [46].Furthermore,an obvious advantage of RS3 was associated with the low calorie load because SCFAs generated less than 10% of body energy reserves [21].The results demonstrated that RS3 intervention could notably increase the SCFA concentrations in the colon (Table 3)in mice fed an HFD diet,which was in line as described by Qian et al.[47].Corresponding to the changes in SCFA contents,the HFD enhanced the pH values of cecal contents,whereas the RS3 diet changed this situation significantly by lowering the cecal pH to normal levels (Table 3).It was inferred that the positive effects of SCFAs were attributed to the production and utilization of RS3 fermentation by gut microbiota,thus leading to the alteration in microbial composition and regulation of lipid metabolism.In addition,the liver TC of mice in the RS3 group significantly reduced(P<0.05),and the fecal TC increased compared with that in the BC group (Table 3).These results suggested that RS3 promoted cholesterol excretionin vivo,which might be because cholesterol decomposition conjugated cholesterol co-precipitation with the excretion of feces.Additionally,this study found that the consumption of the RS3 diet effectively increased the cecal weight,surface area,and water contents,suggesting that an increase in the rate of gut fermentation was linked to an increase in tissue weight and lowering of pH values [48,49].These phenotypic changes were associated with a larger cecum [41]and altered colon microbiota [50].In short,SCFA production and the physiological environment of the cecum play an important role in anti-obesity and hyperlipidemia induced by an HFD;the mechanism might be closely associated with the moderating effect of lipogenesis and lipolysis.
Gut microbiota is a considerable hidden metabolic organ of the body;an imbalance in gut microbiota is closely related to overweight and obesity [51].The high-throughput sequencing data elucidated thatα-diversity indexes were enhanced by the RS3 intervention in mice fed an HFD diet.This finding might be attributed to the changes in dominant bacterial composition and metabolites in the colon after the administration of RS3.Previous studies found that RS entering the gut could alter the colonic physiology via fermentationcoupled mechanisms,thus influencing lipid metabolism [4].The result indicated that the B/F ratio was higher in the RS3 group than in the BC group (Fig.5C),which was in accordance with the observed trend in a previous report [52].An increased ratio of B/F might make a significant contribution to reducing overall energy consumption,further lowering the lipid and cholesterol levels [53].It was hypothesized that Novelose 330 might influence some specific bacteria,thereby promoting SCFA production and facilitating lipid metabolism.The relative abundance of top 10 identified genera and related SCFA producers were presented to identify the potentially specific bacteria (Fig.5).The relative abundance ofBacteroides,Ruminococcus_2,andBifidobacteriumsignificantly increased and the relative abundance ofFusicatenibactermarkedly decreased after feeding RS3 diet.This alteration in bacterial composition was caused by the RS3-diet intervention,which also ascribed to the fine structure of starch obtained for analyzing structural characteristics,thus potentially alleviating the lipid metabolic disturbance of the host.In addition,Bacteroideseffectively improved carbohydrate fermentation to produce the total SCFAs,which also affected the metabolism of bile acid and steroids [54].RS3 intervention could increase the relative abundance of beneficialBifidobacteriumfollowing the consumption of RS3,directly inducing the intestinal immunity system.The notable increase inRuminococcus_2 abundance might be attributed to fermenting RS3 substrates because reports suggested thatRuminococcusplayed a key role in the degradation of dietary RS to small molecular fragments [55].The decrease inFusicatenibacterabundance in the RS3 group was associated with improved lipid metabolism after RS3 intervention.In accordance with these findings,Wang et al.[56]revealed that the abundance ofFusicatenibacterincreased in patients with hyperlipidemia compared with healthy volunteers.The main producers of SCFAs are listed in Fig.6.In addition to specific bacterial genera mentioned earlier,RS3 intervention could enhance the relative abundance of Lachnospiraceae,Blautia,Roseburia,andRuminofilibacter.Members of the family Lachnospiraceae are known for their ability to synthesize SCFAs by the fermentation of dietary carbohydrate [57].Another carbohydrateconsuming microbe wasRuminofilibacter,which was associated with the fermentation of cellulose and the production of methane and promoted the fermentation of RS3 substrates.In addition,previous studies indicated thatBlautiaandRoseburiawere the major SCFA producers [5].
Collectively,the results demonstrated the hypolipidemic effects of RS3 and showed that RS3 could contribute to gut health by shortterm (4 weeks) dietary supplementation.Linking the gut microbiota shifts with serum lipid levels and SCFA production further explained the beneficial effects of RS3.Further studies should assess the contribution of dominant bacteria to the hypolipidemic effects of RS3 using a colonization model system.
5.Conclusions
This study was novel in investigating the influence of Novelose 330 intervention on the KM mice model fed an HFDin vivo,in terms of hypolipidemic effect,production of beneficial SCFAs,and shifts of the gut microbial community.Remarkably,RS3 intervention could decrease body weight and food intake and lower the serum lipid profiles and accumulation of liver adipose.RS3 could ameliorate the cecum environment of mice fed an HFD and increase the levels of SCFAs in the colon.Moreover,the administration of RS3 could increase the abundance of SCFA-producing bacteria.The RS3 degradation in the gut was associated with the ratio of B/F.In addition,the relative abundance ofBacteroides,Ruminococcus,andBifidobacteriumand main SCFA producers increased at the genus level,which might be ascribed to the regulatory effect of RS3 on lipid metabolism.Thus,this study might be of great significance in modulating the lipid metabolism of RS3 and instrumental to the development of RS3-based functional food.
Conflicts of interest
There are no conflicts of interest to declare.
杂志排行
食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil
