Structural characterization and immunostimulatory activity of a water-soluble polysaccharide from abalone (Haliotis discus hannai Ino) muscle
2023-01-21LinfnShiGengxinHoJunChenJunlingWngWuyinWeng
Linfn Shi,Gengxin Ho,Jun Chen,Junling Wng,Wuyin Weng,b,*
a College of Ocean Food and Biological Engineering,Jimei University,Xiamen 361021,China
b Engineering Research Center of the Modern Technology for Eel Industry,Ministry of Education,Xiamen 361021,China
Keywords:Polysaccharide Abalone muscle Structural characterization Immunostimulatory activity Nuclear magnetic resonance spectra
ABSTRACT A water-soluble polysaccharide from abalone muscle (AMPP) was isolated.The contents of carbohydrate,protein,uronic acid,and sulfate in AMPP were 83.5%,0.5%,2.7%,and 2.6%,respectively.High-performance liquid chromatography analysis indicated that AMPP was homogeneous and had an average molecular weight of approximately 3.2 kDa.The main monosaccharides of AMPP were glucose (Glc) and mannose with a molar ratio of 99.7:0.3.The structural characteristics of AMPP were elucidated through methylation analysis,Fourier transform infrared spectroscopy,and nuclear magnetic resonance spectroscopy.The linkages of AMPP consisted of terminal,1,4-linked,1,6-linked,and 1,4,6-linked Glcp with a molar ratio of 3.1:7.2:1.0:2.5.In one repeat unit of the proposed AMPP structure,the backbone chain was composed of eight 1→4 glycosidic bonds and one 1→6 glycosidic bond,with three branch chains linked by 1→6 glycosidic bond.In addition,AMPP was found to possess potent immunostimulatory activity via rising phagocytosis of RAW264.7 cells and promoting secretion of TNF-α.
1.Introduction
Abalone is an “archeogastropod” mollusk with a characteristic single shell,and it is considered a seafood delicacy with a high commercial value in Asian countries [1,2].Abalone is also regarded as “ginseng in the ocean” and has health-promoting effects because of its nutrients and bioactive components,including polysaccharides [3].Various polysaccharides isolated from the abalone muscle and viscera have also been studied.Zhu et al.[4]purified a neutral polysaccharide consisting of 1-,1,4-,1,6-,1,4,6-linked Glc and 1-,1,3-,1,6-,1,4,6-linked galactose (Gal) from the abalone muscle.Furthermore,several types of polysaccharides extracted from the gonad or viscera of abalone have been reported [5-8].Differences have also been found in the monosaccharide compositions of abalone polysaccharides from different regions [9].
In a previous study [10],a crude polysaccharide was extracted from abalone (Haliotis discus hannaiIno) muscle (AMP) through protease hydrolysis and ethanol fractional precipitation.Three subfractions (AMP-1,AMP-2,and AMP-3) eluted with 0,0.1,and 0.3 mol/mL sodium chloride solutions were separated from AMP via DEAE-52 column chromatography.Among the subfractions,AMP-1 shows the highest inhibitory activities against the growth of human breast cancer MDA-MB-231 and hepatocellular carcinoma HepG2 cells.Furthermore,the carbohydrate and Glc contents (percentage of the carbohydrate) of the obtained AMP-1 reach 83.8% and 99.3%,respectively [10],which differ from those of the reported polysaccharide extracted from an abalone pleopod [6].The role of the immune system is to eliminate potentially harmful substances and prevent the growth of cancer cells [11].Thein vitrocell culture studies are often used to evaluate the immunomodulatory effects of seafood derived polysaccharides.A glucan fromCyclina sinensisexhibited significant ability to stimulate RAW264.7 cells to release tumor necrosis factor-alpha (TNF-α) [12].The release of TNF-α could be significantly promoted by a polysaccharide purified from jellyfish [13].However,information on the molecular structure and immunomodulatory activity of these polysaccharides has not been published.
In the present study,an abalone polysaccharide (AMPP) was further purified from AMP-1 by using a Sephadex G-25 column.Then,the molecular weight and monosaccharide composition of AMPP were investigated.The structural characteristics of AMPP were revealed through methylation analysis,Fourier transform infrared spectroscopy (FTIR),and 1D (1H and13C),and 2D nuclear magnetic resonance (NMR) spectroscopy,including COSY,TOCSY,HSQC,HMBC,and ROESY.Last,the immunomodulatory activity of the obtained polysaccharide was also investigated to elucidate the antitumor mechanism.Our results indicated that the abalone muscle was a valuable source for polysaccharide,which exhibits immune function.
2.Materials and methods
2.1 Materials and chemicals
Glc,Gal,glucuronic acid,galacturonic acid,glucosamine,galactosamine,sodium tetraborate,sodium borohydride,acetic anhydride,pyridine,and 1-phenyl-3-methyl-5-pyrazolone (PMP)were purchased from Sigma Chemical Co.(St.Louis,MO,USA).DEAE-52 cellulose and Sephadex G-25 were procured from Shanghai Yuanye Biological Technology Co.,Ltd.(Shanghai,China).All other chemicals used in the study were analytical grade.
2.2 Purification of polysaccharide
AMP-1,which was extracted in accordance with our previous study [10],was purified on a Sephadex G-25 column (400 mm ×16 mm) and eluted with distilled water at a flow rate of 0.5 mL/min.The collected eluent was monitored using the phenol-sulfuric acid method [14].The obtained polysaccharide fraction was dialyzed and lyophilized for further analyses.
2.3 Component analysis
Carbohydrate content was determined by phenol-sulfuric acid method by usingD-Glc as a reference [14].Protein content was determined with the Lowry method by using bovine serum albumin as a reference [15].Uronic acid content was measured with them-hydroxydiphenyl method withD-glucuronic acid as a reference [16].Sulfate content was determined with the barium chloride-gelatin method by using K2SO4as a reference [17].
2.4 Monosaccharide composition
Monosaccharide composition was analyzed in accordance with the reported method through reversed-phase high-performance liquid chromatography (HPLC) [18].Briefly,2 mg of each sample was hydrolyzed with 1.0 mL of 2.0 mol/L trifluoroacetic acid at 110 °C for 6 h.The resulting hydrolysate was derivatized with PMP.Then,the PMP-labeled carbohydrates were analyzed using an HPLC system(Agilent Technologies) equipped with an Atlantis®dC18column(4.6 mm × 250 mm,5 μm) and detected at UV 245 nm and a flow rate of 0.5 mL/min.The mobile phase was 0.1 mol/L phosphate buffer(pH 6.7) containing 17% acetonitrile.Mannose (Man),rhamnose,glucosamine,galactosamine,glucuronic acid,galacturonic acid,Glc,Gal,arabinose,and frucose were used as monosaccharide standards.
2.5 FTIR spectroscopy
FTIR spectroscopy was carried out at room temperature by using a Thermo Nicolet iS50 FTIR spectrometer (Thermo Scientific,Waltham,MA,USA).The spectra in the range of 4 000–400 cm−1were rationed,and the automatic signals gained were collected in 32 scans at a resolution of 4 cm−1.
2.6 Homogeneity and molecular weight
The molecular weight of the purified polysaccharide sample was determined through high-performance gel filtration chromatography(HPGFC) on a Waters 1525 instrument equipped with a 2410 refractive index detector.The samples dissolved in the mobile phase were loaded onto an HPGFC system with tandem analytical UtrahydrogelTMLinear (300 mm × 7.8 mm,2 μm).Elution was carried out with 0.1 mol/L NaNO3at a flow rate of 0.8 mL/min.Dextran T-150,Dextran T-40,Dextran T-10,and Dextran T-5 were used as standards.
2.7 Methylation analysis and gas chromatography-mass spectrometry (GC-MS) assay
Methylation analysis was carried out in accordance with the method of Needs and Sevendran [19]with minor modifications.The purified polysaccharide sample (5 mg) was decomposed in 2 mL of dimethyl sulfoxide before NaOH was added,and the mixture was stirred at room temperature for 90 min.The mixture was added with 1.5 mL of methyl iodine and stirred in darkness for 1 h.Then,methylation was terminated by adding 4 mL of distilled water.The methylated polysaccharides were extracted with chloroform and dried through evaporation under reduced pressure.The completely methylated polysaccharides were hydrolyzed with 2 mol/L trifluoroacetic acid at 110 °C for 6 h.The obtained hydrolysates were dissolved in 2 mL of 0.2 g/100 mL NaOH,and 10 mg of NaBH4was added to reduce uronic acid.After the obtained mixture was incubated at 25 °C for 4 h,glacial acetic acid (100 μL) was added to terminate the reduction.The reaction products were dried under reduced pressure and acetylated by adding 2 mL of acetic anhydride and 2 mL of pyridine at 100 °C for 1 h.After the remaining acetic anhydride in the reaction mixture was decomposed with distilled water,the acetylated derivatives were extracted with methylene chloride.The glycosidic linkage of the acetylated derivatives was analyzed through gas chromatography-mass spectrometry (GCMS-QP 2010,Shimadzu,Kyoto,Japan) equipped with an RTX-5 capillary column (60 m ×0.32 mm,0.25 μm,Restek Co.,Bellefonte,PA,USA).The temperature program was set as follows: the initial column temperature was 150 °C and held for 1 min,increased to 180 °C at 5 °C/min,further increased to 210 °C at 1.5 °C/min and held for 5 min,and increased to 280 °C at 40 °C/min.The injection temperature was 230 °C.The ion source of the mass spectrometer was set at 240 °C.
2.8 NMR spectroscopy analysis
The purified polysaccharide sample was dried in a vacuum oven with dried silica gel for several days.Approximately 30 mg of polysaccharide samples were then dissolved in 0.5 mL of D2O.1H and13C NMR experiments were recorded on a Bruker Avance III (400 MHz) spectrometer (Bruker,Rheinstetten,Germany) by using sodium 3-trimethysilyl propionate as a chemical shift reference.The spectra of HSQC,COSY,TOCSY,HMBC,and ROESY were also obtained.Experimental data were analyzed using MestReNOVA 5.3 (Mestrelab Research SL,CA).
2.9 Immunostimulatory activity test in vitro
2.9.1 Preparation of murine peritoneal macrophages
Male BALB/c mice (18-22 g) were injected with 2 mL sterile thioglycollate medium.After 3 days,the peritoneal macrophages were harvested by peritoneal lavage and centrifugation at 4 °C,1 500 ×gfor 5 min.The precipitated cells were resuspended in RPMI-1640 and centrifuged to remove non-adherent cells.The cell viability of obtained macrophages was measured by trypan blue exclusive assay and reached up to 97% .
2.9.2 Cell viability
The peritoneal macrophages (1 × 106cells/mL) were plated in 96-well plates and incubated at 37 °C for 3 h in a 5% CO2incubator.After non-adherent cells were removed,the RPMI-1640 medium(without 10% FBS) containing series of AMPP concentrations (125,250,500,1 000 μg/mL) were added and incubated at 37 °C for 24 h in a 5% CO2incubator.The negative control and positive control groups were treated with RPMI-1640 medium containing 10% FBS and 1 μg/mL LPS,respectively.The cell viability was detected with a MTT kit at a wavelength of 570 nm and calculated using the following equation:
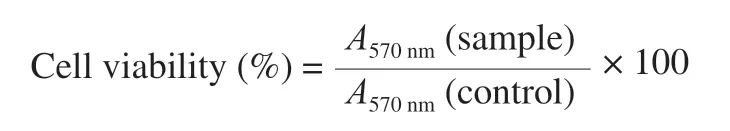
Where the cell viability of peritoneal macrophages from control group was calculated as 100% .
2.9.3 Phagocytosis assay
The effect of AMPP on the phagocytic activity of peritoneal macrophages was measured using neutral red assay according to the method established by Sun et al.[20].The macrophage cells cultured according to the method described in Section 2.9.2 were added with neutral red solution (0.1 g/100 mL) and incubated at 37 °C for 60 min.After discarding supernatant,the neutral red was removed by washing with PBS three times.Then cell lysis buffer (acetic acid/ethanol,1:1;V/V) was added and kept at room temperature for 2 h.The absorbance of resulting solution was measured at 540 nm by a microplate reader.On the other hand,the TNF-α existed in the peritoneal macrophages were measured by ELISA kit according to the manufacturers’ manner.
2.10 Statistical analysis
Results of immunostimulatory activity testin vitrowere recorded as mean ± standard deviation (n=5),and significant differences between groups were tested using one-way ANOVA and Duncan’s test atP<0.05.Statistical analysis was conducted by using SPSS version 18.0 software (SPSS,Inc.,Chicago,IL,USA).
3.Results and discussion
3.1 Purification of polysaccharide
The polysaccharide of AMP-1 was purified with the Sephadex G-25 column,and only one symmetrical fraction of AMPP was obtained from distilled water elution (Fig.1a).The obtained AMPP was white,and its carbohydrate,protein,uronic acid,and sulfate contents were (83.5 ± 3.0)%,(0.5 ± 0.1)%,(2.7 ± 0.3)%,and(2.6 ± 0.1)%,respectively.The recovery rate of AMPP based on the amount of AMP-1 was 77.7% .After HPGFC analysis,AMPP showed a single and symmetrical sharp peak (Fig.1b),indicating that AMPP was a highly homogeneous polysaccharide.The calculated average molecular weight of AMPP was approximately 3.2 kDa based on the calibration curve of dextran.
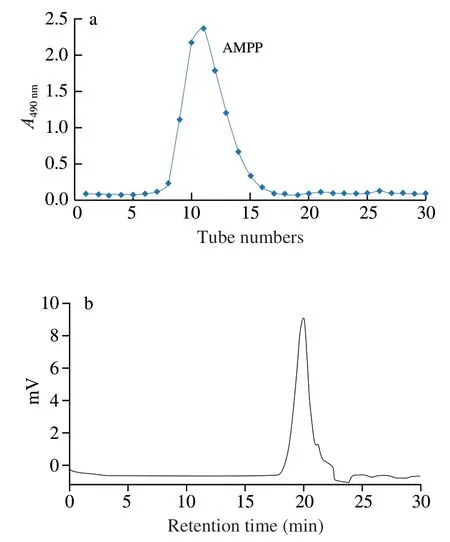
Fig.1 Elution curve of polysacchride on a Sephadex G-25 column chromatography (a) and HPGFC profile of AMPP (b).
3.2 Monosaccharide composition
Fig.2 shows the HPLC profiles of PMP-labeled monosaccharide standards and AMPP.After comparing with the standard monosaccharide,it was found that the obtained AMPP was composed of Glc and Man with a molar ratio of 99.7:0.3.A neutral polysaccharide extracted from abalone pleopods through a proteolytic method with papain and followed by precipitation with ethanol and found that this polysaccharide is composed of Glc and Gal with a molar ratio of 1:1 [6].The obvious difference in the monosaccharide compositions of the polysaccharides between this study (Fig.2) and the previous report [6]may be associated with different extraction methods and cultivated regions of abalone [9].
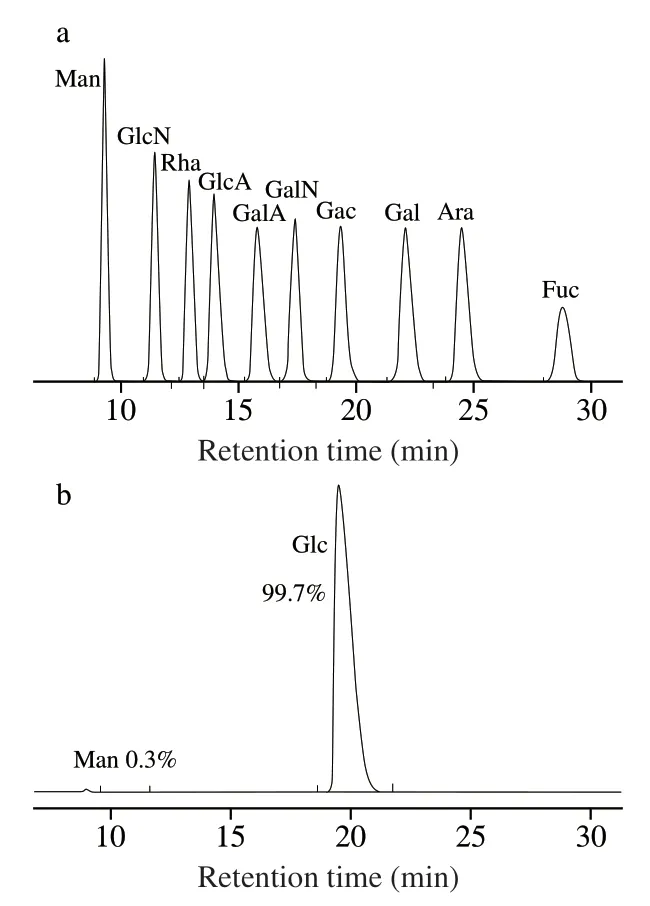
Fig.2 HPLC profiles of PMP derivatives of standard monosachhrides (a) and AMPP from abalone muscle (b).
3.3 FTIR analysis
The FTIR spectrum of AMPP is presented in Fig.3.The absorption peaks in the spectrum of AMPP were similar to those of polysaccharides from abalone pleopods [6].The absorption peaks around 3 335,2 928,1 644,and 1 024 cm−1in this study corresponded to the stretching vibration of O–H,asymmetrical stretching vibration of C–H,asymmetric stretching vibration of C=O,and stretching vibration of C–O,respectively [21].A previous study reported that a monosaccharide in a polysaccharide of Hawk mature leaf tea has a pyranose ring when strong absorption peaks appear in the range of 1 200–1 000 cm−1[22].The weak band at 1 078 cm−1and the intense band at 1 024 cm−1in AMPP were assigned to C–O and C–C stretching vibrations of a pyranose ring (Fig.3a),respectively.This characteristic of AMPP was similar to that of a polysaccharide from edible brown and red seaweeds [23].Furthermore,the characteristic absorption at 849 cm−1indicated thatα-configuration glycosidic bonds existed in the obtained AMPP (Fig.3a).When the polysaccharide is successfully methylated,the OH absorption peak around 3 400 cm−1decreases,whereas the CH3– absorption peak around 2 900 cm−1increases [24].In our study,the absorption band in the range of 3 000–2 800 cm−1strengthened after AMPP was methylated,whereas the absorption band around 3 335 cm−1decreased significantly (Fig.3b).However,when the AMPP was further methylated,a weak peak around 3 335 cm−1could be found due to the slight moisture content.Therefore,the result of Fig.3 suggested that AMPP was methylated completely.
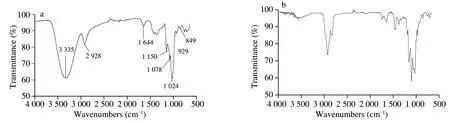
Fig.3 FTIR spectra of AMPP (a) and the methylated polysaccharide (b).
3.4 Methylation and GC-MS spectra
Generally,the position of glycosidic linkage in a polysaccharide can be observed through methylation analysis [25].The methylated AMPP was hydrolyzed with acid,converted into alditol acetate,and analyzed through GC-MS.The partially methylated monosaccharides were analyzed on the basis of retention times and mass spectra(Table 1).The linkages of AMPP consisted of terminal,1,4-linked,1,6-linked,and 1,4,6-linked Glcpwith a molar ratio of 3.1:7.2:1.0:2.5.The number of 1,4-linked and 1,4,6-linked Glcpresidues accounted for 70.18% of the total methylated monosaccharide residues,suggesting that the two residues could be the backbone of AMPP,and the branches were likely attached to the O-6 position of 1,4,6-linked Glcpresidues.This was similar to the report previously described by Hu et al.[26],who elucidated a water-soluble polysaccharide isolated from the stem barks ofAcanthopanax brachypus.Three polysaccharides were isolated fromDictyophora echinovolvata,which were conjectured to (1→4)-linked-glucans branched atO-6 based on the results of methylation and GC-MS analysis [27].The terminal Glcpwas also possibly the branch chain attached to the backbone of AMPP via a 1,6-glycosidic bond in present study.
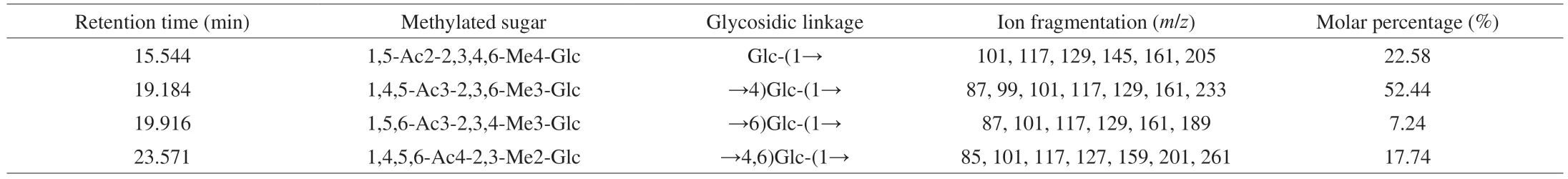
Table 1 Methylation analysis of AMPP from abalone muscle.
3.5 NMR analysis
The structural features of AMPP from the abalone muscle were further established through 1D and 2D NMR spectral analysis.The chemical shift of the anomeric proton inα-configuration residues was atδ4.8–5.3,and the vicinal coupling constant value was in the range of 2–4 Hz [28-30].Meanwhile,the chemical shift ofβ-configuration residues was atδ4.0–4.8,and the vicinal coupling constant value was in the range of 7–10 Hz [28-30].Considering that the sugar residues of AMPP were linked via anα-configuration glycosidic bond based on the results of FTIR analysis (Fig.3a),four anomeric proton signals atδ5.35 (d,3JH-1,H-2=4.13 Hz),5.33 (unresolved),5.24 (d,3JH-1,H-2=4.00 Hz),and 4.98 (d,3JH-1,H-2=4.13 Hz) in the1H-NMR spectrum(Fig.4a) were selected for analysis.
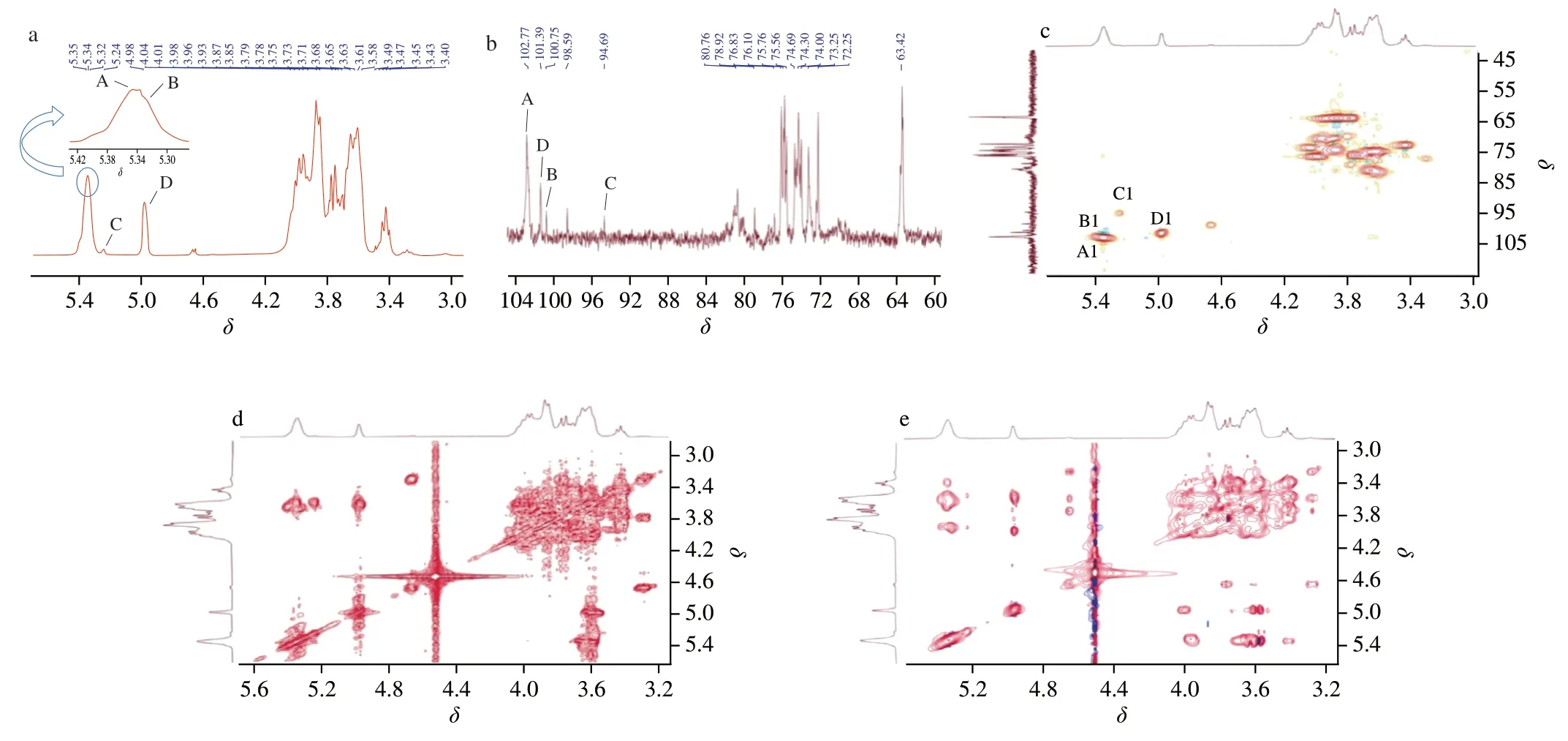
Fig.4 1H NMR (a),13C NMR (b),HSQC (c),COSY (d) and TOCSY (e) spectra of AMPP from abalone muscle.
The anomeric carbon signals atδ102.83,101.63,100.76,and 94.68 were observed in the13C NMR spectrum (Fig.4b),corresponding to theδ5.35,4.98,5.33,5.24 in the anomeric regions of HSQC (Fig.4c).The anomeric carbon signal atδ98.58 corresponds to theδ4.68,indicating that the residue isβ-configuration moiety.This is not consistent with the results of FTIR and1H-NMR analysis.Therefore,four glycosyl residues were assigned atδ5.35/102.83 (residue A),5.33/100.76 (residue B),5.24/94.68 (residue C),and 4.98/101.63 (residue D).The anomeric carbon signals in the13C NMR spectra (Fig.4b) also indicated that the obtained AMPP contained pyranose rings,and this observation was consistent with the FTIR result (Fig.3a).The results of the1H-NMR spectrum (Fig.4a)and methylation analysis (Table 1) suggested that the residues of A,B,C,and D were tentatively assigned to →4)-α-D-Glc-(1→,→4,6)-α-DGlcp-(1→,→6)-α-D-Glcp-(1→,and T-α-D-Glcp-(1→,respectively.
The signals in1H NMR and13C NMR spectra were assigned on the basis of the results of HSQC,COSY,and TOCSY experiments and previous literatures [31-33],and they are and summarized in Table 2.The resonance in the region ofδ80.80 and 80.87 in the13C NMR spectrum indicated that substitutions at position C-4 of residues A and B were present.Similarly,substitutions at position C-6 of residues B and C were also observed.These results further indicated that the assignment of residues A,B,C,and D was reasonable.

Table 2 Chemical shift assignment of 1H NMR and 13C NMR spectra of AMPP based on the data of COSY,TOCSY and HSQC.
The sequence and linkage of the glycosyl residues of AMPP were completely elucidated by the HMBC and ROESY spectra.The cross peaks of both anomeric protons and carbons from the HMBC experiment of the sugar residues were examined,and the residual connectivities were observed (Fig.5a).Cross peaks were found between H-1 (δ5.35) and C-4 of residue A (A H-1/A C-1),H-4 of residue B (δ3.66) and C-1 of residue A (B H-4/A C-1),and H-1 of residue B (δ5.33) and C-4 of residue A (B H-1/A C-4).These results suggested that residue A was linked to the residues of A and B via a 1,4-glycosidic bond.
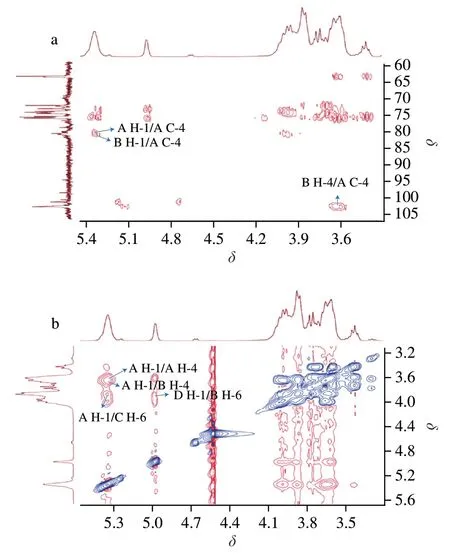
Fig.5 HMBC (a) and ROESY (b) spectra of AMPP from abalone muscle.
ROESY experiment was also carried out (Fig.5b).Cross peaks were identified between H-1 of residue A (δ5.35) and H-6 of residue C(A H-1/C H-6) and between H-1 of residue D (δ4.98) and H-6 of residue B (D H-1/B H-6).This result indicated that 1,6-glycosidic linkages were present in residues A and C and residues D and B of AMPP from abalone muscle.The cross peaks of A H-1/A H-4 and A H-1/B H-4 were also observed in the ROESY spectrum.
A possible molecular structure of AMPP from abalone muscle(Fig.6) was proposed on the basis of the chemical and spectroscopic evidence presented above.In one repeat unit of the proposed AMPP structure,the backbone chain consisted of eight 1→4 glycosidic bonds and one 1→6 glycosidic bond,with three branch chains linked by a 1→6 glycosidic bond.Therefore,it can be concluded that AMPP belongs to theα-type glucan.Special glucans derived from mollusks have beneficial effects on immunomodulatory and antitumor activities and have been focused by many researchers,but it is still unknown how glucans generated in mollusks and what their biological activities are [34].We determined the monosaccharide composition of abalone viscera,connective tissues,pui-muscle and pleopod muscle (Fig.7),suggesting that the seaweeds fed by abalone are digested by various enzymes in the abalone viscera and gradually converted into Glc,which is absorbed and involved in the metabolism,and converted to glucans.Therefore,the sampling parts are very important for the analysis and comparison of abalone polysaccharides from different sources or cultivated regions.The next studies will be focused on the possible generation mechanisms of glucans in the abalone muscle.
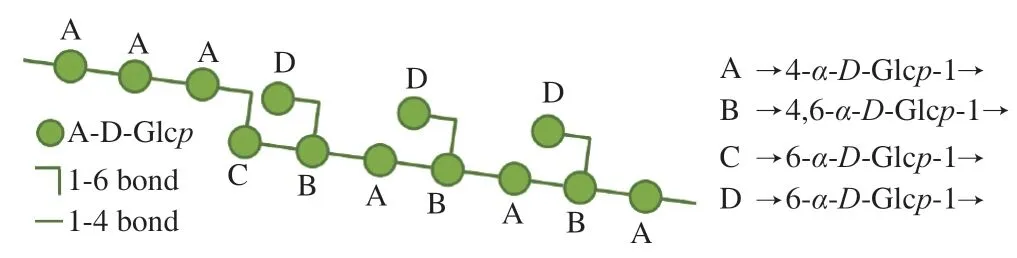
Fig.6 Proposed model for AMPP from abalone muscle.
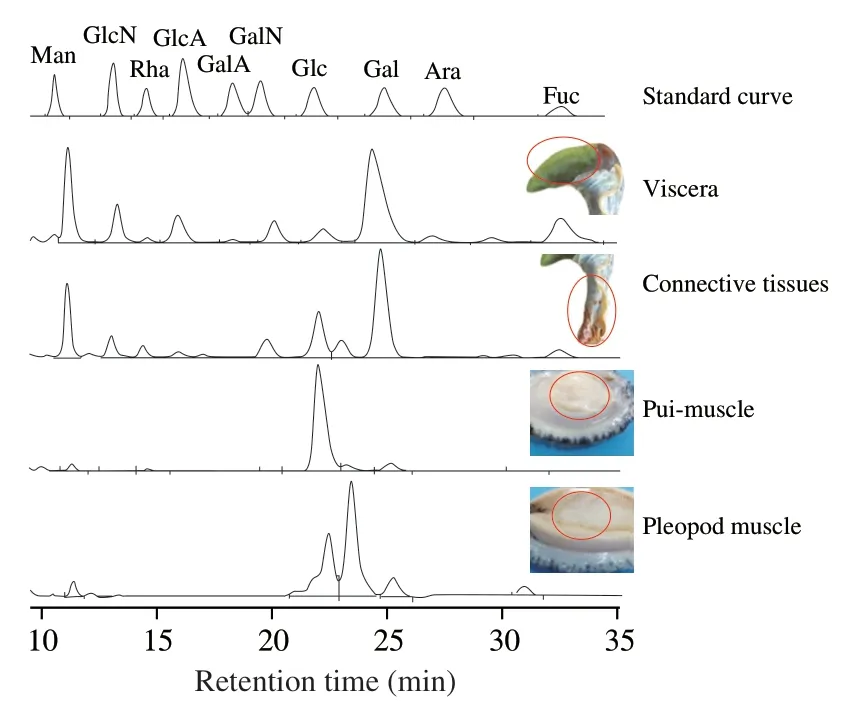
Fig.7 Monosaccharide compositions of abalone viscera,connective tissues,pui-muscle and pleopod muscle.
3.6 Immunomodulatory activity in vitro
The macrophages are important immunomodulatory effector cells,and have been widely used as cell model to investigate the immunomodulatory activity of food derived extracts [35].The effect of AMPP on the proliferation of Raw264.7 cells was investigated and shown in Fig.8a.No significant effects (P>0.05) of cell viability in the AMPP concentration range from 125 to 1 000 μg/mL,which was selected to assess the potential immunomodulatory activity of AMPP.
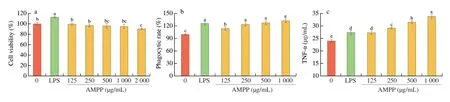
Fig.8 Effect of AMPP on the proliferation (a),phagocytic activity (b),and TNF-α (c) of RAW264.7 cells.LPS (1 μg/mL) was used as positive control.Results were presented as mean ± SD from five replicates (n=5).Means with different superscript letters are significantly different (P <0.05) by Duncan’s test.
The phagocytic activity of peritoneal macrophages RAW264.7 cells treated with AMPP was measured using neutral red assay and shown in Fig.8b.The phagocytosis of RAW264.7 cells treated with AMPP was increased (P<0.05) with increasing AMPP concentration,and all phagocytosis indices for AMPP at the test concentrations were higher than 1.0,indicating that the obtained AMPP had ability to activate phagocytic activity of RAW264.7 cells.A similar trend was also reported by Xiong et al.[36],who investigated inflammatory effect ofCyclocarya paliuruspolysaccharide in RAW264.7 macrophage.
It is reported that TNF-α is a mediator of immune stimulation and inflammatory response,which plays an important role in the resistance of host cells to malignant tumor growth [37].Thus,the effect of AMPP on TNF-α was assayed and presented in the Fig.8c.AMPP could promote the production of TNF-α in a dose-dependent manner(P<0.05),which is similar to a glucan fromCyclina sinensis[8].This result further suggests that AMPP has immunostimulatory activity on macrophages,thus promoting the secretion of TNF-α to enhance the antitumor activity of polysaccharide.
4.Conclusion
In this study,AMPP was purified and the obtained AMPP was a highly homogeneous polysaccharide with an average molecular weight of approximately 3.2 kDa.Based on the results of methylation and NMR spectral analyses,our conclusion was that the main backbone of the proposed AMPP structure was composed of 1→4 and 1→6 linked Glc,with branches consisting ofα-D-Glcp-(1→6).The obtained structural information would be important for further studies on the structure–activity relationship of polysaccharide from abalone muscle.Immunological assaysin vitroshowed that AMPP has the ability to activate phagocytic activity of RAW264.7 cells and stimulate cells to release TNF-α.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors are grateful for financial support received from the National Key R&D Program of China (2021YFD2100200/2021Y FD2100202),National Natural Science Fund (31571835),Fujian Key Project of Natural Science Foundation (2019J02013),and the Opening Project of Fujian Provincial Engineering Technology Research Center of Marine Functional Food (Z820239).
杂志排行
食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil
