Physicochemical and structural properties of starches from non-traditional sources in China
2023-01-21JingyiYngChgmKoteswrReddyZhiliFnBojunXu
Jingyi Yng,Chgm Koteswr Reddy,Zhili Fn,Bojun Xu,*
a Food Science and Technology Program,BNU-HKBU United International College,Zhuhai 519087,China
b Department of Biochemistry and Bioinformatics,Institute of Science,GITAM (Deemed to be University),Visakhapatnam 530045,India
Keywords:Starch Viscosity Morphology Quinoa Sorghum Proso millet
ABSTRACT In this study,we isolated starches from non-traditional sources,including quinoa,lentil,arrowhead,gorgon fruit,sorghum,chickpea,proso millet,and purple potato and investigated their morphology,physicochemical,and functional properties.Significant differences in starch particle morphology,swelling power,solubility,syneresis,crystalline pattern,and pasting viscosity were observed among the starches from these nontraditional sources.Further,all these isolated starches had unique properties because of their characteristic distinct granules when seen under scanning electron microscopy (SEM).The amylose content of the isolated starches shown significant difference (P <0.05),and the values ranged between 11.46% and 37.61% .Results demonstrated that the isolated starches contained between 79.82% to 86.56% starch,indicating that the isolated starches had high purity.X-ray diffraction (XRD) patterns of starches isolated from sorghum,proso millet,quinoa,purple potato,and gorgon fruit presented A-type diffraction pattern;while lentil seeds,arrowhead,and chickpea starches presented C-type diffraction pattern.Overall,these results will promote the development of products based on starch isolated from non-traditional starches.
1.Introduction
Starch,a biodegradable and nontoxic biopolymer,is present richly in different parts of plants including grains,seeds,fruits,roots,and tubers [1].Starch is a major ingredient in human diet,and it has been widely used as functional ingredient in a variety of food,paper,textile,and pharmaceutic,and cosmetic applications [2,3].At the molecular level,starch contains two types of biopolymers: amylose is a linear molecule with a few long-chain branches and amylopectin is highly branched with short-chains [4,5].Besides,the composition,morphology,and structure of starch are significantly influenced by its botanical origin,leading to diverse properties and possible applications [4,6].Over the decades,traditionally,starch has been used as food source and as well as to sustain the quality of stored foods.In recent years,due to its unique characteristics,starch has been used as a coating material for delivering bioactive compounds in non-food industries [5,7].Till now,numerous studies reported that the structural,morphology,thermal,and physicochemical properties of starches from mainstream and commercial sources.However,with the growth of food and non-food industrial applications,there is rising importance in achieving novel starch sources with unique properties.
Proso millet (Panicum miliaceumL.) is an ancient grain commonly grown in Asia,Europe,and U.S.A.Proso millet is rich in protein,carbohydrates,and minerals,and its nutritive parameters are similar or better than traditional cereals [8].As well,starch is the major glycemic carbohydrate component in proso millet and accounts for around 60% of the dry matter [9].Sorghum (Sorghum bicolorL.)is one of the leading crops worldwide and it has been considered as a staple food for humans in at least thirty countries [10].Like traditional cereals,sorghum is rich in starch (around 70%),proteins,and phenolic compounds,and it has been used as functional ingredient in development of functional foods [11,12].Quinoa (Chenopodium quino) is a pseudo-cereal crop,become more popular as a novel food item due to the attractive nutritional benefits.Quinoa is rich in proteins,starch (around 65%,dry basis),fiber,essential amino acids,and vitamins [13].Due to its wealthy nutritious composition,quinoa seeds can be used to produce different foods including infant food,flakes,and bread [14].
Arrowhead (Sagittaria sagittifoliaL.) is a perennial aquatic plant commonly grown in North America,Europe,and Asian countries [15].The tubers of arrowhead are rich source of starch (54.60%,dry basis),protein,macro-and micro-nutrients,and therefore,used in preparation of different dishes [16].Purple potato (Ipomoea batatasL.)(sweet potato) is a one of the leading crops,widely cultivated in many tropical and sub-tropical countries in world.Purple potato is rich in starch,pectin,dietary fiber,minerals,vitamins,and bioactive compounds [17].Because of the rich nutritious composition,purple potatoes are widely used in food industries to produce value-added foods [18].Gorgon fruit (Euryale feroxSalisb.) is an eminent edible and medicinal aquatic plant.The seed of theE.feroxSalisb.,known as Qianshi as well as cock’s head in Chinese,are consumed medicinally or as food [19].
Chickpea (Cicer arietinum) is one of the important legumes mainly cultivated in semi-arid regions of Asian and Africa.Chickpea is rich in high-quality proteins,starch,minerals,vitamins,and other important nutrients [20].Because of the rich nutritious composition and starch content,chickpea is considered commercially important for many processed foods.Lentils (Lens culinaris) are grown annually on a variety of soil types in semi-arid regions worldwide [21].Lentils,which contains high-quality proteins,carbohydrates,and dietary fiber.The presence of nutritious ingredients makes lentil a suitable supplement for nutritional fortification targeting a variety of valueadded food product applications [22].
Till now,the main source of starch is cereals,but the accessibility of cereal starch to the food and non-food industries is shrinking day by day because of increased demand by industries involved in the production of breakfast cereals and snacks.Due to this reason,research taken a turn to initiate another starch source which could achieve feasible requirements in various industrial food applications.However,till now,limited studies have been completed on isolation,characterization,and applications of starches from non-conventional sources.Also,the unique properties of non-conventional starches may help in improving the food and non-food applications.Based on this context,the aim of this study is to explore the morphology,physicochemical,and functional properties of starches isolated from different non-conventional sources cultivated in China.
2.Materials and methods
2.1 Materials
The raw materials,quinoa,sorghum,proso millet, were obtained respectively from Shandong,Jiangxi province,and northeast of China.The flours of chickpea,gorgon fruit and purple potato were purchased respectively from Xinjiang,Guangdong,and Shandong provinces of China.Commercial lentil seed starch (LSS) and arrowhead starch(AHS) were collected respectively from Gansu and Yunnan provinces of China.The morphological features of the original samples are shown in Supplemental Fig.1.
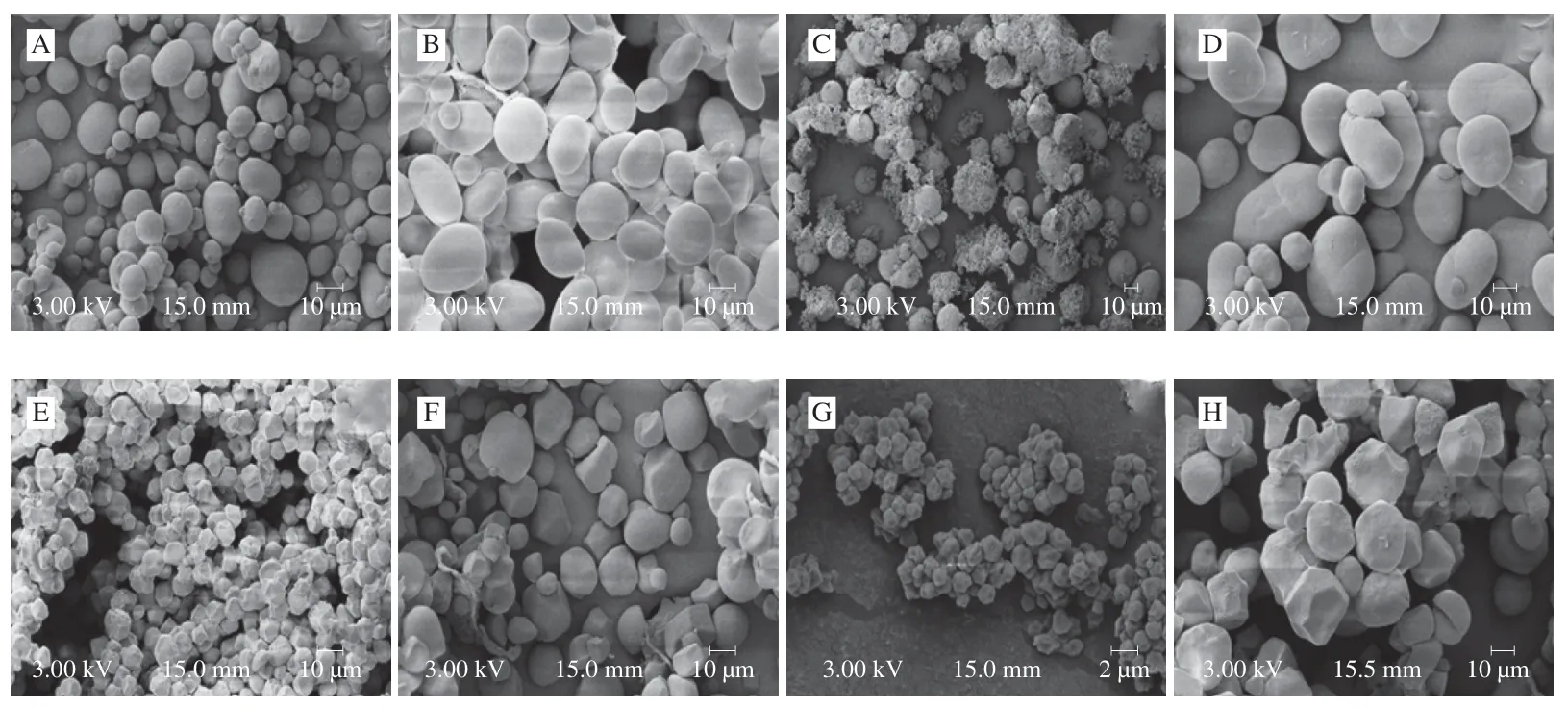
Fig.1 SEM photographs of starches isolated from non-traditional sources in China.A-I,and I indicate arrowhead,chickpea,gorgon fruit,lentil seed,proso millet,purple potato,quinoa,and sorghum,respectively.
2.2 Flour preparation
Flour was prepared from the quinoa,sorghum,and proso millet by grinding using Mini Grain Mill (A11B,IKA Inc,Bangalore,India.),sieved using 100 μm mesh-sieve,and stored at 4 °C for further analysis.
2.3 Extraction of starches
The starches were extracted from chickpea,gorgon fruit,and purple potato samples following procedure described by Guo et al.[23],yet,applying certain changes.Flour sample (10%) was steeped in 0.025 mol/L sodium sulfite solution at 25 °C for 30 min and then blended using the hand blender (EURO-ST 20 D S25,IKA,Guangzhou,China) for 3 min.After 90 min incubation at 25 °C,the precipitate was washed with deionized water and filtered through 250 and 100 μm mesh sieves.The filtrate was centrifuged for 15 min at 3 000 ×gand the pellet containing starch was dried at 30 °C for 24 h.The dried starch was powdered and stored at 4 °C for further analysis.
The starches were extracted from quinoa,sorghum,and proso millet samples following method described by Contreras-Jiménez et al.[24],yet,applying certain changes.Flour sample (10%) was steeped in 0.25% NaOH solution at room temperature for 30 min and then blended using the hand blender for 3 min.The obtained slurry was heated in shaking water bath at 40 °C for 6 h.After incubation,the precipitate was washed with deionized water and filtered through 250 100 μm mesh sieves.The filtrate was centrifuged for 15 min at 3 000 ×gand the pellet containing starch was dried at 30 °C for 24 h.The dried starch was powdered and stored at 4 °C for further analysis.
Other commercially obtained starches such as LSS and AHS were sieved through 100 μm mesh sieve and stored at 4 °C for further analysis.
2.4 Chemical composition analysis
The moisture content of the isolated starches was determined by using rapid moisture analyzer (MA150,Sartorius,Göttingen,Germany).The contents of amylose and starch of the isolated starches were measured using the colorimetric method reported by Guo et al.[23].
2.5 Color analysis
The color analysis of the isolated starches was carried out by using D-25 colorimeter (Hunter Lab Associates Inc.,U.S.A.),following a method described by Reddy et al.[6].
2.6 Scanning electron microscopy (SEM)
Granular morphology of the isolated starches was viewed by using an EVO18 SEM (Zeiss,Oberkochen,Germany) at an accelerating voltage of 5 kV according to the procedure described by Reddy et al.[6].Prior to viewing,all the samples were mounted on a sample holder using double-sided sticky tape,coated with a thin film of gold.
2.7 X-ray diffraction (XRD)
The XRD pattern of starch samples were recorded using an X-ray diffractometer (SmartLab9,Rigaku Miniflex,Japan) as described in a previous publication [25].The diffractograms were obtained in a scanning range from 5° to 35° in 2θscale,with a speed of 1.5 °/min,operating at 40 kV and 30 mA with Cu-Kα radiation at 0.154 2 nm.
2.8 Fourier transform infrared spectroscopy (FTIR)
FTIR spectra of the isolated starches were obtained using a Fourier transform infrared spectrometer (Bruker Optics Inc,U.S.A.).Prior to analysis,all the samples were made as pellets with potassium bromide and dried.Transmittances were recorded at wave numbers between 4 000 and 500 cm−1at a resolution of 2 cm−1.
2.9 Pasting properties
The pasting properties of the isolated starches were determined using a Rapid Visco Analyzer (RVA;Perten Instruments Ltd.,Australia) as described in a previous publication [26].Briefly,starch (3.0 g) was added to 25 mL of distilled water placed into the aluminum canister.Slurries underwent a controlled heating and cooling cycle as follows: the slurries were first held at 50 °C for 1 min,heated to 95 °C within 7.5 min,and then held at 95 °C for 5 min.The hot samples were subsequently cooled to 50 °C within 7.5 min,and maintained at 50 °C for 2 min.
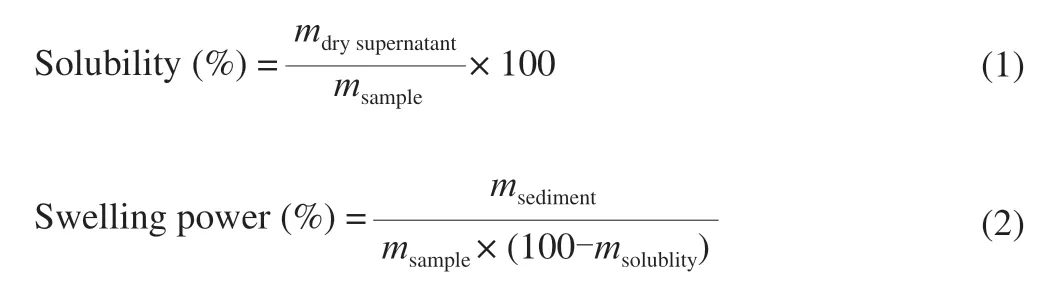
2.10 Swelling power and solubility
Swelling power and solubility of the isolated starches were determined based on a previously published process with minor modifications [27].Briefly,starch sample (0.5 g) and distilled water(30 mL) were mixed and then heated at 90 °C in a water bath for 30 min with interval agitation.After heating,the samples were centrifuged at 3 000 ×gfor 15 min.The supernatant was dried at 105 °C overnight,and the precipitate was weighed.The solubility and swelling power of the isolated starches were calculated by the following equations,respectively:
2.11 Syneresis
Syneresis of the isolated starches were determined based on a previously published process with minor modifications [28].Starch suspensions (2%,m/mdry basis) were heated at 90 °C for 30 min with interval agitation.Then the samples were stored at 4 °C for 24 h and were centrifuged at 3 000 ×gfor 15 min.The weight of suspension and the released water were measured and the syneresis of starch can be determined by the following equation:
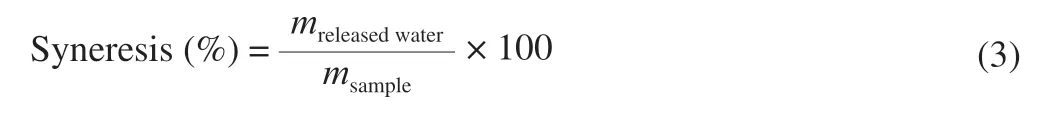
2.12 Statistical analysis
The data reported in the tables were carried out in triplicate.SPSS 20 software (IBM,Chicago,IL,U.S.A.) was used to analyze the variance (ANOVA) by Duncan’s test (P<0.05).
3.Results and discussion
3.1 Moisture content of isolated starches
Results of moisture content of isolated starches from different botanical sources are given in Table 1.The moisture content of the isolated starches showed significant difference (P<0.05),and the values ranged between 10.45% and 13.51% .Among the isolated starches,LSS was found the highest (13.56%) and that of proso millet starch (PMS) was found to have the lowest moisture values of 10.45% .Moisture content of the isolated starches were comparable to the findings of previous studies [1,6].Further,the discrepancy in the moisture content of isolated starches could be possible due to the applying usage of different types of extraction techniques for starch isolation.
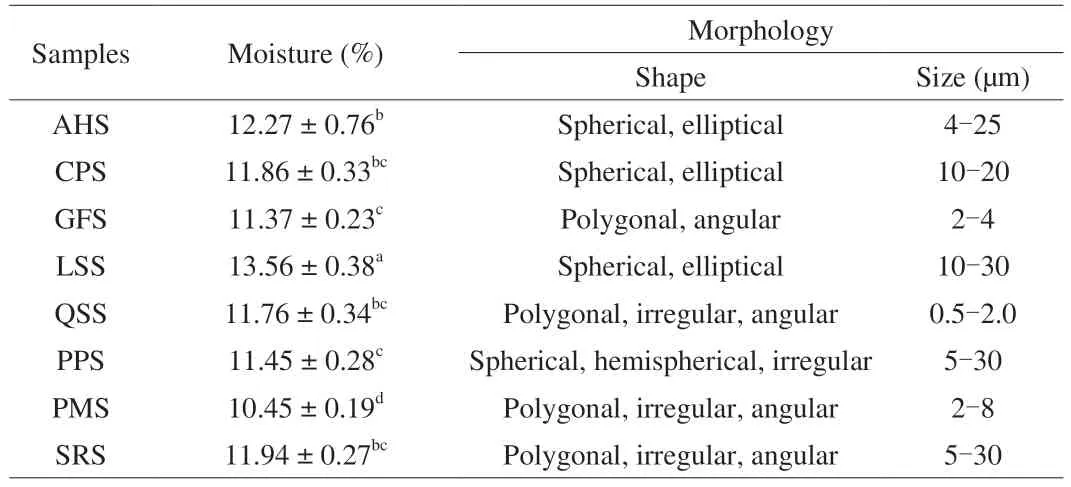
Table 1 Moisture content,and morphological features of isolated starches from nontraditional sources in China.
3.2 Amylose and starch contents of isolated starches
Results of amylose and starch contents of isolated starches from different botanical sources are presented in Table 2.The amylose contents of the isolated starches showed significant difference(P<0.05),and the values ranged between 11.46% and 37.61% .Among the isolated starches,LSS was found the highest (37.61%) and that of PMS was found to have the lowest amylose values of 11.46% .The differences in the amylose content of isolated starches can be attributed to the procedure,type of variety,maturity,geography,and culivation conditions [28].Results demonstrated that the isolated starches contained between 79.82% to 86.56% starch (wet basis)and approximately 10.45% to 13.56% moisture,indicating that the isolated starches had high purity.
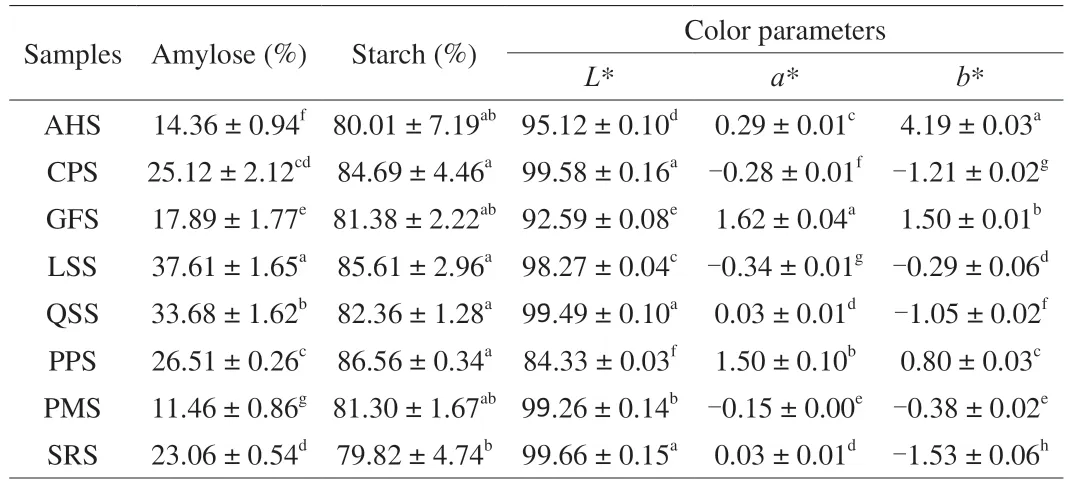
Table 2 Amylose content,starch content,and color parameters of isolated starches from non-traditional sources in China.
3.3 Color analysis
Table 2 illustrates the color parameters such asL*,a* andb* of starches isolated from different botanical sources.Color parameters is one of the vital characteristics,which improves consumer interest about selection of food products,and it is applied in industrial applications,particularly in textile industries [29].As shown in Table 2,the color values of the isolated starches showed significant(P<0.05) difference.Among the isolated starches,the lowest lightness (L*) values were observed in gorgon fruit starch (GFS;92.59) and AHS (95.12);while the chickpea starch (CPS) was found to have the highestL* value of 99.58.The results can be credited to the leaching of color pigments,such as anthocyanin in purple potato starch (PPS) and carotene in GFS [23].Hence,native starch with higher lightness value can improve its application in food industries where fairness in color is demanding.The highest and the lowesta* values were found in GFS (1.62) and LSS (−0.34),indicates the highest and the lowest values of redness.Theb* values of AHS was maximum (4.19) i.e.higher yellowness while sorghum starch (SRS)was minimum (−1.53).The appearance of varying degree of redness and yellowness in the starch may owe to the genetic makeup of starch itself and ageing of the raw material before starch extraction [23].
3.4 SEM
SEM plays a vital role in understanding of starch granule structure.The SEM images of isolated starches from different botanical sources are shown in Fig.1.Granules of AHS presented round,elongated,elliptical,irregular,and oval shaped with smooth surfaces;and the size typically ranged between 4 to 25 μm (Fig.1A).CPS granules had an average size of around 10 to 20 μm and the surface of granules appeared smooth with no evidence of any fissures,and most of the granules were elliptical shaped,whereas others were spherical (Fig.1B).The starch granules of GFS exhibited polygonal,irregular,and spherical shapes with some debris on the surface(Fig.1C) and the sizes of GFS granules varied between 2 and 4 μm.
The granules of LSS exhibited oval to spherical shapes with smooth surfaces and did not exhibit any cracks (Fig.1D).The size of LSS granules varied between 10 and 30 μm.The granules of PMS exhibited a mixture of shapes such as spherical,irregular,and polygonal,with a granule size between 2 and 8 μm (Fig.1E) and there were some small pores randomly distributed over the granule surface.The granules of PPS exhibited round,spherical,hemispherical,and polygonal shapes with smooth surface and no evidence of cracks (Fig.1F).The sizes of PPS granules varied between 5 and 30 μm.Quinoa starch (QSS) granules had an average size of around 0.5 to 2.0 μm and the granules appeared polygonal and irregularly shaped,and some starch granules showed coalescence (Fig.1G).The granules of SRS exhibited polygonal,irregular,angular,and spherical shapes (Fig.1H)and the sizes of SRS granules varied between 5 and 30 μm.
As shown in Fig.1,among the isolated starches,difference in starch granule size and shape might be possible due to the difference in botanical source,cultivation,and origin of sources.According to literature,starch granule size plays a crucial role in defining the quality traits of isolated starches [30].In addition,granule size of starches is relative to the digestibility and mouth feel of starchy products.Sukhija et al.[31]described the link between starch granule size and digestibility.Starches with small granule size contains high digestibility because smaller granules have greater surface area and are readily digested by enzymes [30].Further,starch granules with smaller size can act as substitute of lipid molecules in food industrial applications.Therefore,among the isolated starches,QSS is the smallest and has the potential to replace fat in food applications.
3.5 XRD
In general,starch granules exist in nature in the form of semicrystalline particles,with both crystalline and amorphous structural components.The crystallinity of starch is related to the well-ordered structure of the amylopectin molecules inside the granules [29].According to literature,crystalline structure of starch granules was categorized in to three types: A,B,and C based on the XRD pattern.In general,A-type starch is mainly found in the seeds of cereal crops,while B-type starch plant is associated mainly with tubers and highamylose crop seeds [4].As for C-type crystals,it is a combination of A-type and B-type crystals,which can be observed in legume and rhizomes [1].Crystalline structures of isolated starches from different botanical sources are given in Fig.2.Starches from SRS,PMS,QSS,PPS,and GFS presented A-type crystalline pattern with strong diffraction peaks including a solid doublet peak at around 17.11° and 17.99° (2θ) and other main peaks at around 15.08° and 23.06° (2θ) [32].Starches from LSS,AHS,and CPS presented C-type crystalline pattern with strong diffraction peaks including a solid single peak at around 17.10° and other main peaks at around 15.08°,15.16°,22.15°and 23.90° (2θ) [33].The variance in the diffraction pattern and degree of crystallinity of starch granules was primarily influenced by cultivar genotype,extraction process,agronomic and cultivation conditions [34].
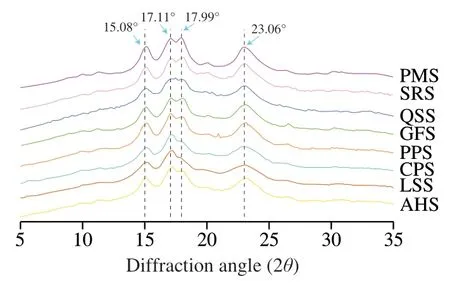
Fig.2 XRD patterns of starches isolated from non-traditional sources in China.AHS,CPS,GFS,LSS,PMS,PPS,QSS,and SRS indicate arrowhead,chickpea,gorgon fruit,lentil seed,proso millet,purple potato,quinoa,and sorghum starches,respectively.Same as Fig.3.
3.6 FTIR analysis
The FTIR spectra of the isolated starches from different botanical sources illustrated in Fig.3.As shown in Fig.3,all the isolated starches exhibited a broad band between 3 000 and 3 600 cm−1with a peak at around 3 250 cm−1which corresponds to complex vibration stretching of O–H including free,inter-,and intra-molecules.Joshi et al.[35]described that the broad region located at 3 500 cm−1indicating the contribution of the water molecule.The peak at 2 926 cm−1was attributed to the asymmetric stretching of C−H,related with the ring methine hydrogen atoms.The bending vibration of H−O−H in bound water leaded to the peak around 1 617−1 649 cm−1.The peak shown at 1 315−1 337 cm−1indicated the twitching of CH2.According to Jiang et al.[36],the appearance of intense peaks at 1 153 cm−1and 1 077 cm−1are due to the anhydroglucose ring C–O stretch of C–O–H in starch.In addition,the peak at 1 012−1 021 cm−1was attributed to the stretching of C–O of C–O–C in starch molecule.The absorption band at 932,850 and 762 cm−1,can be assigned to the skeletal mode vibrations ofα-1,4 glycosidic linkage,C−H bending and C−C stretching,respectively [37].In the spectra,the impurity peaks ranged 3 246−3 445 cm−1,and the noise present at around 1 617−1 649 cm−1might be caused by the impurity of samples and low scans during sample scanning,respectively.
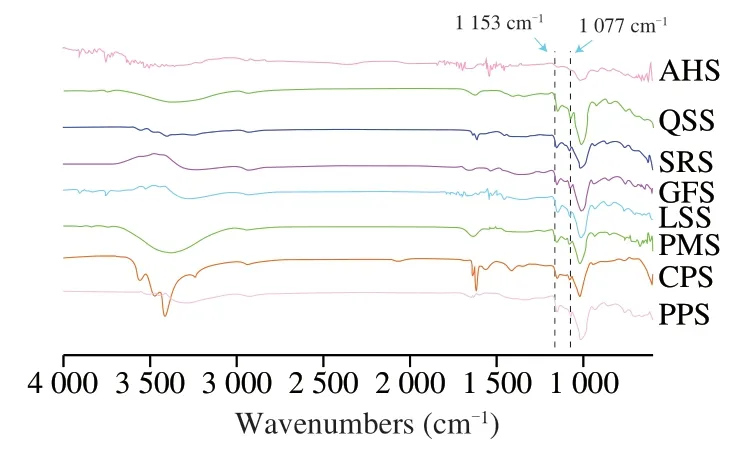
Fig.3 FTIR spectra of starches isolated from non-traditional sources in China.
3.7 Pasting properties
Pasting properties are directly related to functionality of starch and commonly play a vital role in determining the application of starchbased products.Table 3 shows the pasting parameters of the isolated starches from different botanical sources were analyzed by rapid visco analyzer (RVA).Significant differences (P<0.05) in pasting parameters among isolated starches were observed.As shown in Table 3,the pasting temperature (PT) of isolated starches ranged from 73.18 to 93.65 °C,the lowest PT for CPS and the highest for QSS.In general,PT provides a sign of the least temperature required to prepare of the starchy foods.Du et al.[38]described that the starches with higher PT are difficult to swell and rupture.From the findings,the peak viscosity (PV) of isolated starches ranged between 1 593 and 5 259 cP,the lowest for GFS and the highest for AHS.According to literature,the PV of starches are usually controlled by structure of starch,content of amylose and crystallinity [1].These results display that the starch granule from GFS contain the highest amylose content,which illustrates the lowest PV of GFS [39].Previous studies reported that the content of amylose in isolated starches is directly related to the PV,high quantity of amylose lead to reduction of starch PV because swelling capacity of starch granules were mainly controlled by amylose molecule.Further,high PV values are related with the high degree of granule swelling or water binding capacity of isolated starches [40].
From the findings,trough viscosity (TV) of isolated starches were ranged between 1 282 and 2 463 cP,the highest for AHS and lowest for SRS.In general,the TV of starches indicates the disintegration of starch granules from the effects of shear and heat.Further,the breakdown viscosity (BD) of isolated starches were ranged from 83 to 2 615 cP,which were belonged to GFS and AHS,respectively.BD indicates the stability of the starch paste and disruption of starch granules [38].Among the isolated starches,GFS with the lowest BD value had high thermal stability,which can be used as a good ingredient in food processing.In terms of the setback viscosity (SB),the lowest value was observed in PMS (317 cP) and the highest one was observed in CPS (2 970 cP).SB indicates the stability of starchcold paste and starch retrogradation tendency after gelatinization and cooling at 50 °C [23].From the findings,it can be observed that PMS had the less tendency to retrograde,while CPS was the most unstable starch after heating.As shown in Table 3,the final viscosity (FV)of isolated starches ranged from 1 632 to 5 345 cP,the highest for PMs and the lowest for CPS.Mostly,the FV of starch granules was influenced by the aggregation of the amylose molecules.Previous studies described that the pasting properties of isolated starches were controlled by various factors such as ratio of amylose and amylopectin,structure of starch,morphology of granules,architecture and integrity,granule swelling,crystallinity and chain length of amylose and amylopectin [38].
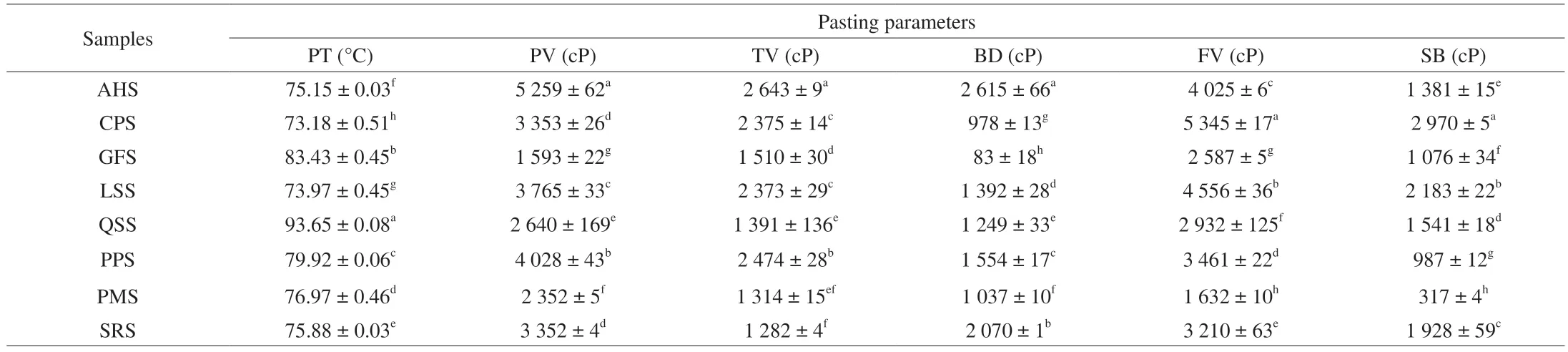
Table 3 Pasting parameters of isolated starches from non-traditional sources in China.
3.8 Swelling power and solubility
The swelling power and solubility properties reflects the mutual bonding ability and water holding capacity of the starch granules.When starch is heated and gelatinized in adequate water,hydrogen bonds controlling the structure of the double helices in crystallites are broken and replaced by hydrogen bonds with water,the starch granule then swells and its volume increases [41].Subsequently,increases the leaching of amylose and amylopectin chains,which can improve the solubility of starch granules [42].The swelling power and solubility of the isolated starches were measured at 90 °C,and the results are summarized in Table 4.Significant differences (P<0.05) in swelling power and solubility among isolated starches were observed.In the isolated starches,the maximum swelling power was obtained in SRS (23.89%),and the minimum values in GFS (7.97%).Previous studies described that the starch granule swelling is largely regulated by structural integrity [43].As shown in Table 4,in the isolated starches,the maximum solubility was obtained in SRS (12.61%),and the minimum values in QSS (3.04%).Tester et al.[44]descried that the solubility of starch granules is related to the existence of soluble particles such as amylose,which specifies the ability of starch solids to dissolve or disperse in an aqueous solution.These results show that the lowest solubility of QSS granules might be possible due to the integrity of the starch granules where controlled swelling and solubility were present.The differences in solubility among the isolated starches may be possible due the difference among their content of starch components and chain length distributions.
3.9 Syneresis of starches
Syneresis is a process in which the water molecules released from the gel because the starch chains are starting to form a thick structure.Degree of syneresis be directly proportional to the storage time in low temperatures [45].Syneresis of isolated starches from different botanical sources for 24 h storage at 4 °C are shown in Table 4.In the isolated starches,the maximum syneresis value was obtained in CPS (79.72%),and the minimum value in PMS (26.01%). Therefore,compared with other analyzed starches,PMS is more suitable in frozen food processing.The differences in the degree of syneresis of isolated starches maybe possible due to the difference in chemical composition as well as intermolecular interactions of amylose chains in starch structure [46].
4.Conclusions
This study investigated the structure and physicochemical properties of starches isolated from different non-traditional sources.The differences in the physicochemical characteristics of the isolated starches studied maybe due to their geographically different habitats.In addition,all these isolated starches had unique properties because of their typical granule morphology.Results of XRD demonstrated that starches isolated from sorghum,proso millet,quinoa,purple potato,and gorgon fruit presented A-type crystalline pattern;while lentil seeds,arrowhead,and chickpea starches presented C-type pattern.The high viscosity of starches isolated from arrowhead and purple potato can be used to have an advantage in instant soups and sauces.In summary,the results reported by the present study should inspire further studies to explore the potential of non-traditional starches for utilization in food and non-food industries.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This research was jointly supported by two research grants(R202016 and R202017) from Beijing Normal University-Hong Kong Baptist University United International College,China.
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.1016/j.fshw.2022.07.043.
杂志排行
食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil
