Structural characteristics,anticoagulant and antithrombotic mechanism of a novel polysaccharide from Rosa Chinensis Flos
2023-01-21XiofengZhngZhenhuLingGeoffreyIvnNeilWterhouseShengjunJingDongxioSunWterhouseJinmeiWngChngyngWenyiKng
Xiofeng Zhng,Zhenhu Ling,Geoffrey Ivn Neil Wterhouse,Shengjun Jing,Dongxio Sun-Wterhouse,Jinmei Wng,b,*,Chngyng M,d,*,Wenyi Kng,b,d,*
a National R&D Center for Edible Fungus Processing Technology,Henan University,Kaifeng 475004,China
b Joint International Research Laboratory of Food&Medicine Resource Function,Henan Province,Kaifeng 475004,China
c School of Chemical Sciences,University of Auckland,Auckland 1142,New Zealand
d Kaifeng Key Laboratory of Functional Components in Health Food,Kaifeng 475004,China
Keywords:Rosa Chinensis Flos Polysaccharide isolation Antithrombotic activity Intestinal f lora
ABSTRACT This is the first report on a polysaccharide (RCJ2-Ib) isolated from Rosa Chinensis Flos.RCJ2-Ib was obtained through the extraction with water,precipitation with ethanol,separation with DEAE-52 column and purification with DEAE-Sepharose Fast Flow column and Sephadex G100 column.GC,FT-IR and NMR analyses revealed that RCJ2-Ib (3.3 kDa) was a 1,4-linked polymannuronic acid containing substantial β-Danomers units.The anticoagulant effect of RCJ2-Ib evaluated by using rabbit ear venous blood and an acute blood stasis rat model showed that RCJ2-Ib had obvious anticoagulant activity in regulating endogenous and exogenous coagulation pathways and reducing serum thromboxane B2 and endothelin-1.In addition,RCJ2-Ib could also increase the number of Lactobacillus and Escherichia coli.As a result,RCJ2-Ib has the potential to inhibit thrombosis and maintain the intestinal environment.
1.Introduction
Natural plant products represent an important segment of the food and beverage market.This market is subdivided into the categories of produce (fruits and vegetables),herbs,f lowers and spices [1].Edible flower-based foods and beverage recipes are attracting growing interest of consumers for the health benef its as well as distinct floral flavours [2-4].
Rosa Chinensis Flos,an edible flower ofRosa chinensisJacq.(Rosaceae),originates from China and grew widely in the world.It is commonly used in the food,pharmaceutical,and cosmetic industries as a traditional medicine,raw material or food additive such as “Rosa Chinensis Tea” and “Rosa Chinensis Yogurt” [2,5].Rosa Chinensis Flos or its derived products have signif icant antioxidant properties and antifungal activity [6]as well as historically known therapeutic effects including blood circulation-promoting effect,detumescence function,detoxifying activity,and hepatic depression-relieving effects [7].These bioactivities are closely related to the constituents in Rosa Chinensis Flos such as flavonoids (e.g.quercetin,kaempferol,and their derivatives),triterpenes,volatile oil,proteins and amino acids,vitamins and trace elements [8].However,no studies have been reported examining polysaccharides fromR.chinensis,motivating the current study which aimed to fill this knowledge gap.
A variety of plant polysaccharides have been shown to promote intestinal mucosal immunity.For example,Fermented Yupingfeng polysaccharides (FYP) have shown greater beneficial effects in improving intestinal flora,including increasing the abundance and diversity of flora [9].At present,there is no report about Rosa Chinensis Flos polysaccharide on intestinal flora.
Over the past few decades,polysaccharides from various nonplant and plant sources have attracted a great deal of interest because of their broad spectrum bioactivities,such as antioxidant,anti-viral,anti-tumor,anticoagulant,antidiabetic and immunomodulatory activities [10,11].In recent years,our research has found that the polysaccharides fromAngelica dahuriceroot,blackberry (Rubusspp.)seeds,Apocynum venetumL.andMalus hallianaKoehne exhibited significant anticoagulant and antithrombotic activities [12,13].Here we aimed to extend this work further,by isolating polysaccharides from Rosa Chinensis Flos and then examining the effects on the relative abundance and composition of the intestinal flora,as well as the anticoagulant and antithrombotic activities.Fractionation and purification of a crude polysaccharide extract from Rosa Chinensis Flos were conducted via dialysis,then DEAE-cellulose 52 and Sephadex G100 column chromatography.The monosaccharide composition and the characteristic groups of polysaccharide were determined by gas chromatography (GC),Fourier transform infrared(FTIR) spectroscopy,the configuration of the glycosidic bond of the polysaccharide,the substitution position and branch point of the polysaccharide are determined by Nuclear magnetic resonance (NMR)spectroscopy (including1H NMR,13C NMR,1H-13C heteronuclear single quantum coherence (HSQC) NMR,1H-1H correlated spectroscopy(COSY) NMR,and1H-13C heteronuclear multiple bond correlation(HMBC) NMR).Our study showed that the polysaccharide components of Rosa Chinensis Flos had anticoagulant activity and could regulate the balance of intestinal flora at the genus level.
2.Materials and methods
2.1 Materials and chemicals
Dried Rosa Chinensis Flos was obtained from Hebei Chufeng Traditional Chinese Medicine Pieces Co.,Ltd.(production batch number: B611141),and was identified by Professor Changqin Li,Henan University.
Monosaccharide standards (Dr.Ehrenstorfer GmbH,Germany),trifluoroacetic acid (TFA) (Shanghai Aladdin Biotechnology Co.,Ltd.,China),breviscapine (Yunnan Bio Valley Pharmaceutical Co.,Ltd.,China),and adrenaline (Tianjin Pharmaceutical Group Xinzheng Co.,Ltd.,China) were purchased from commercial suppliers.Prothrombin time (PT) enzyme-linked immunosorbent assay (ELISA) kit(20181225),activated partial thromboplastin time (APTT) ELISA kit(20190307),and thrombin time (TT) ELISA kit (2019010702) were all purchased from Shanghai Sun Biotechnology Co.,Ltd.6-Ketoprostaglandin F1α (6-keto-PGF1α) ELISA kit (201908),thromboxane B2 (TXB2) ELISA kit (201908),endothelial nitric oxide synthase(eNOS) ELISA kit (201908),and endothelin-1 (ET-1) ELISA kit(201908) were obtained from Nanjing Senbeijia Biotechnology Co.,Ltd.(China).DEAE-cellulose 52 was purchased from Whatman Ltd.(USA).Sephadex G-100 was purchased from GE Healthcare Bio-Sciences AB (Sweden).
2.2 Animals
Male Sprague-Dawley rats (190-220 g) and rex rabbit (2.0-2.5 kg)were purchased from Zhengzhou Animal Experimental Center,China.All animal experiments gained approval with the license numbers for animal experiments were SCXK (Yu) 2017-0001 and SCXK (Yu)2015-0005.The animals were kept in a temperature-and humiditycontrolled room (25 °C and 45% -65% relative humidity) on a 12 h light-12 h dark cycle,and were fed a standard rodent diet withad libitumaccess to distilled water.
2.3 Extraction and purification of RCJ2-Ib
The dried Rosa Chinensis Flos (5 kg) was the first ground to powder,then depigmented and defatted with petroleum ether (twice;10 L and 48 h each time) and 70% aqueous ethanol (thrice;10 L and 48 h each time) at room temperature.The depigmented and defatted Rosa Chinensis Flos was then extracted three times with distilled water (sample-water ratio: 1:25 (m/V);3 h each time) at 80 °C,with each extract subjected to filtration using a filter paper.The obtained filtrates were combined and concentrated to 1 000 mL by a vacuum evaporator.The resulting solution was then mixed with 2.8 L of 95% ethanol solution and stored at 4 °C overnight.The precipitate was centrifuged at 1 884 ×gand 25 °C for 15 min using a highspeed refrigerated centrifuge,then collected by water filter (0.22 μm,PALL corporation,American) and washed successively with anhydrous ethanol,acetone and petroleum ether.The obtained solid was then deproteinized ten times using the Sevag reagent (chloroform:butanol=4:1).Next,the deproteinized solution was dialyzed against deionized water with a dialysis bag for 3 days (8 000-14 000 Da,Viskase,American),and the water was changed every 12 h.Finally,the retentate was freeze-dried for 48 h using a freeze dryer to yield the crude polysaccharide extract from Rosa Chinensis Flos (termed “RCJ”).
The dried RCJ (360 mg) was reconstituted in distilled water(15 mL).The resulting solution was then injected into the DEAE-52 column (2.6 × 40 cm) of a chromatography system,and eluted with 0.0-0.2 mol/L NaCl gradient at a flow rate of 1 mL/min using constant flow pump (HL-1D;Shanghai Qingpu Huxi Instrument Factory,China).Product elution was monitored using the UV-visible absorbance at 490 nm.The fraction eluted with 0.1 mol/L NaCl was further purified by DEAE-Sepharose Fast Flow column,via gradient elution with 0.0-5.0 mol/L NaCl at an elution rate of 1 mL/min,and the fraction eluted with 0.2 mol/L NaCl collected using a fraction collector and then purified by a Sephadex G100 column,via elution with distilled water at a flow rate of 0.5 mL/min.The resulting eluate was collected and termed “RCJ2-Ib”,as described in Fig.1a.
The total carbohydrate content of the collected fractions was measured by the phenol-sulfuric acid assay [13].The UV-vis absorbance spectrum (200-800 nm) of RCJ2-Ib (0.1 mg/mL,in distilled water) was acquired at 25 °C on a SP2600 UV spectrophotometer (Shimadzu,Japan).
2.4 Molecular weight analysis
The molecular weight of RCJ2-Ib was determined by high-pressure size exclusion chromatography (HPSEC) (National Pharmacopoeia Commission,2015) coupled with a Dawn Heleos-II multiangle light scattering system (Wyatt,USA) and an HPLC system (USA,Waters).RCJ2-Ib solution (1 mg/mL,in distilled water) was loaded on the column and the elution was performed with an aqueous 0.1 mol/L NaNO3solution at a flow rate of 0.5 mL/min.A glucan standard(38 000 Da) was used as a calibration standard.
2.5 Analysis of monosaccharides
The content of uronic acid in RCJ2-Ib was determined according to a published method [13].
The identification and quantification of the neutral monosaccharides in RCJ2-Ib were achieved by GC [13].Dried RCJ2-Ib(10 mg) was hydrolyzed with 4 mL of 2 mol/L TFA (110 °C,5 h)and H2SO4(72%,30 °C,2 mol/L,then: 1 mol/L,100 °C,3 h),before reduction with NaBH4and then acetylation with acetic anhydride [14].The resulting alditol acetates were analyzed by GC using an Agilent 7890N instrument equipped with an HP-5 capillary column (Agilent Technologies,Inc).The following temperature program was used:The initial column temperature was 110 °C and held for 5 min,then increased to 190 °C at 5 °C/min and held for 4 min,and finally increased to 210 °C at 3 °C/min and held for 8 min.The temperature of the injector was 250 °C.Helium was used as the carrier gas (flow rate 0.8 mL/min).The same procedure was used to derivatize a mixture of several monosaccharide standards (rhamnose,arabinose,xylose,mannose,glucose and galactose;all were purchased from Dr.Ehrenstorfer GmbH,Germany).
2.6 FTIR analysis
RCJ2-Ib powder was prepared for analysis using the KBr presseddisk method.Briefly,1 mg dried RCJ2-Ib was ground with potassium bromide (KBr;150 mg) powder,with the resulting powder then pressed into a thin pellet that was then transferred to the disk holder of the FTIR spectrometer (UV-2600;Shimadzu,Japan).The FTIR spectrum of RCJ2-Ib was collected over the wavenumber range 400-4 000 cm−1at 2 cm−1resolution.
2.7 NMR analysis
RCJ2-Ib was dissolved in deuterium oxide (D2O;0.5 mg/mL) for NMR analyses at 25 °C.The1H-NMR,13C-NMR,COSY,HMQC,and HMBC spectra were acquired using a Bruker spectrometer(Am-400;Bruker,USA) at a probe temperature of 298 K and field strengths of 600 MHz and 125 MHz respectively.Chemical shifts were reported in parts per million (δ).
2.8 Evaluation of the anticoagulant activity of RCJ2-Ib using the blood from rabbit auricular vein
Blood samples were taken from rabbits’ auricular veins following our previously reported method [15].4 mL of blood was collected from the marginal ear vein of the rabbit,placed in an EP tube,centrifuged at 3 000 r/min for 5 min,and plasma was taken for use.And then randomly divided into 5 groups: 1) a blank control group(also called the negative control;in which the mixture of 20 μL DMSO,80 μL of Tween and 170 μL of saline were used as the blank medium);2) two positive control groups (in which Yunnan Baiyao (20 mg/mL) and breviscapine (13.3 mg/mL) were used,respectively);3) two treatment groups (in which RCJ and RCJ2-Ib were used,respectively,at a concentration of 5 mg/mL.We adopted our previously reported method and it had been confirmed by our pre-test) [10].PT,APTT and TT tests were conducted following the kit instructions with a RAC-030 semi-automated coagulation analyzer(Rayto,USA).
2.9 Evaluation of the anticoagulant effect of RCJ2-Ib using an acute blood stasis rat model
Rats (n=42) were randomly divided into 7 groups of a blank group (saline,intragastric administration),a model group(saline,intragastric administration),two positive group (40 mg/kg breviscapine+0.57 mg/kg Warfarin sodium,intragastric administration) and three RCJ2-Ib-treated groups (150,100 and 50 mg/kg RCJ2-Ib,intragastric administration).Each group of rats was administered once a day for 5 consecutive days.
The acute blood stasis model was established after the fifth administration,slightly modified according to the literature method [16].The model rats were injected twice with epinephrine hydrochloride (0.8 mg/kg) at an interval of 4 h.Two hours after the first injection of epinephrine hydrochloride,the rats were placed in an ice-cold water bath (0-2 °C) for 5 min.After the second injection,rats were fasted (water only).Thirty minutes after the last administration on the seventh day,the rats were anesthetized with 10% chloral hydrate (300 mg/kg).Then,blood samples were collected from the abdominal aorta,with some blood being centrifugated at 1 500 ×gand 25 °C for 15 min to obtain serum for analyses.The plasma TXB2,6-keto-PGF1α,eNOS and ET-1 were performed using the corresponding ELISA kits according to the manufacturer’s recommended procedures.Whole blood viscosity (WBV) and plasma viscosity (PV) were measured by an Auto-Viscometer.Besides,0.3 g of cecal contents were collected in the ultra-clean working table and placed in a 4 mL sterile centrifuge tube.The cecal contents of every 3 rats were collected and stored at −80 °C and sent to Suzhou Jinweizhi Biotechnology Co.,Ltd.for sequencing and analysis.
2.10 Differences in intestinal flora at the level of genus
Sequencing of the amplification of the 16S rRNA v3-v4 region was performed by Suzhou Jinweizhi Biotechnology Co.,Ltd.The reverse and reverse reads obtained by double-end sequencing were the first spliced in pairs,and the sequences with the sequence length greater than 200 bp were retained.After quality filtering and removal of chimeric sequences,the final obtained sequences were used for OTU clustering.Sequence clustering was performed by VSEARCH(1.9.6) (sequence similarity was set at 97%).The reference database of 16S rRNA for comparison was Silva 132.Then,the Bayesian algorithm of RDP classifier (Ribosomal Database Project at Michigan State University) was used for taxonomic analysis of the representative sequences of OTU,and the community composition of each sample was counted at the genus level.
2.11 Statistical analysis
The results were expressed as “mean ± standard deviation”.The data analysis was performed by SPSS 19.0 software with the single factor analysis of variance (ANOVA One-Way) to compare the significant difference.GraphPad Prism 5 software was used for drawing.The differences between groups atP<0.05 andP<0.001 were regarded as significant and highly significant,respectively.
3.Results and discussion
3.1 Preparation of crude polysaccharide extracted (RCJ)and derived fraction (RCJ2-Ib)
The total extraction process of RCJ2-Ib is shown in Fig.1a.The extraction yield of crude polysaccharide (RCJ) was 3.2% (m/m) of the raw material, Rosa Chinensis Flos. (on dry).As shown in Fig.1b,RCJ was separated by the DEAE-52 column to obtain four fractions of RCJW,RCJ1,RCJ2 and RCJ3.RCJ2 with the highest yield(20.6% of RCJ,by dry mass) was selected for further purification by the DEAE-Sepharose FF column,yielding two fractions (Fig.1c) of RCJ2-I (16.7% of RCJ2,by dry mass) and RCJ2-II (2.5% of RCJ2 by dry mass).RCJ2-I was further purified to obtain RCJ2-Ib (58.7% of RCJ2-I,by dry mass).
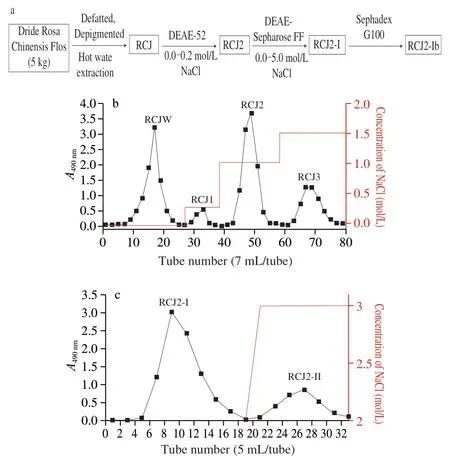
Fig.1 (a) The total extraction process of RCJ2-Ib;Elution of (b) RCJ and(c) RCJ2 by ion exchange column chromatography.RCJ represents the crude polysaccharide extract from Rosa Chinensis Flos.
3.2 Molecular weight,monosaccharide analyses and UV-VIS absorption of RCJ2-Ib
The average molecular weight of RCJ2-Ib was 3.3 kDa,with a dispersion index (Mw/Mn) of 1.117 (Fig.2a),indicating that the molecular weight distribution of RCJ2-Ib was relatively narrow.Analysis of RCJ2-Ib by UV-vis spectroscopy revealed no obvious absorption at 260 or 280 nm,suggesting that there were negligible proteins or nucleic acids in RCJ2-Ib [17].
Relatively high content of uronic acid ((63.59 ± 0.23)%) was found in RCJ2-Ib,indicating that RCJ2-Ib was likely an acidic polysaccharide.The comparison between the GC spectrum of the mixture of monosaccharide standards and the spectrum of RCJ2-Ib(Fig.2b) revealed that mannose was the dominant neutral monosaccharide in RCJ2-Ib.
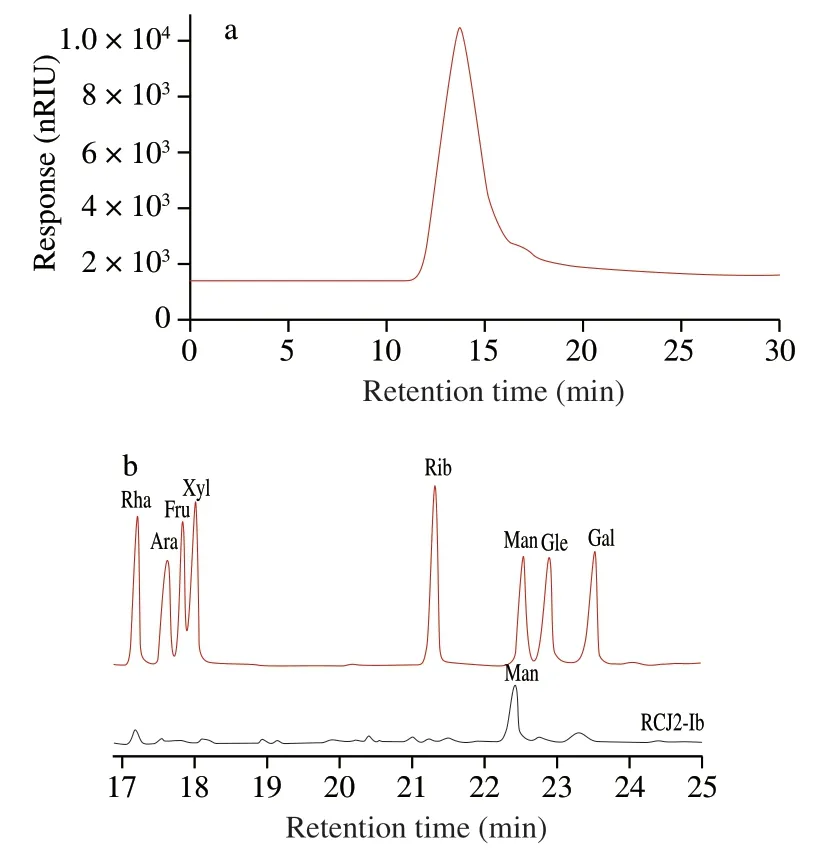
Fig.2 (a) HPSEC chromatograms of RCJ2-Ib.(b) GC chromatograms showing the monosaccharide profiles of a reference mixture of standard monosaccharides and RCJ2-Ib.RCJ2-Ib represents a fraction of the crude polysaccharide extract from Rosa Chinensis Flos.
3.3 FTIR analysis of RCJ2-Ib
The infrared spectrum of RCJ2-Ib is shown in Fig.3.The intense and broad absorption peak at 3 393 cm−1was due to the O–H stretching vibration of hydroxyl groups [18,19].The weaker feature at 2 934 cm−1originated from the C–H stretching vibration [9].The polysaccharide is constructed by plenty of bonds of O–H and C–H,so the absorption peaks at 3 393 and 2 934 cm−1are the characteristic peaks of polysaccharide.The intense absorption peak at 1 608 cm−1was assigned to the stretching vibration of C=O [20].Uronic acid was characterized by C=O stretching vibrations at 1 625 cm−1(for deprotonated–COO−groups) and 1 740-1 760 cm−1(for protonated carboxylic acid–COOH groups) [20].Therefore,the absorption feature saw at 1 608 cm−1in the current work was likely an asymmetric carboxylate (–COO−) stretching mode.This assignment was supported by the presence of an additional peak at 1 414.5 cm−1(a symmetric stretching mode of deprotonated–COO−groups) [21].Results suggested that RCJ2-Ib was rich in uronic acid residues [22,23].
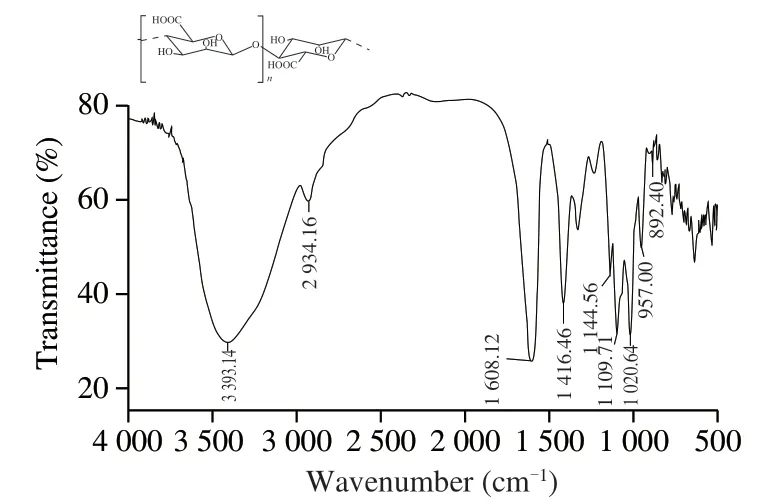
Fig.3 Infrared spectrum of RCJ2-Ib.The inset shows the partial structure of RCJ2-Ib.
The absorption peak at 1 145.5 cm−1was likely derived from the angular vibration of C–C–H and O–C–H on the pyran ring [24],all three signal peaks (1 145.5,1 109.7 and 1 020.6 cm−1) could make contributions to the stretching vibration of C–O in the form of a pyranose ring [25,26].The absorption at 1 145.5 and 1 020.6 cm–1might come from the C–C stretching vibration of polysaccharides.The signals around 1 109.7,1 020.6 and 957.0 cm−1indicated the presence of C–O–C and C–O–H structures (like those of the pyranose rings),with the signals at 1 020.6 and 957.0 cm−1particularly associated with the C–O–H bending and C–O–C bond stretching mode of the glycosidic link andβ-(1,4) linkages for polysaccharides [20,26].The small band around 892 cm−1was derived from the glycosidic C–H deformation mode along with OH bending and (pyranoid) ring vibration,and often associated with theβ-glycosidic linkage between monosaccharide residues [27].Therefore,the spectrum in the region 1 200-800 cm−1is typical for glycosidic linkages includingβ-(1,4)linkages in polysaccharides.
3.4 NMR analysis of RCJ2-Ib
1H-NMR spectra and13C-NMR spectra of RCJ2-Ib are shown in Fig.4a and 4b.In Fig.4a,the signal atδ4.63 was the solvent D2O,withδ5.07 for Man,and anO-methyl group (δ3.55) [28].In agreement with published results [28,29],the proton signals of RCJ2-Ib were mainly located in the region ofδH3.4-5.3 (a typical feature of polysaccharides).The signals atδH4.5-5.8 (Fig.4a) were derived from the anomeric protons.Previous studies have shown that anomeric OH in theαposition (α-glycosidic form containing an equatorial H) resonates atδ4.9-5.6 (δH>4.9),whereas theβcounterpart (β-anomer containing an axial H) exhibits resonances atδ4.3-4.9 (δH<4.9),with a ring proton region atδH4.5-3.0 [28,29].
In Fig.4b carbon signals were seen aroundδ178.3,102.0,80.7,74.1,71.7 and 70.9,which are within those typical for polysaccharides (δ50-110) [30,31].The signal atδ178 is identified as C-6 of esterified residues in polysaccharides [32,33].The resonances for anomeric C-1 and C-2 to C-5 were assigned mainly atδ90-110 andδ65-78,respectively [30,34].The signal atδ102.0 indicated the presence of a C-1 carbon of(1,4)-β-D-glucopyranosyl residues,with signals around C-2,C-3,C-4 and C-5 was arranged atδC70.9,71.7,80.7 and 74.1,respectively [35,36].The chemical shift atδ102 was assigned as the only anomeric carbon.Theβ-anomeric configuration mostly occurs in the upfield region (typically atδ97-101) with theα-anomeric configuration located in the downfield region (typically atδ103-105) in a13C NMR spectrum [31,36,37].Under the current1H-NMR and13C-NMR conditions,the anomeric proton signals inδ5.07 for Man indicated that RCJ2-Ib had just one type of monosaccharide residues,which was consistent with carbon signals.The presence of a substituted group (e.g.,O-methyl group) can cause a shift ofδ0.5 for1H orδ6-7 for13C.According to the current1H-NMR and13C-NMR experiments,the presence ofβ-anomeric configuration of mannans was confirmed,although the co-occurrence of glycosidic bonds inαandβ-forms could not be excluded.
In Fig.4c (1H-13C HSQC spectrum),the chemical shifts of C-2,C-3,C-4 and C-5 were atδC70.9,71.7,80.7 and 74.1,respectively.In Fig.4d (1H-1H COSY spectrum),the chemical shifts of H-2,H-3,H-4 and H-5 wereδH3.77,δH4.01,δH4.42,δH4.77,respectively.The chemical shift atδC178.3 was derived from C-6 of an esterified residue (uronic acid group) (Fig.4b),and the corresponding chemical shift of H-5 wasδ4.77 through the correlation from H-5 (δH4.77) to C-6 (δC178.3) in the1H-13C HMBC spectrum (Fig.4e) [13].In the HMBC experiments(Fig.4e),the possible linkage sites and sequence of monosaccharide residues in RCJ2-Ib were elucidated.The correlation found between H-1(δH5.07) and C-4 (δC80.7) suggested that the monosaccharide residues were linked at the 1,4-position.According to GC analysis of the neutral monosaccharide profile,RCJ2-Ib was composed mostly of mannose,which indicated that RCJ2-Ib may be a 1,4-linked polymannuronic acid containing substantialβ-D-anomers with someα-D-anomer units(its possible partial structure is shown in the inset chemical structure of Fig.3) [23].Theα-D-anomer units glycosidic bond is unstable,so it was preliminarily judged that RCJ2-Ib was 1,4-linked polymannuronic acid mainly containing substantialβ-D-anomers.
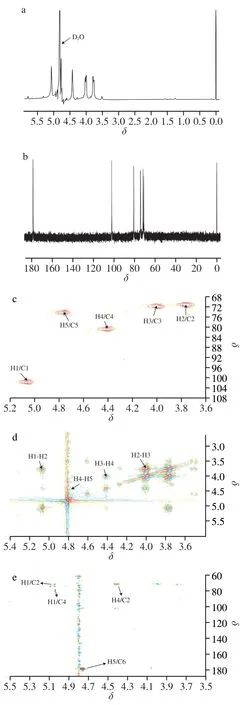
Fig.4 NMR analyses of RCJ2-Ib.(a) 1H NMR spectrum (600 MHz);(b)13C NMR spectrum (125 MHz);(c) 1H-13C HSQC spectrum;(d) 1H-1H COSY spectrum;(e) 1H-13C HMBC spectrum.
3.5 Evaluation of the anticoagulant effect of RCJ and RCJ2-Ib using the blood from rabbit auricular veins
Compared with the blank group,RCJ,RCJ2-Ib and breviscapine significantly (P<0.001) increased APTT for the bloods from rabbit auricular veins,and the PT had also increased to a certain extent(Table 1).These results indicated that both RCJ and RCJ2-Ib had a significant anticoagulant activity and could inhibit coagulation through influencing an endogenous and exogenous coagulation pathway [10,38].
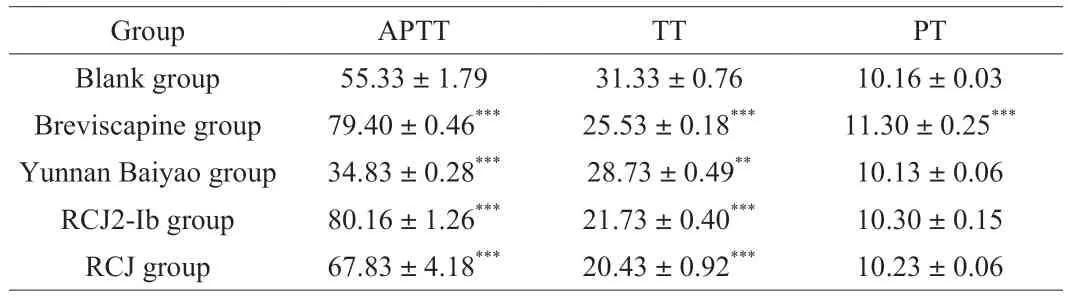
Table 1 Evaluation of plasma coagulation parameters (affected by RCJ or RCJ2-Ib)using the blood from rabbit auricular veins (mean ± standard deviation,n =3)
3.6 Evaluation of anticoagulant effect of RCJ2-Ib using an acute blood stasis rat model
3.6.1 Effect of RCJ2-Ib on blood coagulation factors
Compared with the blank group,APTT,PT and TT values in the model group were significantly (P<0.001) lower (Table 2).These results indicated that the acute blood stasis model was successfully established.Compared with the model group,the administration with a high or medium dose of RCJ2-Ib could significantly (P<0.001;P<0.01) increase APTT,PT and TT.The results of Section 3.8 already showed that RCJ2-Ib inhibited the coagulation of blood from rabbit auricular veins via endogenous and exogenous coagulation pathways.All these results were consistent with those published in the literature [8,39].Therefore,it was speculated that RCJ2-Ib could inhibit thrombosis through influencing endogenous and exogenous coagulation pathways.
3.6.2 Effects of RCJ2-Ib on TXB2 and 6-keto-PGF1α in blood stasis model rats
Compared with the blank group (Table 2),the content of 6-keto-PGF1α in the model group decreased significantly (P<0.001),whilst the content of TXB2 increased significantly (P<0.001),indicating that the acute blood stasis model was successfully established.Compared with the model group,high and medium doses of RCJ2-Ib could significantly (P<0.001) increase the content of 6-keto-PGF1α,significantly (P<0.001) reduce the content of TXB2 and adjust the balance of 6-keto-PGF1α/TXB2.Previous studies reported that TXB2 and 6-keto-PGF1α were metabolites of thromboxane A2 (TXA2) and prostacyclin (PGI2) [12],and that afzeloside could inhibit platelet aggregation and dilate blood vessels by reducing the TXA2 content and increasing the PGI2content [40,41].The imbalance between TXA2 and PGI2was believed to change the ratio of TXB2/6-keto-PGF1α,then stimulate platelet aggregation,vascular contraction and thrombosis formation [40].Accordingly,RCJ2-Ib may be an effective regulator for the balance of 6-keto-PGF1α/TXB2,as the polysaccharide could inhibit thrombus formation by reducing TXB2 and increasing PGI2.
3.6.3 Effect of RCJ2-Ib on the contents of eNOS and ET-1 in blood stasis model rats
ET and NO are bioactive factors with opposite functions.Both play important roles in the regulation of the cardiovascular system and the pathological processes of various cardiovascular diseases [42].NO,a signaling molecule produced by vascular endothelial cells under the action of nitric oxide synthase (eNOS),exhibits a strong vasodilating effect,an inhibitory effect against platelet aggregation and adhesion,and a preventive effect on thrombus formation [10].The vascular factor,ET-1,has shown a strong vasoconstrictive function and thrombosis-promoting effect,and plays an important role in a variety of vascular diseases in humans [43].In this research,the contents of eNOS and ET-1 in the model group increased significantly(P<0.001) compared with the blank group (Table 2),indicating that the acute blood stasis model was successfully established.Comparedwith the model group,the levels of ET-1 in all the three RCJ2-Ibtreated groups were all significantly lower (the high-and mediumdose groups atP<0.001;the low-dose group atP<0.01),whilst the contents of eNOS in the three RCJ2-Ib-treated groups increased significantly (the high-dose group atP<0.01;the medium-dose group atP<0.001;the low-dose group atP<0.05) These results indicated that RCJ2-Ib regulated the tension of vascular smooth muscle by down-regulating ET-1 and up-regulating eNOS.
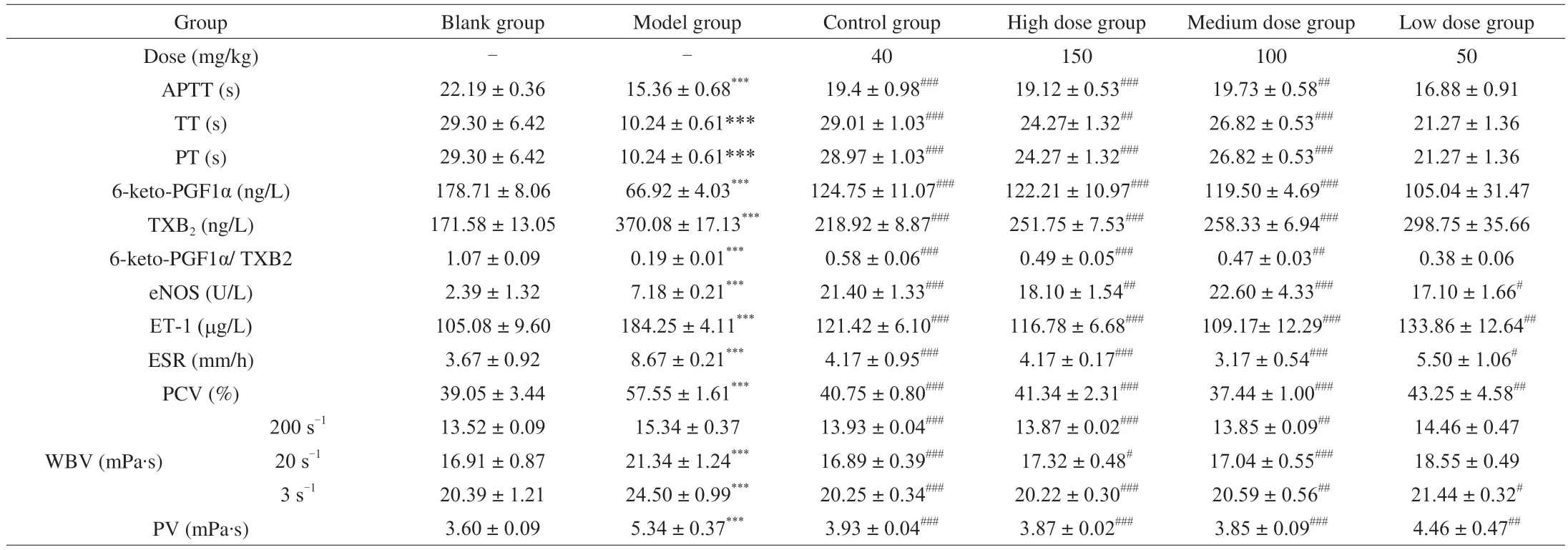
Table 2 Evaluation of anticoagulant/antithrombotic effect of RCJ2-Ib using an acute blood stasis rat model (mean ± standard deviation,n =6)
3.6.4 Effect of RCJ2-Ib on ESR and PCV in blood stasis model rats
The aggregation of red blood cells was the most important factor affecting ESR speed.The greater the aggregation force between the red blood cells,the faster ESR,and vice versa [22].PCV was the most important factor affecting blood viscosity.Compared with the blank group,the contents of ESR and PCV in the model group increased very significantly (P<0.001) (Table 2),indicating that the acute blood stasis model was successfully established.ESR and PCV in the high-dose and medium-dose groups were significantly (P<0.001) lower than those in the model group,with PCV in the low-dose groups being significantly (P<0.01) lower.These results indicated that RCJ2-Ib might inhibit thrombus formation by decreasing PCV and ESR.
3.6.5 Effect of RCJ2-Ib on WBV and PV in blood stasis model rats
Hemorheology detection is widely used in clinical investigations,especially for studies on the mechanism of thrombosis and the determination at the early stage of thromboembolism [43,44].WBN reflects the sum effect of PV,hematocrit (PCV),red blood cell deformability and aggregation,and platelet and leukocyte rheological properties.A higher blood viscosity results in a slower blood flow,causing a higher susceptibility to hyperviscosity,thrombosis and embolic disease [45].In this study (Table 2),PV and WBV in the model group increased significantly (P<0.001) compared with the blank group at frequencies of 200,30 and 3 s-1.These results indicated that the acute blood stasis model was successfully established.Compared with the model group,the high-and medium-dose groups had significantly lower PV (P<0.001) and WBV (P<0.01) at all frequencies.The low dose of RCJ2-Ib exhibited significantly (P<0.01)lower efficacy on PV but exerted little effect on WBV (P>0.05).Accordingly,one can speculate that RCJ2-Ib might decrease WBV by lowering PCV,decrease PV by lowering ESR,and finally cause the inhibition of thrombus formation.
3.7 Analysis of the differences of intestinal flora at the level of genus
The relative abundance distribution histogram (Fig.5a) and the heat map (Fig.5b) were drawn according to the horizontal abundance information and species annotation of the samples in the genus.According to Fig.5,intestinal flora of rats in the experimental group mainly included four genera,includingLactobacillus,Escherichia-Shigella,Lachnospiraceae-unclassified and Lachnospiraceae_NK4A136_group,accounting for more than 50% .In general,Escherichia coliis harmless to human body,while,it will become toE.pathogenicbacteria with the reduced immunity,intestinal chronic lack of stimulation and other special circumstances [46].It was reported that plasma coagulase was a major pathogenicE.colimaterial [47].Lactobacillusis a bacterium,and it that ferments sugars to lactic acid.Both of them are the important bacteria of intestinal polysaccharide fermentation [48].Compared with the blank group,Escherichia-ShigellaandLactobacilluswere significantly increased in the high-,medium-and low-dose groups.We speculated that the reason may be that RCJ2-Ib had good anticoagulant activity,so it could inhibit the plasma coagulation enzymes activity,and promote the growth ofE.coli.It’s also possible that with the increase ofE.coli,RCJ2-Ib,as a lactobacillus starter,increased the number ofLactobacillusandE.coliin the intestinal tract in order to maintain the balance of intestinal flora [49].As shown in Figs.5a and 5b,the intragastric administration of RCJ2-Ib in Lachnospiraceae_NK4A136_ group was significantly reduced,suggesting that the medium-dose group could maintain the intestinal flora balance of rats.
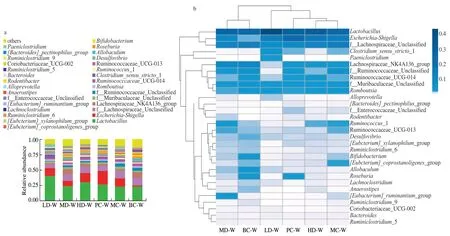
Fig.5 (a) Phylogenetic tree of fungi and (b) heatmap at the genus level for RCJ2-Ib
4.Conclusions
A novel acidic polysaccharide (RCJ2-Ib) isolated from Rosa Chinensis Flos was identified and showed anticoagulant and antithrombotic properties for the first time.RCJ2-Ib was determined to be 1,4-linked polymannuronic acid mainly containing substantialβ-D-anomers.RCJ2-Ib exerted a potent antithrombotic effect in an acute blood stasis rat model, which was achieved through three mechanisms: 1) regulation of endogenous and exogenous coagulation pathways;2) inhibition of thrombus formation by reducing TXB2 and ET-1 in the serum,increasing the eNOS and 6-keto-PGF1αcontents,while maintaining the balance of 6-keto-PGF1α/TXB2;3) reduction of both WBV (by reducing PCV) and PV (by reducing ESR).As an anticoagulant,RCJ2-Ib could increase the number ofLactobacillusandE.coliand maintain the balance of intestinal flora.Thus,RCJ2-Ib might be an effective antithrombotic agent and the maintenance of the intestinal tract,playing an important role in the antithrombotic activity of Rosa Chinensis Flos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
This work was supported by Research on Precision Nutrition and Health Food,Department of Science and Technology of Henan Province (CXJD2021006),and Key Project in Science and Technology Agency of Henan Province (202102110283 and 202102110149).
杂志排行
食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil
