Desertification Drives the Shift in Egg Size-Number Trade-Off in an Agamid Lizard
2023-01-05ZhigaoZENGZhenshengLIUJinyunWEIXiaoleiZHANGandWeiguoDU
Zhigao ZENG ,Zhensheng LIU ,Jinyun WEI,2 ,Xiaolei ZHANG,2 and Weiguo DU*
1 Key Laboratory of Animal Ecology and Conservation Biology,Institute of Zoology,Chinese Academy of Sciences,Beijing 100101,China
2 College of Wildlife Resources,Northeast Forestry University,Harbin 150040,Heilongjiang,China
3 Key Laboratory of Conservation Biology,State Forestry Administration,Harbin 150040,Heilongjiang,China
Abstract Desertification is a serious ecological problem leading to significant biodiversity loss,but how desertification drives shifts in life history and fitness of animals remains understudied.To clarify whether habitat desertification causes shifts in life history strategies,we compared ecological factors and reproductive traits of Phrynocephalus przewalskii from three different desertification habitats– fixed dune,semi-fixed dune and mobile dune of a semi-arid region of Inner Mongolia,at the eastern edge of Hobq Desert,China.Our results showed a significant shift in the egg size-number trade-off of P.przewalskii in response to desertification,with lizards from the mobile dune habitat producing smaller clutches of larger eggs than lizards from the fixed and semi-fixed dune habitats.This life history shift is likely adaptive and driven by abiotic factors (temperature and precipitation)rather than biotic factors (food availability and lizard population density).Our study demonstrates that habitat desertification drives the shift in egg size-number trade-off in a lizard and highlights the importance of exploring the life history responses of animals to habitat desertification as well as to other traditionally well-studied factors like temperature,especially in the context of future global climate change.
Keywords desert adaptation,egg,life history,Phrynocephalus przewalskii,reptile,trade-off
1.Introduction
Life history variation and the ecological forces driving that variation have been a central topic in the study of life history evolution (Stearns,1992;Roff,2002).Traditional life history studies have focused on variation along geographic (e.g.,latitudinal and altitudinal) clines differing in temperature,humidity,precipitation,food availability or population density (Angillettaet al.,2004;Denno and Dingle,1981;Duet al.,2005;Hille and Cooper,2015;Yu and Deng,2020).Recently,life history variation in response to climate change has received increasing attention in ectothermic animals because the behavior and physiology of these animals are highly temperature-dependent (Angillettaet al.,2002;Haoet al.,2021;McLeanet al.,2018;Radchuket al.,2013).However,life history variation in response to other important global changes,such as desertification,has attracted much less attention.
Desertification means land degradation in arid,semi-arid and dry sub-humid areas caused by various factors including climatic variation and human activities (Heshmati and Squires,2013).Climate change can accelerate land degradation processes and trigger desertification by altering the spatiotemporal patterns in temperature,rainfall and wind,and resulting in extreme weather events (Heshmati and Squires,2013;Huanget al.,2020).Desertification mainly occurs in arid,semi-arid and dry sub-humid areas,and is increasingly becoming one of the most serious ecological problems responsible for biodiversity loss (Heshmati and Squires,2013;Huanget al.,2020;Malavet al.,2020;Tanget al.,2018).The environmental extremes present in areas of desertification include sparse vegetation,high temperatures and low precipitation which threaten the survival of animals in these degraded habitats.For example,in the arid desert steppe of China,habitat desertification has reduced lizard species diversity and simplified lizard community structure(Weiet al.,2019;Zenget al.,2014).Generalist desert reptile species are not able to persist in degraded sites due to vegetation loss,whereas some species specialized for open and sandy environments survive well (Attumet al.,2006).This implies that desert specialists may adopt unique life history strategies adapting them to desertification.Comparing variation in life history traits among different animal populations along the desertification gradient may help us understand the driving forces responsible for life history adaptation to desert environments.
Life history trade-offs that are negatively associated with each other play an important role in life history adaptation in response to environmental change (Roff,2002;Stearns,1992).The trade-off between size and number of offspring is wellknown among and within populations of reptiles and other animal lineages (Jiet al.,2009;Liet al.,2014;Ljungströmet al.,2016;Roff,2002;Stearns,1992).Some high-latitude lizards produce larger clutches with bigger eggs than their low-latitude counterparts (Duet al.,2014).Compared with low-elevation populations,Qinghai toad-headed lizards (Phrynocephalus vlangalii)from high-elevation populations produce fewer and larger offspring (Liet al.,2014),while two populations of Mediterranean lizard species (Psammodromus algirus) separated by a 600-m altitudinal gradient show the opposite pattern(Iraetaet al.,2013).This kind of geographic variation in life history traits largely reflects intrinsic (presumably genetic)influences (Duet al.,2005),however maternal effects can also significantly affect the trade-off between size and number of offspring (Maet al.,2014;Olssonet al.,2002).For example,females of the oviparous lizard (Scincella modesta) maintained at low temperatures produce larger eggs and heavier hatchlings than those at warm temperatures (Maet al.,2014).Accordingly,life history responses to environmental changes may be a result of evolutionary adaptation and/or environmentally induced phenotypic variation.
The desert toad-headed agama (Phrynocephalus przewalskii)is a desert specialist and widely distributed in desert,semidesert and desert steppe regions of northern China and adjacent Mongolia (Urquhartet al.,2009;Zhaoet al.,1999).P.przewalskiiis dominant in habitat with sparse vegetation,and its abundance increases with decreasing vegetation cover (Zenget al.,2014,2016).However,some life history traits ofP.przewalskiivary significantly among different geographical populations due to the influence of food availability and population density(Wanget al.,2011;Zenget al.,2013).In this study,we compared differences in reproductive life history traits ofP.przewalskiifrom three desertification habitats (i.e.,fixed dune,semi-fixed dune,and mobile dune) at the same latitude on the eastern edge of the Hobq Desert,China,to clarify whether habitat desertification is responsible for driving adaptive shifts in their life history strategies.We predicted that females from the mobile dune population would produce smaller clutches of larger eggs than the fixed dune population,either because increased population density may induce this population of lizards to produce smaller clutches (Wanget al.,2011),or because females exposed to low precipitation/humidity,high temperature and low food availability produce smaller clutches of larger offspring,as seen in some other lizards (Abell,1999;Díazet al.,2012;Wanget al.,2016).
2.Materials and Methods
2.1.Study speciesThe desert toad-headed agama(Phrynocephalus przewalskii) is a small diurnal oviparous lizard[up to 60 mm snout-vent length (SVL)] from the Jungar Banner and Dalad Banner of Inner Mongolia (40°07′–40°25′ N,109°20′–111°09′ E),at the eastern edge of the Hobq Desert,China (Figure 1),and was formerly named the steppe toad-headed agama (P.frontalis) (Gozdzik and Fu,2009).P.przewalskiiinhabits selfexcavated burrows at night and during its winter hibernation period,and gravid females dig burrows in the sand to lay their eggs in late spring or early summer (Liet al.,2017;Zhaoet al.,1999;Zhaoet al.,2011).Most females produce a single clutch of up to six eggs with an average of 3 eggs,while the minority(5–19%) of females can produce two clutches from late May to late July (Liet al.,2017;Zenget al.,2013;Zhaoet al.,2011).Reproductive traits of the species differ among geographicallyseparated populations in response to environmental factors such as temperature,precipitation and food availability (Zenget al.,2013;Zhaoet al.,2011;Sunet al.,2018).
2.2.Study sites and survey methodsThe study area was predominately desert steppe with low to moderate levels of sparse vegetation.During June–July of 2017,we investigated lizard population density and associated environmental factors at three sites,comprising three desert habitats,with different vegetation cover.The three study sites (20–150 km apart) were characterized as (i) fixed dunes (40°11′–40°14′ N,111°05′–111°09′ E;elevations 1030–1090 m;vegetation cover >50%),(ii) semi-fixed dunes (40°07′–40°09′ N,111°00′–111°05′ E;elevations 1080–1160 m;vegetation cover 20–40%) in the Jungar Banner,and (iii) mobile dunes (40°12′–40°18′ N,109°46′–109°52′ E;elevations 1080–1190 m;vegetation cover <10%) in the Dalad Banner (Figure 1).There should be no chance for population crossover of the lizards at the three sites,because their weekly home ranges were small with less than 600 m2(S.R.Li,unpublished data).The fixed dune,semi-fixed dune and mobile dune are three major stages of desertification process (Heshmati and Squires,2013;Zhaoet al.,2007).Desertification caused by climate change and human activities can transform fixed dune into mobile dune,and semifixed dune is its transitional type.On the contrary,mobile dune can be gradually stabilized and converted to semi-fixed and fixed dunes due to vegetation restoration and improvement of the soil environment (Heshmati and Squires,2013;Zhaoet al.,2007).
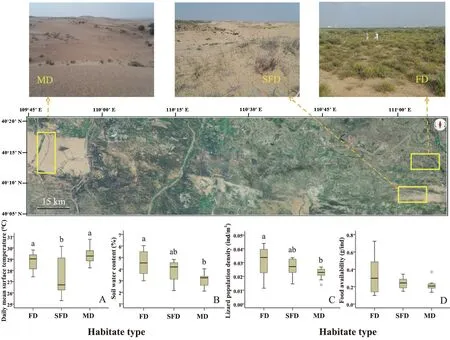
Figure 1 Photographic images show the study area on the eastern edge of Hobq Desert,and location of the three habitat types (FD:fixed dune;SFD: semi-fixed dune;MD: mobile dune).Panels (A)–(D) show differences in environmental factors,population density and food availability of the desert toad-headed agama (Phrynocephalus przewalskii) during the study period.Boxplots display a median line,interquartile range (IQR) boxes,1.5IQR whiskers and data points (open circle).Different letters above boxplots indicate statistically different mean values (Tukey’s test).Sample sizes all for the fixed dune,semi-fixed dune and mobile dune populations were 12,12 and 12 transects,respectively.
We surveyed lizards along 12 transects (200 m in length,with >500 m interval between transects) at each study site (N=36 transects).We searched for lizards while walking (1.5 km/h)from 10:00 to 12:00 h on 9 sunny days during June and July,and recorded the number of lizards sighted visually within 2 m either side of the transect,to estimate relative population density.Lizard surveys were conducted by three persons in the three sites at the same time.We completed lizard investigation of four transects at each site on a day,and each transect was repeated three times.Population density was calculated as the number of lizards encountered per square meter of transect.
Phrynocephalus przewalskiimainly preys on insects (Zhanget al.,2018).Insect abundance was surveyed by placing one tray trap and one sticky trap at each transect for one week.Tray traps (43 cm × 30 cm × 4 cm) were buried up to their top edge in soil and half-filled with a solution of water and detergent.We checked the tray traps once a day and collected the available food of lizards in the traps.Sticky traps (30 cm × 25 cm) were yellow sticky cards suspended on a bush 0.5 m above each transect and recovered after one week.Total mass of available insects from each trap was weighed to the nearest 0.01 g.An estimate of relative food availability was calculated as insect mass divided by the number of lizards counted in each transect.
We also recorded ground surface temperature every 30 min from June to July by randomly placing three DS1921 iButton temperature loggers (Dallas Semiconductor,Dallas,Texas,USA;diameter,15 mm;height,6 mm) along each transect.To measure soil water content,we used a foil sampler to take three intact soil cores at the subsurface layer (15–20 cm) along each transect,and oven-dried the cores at 105 °C for 24 h.Soil water content was calculated as a percentage of dry weight.
2.3.Animal collection and captive husbandryThe lizards were palpated around the abdomen to determine their pregnancy status.We collected 83 gravid females from fixed(30♀),semi-fixed (24♀) and mobile (29♀) dune habitats in June 2017 and transferred them to the Shierliancheng Field Station,Institute of Grassland Research of the Chinese Academy of Agricultural Sciences (40°12′17′ N,111°07′43′ E),Inner Mongolia,China.They all survived the transportation.Gravid females were measured (SVL to ±0.5 mm),weighed (mass to ±0.01 g),toe-clipped for individual identification,and then individually housed in plastic terraria (500 mm × 300 mm × 300 mm,L mm ×W mm × D mm) with 50-mm deep substrate of moist sand.Terraria were kept in a temperature-controlled room (24 ±1 °C) with a light cycle of 12L:12D (08:00 h on,18:00 h off).A 60 W light bulb was suspended 50 mm above each terrarium to provide opportunities for behavioral thermoregulation from 08:00 to 16:00 h.Each terrarium was checked at least six times a day.When freshly-laid clutches were found,eggs were promptly counted and weighed (±0.001 g) to minimize potential changes in mass due to water exchange.Postpartum females were measured again for SVL and mass,and then returned to their original terraria.Food [mealworms (Tenebrio molitor) and crickets (Acheta domesticus) dusted with mixed vitamins and minerals] and water were provided ad libitum for adult lizards.
2.4.Statistical analysisWe only used data from the first clutch to test for among-population differences in reproductive traits,because the majority of females only laid one clutch in the laboratory (95.7% for the fixed dune population,100% for the semi-fixed dune population,and 100% for the mobile dune population).Relative clutch mass (RCM) was calculated as the ratio of clutch mass to maternal post-oviposition mass.We used one-way ANOVAs to determine among-habitat differences in daily mean ground surface temperature,soil water content at 15 cm depth,lizard population density and food availability,and among-population differences in female body size,time in captivity before oviposition,clutch size,egg size,clutch mass,and RCM.Chi-square test was used to analyze the interpopulation difference in the reproductive success rate of gravid females.ANCOVAs were conducted to determine amongpopulation differences in clutch size,egg size,and clutch mass with female body size (i.e.,SVL) as a covariate.We analyzed all data with the software package IBM SPSS Statistics 25.
3.Results
3.1.Environmental factors and population density of lizardsAbiotic factors (i.e.,temperature and soil water content)and biotic factors (i.e.,lizard population density and food availability) were compared among the three study sites.Daily mean ground surface temperature was higher in fixed and mobile dune habitat than in semi-fixed dune habitat (F2,33=8.759,P=0.001;Figure 1A),while soil water content at 15 cm depth decreased with increasing habitat desertification (F2,33=8.849,P=0.001;Figure 1B).Population density of lizards also decreased with increasing habitat desertification (F2,33=4.300,P=0.022;Figure 1C).Relative food availability did not differ among the three habitat types (F2,33=2.399,P=0.106;Figure 1D).
3.2.Lizard body size and reproductive traitsEgg-laying females for the fixed,semi-fixed and mobile dune populations were 23,18 and 22 individuals respectively,showing no interpopulation difference in the reproductive success rate (χ2=0.020,df=2,P=0.990).Body size was greater in the fixed dune and mobile dune populations than in the semi-fixed dune population (SVL:F2,60=5.933,P=0.004;Body mass:F2,60=3.738,P=0.030;Figure 2).Females produced a clutch of eggs at an average of 14.7,16.3 and 16.2 days following capture for the fixed,semi-fixed and mobile dune populations respectively,showing no inter-population difference (F2,60=0.369,P=0.693).Clutch size did not differ among the three lizard populations(F2,60=1.498,P=0.232).After maternal body size effects were statistically removed,clutch size was greater in fixed dune and semi-fixed dune populations than in the mobile dune population (F2,59=5.391,P=0.007) (Figure 3A).Females from the mobile dune population laid larger eggs than those from the fixed dune and semi-fixed dune populations before (F2,60=6.909,P=0.002) and after (F2,59=6.193,P=0.004) maternal body size effects were removed (Figure 3B).Clutch mass did not differ among the populations before (F2,60=0.127,P=0.881) or after(F2,59=2.520,P=0.089) maternal body size effects were removed(Figure 3C).As a result,reproductive effort (measured as relative clutch mass,RCM) did not differ among the populations (F2,60=1.171,P=0.317) (Figure 3D).

Figure 2 Snout-vent length and post-partum body mass of the desert toad-headed agama,Phrynocephalus przewalskii,from three populations in fixed,semi-fixed and mobile dune habitats of a semi-arid region of Inner Mongolia,China.Boxplots display a median line,interquartile range (IQR)boxes,1.5IQR whiskers and data points (open circle).Different letters above the boxplots indicate statistically different mean values (Tukey’s test).Sample sizes for the fixed dune(FD),semi-fixed dune (SFD) and mobile dune (MD) populations were 23,18 and 22 lizards,respectively.
4.Discussion
Consistent with our prediction,our results show a shift in the egg size-number trade-off ofP.przewalskiiin response to desertification,with lizards from the mobile dune habitat producing smaller clutches with larger eggs than those from desert steppe populations (fixed and semi-fixed dune habitats).Reproductive life history traits have been quantified in some desert lizard species both in our study regions (Quet al.,2011;Wanget al.,2011),as well as in other desert areas of the world(Mata-Silvaet al.,2010;Pianka,1986;Van Loben Sels and Vitt,1984).Nonetheless,as far as we know,this is the first study to show that habitat desertification drives the shift in egg sizenumber trade-off in a desert lizard.
Both biotic and abiotic factors can influence the shift in egg size-number trade-off in response to desertification.Our study demonstrates that the shift in egg size-number tradeoff inP.przewalskiiwas associated with high temperatures and drought due to desertification,with the mobile dune habitat having higher surface temperatures and lower soil water content than the less desertified habitats.Our findings are comparable to previous studies showing that oviparous lizards tend to produce fewer but larger offspring under conditions of high temperatures and drought.For example,the lacertid lizard (Psammodromus algirus) from drier and warmer sites produced larger hatchlings (Díazet al.,2012),and the striped plateau lizard (Sceloporus virgatus) produced smaller clutches of larger offspring under low rainfall conditions (Abell,1999).In contrast,we found no evidence to suggest that the shift in egg size-number trade-off in our study was associated with food availability or population density of lizards,because food availability did not differ among the three habitats,and the population density of lizards decreased rather than increased with increasing habitat desertification.Accordingly,our study shows that the shift in egg size-number trade-off is likely driven by abiotic factors (e.g.,temperature and precipitation) rather than biotic factors (e.g.,food availability and lizard population density).Interestingly,in contrast to our results,a comparative study onP.przewalskiipopulations from Badan Jaran and Tengger deserts showed that females produced fewer but larger eggs with increasing population density (Wanget al.,2011),probably because the increased population density intensified the pressure of intraspecific competition,and therefore induced smaller clutch size,delayed maturity,and low laying frequency(Case,1983).The discrepancy with our results implies that life history evolution may also be driven by biotic factors in desert populations which experience similar abiotic but different biotic factors.High-density population ofP.przewalskiiin the fixed dune of this study produced more but smaller eggs,which might be related to the relatively low population density compared with other studied populations.Clutch sizes in the fixed dune population are similar to those in many lowdensity populations (4/9=44.4%) from the Badan Jaran and Tengger deserts (Wanget al.,2011).In low-density populations,lower levels of intraspecific competition and selection for large offspring should be weaker and more easily overridden by direct selection for increased fecundity (Schrader and Travis,2012).In addition,food availability can also be a driving factor of life history variation among different geographical populations ofP.przewalskii,because females produce more and larger eggs in localities with high food availability than those with low food availability (Zenget al.,2013).

Figure 3 Regional variation in clutch size (A),egg mass (B),clutch mass (C),and relative clutch mass (RCM) (D) of the desert toad-headed agama,Phrynocephalus przewalskii.Boxplots display a median line,interquartile range (IQR) boxes,1.5IQR whiskers and data points(open circle).Different letters above the boxplots indicate statistically different mean values (univariate pairwise analysis of general linear models with post-partum snout-vent length as the covariate).Sample sizes for the fixed dune (FD),semi-fixed dune (SFD) and mobile dune (MD) populations were 23,18 and 22 lizards,respectively.
Our study demonstrates that desert specialist reptiles can adjust their life history strategies to adapt to desertification.The eastern edge of modern Hobq Desert was only desertified in the past 120 years due to intensive human activities such as over-grazing (Ai,2009;Zhanget al.,2018).Our commongarden experiment partly controlled for the environmental effect on reproductive life history,and thus,the shift in egg size-number trade-off in response to desertification is likely intrinsic and adaptive.Nonetheless,it is noteworthy that our short-term common-garden experiments cannot completely exclude the contribution of phenotypic plasticity (e.g.,maternal effects) to life history responses.More rigorous experiments are needed to fully understand the contribution of phenotypic plasticity or genetic determination to life history shifts.More generally,our study highlights the importance of exploring life history responses of animals exposed to other environmentally threatening processes (e.g.,desertification,pollution) rather than traditionally well-studied factors (e.g.,temperature),especially in the context of future global climate change.Such information is critical for evaluating the vulnerability of animals to the ongoing environmental perturbations induced by human activities (Marshallet al.,2016;Pearsonet al.,2014).
AcknowledgementsThis work was supported by grants from the National Natural Science Foundation of China(31861143023,31821001,and 31570526) and China’s Biodiversity Observation Network (Sino-BON).We thank X.HAO,X.Z.HAN,S.R.LI,Y.J.YANG and X.L.ZHANG for their assistance in the field and laboratory.We are also very grateful to S.R.LI for providing the home range data.Ethics approval(IOZ14001) for the collection,handling,and husbandry of the study animals was given by Animal Ethics Committees at Institute of Zoology,Chinese Academy of Sciences.
杂志排行
Asian Herpetological Research的其它文章
- Endocast Morphological Variation and Its Driving Forces in Scutiger boulengeri
- Metagenomic Analysis of Mangshan Pit Viper (Protobothrops mangshanensis) Gut Microbiota Reveals Differences among Wild and Captive Individuals Linked to Hibernating Behaviors
- Lineage Diversification and Niche Evolution in the Chinese Cobra Naja atra(Elapidae)
- Phylogenetic Relationships among Chinese Rice Frogs within the Fejervarya limnocharis Species Complex (Amphibia: Dicroglossidae)
- Application of eDNA Metabarcoding for Detecting Anura in North China
- Three New Species of Diploderma Hallowell,1861 (Reptilia: Squamata:Agamidae) from the Shaluli Mountains in Western Sichuan,China
