A flexible hard carbon microsphere/MXene film as a high-performance anode for sodium-ion storage
2022-12-13CAOHailiangYANGLiangtaoZHAOMinLIUPeizhiGUOChunliXUBingsheGUOJunjie
CAO Hai-liang, YANG Liang-tao, ZHAO Min, LIU Pei-zhi,GUO Chun-li, XU Bing-she,3, GUO Jun-jie
(1.Key Laboratory of Interface Science and Engineering in Advanced Materials, Ministry of Education,Taiyuan University of Technology, Taiyuan 030024, China;2.Shenzhen Institute of Advanced Technologies, Chinese Academy of Sciences, Shenzhen 518055, China;3.Materials Institute of Atomic and Molecular Science, Shaanxi University of Science & Technology, Xi'an 710021, China)
Abstract: Hard carbon is considered the most promising anode material for sodium-ion batteries, but its volume change during sodiation/desodiation limits its cycle life.Hard carbon microspheres (HCSs) with no binder were composited with a MXene film to form an electrode and its sodium storage properties were studied.The microspheres were prepared using Shanxi aged vinegar as a liquid carbon source.Two-dimensional Ti3C2Tx MXene (T is a functional group) was used as a multifunctional conductive binder to fabricate the flexible electrodes.Remarkably, because of the three-dimensional conductive network, the HCS/Ti3C2Tx film electrode has a high capacity of 346 mAh g−1, excellent rate performance and outstanding cycling stability over 1 000 cycles.This remarkable electrochemical performance indicates that the flexible film is a very promising anode for next-generation sodium-ion batteries.
Key words: Sodium-ion batteries;Hard carbon microspheres;MXene;Anode;Flexibility
1 Introduction
Lithium-ion batteries (LIBs) have been the leading chemical power source because of their advantages in energy density, power density, and cycling life[1–2].However, the uneven distribution of lithium resource risks the supply chain of raw materials for LIBs, especially for stationary energy storage[3].Consequently, sodium-ion batteries (SIBs) have been considered as an important complement system to LIBs due to the earth abundant sodium resource and the same rocking-chair storage mechanism[4–6].However,the advanced materials of LIBs are not effectively in accordance with that of SIBs because of the difference in size and local environment between Li+and Na+.The sodium ion (0.102 nm) has a larger radius than that of lithium ion (0.076 nm), resulting in the sluggish diffusion kinetics[7–8].To date, extensive efforts have been devoted to explore cathode materials for SIBs, including layered transition metal oxides,prussian blue analogs, and polyanionic compounds[9–11].However, exploring high-performance anode materials is still challenging.
Several materials have been studied as negative electrode for SIBs, such as carbonaceous materials, alloys, metal oxides/sulfides and phosphates[12–16].Metal oxide and alloy electrodes usually show poor cycling durability because of their large volume expansion during the sodiation/desodiation processes[17–18].Among various negative electrode materials, hard carbon (HC) has been recognized as a promising negative electrode material for sodium ion storage[19–20].Until now, HCs from different precursors have been reported, including biomass wastes, carbohydrates and polymers[21–23].Our group reported a hard carbon microfiber derived from renewable papers, which showed a specific capacity of 319.6 mAh g−1[24].Tirado et al.reported microspherical carbon particles prepared using mixture precursors of resorcinol and formaldehyde, which showed a capacity of 285 mAh g−1[25].However, the undesirable and inactive impurities derived from these precursors as well as irregular geometric morphologies compromise the sodium ion storage performance of HCs[26].
In addition, MXenes, a family of two-dimensional transition metal carbides and nitrides, have received attractive attentions in energy storage and conversion[27–28].MXenes are considered as promising candidate materials for supercapacitors and secondary rechargeable batteries because of their tunable surface terminations, metallic conductivity, and surface hydrophilicity[29].Moreover, MXenes flakes can be adopted to fabricate free-standing, flexible electrodes,holding a great promise for fabricating flexible devices.The flexible MXene film electrodes can be easily obtained through rolling or vacuum filtration.Recently, Xu et al.studied MXene as a conductive binder to prepare flexible porous composite electrodes for supercapacitors, which show excellent flexibility and electrochemical performance[30].Therefore,it is reasonable to expect that the combination of MXene and HC can not only fabricate free-standing flexible electrodes, but also promote the electrochemical properties of the electrodes, expanding the application of HC.
We here chose Shanxi aged vinegar as the liquid carbon source to synthesize hard carbon microspheres(HCS) using hydrothermal method followed by subsequent carbonization treatment.The HCS pyrolyzed at 1 400 °C displays the highest specific capacity and good cycling stability.The Ti3C2TxMXene nanosheets were used as multifunctional binder to fabricate flexible and free-standing HCS/MXene(HCS/MX) film electrode with excellent cycling stability.Compared with the conventional PVDF-bonded HCS electrode, the flexible HCS/MX electrode exhibits superior performances in term of capacity,and long cycling ability for SIBs.The results demonstrate that the as-obtained film electrode is a promising negative electrode of SIBs.
2 Experimental
2.1 Materials synthesis
The Shanxi aged vinegar (Donghu) was purchased and used as liquid carbon precursor.80 mL vinegar was placed in a 100 mL autoclave, and treated hydrothermally at 180 °C for 12 h to obtain a black powder.Then, the powder was washed three times using ethanol and DI water, and dried overnight in a vacuum oven.The obtained powder was pyrolyzed at 1 000, 1 200, 1 400 and 1 600 °C respectively, for 2 h with a ramping rate of 3 °C min−1.The resulting materials were labeled as HCS-X, where X is the carbonization temperature.
The Ti3C2TxMXene used in this work was synthesized by etching MAX phase following the reported method[31].Firstly, 1.85 g of LiF was dissolved in 40 mL 9 mol L−1mixed acid solution (V(HCl)∶V(HF)=37∶3) during stirring process.Then, 1.85 g of Ti3AlC2powder was gradually added into the acidic solution in 15 min.Secondly, the etching reaction run for 24 h at 35 °C in an oil bath.After the etching procedure, DI water was adopted to wash the obtained resultant at 3 500 r min−1until a pH value of 6 was achieved.Then, the sediment was collected after the last centrifugation cycle and sonicated for 30 mins under Ar bubbling.The MXene aqueous solution was collected through centrifugation of the supernatant at 3 500 r/min for 30 min.Finally, the concentration of the MXene aqueous solution was adjusted to 1 mg mL−1for further use.
The HCS/MX film electrodes were prepared by a vacuum-assisted filtration of the mixture of HCS-1400 and Ti3C2TxMXenes solution.First of all, the HCS-1400 solution dispersed in N,N-dimethylformamide(DMF) with the concentration of 1 mg mL−1was prepared in advance.Then the Ti3C2Txsolution and HCS dispersion were mixed homogeneously at different ratios through ultrasonic treatment.The HCS/MX films were fabricated by vacuum filtration of the mixed dispersion.The flexible film was obtained after dried overnight.The mass ratio of HCS-1400 and Ti3C2Txsolution is 1∶1, 2∶1, and 4∶1.The corresponding film was labeled as HCS/MX-1, HCS/MX-2 and HCS/MX-4, respectively.
2.2 Materials characterization
The microstructure was characterized using a Japanese science Ultima Ⅳ X-ray diffraction (XRD)with a CuKα radiation source (40 kV, 100 mA,λ=0.154 178 nm).Raman spectra were examined on a Thermo Fischer DXR spectrometer with a 532 nm laser excitation.Morphology and microstructure investigations were carried out by using the transmission electron microscopy (TEM, JEOL JEM-2010)and scanning electron microscope (SEM, LYRA3 XMH TESCAN).
2.3 Electrochemical measurements
CR2032 coin cells were assembled to test the electrochemical properties of HCS and HCS/MX film electrodes.For electrode preparation, a slurry of 80% HCS samples, 10% Super P, and 10% polyvinylidenefluoride (PVDF) binder in N-methylpyrrolidone was casted on the Cu foil, followed by drying at 100 °C for 12 h in a vacuum oven.The HCS/MX films were cut into 12 mm diameter circles, and directly used as working electrodes.A Na foil and glass fiber were used as the counter electrode and separator,respectively.1 mol L−1NaClO4in a mixture of ethylene carbonate and diethyl carbonate (1∶1 in volume)was employed as the electrolyte.The active material of the electrode is ~1.0 mg cm−2.A LAND CT2001 battery test system was used to conduct the charge and discharge tests.Cyclic voltammetry (CV) measurements were carried out on Princeton Applied Research Versa STAT 3 electrochemical workstation.
3 Results and discussion
3.1 Morphology and structure of HCS
In this work, Shanxi aged vinegar was used as liquid carbon source.The HCS material can be obtained through hydrothermal treatment of aged vinegar solution, followed by carbonization treatment.The scanning electron microscope (SEM) image in Fig.1a displays the spherical morphology of HCS-1400 with the size ranges from 2 to 5 μm.Transmission electron microscope (TEM) image (Fig.1b) demonstrates the microstructure of HCS-1400 is primarily amorphous with a few “graphitic” domains.X-ray diffraction (XRD) and Raman spectra were further carried out to study the crystallinity of the samples.Fig.1c shows 2 broad peaks at about 23° and 43°which are assigned to (002) and (100) reflections of graphite, respectively, indicating the disordered structure characteristic of HC[24,32].It is worth noting that the (002) peak shifts to higher angle with the rise of carbonization temperature, demonstrating the smaller interlayer distance (d002).The calculated interlayer distances (d002) by Scherrer equation are 0.389, 0.378,0.371 and 0.362 nm for HCS-1000, HCS-1200, HCS-1400 and HCS-1600, respectively.The Raman spectra (Fig.1d) of disordered carbons normally exhibits a broad peak at around 1 345 cm−1calledD-band(amorphous carbon) and a hump peak at about 1 590 cm−1referred to theGband (crystalline graphite)[33–34].TheID/IGratios of HCS-1000, HCS-1200, HCS-1400 and HCS-1600 were 1.10, 1.15, 1.25 and 1.35, respectively, showing an increasing trend.Moreover,the half width of the 2 bands decreases with increasing carbonization temperature, indicating that the degree of order of carbon layer rises.These Raman results match well with previous reports.
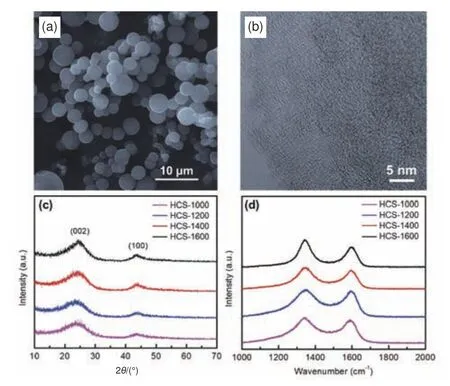
Fig.1 (a) A representative SEM image of HCS-1400.(b) TEM image of HCS-1400.(c) XRD patterns and (d) Raman spectra of HCS carbonized at different temperatures
3.2 Electrochemical performance of HCS
The electrochemical properties of HCS were tested in half cells.Fig.2a shows the first galvanostatic charge/discharge curves of HCS-1000, HCS-1200,HCS-1400, HCS-1600 at a current density of 30 mA g−1with voltage window of 0.01-3.0 V (vs.Na+/Na).HCS-1000 and HCS-1200 exhibit discharge and charge specific capacities of 318.2 and 218.6 mAh g−1, 318.4 and 241.5 mAh g−1, respectively.However, the specific capacities of HCS-1400 electrode increase to 401.8 and 298.2 mAh g−1, corresponding to an initial coulombic efficiency (ICE) of 74.2%.Nevertheless, the capacity of HCS-1600 electrode is only 259 and 201.8 mAh g−1, despite the ICE increases to 78 %.The irreversible capacity mainly assigned to the formation of a solid-state interface film by the side reactions between the electrolyte and the surface functional groups.The discharge capacity is composed of two parts: the plateau capacity below 0.1 V and slope capacity above 0.1 V.As shown in Fig.2b, the slope capacity of HCS-1000 and HCS-1200 is much higher than the plateau capacity.By contrast, the plateau capacity raises from 71 (HCS-1000) and 86 (HCS-1200) mAh g−1to 171.5 mAh g−1for HCS-1400, and then decreased to 100.4 mAh g−1for HCS-1600.According to the previous research,the slope capacity refers to the adsorption and desorption of sodium ions on surface defects, and plateau capacity corresponds to insertion and desertion of sodium ions into graphitic interlayers[8,24,32].
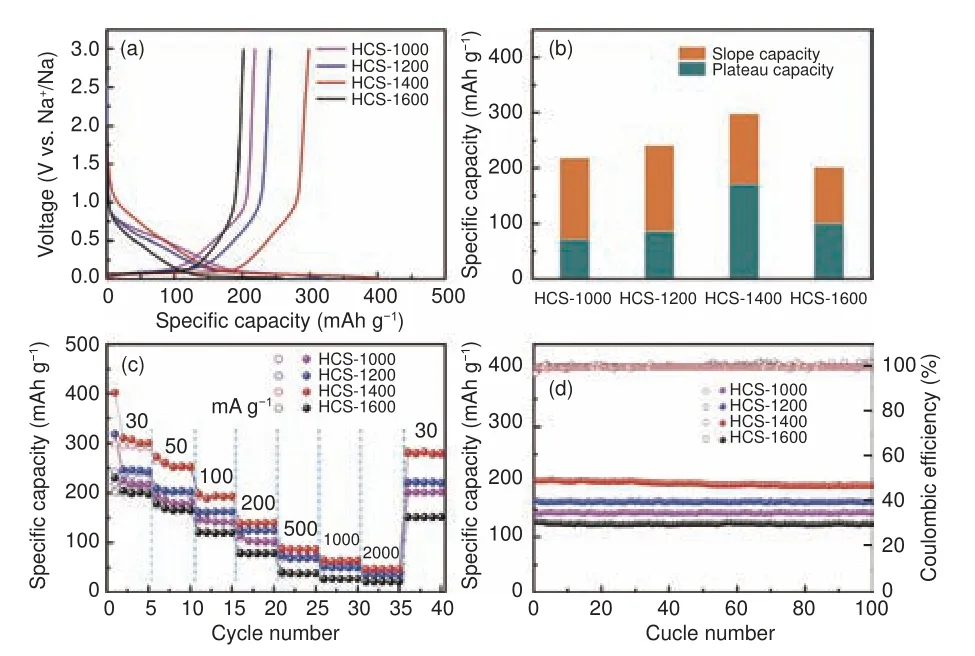
Fig.2 Electrochemical performances of the HCS electrodes.(a) The first charge/discharge profiles.(b) Slope and plateau capacity contribution.(c) Rate performance of HCS at different current density.(d) Cycling stability of HCS
Fig.2c manifests the rate performance to evaluate the kinetic activity of HCS electrodes.Surprisingly, HCS-1400 displays the best average rate capabilities than that of other HCS samples.HCS-1400 delivers charge capacities of 299, 257.5 and 193 mAh g−1at 30, 50 and 100 mA g−1, respectively.It can still delivers 64 mAh g−1when the rate elevates to 1000 mA g−1.Importantly, when the current density recovers to 30 mA g−1, the reversible capacity is as high as 283.2 mAh g−1, implying the outstanding stability of hard carbon.The cycle life of HCS samples is evaluated at the current density of 100 mA g−1for 100 cycles.As shown in Fig.2d, the HCS-1400 demonstrated outstanding cycling durability, and its specific capacity can be retained as 193.5 mAh g−1after 100 cycles, corresponding to a capacity retention of 95.8%.It can be found that the HCS-1400 shows excellent electrochemical properties among the hard carbon anode materials.
3.3 Characterization of HCS/MX
In addition, Ti3C2TxMXene as a functional binder was adopted to promote the electrochemical properties of HCS.Fig.3a illustrates the fabrication of the HCS/MX film.The MXene-bonded HCS films can be simply prepared by vacuum filtration of the mixture solution of HCS-1400 and Ti3C2Txnanosheets.As expected, the HCS/MX films are flexible, free-standing and can be used as anode electrode directly without binder and current collector.Three ratios of HCS-1400: Ti3C2Txof 1∶1, 2∶1 and 4∶1 were employed, which are labeled as HCS/MX-1, HCS/MX-2,and HCS/MX-4, respectively.Fig.3b displays the TEM image of Ti3C2Txflakes.Ultrathin MXene sheets with size of several micrometers can be observed.Fig.3c presents the XRD patterns of the Ti3C2Txand HCS/MX films.The XRD pattern of pure Ti3C2TxMXene film exhibits a (002) diffraction peak at 2θ=7.24°, corresponding to an interlayer distance of 1.22 nm[35].For the HCS/MX films show the features of both HCS-1400 and MXene.Importantly, the Ti3C2Tx-bonded HCS films are flexible and free-standing, as shown in the inset in Fig.3d.Fig.3d and e show the top and cross-sectional SEM images of the HCS/MX-2 film, respectively.The HCS-1400 microspheres are evenly embedded in the three-dimensional (3D) networks fabricated by Ti3C2Txsheets.This structure is beneficial for the rapid transportation of electron and boosts the stability of the electrode.
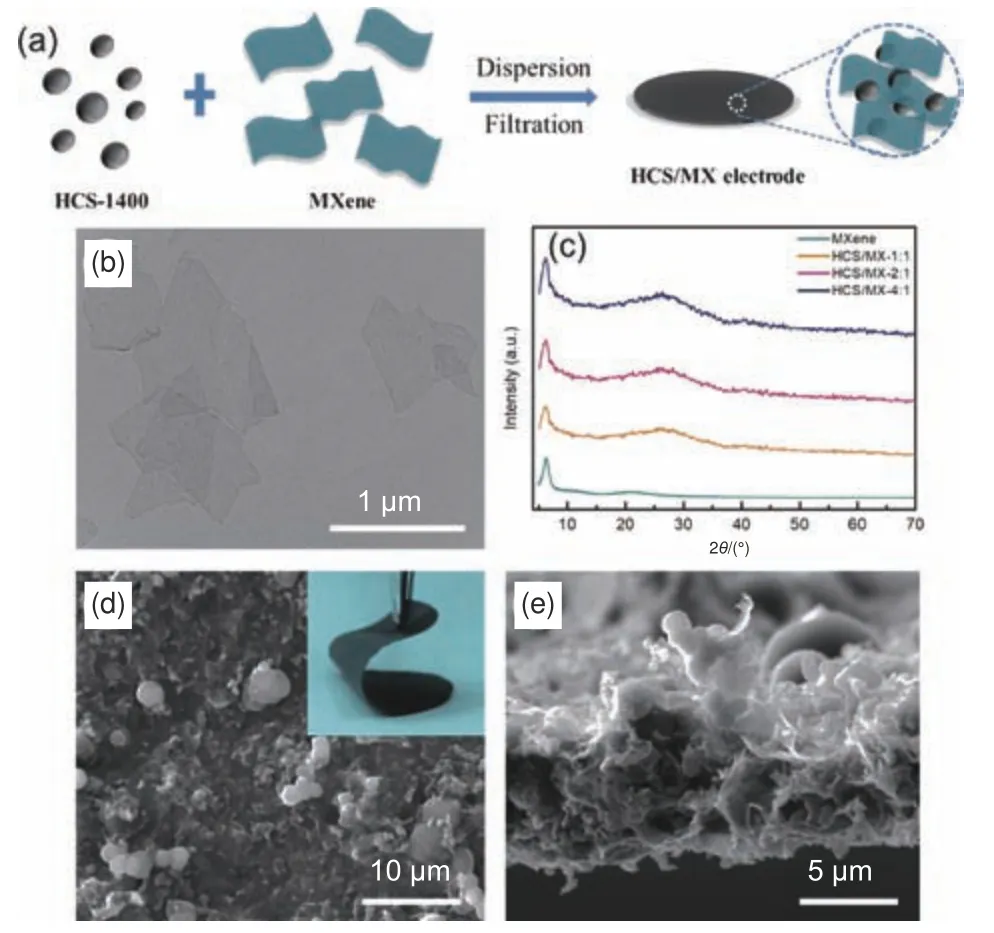
Fig.3 (a) Schematic for the preparation of HCS/MX film.(b) TEM image of MXene nanosheets.Structure characterization of the HCS/MX electrode.(c) XRD patterns, (d) SEM images from top view and (e) cross-sectional view.The insert in (d) is a photo of the flexible HCS/MX film
3.4 Electrochemical behaviors of HCS/MX
To evaluate the electrochemical properties of the HCS/MX films electrodes, the flexible films were directly used as working electrodes.Fig.4a and b show the cyclic voltammetry (CV) curves of the initial three cycles at 0.1 mV s−1for the HCS-1400 and HCS/MX-2 electrode.A pair of cathodic and anodic peaks located at 0-0.2 V, corresponding to the insertion/extraction of Na+in the carbon interlayers[24].The overall peak intensity and the stability of the film electrode is better than that of the HCs electrode.Fig.4c presents the first galvanostatic charge/discharge profiles of HCS/MX film electrodes conducted at a 30 mA g−1.The charge and discharge specific capacity of HCS/MX-1 and HCS/MX-4 is 208.7 and 374.7 mAh g−1, 310.8 and 475 mAh g−1, respectively.However, the discharge and charge capacity of HCS/MX-2 is as high as 596.1 and 346 mAh g−1, corresponding to an ICE of 58%.
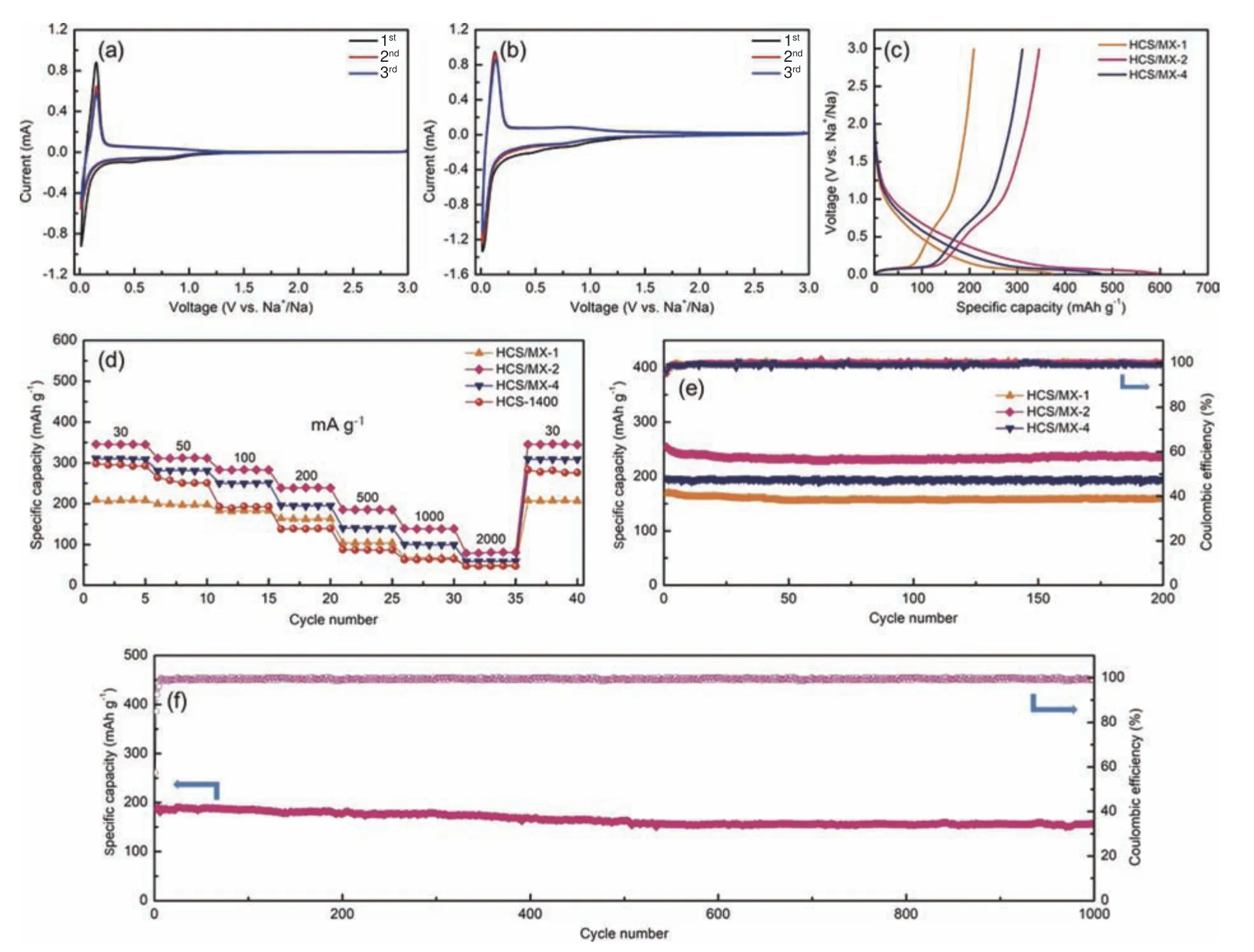
Fig.4 Na-storage behavior of HCS/MX film electrodes.(a) CV curves for initial three cycles of HCS-1400 and (b) HCS/MX-2 film.(c) Charge/discharge performance at 30 mA g−1.(d) Rate capability and (e) cycle performance at 200 mA g−1 for all the film electrodes.(f) Cycling stability of HCS/MX-2 film at 500 mA g−1
The rate capability of the HCS/MX electrodes was investigated at different current rates ranging from 30 to 2 000 mA g−1( Fig.4d).Apparently,HCS/MX-2 presents the best rate capability compared to other electrodes.It shows capacity of 346,313, 283, 239, 185, 139, and 81 mAh g−1at 30, 50,100, 200, 500, 1 000 and 2 000 mA g−1, respectively.Importantly, when the current density gets back to 30 mA g−1, reversible capacity again reaches to 345 mAh g−1, demonstrating the remarkable reversibility.Subsequently, the cycling stability of HC/MX film electrodes is measured at 200 mA g−1for 200 cycles.As shown in Fig.4e, HCS/MX-2 shows superior cycling stability.The 200.6 mAh g−1reversible capacity can be retained after 200 cycles, corresponding to a capacity retention of 99.3%.More importantly,the HCS/MX-2 can even cycle over 1 000 cycles at a high current density of 500 mA g−1(Fig.4f).The obtained reversible capacity at the end of 100, 200, 500,800 and 1 000 cycles is 185, 177, 162, 156 and 155 mAh g−1, respectively, corresponding to a capacity retention of 83.3%.The outstanding cycling durability of the flexible HCS/MX electrode can be mainly attributed to the 3D conductive networks constructed by MXene nanosheets.Furthermore, the SEM images of HCS/MX-2 after 100 cycles also demonstrate the structural stability of the film electrode during the insertion and extraction of Na+(Fig.5).
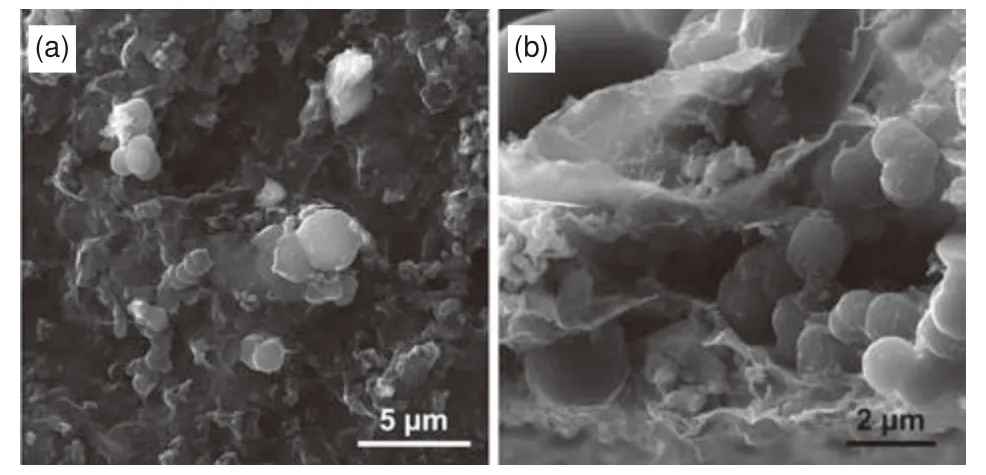
Fig.5 SEM images of HCS/MX-2 film from (a) top view and (b) crosssectional view after 100 charge/discharge cycles
To investigate the kinetics of the electrodes, the HCS/MX-2 electrode was scanned at different scan rates ranging from 0.1 to 1 mV s−1.As presented in Fig.6a, the CV profiles generally behave a similar shape.The integral capacity includes contribution from two parts, namely, Na+insertion/extraction and pseudo-capacitance.The equationi=avbis employed to determine the dominant mechanism, in whichaandbare 2 adjustable parameters values, andvis a scan rate.According to previous report[36], the value ofbcan be obtained by the slope oflog(i) vs.log(v).A value of b close to 0.5 reveals a diffusion-controlled process, while a value of 1.0 suggests an ideal capacitive behavior.As shown in Fig.6b, the value ofbwas calculated to be 0.50 and 0.56 for anodic and cathodic process, respectively, demonstrating a diffusion-controlled process.Based on the reported results, the ratio of capacitive contribution can be calculated using equation[37–38]:i=k1v+k2v1/2, wherek1vandk2v1/2correspond to capacitive and diffusion-controlled process,respectively.As shown in Fig.6c, the HC/MX-2 electrode shows a 44% capacitive contribution at 0.1 mV s−1.However, the proportion of capacitive contribution gradually increases to a maximum value of 75% at 1.0 mV s−1, implying that the most of capacities are mainly controlled by capacitive process at high current density.
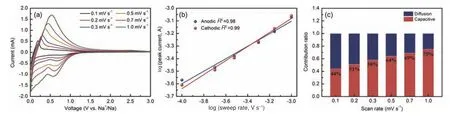
Fig.6 (a) CV curves of HCS/MX-2 film electrode at different scan rates.(b) Relationship between the scan rates and peak currents in logarithmic format.(c) Diffusion and capacitive- controlled contributions
4 Conclusion
Monodispersed hard carbon spherules were successfully prepared using Shanxi aged vinegar as the carbon source.The electrochemical properties of the HCS materials as SIBs negative electrodes were investigated.The results suggest that the carbonization temperature has an impact on the electrochemical performances of HCS electrodes.HCS-1400 showed the best electrochemical performance.In addition, we also successfully fabricated the flexible HCS/MX film electrodes using Ti3C2TxMXene as a multifunctional binder.Notably, HCS/MX-2 film electrode exhibits a high specific capacity of 346 mAh g−1, outstanding rate performance and cycling stability.Encouragingly,it retains 200.6 mAh g−1(99.3% capactiy retention)after 200 cycles at a current density of 200 mA g−1.Importantly, the capacity retention reaches 83.3%after 1 000 cycles even at a high current density of 500 mA g−1.These results indicate that the uniquely structured MXene can be empolyed as a binder and structural stabilizer.Such electrodes can be used for flexible SIBs andenhace of the energy density of the devices.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U1810204,U1910210, U21A20174), Natural Science Foundation of Shanxi Province (201901D211046,20210302123115), Special Foundation for Youth San Jin scholars.
杂志排行
新型炭材料的其它文章
- Ni(OH)2/石墨相氮化碳/石墨烯三元复合材料的制备及电化学性能
- 炭纸衬底上化学气相沉积直立型二维过渡金属硫化物及其电催化产氢性能
- Coal-based graphene as a promoter of TiO2 catalytic activity for the photocatalytic degradation of organic dyes
- Preparation and lithium storage of anthracite-based graphite anode materials
- 咖啡渣成型制备生物质炭及其CH4/N2分离性能
- Oxygen-incorporated carbon nitride porous nanosheets for highly efficient photoelectrocatalytic CO2 reduction to formate
