Microstructures and electrochemical properties of coconut shell-based hard carbons as anode materials for potassium ion batteries
2022-12-13HUANGTaoPENGDachunCHENZuiXIAXiaohongCHENYuxiLIUHongbo
HUANG Tao, PENG Da-chun, CHEN Zui, XIA Xiao-hong,2, CHEN Yu-xi,2, LIU Hong-bo,2,*
(1.College of Material Science and Engineering, Hunan University, Changsha 410082, China;2.Hunan Province Key Laboratory for Advanced Carbon Materials Applied Technology, Hunan University, Changsha 410082, China)
Abstract: Hard carbons have recently attracted wide interest as anode materials for potassium ion batteries (PIBs) because of their high reversible capacity.But, their high preparation cost and poor cycling stability prevent their practical use.Coconut shell-derived hard carbons (CSHCs) were prepared from waste biomass coconut shell using a one-step carbonization method, and were used as anode materials for potassium ion batteries.The effects of the carbonization temperature on the microstructures and electrochemical properties of the CSHCs were investigated by X-ray diffraction, nitrogen adsorption, Raman spectroscopy, scanning electron microscopy, transmission electron microscopy, and cyclic voltammetry, etc.Results indicate that the CSHC carbonized at 1 000 °C(CSHC-10) has a suitable graphite microcrystal size, pore structure and surface defect content, and has the best electrochemical performance.Specifically, it has a high reversible specific capacity of 254 mAh·g−1 at 30 mA·g−1 with an initial Coulombic efficiency of 75.0%, and the capacity retention rates are 87.5% after 100 cycles and 75.9% after 400 cycles at 100 mA·g−1, demonstrating its excellent potassium storage performance.
Key words: Potassium ion battery;Coconut shell-based hard carbon;Carbonization temperature;Microstructure;Electrochemical performance
1 Introduction
In recent years, biomass-derived hard carbons have attracted great interest as anode materials of potassium ion batteries (PIBs) owing to their high potassium storage capacity and widespread precursor sources.However, some unfavorable factors of biomass-derived hard carbons deviate from the requirements of PIBs for green and low cost, which may limit their large-scale applications in PIBs[1].For example, hard carbon materials derived from ganoderma lucidum spore[2]or walnut diaphragm[3]exhibited excellent potassium storage properties, but these precursors are high cost because of their low carbon yields and difficulty in collection.Synthesis processes of hard carbon materials derived from corn husk[4], luffa[5]or lignin[6]involved a lot of strong acid/alkali treatments, which not only caused pollution but also increased the subsequent treatment cost.Moreover, the synthesis processes of hard carbon materials derived from hard-wood[7], chitosan[8]and skimmed cotton[9]are too complicated, which lead to high production cost.In addition, the uncertainty in the potassium ion storage mechanism in hard carbon materials limits the development of hard carbon anode materials for PIBs[10].
Coconut shell is the main by-product of coconut,which has the characteristics of extensive source and low price.Owing to the high tap density, low ash contents and low H/C and O/C molar ratios[11], hard carbon derived from coconut shell exhibits high energy densities and superior electrochemical properties in lithium ion batteries (LIBs) and sodium ion batteries(SIBs).Hwang et al.[12]reported a porous carbon derived from coconut shell by activation with KOH,which exhibited a high reversible lithium storage capacity of 565.6 mAh·g−1.Miao et al.[13]prepared a coconut shell-based hard carbon by direct pyrolysis for SIBs anode, which achieved a high specific capacity of 274 mAh·g−1at 100 mA·g−1and showed a high energy density and cycle stability in a full-cell.In addition to being directly used as an anode material,coconut shell-derived carbon material is also an ideal carrier for phosphorus and sulfur or other active substances due to its high mechanical strength, good conductivity and well-developed pore structure.For example, Sun et al.[14]loaded red phosphorus on a coconut shell porous carbon for a LIB anode, which exhibited a high capacity of 1 523 mAh·g−1and a stable cycling performance, implying the extremely improved capacity attenuation caused by the expansion of red phosphorus.Li et al.[15]prepared the red phosphorus/carbon composite material based on the coconut shell-derived hard carbon, which showed an excellent performance in SIBs.Besides, Yuan et al.[16]loaded sulfur on a coconut shell porous carbon and displayed a high specific lithium storage capacity of up to 1 121 mAh·g−1in lithium/sulfur batteries.Up to date, the reports on coconut shell-based hard carbons used in potassium ion batteries are much limited.
Graphite experiences a large volume expansion of up to 61% during the insertion of potassium ions,resulting in rapid capacity fading and poor rate capability[17].Coconut shell-based hard carbon possesses a high mechanical strength and a large graphite interlayer spacing, which is expected to buffer the volume expansion during potassiation.In addition, the abundant surface defects and pores of coconut shell-based hard carbon could provide sufficient reaction sites for potassium ions and increase the diffusion coefficient,which make it an ideal anode material for potassium ion batteries.
Herein, low-cost biomass coconut shell was used as the precursor to prepare the coconut shell-based hard carbons (CSHCs) through an environmentally friendly and simple method.It is found that the CSHC-10 anode material possess outstanding electrochemical performance in PIBs, which offers a novel way for the green utilization of coconut shell.Besides,we systematically investigated the relationship between potassium storage behavior and the microstructures of CSHCs carbonized at different temperatures, which provides an experimental basis for revealing the potassium storage mechanism of hard carbons.
2 Experimental
2.1 Preparation of CSHCs
As shown in Fig.1, the coconut shell was first broken into particles of 2 to 10 mm and washed 3 times with deionized water, then dried in a vacuum oven at 120 °C for 36 h.After that, the obtained particles were carbonized at various temperatures(800, 1 000 and 1 200 °C) under nitrogen atmosphere for 5 h with a heating rate of 5 °C·min−1in a tube furnace, the nitrogen flow rate is 150 mL·min−1.Finally,the carbonized particles were subjected to ball milling for 12 h at a rotation speed of 270 r·min−1, and then the products were passed through a 325 mesh sieve.The final obtained coconut shell-based hard carbons synthesized at 800, 1 000 and 1 200 °C were denoted as CSHC-8, CSHC-10 and CSHC-12, respectively.
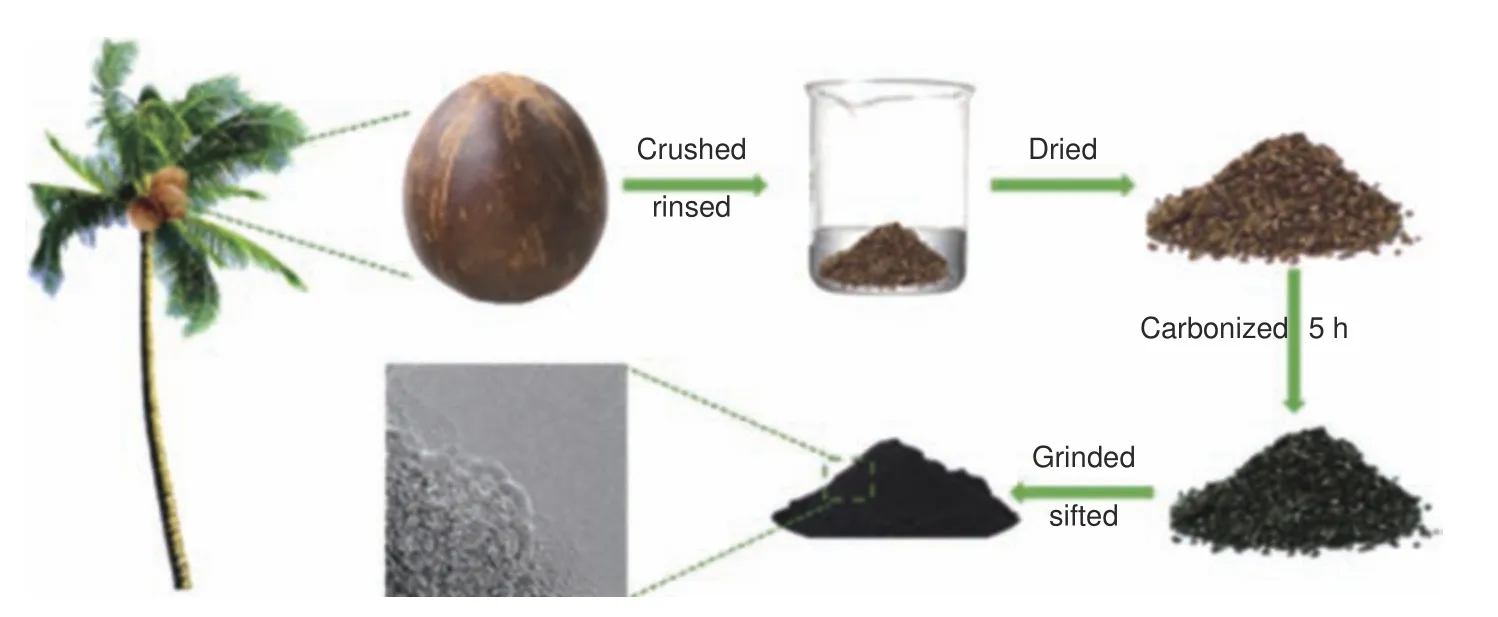
Fig.1 Schematic illustration for the synthesis of coconut shell-derived hard carbons
2.2 Material characterization
The morphology and structure of CSHC samples were characterized by scanning electron microscopy(SEM, TESCAN MAIA3) and transmission electron microscopy (TEM, Tecnai F20).The nitrogen adsorption-desorption isotherms were obtained by a surface area and pore size analyzer (Tristar Ⅱ 3020).The microcrystallite structure of CSHC samples were characterized by XRD (Bruker D8-Advance).XPS analysis of CSHC samples was preformed in an ESCALAB 250Xi.Raman measurements were performed in a Raman spectrometer (Renishaw Centrus 05TC19).
2.3 Electrochemical measurement
The electrodes were obtained by mixing the hard carbon materials (CSHC-x), acetylene carbon black and sodium carboxymethyl cellulose (CMC-Na) with a mass ratio of 90∶5∶5 in deionized water.The slurry was coated on the Cu foil and dried for 6 h at 60 °C under a blast drying oven.The electrodes were cut into disks with a diameter of 14 mm and dried in a vacuum oven at 110 °C for 12 h.
The K metal was used as the counter electrode,the electrolyte was 0.8 mol L−1KPF6in ethylene carbonate and diethyl carbonate (EC∶DEC = 1∶1 by volume).The CR2025 type coin cells were fabricated in an Ar-filled glove box.Cyclic voltammetry (CV)and electrochemical impedance spectroscopy (EIS)tests were executed by the CHI660E workstation at ambient temperature with a CV test voltage range of 0.001-3.0 V and a scan rate of 0.1 mV·s−1.The EIS test frequency was set between 100 kHz to 0.1 Hz with an amplitude of 5 mV.Galvanostatic charge-discharge tests were conducted using a Land CT2001A battery testing system under a voltage range of 0.001-3.0 V vs.K/K+in a 30 °C incubator.
3 Results and discussion
3.1 Morphology and structural characterization
Fig.2a–c display SEM images of CSHC samples obtained by carbonization at 800 °C, 1 000 °C and 1 200 °C, respectively.The annealed coconut shells are found to possess uneven particle size distribution with irregular shape.The corresponding high-resolution transmission electron microscopy (HRTEM) images are exhibited in Fig.2d–f.It can be observed that CSHC particles are basically with an amorphous structure with some apparent short-range ordered graphite microcrystallites inside and at the edges of the particles.Upon increasing the carbonization temperature, it can be seen that the microcrystallite size along theab–plane (La) and the number of graphite layers increase, implying a gradual growth of the graphite microcrystallites.Generally, the growth of the graphite microcrystallites can be attributed to the rearrangement of carbon atoms and the defect reduction in carbon edges[10].
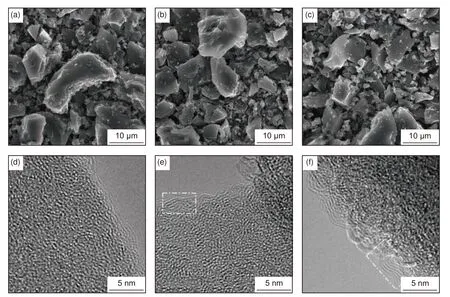
Fig.2 SEM images of (a) CSHC-8, (b) CSHC-10 and (c) CSHC-12.HRTEM images of (d) CSHC-8, (e) CSHC-10 and (f) CSHC-12
According to N2adsorption-desorption isotherms (Fig.3a) and pore distribution curves (Fig.3b),there are many pores in CSHC samples, which are mainly micropores, macropores, and a small number of mesopores.Generally, micropores may be caused by the volatilization of small molecular organics,mesopores and macropores may be inherited from the natural structure of coconut shell precursors[18].The pore size decreases with the advances of carbonization process.As shown in Table 1, the BET surface area and pore volume of CSHC samples are strongly related to the carbonization temperature.Specifically,the specific surface area decreases sharply from 217 m of CSHC-8 to 9 m2·g−1of CSHC-12, and the pore volume reduces from 0.149 of CSHC-8 to 0.011 cm3·g−1of CSHC-12 with increasing the carbonization temperature.
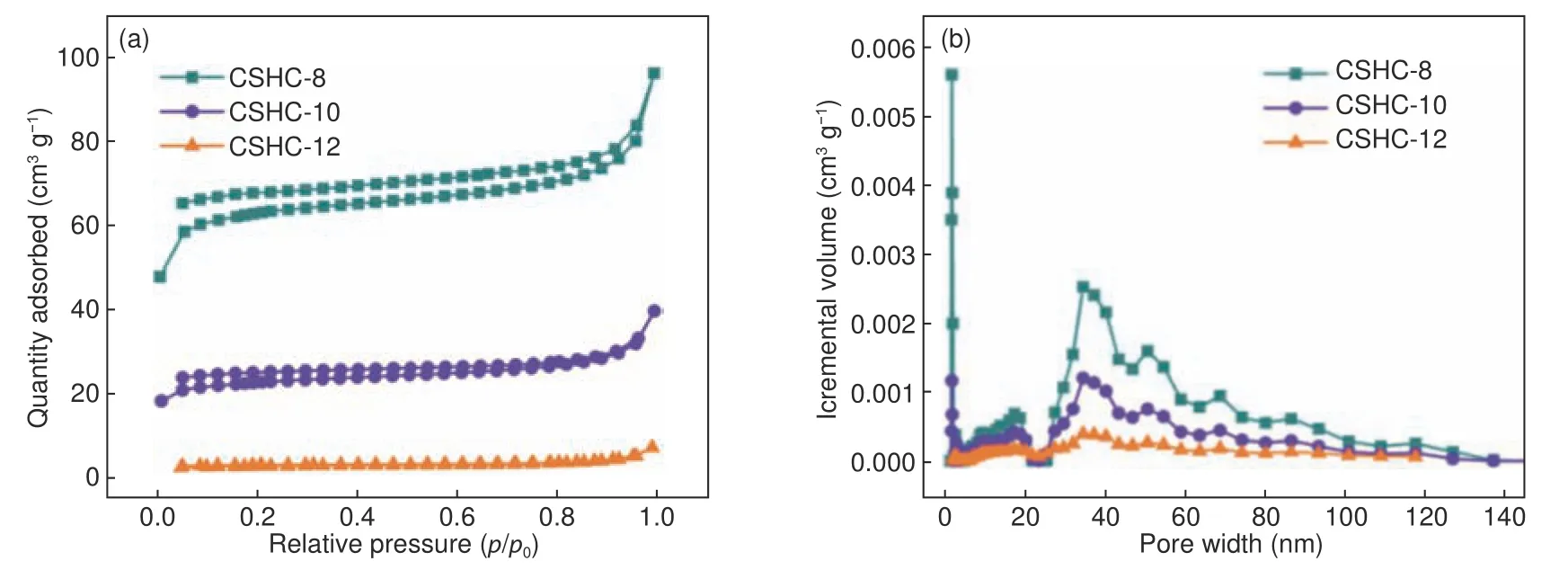
Fig.3 (a) N2 adsorption-desorption isotherms and (b) BJH pore size distributions of the CSHCs prepared at different carbonization temperatures
In order to further explore the structural characteristics of CSHCs, XRD and Raman spectroscopy were adopted.As displayed in Fig.4a, XRD patterns of CSHC samples show two broad diffraction peaks around 2θ= 24° and 44°, which can be indexed as(002) and (100) planes of graphite, respectively.The broad diffraction peaks indicate that the prepared CSHCs possess an ordered crystalline structure in short-range and an amorphous structure in long-range.As shown in Table 1, the average interlayer spacingd002and the size along thec-axisLcof the CSHCs are calculated by using the Bragg diffraction equation and Scheler equation.The diffraction angles of (002)plane shift to high value with increasing the carbonization temperature.The corresponding average interlayer spacingd002decreases from 0.389 for CSHC-8 to 0.386 and 0.377 nm for CSHC-10 and CSHC-12, respectively.The average interlayer spacings of CSHCs are bigger than that of graphite (0.335 nm), which is beneficial for the storage and diffusion of potassium ions.Raman spectra of CSHCs (Fig.4b) exhibits 2 board peaks, which can be further divided into 4 Lorentzian peaks by the Origin software (Fig.4c).The TPA peak (~1 170 cm−1) and a peak (~1 520 cm−1)are related to the carbon formed by sp3hybridization[19].TheDpeak (~1 340 cm−1) corresponds to the vibration of a defective graphitic structure or disordered carbon atoms, and theGpeak (~1 590 cm−1)represents the stretching vibration of C―C bond in sp2hybrid carbon atom plane.The integrated intensity ratioID/IGreflects the defect density[20].TheID/IGvalues of CSHC-8, CSHC-10 and CSHC-12 are 3.26,2.95 and 2.60, respectively, revealing that CSHC-8 and CSHC-10 have more defect sites than that of CSHC-12.In addition, theLaof graphite microcrystallites can be calculated according to theID/IGratio by using the following formula[21]:
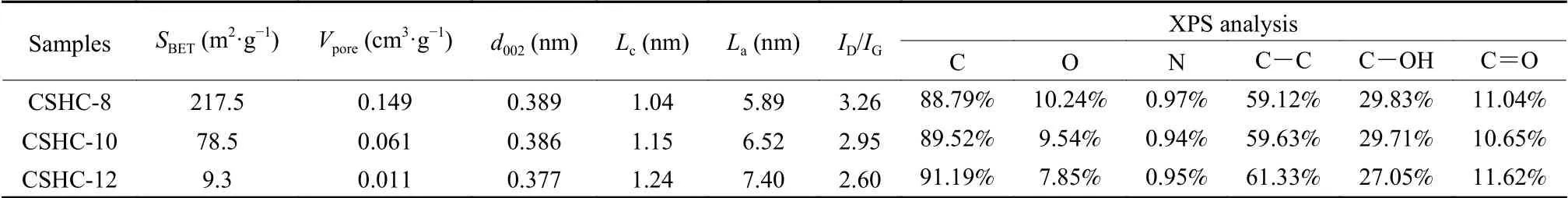
Table 1 The structural parameters and XPS element analysis of the CSHCs prepared at different carbonization temperatures
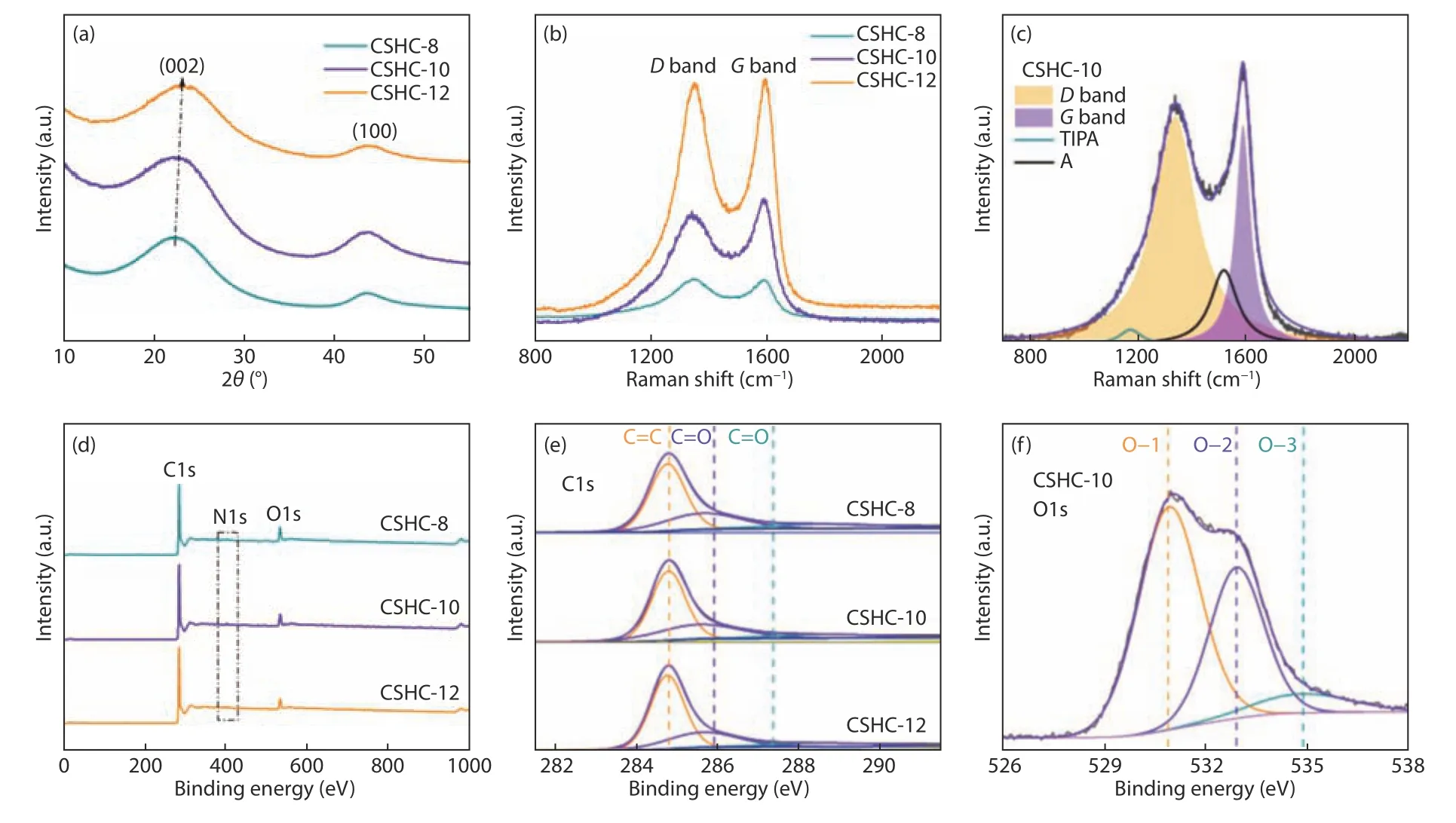
Fig.4 (a) XRD patterns, (b) Raman spectra, (d) XPS spectra, and (e) high-resolution C1s spectra of the CSHCs prepared at different carbonization temperatures.(c) The deconvoluted Raman spectrum and (f) high-resolution O1s spectrum of CSHC-10
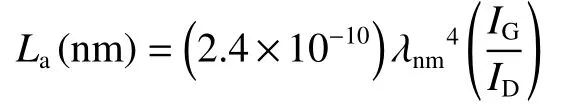
Whereλrepresents the laser wavelength (532 nm).As shown in Table 1, the values ofLaandLcincrease with increasing the carbonization temperature, demonstrating a gradual growth of the graphite microcrystallites.
As shown in Fig.4d, the XPS survey spectra of CSHCs demonstrate the existence of C and O elements as well as a tiny amount of N element.The content of C element increases gradually with increasing the carbonization temperatures, and the O and N contents decrease accordingly (Table 1).Fig.4e shows the high-resolution C1s spectra of the three CSHC samples, including a C―C peak at 284.8 eV,a C=O peak at 287.1 eV and a C―OH peak at 285.9 eV[22].Fig.4f shows the high-resolution O1s spectrum of CSHC-10, in which the O―1, O―2 and O―3 peaks represent C=O, C―OH and C―O―C,and COOH or H2O, respectively[23].The XPS analysis reveals the existence of numerous oxygen-containing functional groups in CSHCs, which can provide the reaction sites of potassium ions[24].
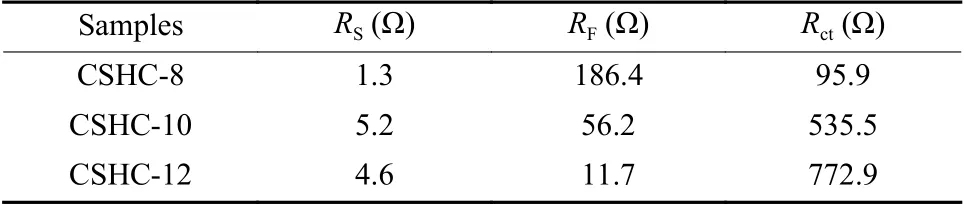
Table 2 The fitting values of the resistance components in the equivalent circuit
3.2 Electrochemical performance of CSHCs
The electrochemical properties of the CSHCs were measured.Fig.5a displays the initial galvanostatic charge/discharge profiles of the CSHC electrodes at a current density of 30 mA·g−1.There is no obvious difference in the capacities of the three CSHC materials.CSHC-10 possesses a reversible specific capacity of 254.5 mAh·g−1and a high initial Coulombic efficiency of 75.0%.CSHC-8 and CSHC-12 have lower reversible specific capacities/the initial Coulombic efficiencies, delivering 245.9 mAh·g−1/72.7% and 250.7 mAh·g−1/74.4%, respectively.The lowest initial Coulombic efficiency of CSHC-8 is mainly attributed to the large surface area, which lead to more SEI films via irreversible reaction of the electrolyte.Paradoxically, CSHC-12 possesses a smaller surface area and exhibites a lower initial Coulombic efficiency than that of CSHC-10.A possible explanation is that the initial irreversible capacity is not only associated with the formation of SEI film, but also related to some non-SEI factors such as irreversible adsorption of active ions at high-energy defect sites, intercalation in the dense structure, and metal underpotential deposition[25].Therefore, the dense and compact structure of CSHC-12 may lead to the irreversible insertion of potassium ions[26].
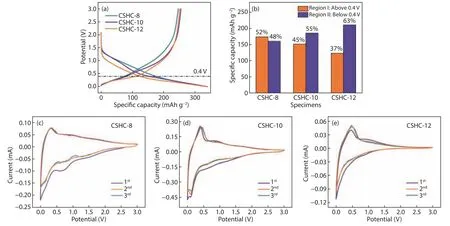
Fig.5 (a) The first charge/discharge profiles, and (b) capacity proportion contributed by different voltage regions during the first discharge process of CSHCs prepared at different carbonization temperatures.CV curves of (c) CSHC-8, (d) CSHC-10 and (e) CSHC-12
To explore the relationship between the shape of the charge/discharge profiles and the carbonization temperatures, the initial discharge curves of the CSHCs have been divided into two regions with 0.4 V as the boundary: Region I (above 0.4 V) and Region Ⅱ (below 0.4 V).As shown in Fig.5b, the capacity ratio of Region I decreases from 52% of CSHC-8 to 45% of CSHC-10 and 37% of CSHC-12.The capacity ratio of Region Ⅱ shows an opposite trend, increasing from 48% of CSHC-8 to 55% of CSHC-10 and 63% of CSHC-12.The previous studies[10,27]suggest that potassium ions be mainly adsorbed on surface defects of hard carbons in the voltage range of Region I (above 0.4 V), attributing to adsorption-induced potassium storage.In the voltage range of Region Ⅱ (below 0.4 V), potassium ions mainly intercalate into the graphite crystallite layers of hard carbons, corresponding to the insertion mechanism.Therefore, the charge/discharge curves of CSHCs shift towards lower voltage with an increase of the carbonization temperatures.Specifically,CSHC-12 possesses the larger size of graphite microcrystallines than those of CSHC-8 and CSHC-10,which allows potassium ions to insert more at the low voltage range, achieveing the largest capacity in Region Ⅱ and the lowest capacity in the charge/discharge curve of CSHC-12.
Cyclic voltammetry (CV) curves of the CSHC electrodes in the voltage range between 0.001 and 3 V are shown in Fig.5c–e.In the initial cathodic scan of CSHC-8, a strong reduction peak can be found at around 0.7 V, which can be attributed to the decomposition of electrolyte and the formation of the SEI films[28].All the three CSHC electrodes show the protruding irreversible region of between 0.2 and 2 V,due to the irreversible potassium ion storage caused by defects.The area of irreversible regions of the CSHC-12 and CSHC-10 electrode are smaller than that of the CSHC-8 electrode, which is consistent with the change law of defects and pores of samples.Meanwhile, the third cycle CV curves of all the three CSHC electrodes display a well coincided curve with the second one, illustrating that CSHCs have a good reversibility.
Fig.6a displays the rate performance of the CSHCs at different current densities from 30 to 1 000 mA·g−1.The rate performance of CSHC-8 and CSHC-10 are similar, both of which are better than that of CSHC-12.CSHC-10 exhibits reversible specific capacities of 254.5, 211.9, 175.5, 124.7 and 84.2 mAh·g−1at the current densities of 30, 100, 200,500 and 1 000 mA·g−1, respectively.When the current density restores to 100 mA·g−1, the reversible specific capacity maintains at 200.5 mAh·g−1, illustrating that CSHC-10 has good rate capability and stability.CSHC-12 displays poor rate performance in the large current densities.The possible reasons are that the dense structure and the lowest content of defect sites hinder the fast transfer of potassium ions.Notably,CSHC-8 possesses a higher retention ratio of initial capacity than CSHC-10 at a large current density of 1 000 mA·g−1(Fig.6b).The cycling performance of the three CSHCs prepared at different carbonization temperatures is depicted in Fig.6c.All the three samples show good capacity retention.Moreover,CSHC-8, CSHC-10 and CSHC-12 remain reversible specific capacities of 189.8, 201.7 and 165.4 mAh·g−1after 100 cycles at 100 mA·g−1, respectively.CSHC-10 shows the highest capacity retention of 87.5%, while those of CSHC-8 and CSHC-12 are 82.1% and 83.1%, respectively.The Coulombic efficiencies of the three CSHCs are higher than 94% in the second cycle, and increase to about 99% in the eighth cycle, indicating that the SEI layers formed in the initial cycle are stable in the subsequent cycles.Besides, the insertion and extraction of potassium ions in hard carbons may cause the expansion and contraction of graphite microcrystallines, resulting in the destruction and exfoliation of these structures, while the larger interlayer spacing of the CSHCs may lead to a smaller volume expansion during potassiation, its high mechanical strength and developed pore structures could further alleviate the expansion, resulting in their good reversibility.Particularly, the average Coulombic efficiencies in the subsequent 100 cycles of CSHC-8, CSHC-10 and CSHC-12 are 99.58%,99.61% and 99.66%, respectively.The long-term cycle capability of CSHC-10 at a current density of 100 mA g−1was further examined and shown in Fig.6d.The reversible specific capacity maintains at 175.1 mAh g−1after 400 cycles with a retention of 75.9% (based on the initial capacity at 100 mA·g−1),which is the best among those of the previously reported KIB anode materials[7,29,33].
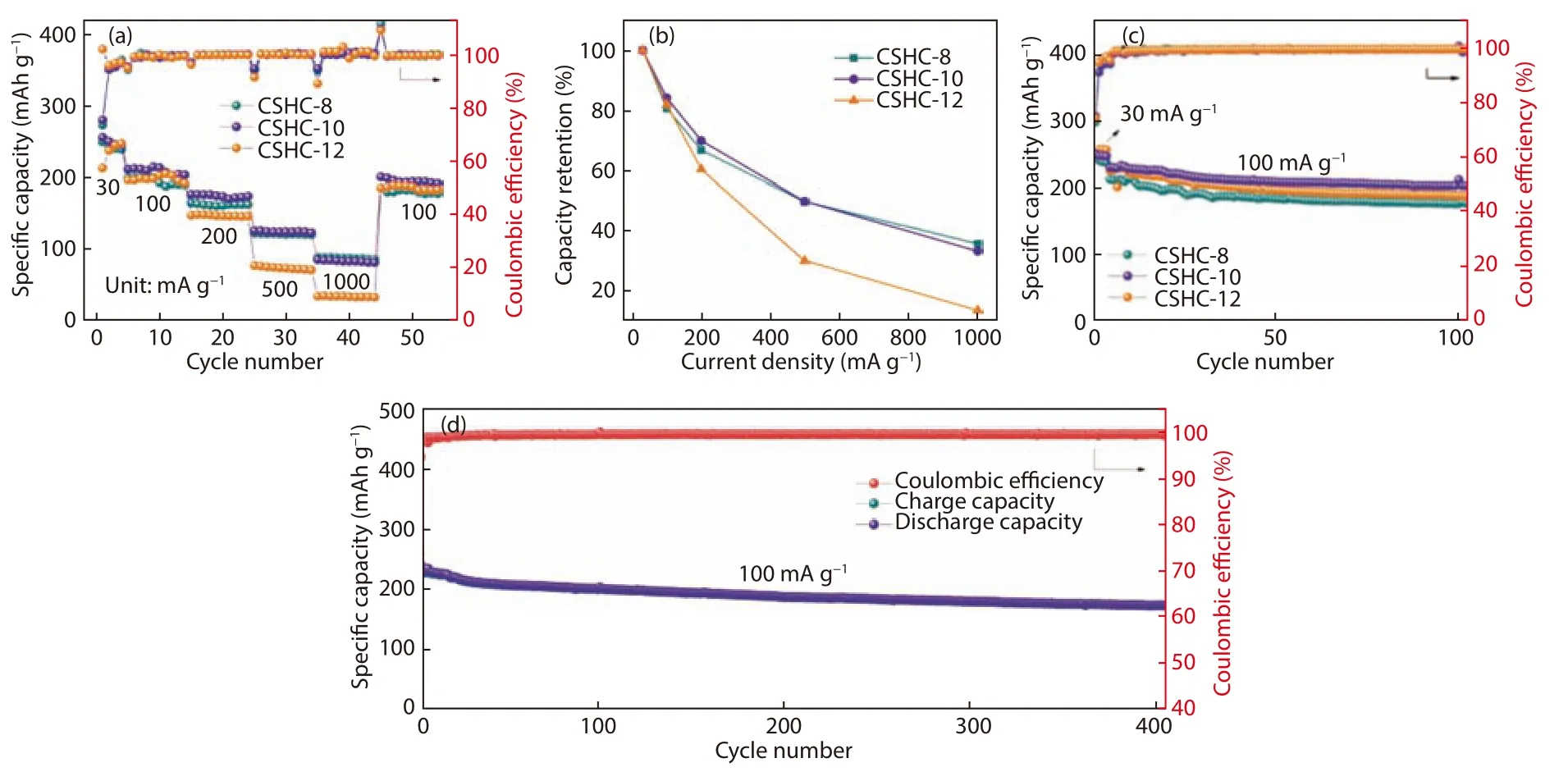
Fig.6 (a) Rate capability and (b) capacity retention at various current densities.(c) Cyclic performance of the CSHCs prepared at different carbonization temperatures.(d) Long-term cyclic performance of CSHC-10 at 100 mA·g−1
To further investigate the difference in the electrochemical kinetics of the CSHCs, the electrochemical impedance spectroscopy (EIS) was performed after the CV tests, as shown in Fig.7.The inset shows the equivalent circuit and the fitted results are listed in Table 2, whereRS,RF, andRctindicate the electrolyte resistance, SEI film resistance, and charge-transfer resistance, respectively.The charge-transfer resistanceRctcontains two parts, i.e., the ionic resistance and the electronic resistance.The ionic resistance refers to the transfer resistance of ionic electrolyte between the pores inside a material.The electronic resistance includes the inherent resistance of an active material and the contact resistance between the material and the current collector[30,31].The charge-transfer resistanceRctof CSHC-8 is much lower than that of CSHC-10 and CSHC-12, while the rate capability of CSHC-8 is slightly better than CSHC-10 at a high current density of 1 000 mA·g−1.Namely, the low chargetransfer resistance of CSHC-8 is inconsistent with its rate capability.The possible reason is that the low charge-transfer resistance of CSHC-8 is mainly contributed by the ionic resistance.Multitudinous pores and a large graphite interlayer spacing of CSHC-8 result in a low ionic resistance, while the inherent resistances (electronic resistances) of CSHCs decreases with an increase of the pyrolysis temperature, implying the highest electronic resistance of CSHC-8[10,32].Therefore, the low electronic conductivity and the high ionic conductivity of CSHC-8 cause the similar rate capability with that of CSHC-10.Moreover, the SEI film resistanceRFis found to decrease with increasing the carbonization temperature, illustrating that fewer pores and defects make the formed SEI film more stable.
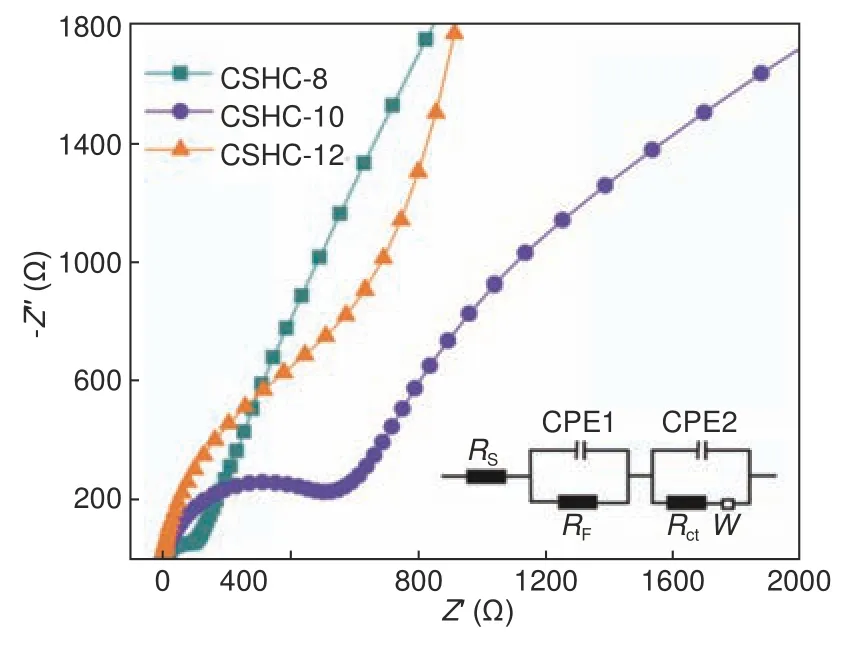
Fig.7 The EIS curves and corresponding equivalent circuit model of the CSHCs prepared at different carbonization temperatures
4 Conclusion
A simple and effective method to prepare CSHCs has been demonstrated.The size of the graphite microcrystalline increases with increasing the carbonization temperature.Meanwhile, the interlayer spacing and the content of active sites including defects and oxygen-containing functional groups decrease with increasing the carbonization temperature.The CSHC carbonized at 1 000 °C (CSHC-10) displays excellent potassium ion storage performance.A reversible specific capacity of 254.5 mAh·g−1and a high initial Coulombic efficiency of 75.0% at a current density of 30 mAh·g−1have been achieved.After 400 cycles at a current density of 100 mA·g−1, the reversible specific capacity remains at 175.1 mAh·g−1with a retention of 75.9%.The high cyclic capacity and stability of the CSHC-10 make it an environmentally friendly and promising anode material for potassium ion batteries.
Acknowledgements
National Natural Science Foundation of China(51772083, 51402101); Science and Technology Planning Project of Hunan Province (2018GK1030).
杂志排行
新型炭材料的其它文章
- Coal-based graphene as a promoter of TiO2 catalytic activity for the photocatalytic degradation of organic dyes
- 咖啡渣成型制备生物质炭及其CH4/N2分离性能
- A flexible hard carbon microsphere/MXene film as a high-performance anode for sodium-ion storage
- Preparation and lithium storage of anthracite-based graphite anode materials
- Oxygen-incorporated carbon nitride porous nanosheets for highly efficient photoelectrocatalytic CO2 reduction to formate
- 炭纸衬底上化学气相沉积直立型二维过渡金属硫化物及其电催化产氢性能
