Incorporating TiO2 nanoparticles into the multichannels of electrospun carbon fibers to increase the adsorption of polysulfides in room temperature sodium-sulfur batteries
2022-12-13YEXinLIZhiqiSUNHaoWUMingxiaANZhongxunPANGYuepengYANGJunheZHENGShiyou
YE Xin, LI Zhi-qi, SUN Hao, WU Ming-xia, AN Zhong-xun, PANG Yue-peng,YANG Jun-he, ZHENG Shi-you,*
(1.School of Materials Science and Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China;2.Shanghai Aowei Technology Development Co., Ltd, Shanghai 201203, China)
Abstract: With the rapid development of electric vehicles and large-scale power grids, lithium-ion batteries inevitably face the problem that their limited energy density and high cost cannot meet the growing demand.Room temperature sodium-sulfur (RT Na-S) batteries, which have the potential to replace lithium-ion batteries, have become a focus of attention.However, the challenging problem of their poor cycling performance cause by the “shuttle effect” of the reaction intermediates (sodium polysulfides) needs to be addressed.We report a method to incorporate TiO2 nano particles into the multichannels of electrospun carbon fibers (TiO2@MCCFs) to stabilize the sulfur compounds and produce high-performance RT Na-S batteries.The TiO2@MCCFs were prepared by electrospinning followed by heat treatment, and were infiltrated by molten sulfur to fabricate S/TiO2@MCCF cathode materials.The addition of the TiO2 nanoparticles increases the affinity of cathode materials for polysulfides and promotes the conversion of polysulfides to lower order products.This was verified by DFT calculations.A S/TiO2@MCCF cathode with a S content of 54% has improved electrochemical rate and cycling performance, with a specific capacity of 445.1 mAh g−1 after 100 cycles at 0.1 A g−1 and a nearly 100% Coulombic efficiency.Even at 2 A g−1, the cathode still has a capacity of 300.5 mAh g−1 after 500 cycles.This work provides a new way to construct high performance RT Na-S battery cathodes.
Key words: Sodium-sulfur battery;Cathode;TiO2;Electrospinning
1 Introduction
The growing markets for hand-held electronics,electric vehicles, and large-scale electrochemical energy storage systems have promoted the development of rechargeable lithium-ion batteries[1–5].However,traditional lithium-ion batteries using LiCoO2or LiFePO4cathodes and graphite anodes suffer from low energy density and high cost due to the limited theoretical specific capacity and the ever-rising price[6–9].Therefore, preparation of high energy density batteries with low cost is an indispensable way to meet the requirements for practical applications.Room temperature sodium-sulfur (RT Na-S) batteries are considered to be a promising energy storage system because of their high theoretical energy density and specific capacity, which are about 2 600 Wh kg−1and 1 675 mAh g−1, respectively.They have very low cost and small impact on the environment[10–13].In addition, sodium anode possesses a similar electrode potential to lithium and has the advantage of reserve abundant and pollution-free[14,15].However, the commercialization of RT Na-S batteries has been plagued by poor electrical conductivity of sulfur, large volume expansion during the sodiation process, and dissolution of polysulfide intermediates.The electronic conductivity of sulfur and the intermediate sodium polysulfides is poor (5×10−30S cm−1at 25 °C)[16–18].Large volume expansion (>80%) in the process of sodiation/desodiation causes the destruction of the electrode structure[19–21].Sodium polysulfides, the intermediate products of charging and discharging, dissolve in the electrolyte to produce the “shuttle effect”,leading to the loss of active materials, the reduction of Coulombic efficiency and rapid capacity decay[22–25].
These issues can be tackled by well-designed sulfur matrix materials, multifunctional interlayers, novel electrolytes and binders[26,27].The carbon-based materials have the best electrical conductivity and strong affinity to sulfur, which become the most common and effective materials to encapsulate sulfur, especially for hollow/porous/nanostructured carbons[28].In the past few years, many carbon nanostructures have been used to host sulfur in RT Na-S battery cathodes,including carbon fibers, porous carbon spheres,graphene, etc[29–31].Although the sulfur/carbon composite cathodes can effectively enhance the capacity and cycle life of RT Na-S batteries, the key problem of dissolution of sodium polysulfides still remains.The weak binding energy between non-polar carbonbased materials and polar polysulfides is the main reason.Therefore, single carbonaceous material cannot effectively inhibit the dissolution of sodium polysulfides.Recently, studies have shown that polar metal oxides (TiO2as a representative) have strong chemical affinity and high adsorption capacity for sodium polysulfides.However, most of metal oxides have poor conductivity, resulting in a relatively low utilization rate of sulfur[32].Therefore, a large amount of conductive carbon agents are required for the preparation of electrodes to composite carbon-based nanomaterials with metal oxides to improve the electrical conductivity and inhibit the shuttle effect of polysulfides.
In the work, a RT Na-S battery cathode fabricated by first incorporating polar TiO2nanoparticles into multichannel carbon fibers and then loading sulfur(denoted as S/TiO2@MCCFs) is reported.Multichannel carbon fibers with abundant hollow pore channels improve the electrical conductivity and volume expansion problems of insulating sulfur and provide physical adsorption of sodium polysulfides, resulting in efficient electron and ion transport.The addition of polar TiO2can effectively trap the intermediate polysulfides by strong chemical binding and the carbon-based materials can improve the adsorption capacity and reaction kinetics.Therefore, the S/TiO2@MCCF cathode with a ~54% sulfur content exhibits outstanding electrochemical performance.At 0.1 A g−1, the S/TiO2@MCCF cathode shows superior cycle stability with a specific capacity of 445.1 mAh g−1after 100 cycles and a high Coulombic efficiency close to 100%.Even at a high current density of 2 A g−1, the cathode still exhibits a capacity of 300.5 mAh g−1after 500 cycles, demonstrating excellent rate performance.This strategy of incorporating polar additives in carbon matrix to stabilize S provides new ideas to construct metal-sulfur cathodes.
2 Experimental
2.1 Synthesis of TiO2@MCCFs
Multichannel carbon fibers containing with TiO2were synthesized by an electrospinning method.800 mg of polyacrylonitrile (PAN, 15 w), 1 600 mg of polymethyl methacrylate (PMMA, 12 w) and 160 mg of TiO2(rutile, 20-30 nm) were added to 10 mL of N,N- dimethylformamide (DMF) under vigorous magnetic stirring.The electrospinning solution was heated to 60 °C and ultrasonicated during the stirring process to allow complete dissolution.Afterwards, the solution was poured into a 10 mL syringe for electrospinning.The pumping speed of the syringe pump was 2 mL h−1, the voltage of the power supply was 18 kV,and the distance between the needle of the syringe and the aluminum foil collector was 15 cm.The obtained fibers were then put in corundum crucibles for stabilization in air atmosphere at 270 °C for 1 h and carbonization in Ar atmosphere at 800 °C for 1 h.The electrospun multichannel carbon fibers containing TiO2were denoted as TiO2@MCCFs.Besides, blank multichannel carbon fibers without TiO2were synthesized by the same method.
2.2 Synthesis of S/TiO2@MCCFs
In a typical synthesis process, 150 mg of sublimated sulfur was dissolved into carbon disulfide (CS2)and then added by drops to 50 mg of TiO2@MCCFs(the mass ratio of fibers to sulfur is 1∶3).The fibers were placed in a drying cabinet to evaporate naturally and then transferred to a vacuum-sealed glass tube that was held at 155 °C for 12 h with a heating rate of 2 °C min−1to allow infiltration of molten sulfur.The obtained composite was named as S/TiO2@MCCFs.S/MCCFs was synthesized by the same way.
2.3 Material characterization
Scanning electron microscopy (SEM, FEI Quanta FEG 450, USA) and transmission electron microscopy (TEM, JEM-2100F JEOL Ltd., Japan) were used to observe the morphology of TiO2@MCCFs and S/TiO2@MCCFs.The X-ray diffraction (XRD) patterns of MCCFs, TiO2@MCCFs and S/TiO2@MCCFs were recorded on a Rigaku D/MAX-2200/PC instrument with CuKαradiation, and the scanning range was 20°-80° (5° min−1).Raman measurements of the TiO2@MCCFs and MCCFs were accomplished by a LabRAM HR Evolution Raman spectrometer (Horiba Jobin Yvon).The thermogravimetric analysis (TG) of S/MCCFs and S/TiO2@MCCFs was carried out with a TA-Q500 instrument in the temperature range of 50-600 °C (5 °C min−1, N2).The bonding states of TiO2@MCCFs and S/TiO2@MCCFs were tested by X-ray photoelectron spectroscopy (XPS) using a ThermoFisher ESCA 250XI instrument.
2.4 Electrochemical measurements
The electrochemical performance of S/TiO2@MCCFs and S/MCCFs in RT Na-S batteries was tested using CR2032-type coin batteries at 25 °C.S/TiO2@MCCFs and S/MCCFs were cut into 1.2 cm diameter discs, dried at 50 °C for 12 h under vacuum and then directly used as positive electrodes (S mass loading: ~1 mg cm−2).CR2032 batteries were assembled with S/TiO2@MCCFs and S/MCCFs as the cathodes, glass fiber (GF/D) as the separator, and commercial sodium as the anode.In addition, batteries used 1 mol L−1NaClO4in EC/PC solution (1∶1 volume ratio) containing 5% FEC as the electrolyte.The instrument used for RT Na-S battery testing is a LAND CT2001A, and the working voltage range is 0.5-2.8 V.The cyclic voltammetry (CV) test was carried out on the electrochemical workstation (Gamry Interface-1000), the working voltage range is 0.5-2.8 V, and the scanning rate is 0.5 mV s−1.
2.5 First-principles calculation
The adsorption binding energies of Na2S and Na2S4on TiO2and MCCF surfaces were calculated using the Vienna Ab Initio Simulation Package(VASP) method.Projector Augmented Wave (PAW)potentials were used to treat the interaction of valence-core electrons.And the generalized gradient approximation (GGA) with the Perdew-Burke-Ernzerhof (PBE) functional was used to describ the electron exchange correlation interactions.
3 Results and discussion
The schematic illustration of the synthesis of TiO2@MCCF electrode is shown in Fig.1.Firstly, the electrospinning solution was obtained by adding polyacrylonitrile (PAN), polymethyl methacrylate(PMMA) and rutile TiO2to N, N-dimethylformamide(DMF).After carbonization of the electrospun fibers,PMMA thermally decomposes and forms abundant hollow channels in the fibers to obtain multichannel carbon fibers containing TiO2(TiO2@MCCFs).Moreover, the thorough stirring of the precursor solution enables nano-TiO2to be uniformly distributed inside MCCFs.Finally, the S/ TiO2@MCCF electrode was prepared by infiltration of molten sulfur.
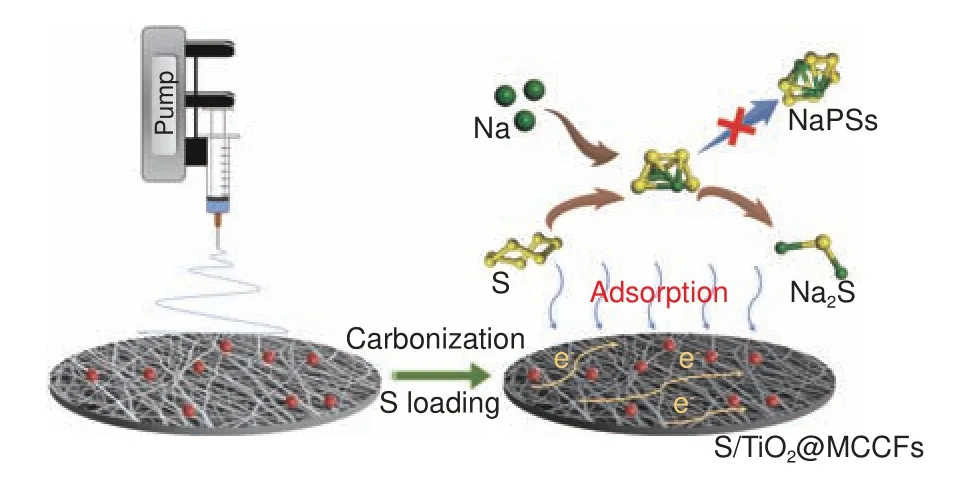
Fig.1 Schematic illustration of the preparation of the S/TiO2@MCCFs
The morphology of S/TiO2@MCCFs was observed by SEM at different magnifications.As shown in Fig.2a-b, a coarse and uniform fiber morphology with a diameter of about 2 μm can be observed.There are abundant hollow channels inside the fiber, which can provide spaces for the volume expansion of polysulfides, and can also ensure fast electron and ion transport[33,34].And after encapsulation by the infiltration method, liquid sulfur can easily diffuse into the pore channels inside the multichannel carbon fibers,so that the internal voids of TiO2@MCCFs can be filled.Fig.2c shows the high-resolution TEM image of TiO2@MCCFs, which clearly shows the lattice fringe spacing of 0.324 nm, corresponding to the(110) crystal plane of TiO2.As shown in the TEM image of Fig.2d,due to sufficient ultrasonic vibration and magnetic stirring during the preparation of the electrospinning solution, the nano-TiO2particles with the diameter of ~25 nm are uniformly dispersed inside the fiber without aggregation, which can chemically combine with polysulfides effectively during charging and discharging, reducing sulfur loss.In addition, in the EDS patterns of S/TiO2@MCCFs taken in the region shown in Fig.2e, S, Ti and O have similar distribution, reflecting the uniform loading of sulfur and nano-TiO2inside the MCCFs.
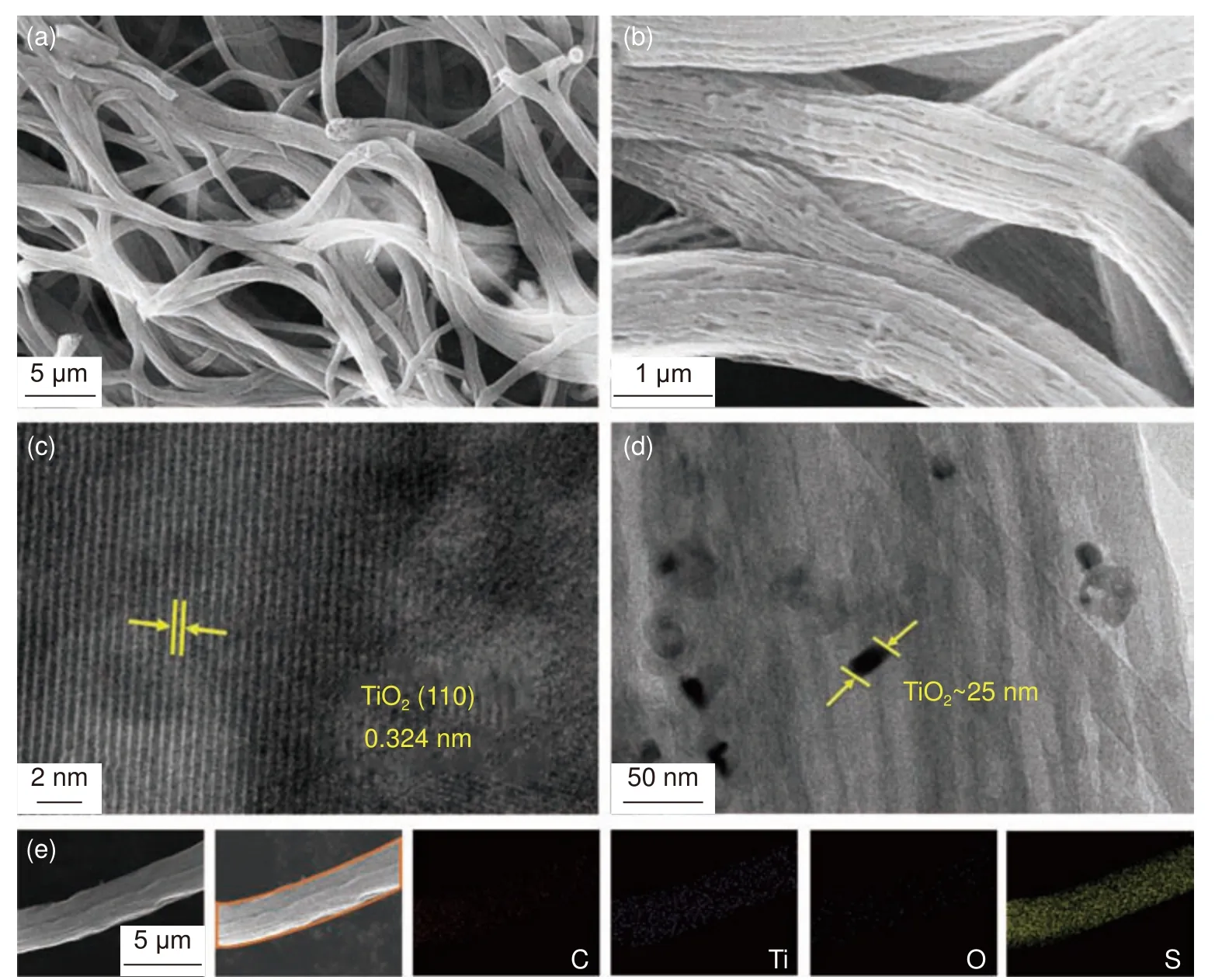
Fig.2 (a-b) SEM images of S/TiO2@MCCFs.(c-d) TEM images of TiO2@MCCFs.(e) Elemental mapping of the S/TiO2@MCCFs
The crystal structure of TiO2@MCCFs before and after sulfur loading was analyzed by XRD.As shown in Fig.3a, a broad peak caused by the accumulation of amorphous carbon in MCCFs can be observed at 20°-30°.And in the TiO2@MCCF sample,the characteristic peak of rutile phase TiO2(PDF#21-1276) can be clearly observed, which is consistent with the results observed in the high-resolution TEM image.In the tetragonal rutile structure, each Ti atom is located at the center of six O atoms, which are located at the four corners of a regular octahedron.After the sulfur encapsulation (Fig.3b), the original TiO2peaks in S/TiO2@MCCFs overlap with the crystalline sulfur peaks.Raman spectroscopy was used to evaluate the graphite structure of MCCFs and their changes after loading TiO2.The results are shown in Fig.3c,where the peaks of TiO2@MCCFs at ~448 and~611 cm−1are caused by theEgandA1gvibrational modes of rutile TiO2, respectively.The positions of these peaks correspond to the standard peaks of rutile TiO2, proving the high purity of TiO2in multichannel carbon fibers in Fig.3d, the two broad peaks at 1 345 and 1 590 cm−1are caused by defects in the carbon atom lattice of MCCFs (D-band) and sp2hybridized in-plane stretching vibration (G-band)[35], respectively.After the TiO2loading, theID/IGratio increases from 1.046 to 1.059, indicating that the introduction of TiO2can cause more defects, which is conducive to the adsorption of polysulfides.Besides, the pore structures of TiO2@MCCFs are consists of micropores and mesopores.After sulfur loading, the number of pores is significantly reduced, indicating that sulfur was loaded into pores (Fig.S1).
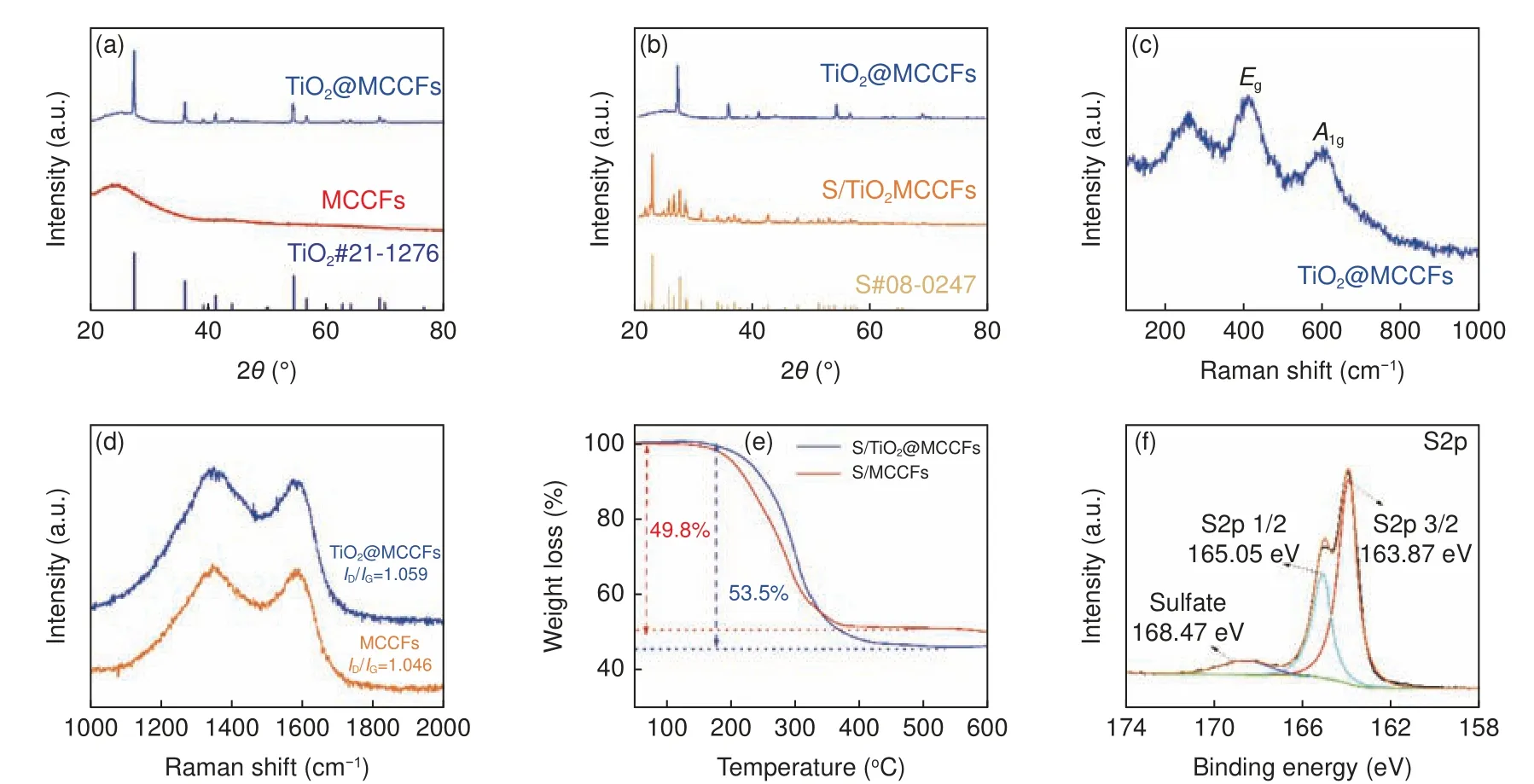
Fig.3 (a) XRD patterns of MCCFs and TiO2@MCCFs.(b) XRD patterns of TiO2@MCCFs and S/TiO2@MCCFs.(c) Raman spectrum of TiO2@MCCFs.(d) Raman spectra of MCCFs and TiO2@MCCFs (1 000-2 000 cm−1).(e) TG curves of the S/MCCFs and S/TiO2@MCCFs.(f) High-resolution XPS spectrum of S2p of S/TiO2@MCCFs
The sulfur contents of S/TiO2@MCCFs and S/MCCFs were measured by the thermogravimetric analysis.The TGA curves (Fig.3e) show that there is only a small amount of mass loss before 200 °C,which is attributed to the evaporation of water adsorbed, while the sulfur is effectively encapsulated inside the carbon fibers without reduction.The measured sulfur content of S/TiO2@MCCFs is 53.5%,which is higher than that of MCCFs (49.8%).This increase in S loading level is mainly attributed to the increased specific surface area after TiO2incorporation in the MCCFs, which can be proved by the comparison between the results in this work and our previous work (59 m2g−1for MCCFs and 158 m2g−1for TiO2@MCCFs)[36].The chemical state of the S/TiO2@MCCFs was determined by XPS.As shown in the high-resolution C1s spectrum of Fig.S2, only a single C―C peak could be observed, indicating that only simple physical adsorption occurs between the MCCF carbon matrix and sulfur.The enhanced adsorption by the addition of TiO2is mainly caused by chemisorption caused by polar interactions.The peaks at 534.60 and 532.37 eV in Fig.S3 correspond to oxygen defects and Ti-O bonds, respectively.Nano-TiO2brings a large amount of oxygen defects into the carbon-based material, which is beneficial to the adsorption of polysulfides.The two peaks at 163.87 and 165.05 eV in Fig.3f are ascribed to S2p 3/2 and S2p 1/2's spin-orbit coupling in the S element.Compared with pure sulfur (S2p 3/2, 164.0 eV), the S2p 3/2 peak of S/TiO2@MCCFs is shifted to the lower part by 0.13 eV.The occurrence of the shift indicates that the addition of TiO2enhances the affinity of the MCCF matrix to sulfur, which can strengthen the redox reaction kinetics[37].In addition, the peak at 168.47 eV is caused by sulfate.As shown in Fig.S4, the main peaks of the Ti2p XPS spectrum of S/TiO2@MCCFs at 464.45 and 458.87 eV correspond to the signals of 2p 1/2 and 2p 3/2, respectively.The difference in binding energy is 5.58 eV, confirming the Ti4+chemical state in S/TiO2@MCCFs[38].
The electrochemical properties of S/TiO2@MCCF and S/MCCF cathodes are characterized in CR2032-type coin batteries.Fig.4a shows the cycling performance of S/TiO2@MCCF and S/MCCF electrodes at 0.1 A g−1.The S/TiO2@MCCFs provides the high initial discharge capacity and the fast capacity decay in the initial ten cycles, which may be contributed by irreversible reactions between sulfur and the polysulfides and side reactions of the electrolyte.After 100 cycles, the reversible specific capacity of the S/TiO2@MCCF electrode (445.1 mAh g−1) is significantly higher than that of S/MCCFs (287.7 mAh g−1).Obviously, the use of MCCFs as a substrate for insulating sulfur can greatly enhance the electrical conductivity of the electrode, and the close contact between sulfur and substrate as well as the efficient spatial trapping effect of multichannel carbon fibers on sulfur contribute to the superior electrochemical performance of both S/TiO2@MCCF and S/MCCF electrodes.The adding TiO2can provide strong polar adsorption for polysulfides and suppress the "shuttle effect" in S/TiO2@MCCF cathode, so the introduction of TiO2can further improve the cycling performance of S/TiO2@MCCFs.Fig.4b shows the discharging/charging curves of the first 3 cycles of the S/TiO2@MCCF cathode at a current density of 0.1 A g−1.The plateau at about 2.0 V during the first cycle of discharge is caused by the dissolution of sulfur and irreversible side reactions.The wide plateaus at 1.7-1.4 V and 1.2-0.9 V during the discharging process correspond to the conversion of sulfur to higherorder polysulfides (Na2Sx,x=4-8) and lower-order sodium sulfides (Na2Sy,y=1-3), respectively.The first discharge shows an extremely high capacity caused by the irreversible side reactions, which is not shown in Fig.4a.The plateaus at 1.8 and 2.0 V during charging process are caused by the conversion of sodium sulfides to polysulfides and sulfur, respectively.The first three cycles of cyclic voltammetry (CV) curves of S/TiO2@MCCFs are shown in Fig.4c for studying the redox behavior of the sulfur cathode.The peak generated at around 2.0 V during the first cycle of discharge is caused by the formation of the solid electrolyte interface (SEI) film and irreversible side reactions.In the subsequent discharge process, the peaks at 1.4 and 1.0 V correspond to the solid-liquid conversion of active sulfur to long-chain sodium polysulfides (Na2Sx,x=4-8) and further reduction to shortchain insoluble sodium sulfides (Na2Sy,y=1-3), respectively.The peaks appearing at 1.8 and 2.0 V during the charging process correspond to the conversion of sodium sulfides to sodium polysulfides and sulfur,respectively, which is consistent with the result of discharging/charging curves.After the first cycle, the electrode exhibits good stability.Almost overlapping curves indicate that the S/TiO2@MCCF electrode has a low degree of electrochemical polarization and good reversibility.
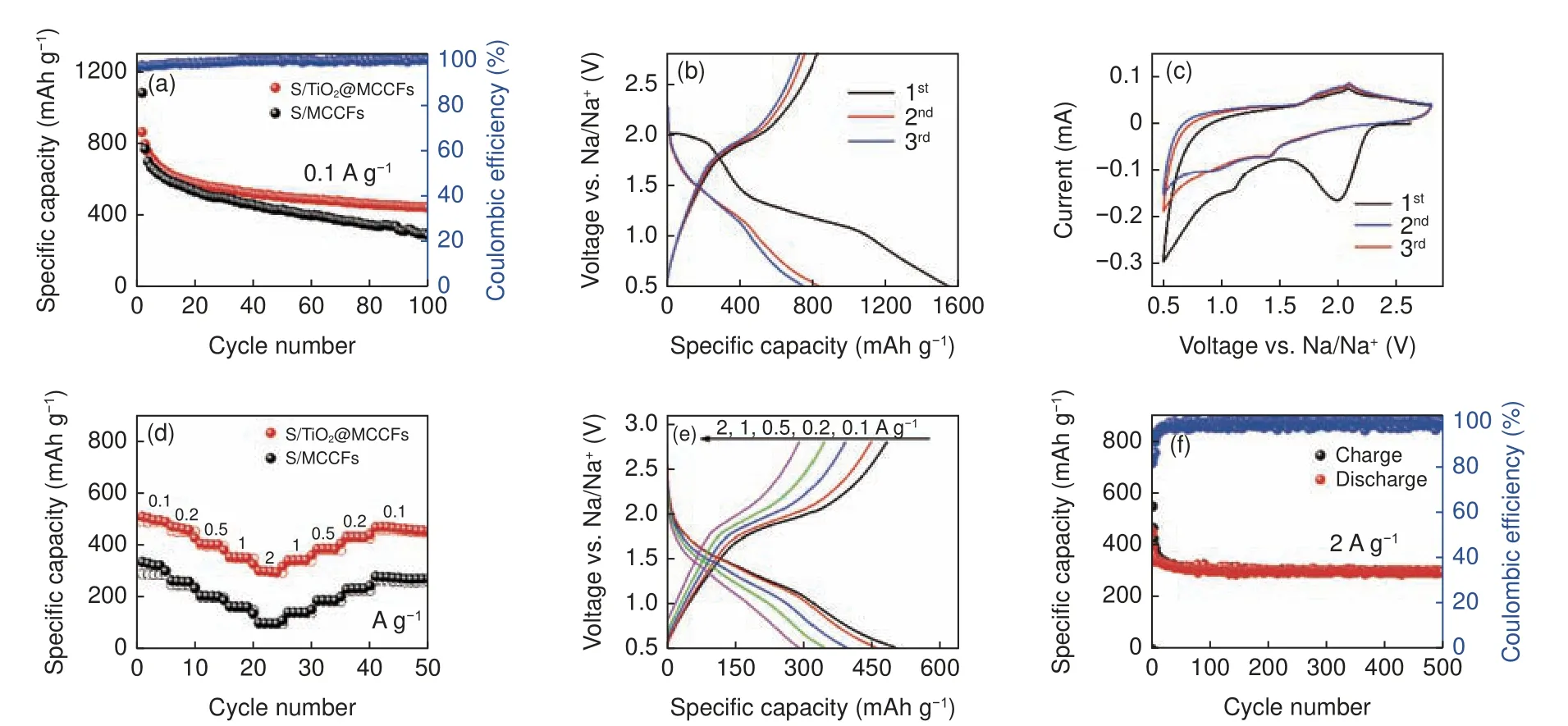
Fig.4 (a) Cycling test of S/TiO2@MCCFs and S/MCCFs at 0.1 A g−1.(b) Discharge/charge profiles of S/TiO2@MCCFs at 0.1 A g−1.(c) CV curves of S/TiO2@MCCFs.(d) Rate capabilities of S/TiO2@MCCFs at different current densities.(e) Discharge/charge profiles of S/TiO2@MCCFs at different current densities.(f) Long-term performance of S/TiO2@MCCFs at 2 A g−1
In order to evaluate the rate performance of the S/TiO2@MCCF cathode, cycling tests at different current densities were performed.At current densities of 0.1, 0.2, 0.5, 1 and 2 A g−1, the reversible capacities reach 521.2, 443.1, 396.8, 362.1 and 308.5 mAh g−1,respectively.When the current density returns to 0.1 A g−1, the reversible specific capacity of the battery is restored to 454.5 mAh g−1.The benefit of nano-TiO2incorporation is also reflected in the change of rate performance with increasing the current density and cycle number, S/TiO2@MCCF electrode exhibits better rate performance than pure S/MCCF electrode.Nano-TiO2have strong affinity to sodium polysulfides, avoid their dissolution, and maintain the structural integrity and stability of the electrode in the redox process.It is worth noting that we performed an activation process before the rate experiment because there is a rapid capacity decay in the initial cycle, which is not good for the rate experiment.Therefore, the capacity in rate performance is lower than that in cycling test.The activation process for S/TiO2@MCCFs before the rating performance test is shown in Fig.S6.Fig.4e shows the corresponding discharging/charging curves of S/TiO2@MCCF electrodes at different current densities (from 0.1 to 2 A g−1).With increasing the current density, obvious charge and discharge platforms can still be observed in each cycle, and the polarization voltage is low, which proves the superb rate performance of the electrode.In addition to rate performance, the S/TiO2@MCCF electrode also performs well in the long-cycle test with high currents(Fig.S5 and 4f).At high current densities of 1 and 2 A g−1, the S/TiO2@MCCF electrode can still maintain high discharge specific capacities of 355.2 and 300.5 mAh g−1, respectively, with the Coulombic efficiency close to 100% even after 500 cycles, indicating that the "shuttle effect" has been effectively suppressed.
To investigate the interaction between sodium polysulfides and TiO2, theoretical simulations of the adsorption energy of Na2S4and Na2S on the surface of MCCFs and TiO2(110) were carried out using density functional theory (DFT)[39,40].The results are shown in Fig.5a-b.The binding energies of Na2S and Na2S4with the TiO2(110) surface are about −3.58 eV and −3.47 eV, respectively, which are significantly stronger than those on the MCCF surface of −0.54 eV and −0.66 eV, indicating the efficacy of nano-TiO2in capturing polysulfides.The addition of TiO2to carbon-based materials can exhibit increased specific discharge capacity and improved cycling performance,inhibit the dissolution of polysulfides in the electrolyte, thereby reducing capacity decay.
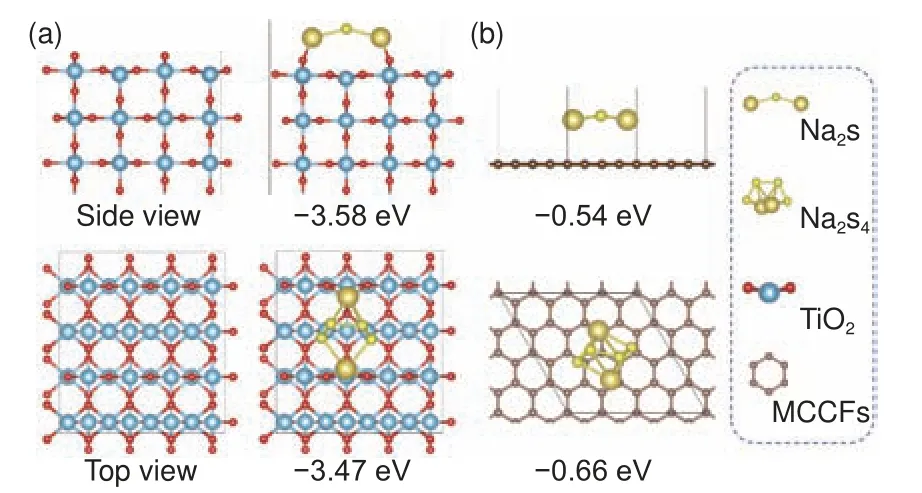
Fig.5 (a) Optimized configurations of Na2S2 and Na2S4 binding to TiO2.(b) Optimized configurations of Na2S2 and Na2S4 binding to MCCFs
4 Conclusion
In summary, a method was provided to incorporate nano-TiO2into multichannel carbon fibers to stablize S for fabricating high performance RT Na-S battery cathode.The synergistic effect of multichannel carbon fibers and nano-TiO2enables the electrode to exhibit superior electrochemical performance with good rate performance and long cycle stability, and the capacity decay is effectively reduced.The battery maintains a discharge specific capacity of 445.1 mAh g−1after 100 cycles at 0.1 A g−1, and can also operate stably at a large current density of 2 A g−1.The effect of TiO2loading on the enhancement of electrochemical performance was analyzed by microscopic morphological observation and structural physical characterization.Due to the unique structure of MCCFs, its internal porous structure can accommodate the volume expansion of sulfur during sodium embedding/desaturation and is beneficial to improve the conductivity of the electrode.The strong interaction between polar TiO2nanoparticles and sodium polysulfides reduces the “shuttle effect” and thus improves the sulfur utilization.In addition, theoretical calculation of the binding energy of adsorption verifies the enhanced effect of the incorporation of nano-TiO2on the adsorption capacity of the carbon-based materials.This method of preparing TiO2@MCCFs by electrospinning provides a simple and low-cost route for the construction of high-performance RT Na-S battery cathode materials.
Acknowledgements
The authors gratefully acknowledge the support of the National Natural Science Foundation of China(51971146, 51971147, 52171218), the Major Program for the Scientific Research Innovation Plan of Shanghai Education Commission (2019-01-07-00-07-E00015), the Shanghai Municipal Science and Technology Commission (21010503100), the Shanghai Outstanding Academic Leaders Plan, the Guangxi Key Laboratory of Information Materials (Guilin University of Electronic Technology, 201017-K), the Shanghai Rising-Star Program (20QA1407100) and the General Program of Natural Science Foundation of Shanghai (20ZR1438400).
杂志排行
新型炭材料的其它文章
- Coal-based graphene as a promoter of TiO2 catalytic activity for the photocatalytic degradation of organic dyes
- 咖啡渣成型制备生物质炭及其CH4/N2分离性能
- A flexible hard carbon microsphere/MXene film as a high-performance anode for sodium-ion storage
- Preparation and lithium storage of anthracite-based graphite anode materials
- Oxygen-incorporated carbon nitride porous nanosheets for highly efficient photoelectrocatalytic CO2 reduction to formate
- 炭纸衬底上化学气相沉积直立型二维过渡金属硫化物及其电催化产氢性能
