Review of chemical recycling and reuse of carbon fiber reinforced epoxy resin composites
2022-12-13TIANZishangWANGYuqiHOUXianglin
TIAN Zi-shang, WANG Yu-qi,3,*, HOU Xiang-lin,3,*
(1.Shanxi Engineering Research Center of Biorefinery, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2.Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China;3.CAS Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China)
Abstract: Carbon fiber-reinforced epoxy resin composites (CFRCs) have been used in the transportation and aerospace fields because of their excellent mechanical properties.The recycling of CFRCs has attracted attention worldwide in recent years.Chemical recycling is a promising method, which can selectively destroy specific resin bonds to achieve controllable degradation.Matrix epoxy resins are degraded into monomers or oligomers, and the high-value carbon fibers can be recycled.First, we summarize progress on chemical recovery methods, mainly super- and subcritical fluids, oxidation, alcoholysis and electrochemical recycling etc.Then, we briefly introduce the synthesis and depolymerization mechanism of recyclable thermosetting resins by the insertion of reversible chemical bonds into the resin to prepare recyclable resins, which is beneficial for the recycling and reuse of components in CFRCs.Finally, possible developments in the chemical recycling of CFRCs and the preparation of high-performance recyclable epoxy resins are proposed.
Key words: Chemical recycling;Chemical degradation;Thermoset epoxy resin;Carbon fiber;Vitrimers
1 Introduction
The carbon fiber reinforced resin composites(CFRCs) are a kind of composites with resin as matrix and fibers as reinforcement[1].Thermosetting epoxy resin is widely used as matrix materials for CFRCs due to its high glass transition temperature(Tg), high modulus, heat resistance and chemical resistance[2,3].The demand for CFRCs as primary structural components of airplanes, wind turbines and automobiles has been increasing in recent years because of their high specific strength, low density, and high rigidity.By 2022, the global demand for carbon fiber and CFRCs is expected to reach 117 kilotons (kt) and 194 kt, respectively[4].In addition, wind turbines and airplanes are the two most extensive applications of CFRCs.If nothing is done, the cumulative total waste of CFRCs will reach 983 kt by 2050[5,6].The CFRCs manufacturing process inevitably produces more waste, up to 40%[7].Therefore, it is urgent to explore the recycling of CFRCs, including the recycling of expensive carbon fibers and thermosetting resins.The main challenge in recycling CFRCs is that the cured epoxy resin has a highly cross-linked network structure, resulting in the insoluble and infusible properties of these materials, which are difficult to recycle.
Recovery methods are mainly divided into three categories, i.e.mechanical recycling, thermal recycling and chemical recycling.Table 1 shows the advantages and disadvantages of the three recycling methods.Mechanical recycling is a process of reducing the size of waste composite materials[8–10].The crushed composites (mixtures of resin, fibers and filler) are used as fillers or reinforcements to reinforce other materials to improve their mechanical properties.However, the long fibers cannot be recovered and the range of applications for shredded composites is limited.Considering the low value of recycling products, it is a huge waste for high-value carbon fibers.Thermal recycling can recover composites and has been widely used in many industrial fields[11].Research on thermal recycling mainly focuses on two types, fluidized bed technique and pyrolysis.Fluidized bed technique has been studied by theUniversity of Nottingham for over 10 years[8,12].Under a stream of hot air (450-550 °C), the composites with a size of about 25 mm were degraded on the bed silica sand with a size of about 0.85 mm.The velocities are usually 0.4-1.0 m/s.In a fluidized bed, the polymer is a volatile substance, and the fibers and fillers are carried as individual particles by air stream.The fibers and fillers are then treated in a high temperature combustion chamber to completely oxidize the polymer, leaving clean fibers.Energy is obtained during this process, and the recovered fibers can be used to make new composites.Pyrolysis is the degradation of combustible materials in the absence of oxygen to organic substances (pyrolysis oil and gas), as well as inorganic fibers and fillers[8].The pyrolysis oil contains aromatic compounds that can be used as fuels.The resulting gas contains hydrogen, methane, and other hydrocarbons with high calorific value to provide energy for the pyrolysis process, accompanied by toxic gases such as CO, NOxand SOx.The carbon fibers recovered (rCFs) by pyrolysis retain relatively high mechanical properties.However, the surface of rCFs was covered with carbonized residue,which could be removed by thermal treatment in air[11,13,14].Unfortunately, rCFs were easy to be oxidized under this condition, resulting in the reduction of mechanical properties[15].Mix gases (oxygen, nitrogen)[13], superheated steam[11]and molten alkali or salt[16–18]are also used to pyrolyze CFRCs.In general,thermal recycling is performed at high temperatures,and toxic gases are produced during the treatment, and the resin cannot be recovered.
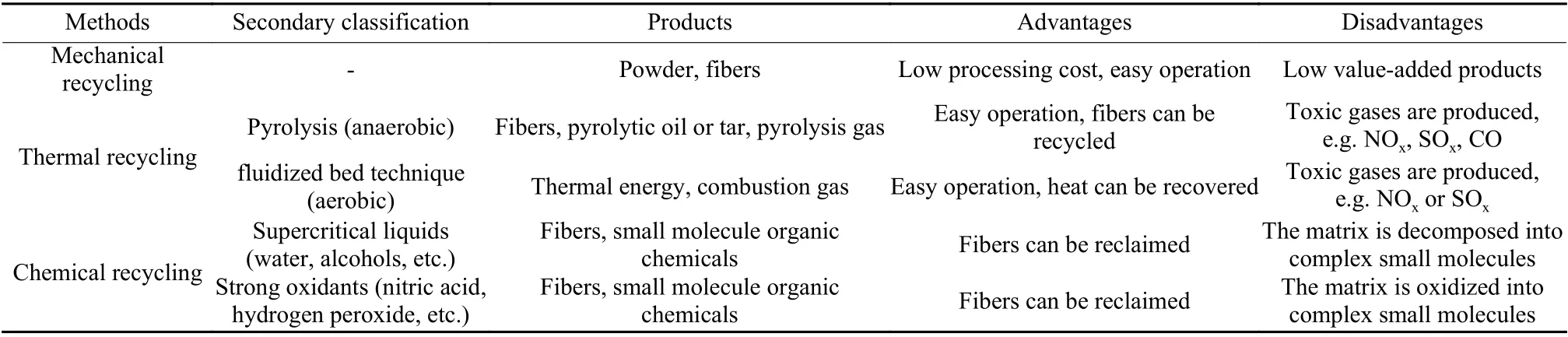
Table 1 The main recycling methods of CFRCs
Chemical recycling, also known as solvolysis,can produce useful chemicals and is considered a more promising approach.Chemical recycling is to degrade and dissolve the resin in composites into monomers, oligomers, or other substances by using solvents with or without catalyst, and finally to recover clean fibers.Based on reaction conditions division,chemical recycling can be carried out in super- and subcritical fluids and mild conditions.Under superand subcritical conditions, the recycling process has high energy consumption, and only carbon fibers are recovered, and the degradation products of epoxy resin are complex[19–21].Catalytic degradation under mild conditions is considered to be the best solution for recovering CFRCs by selectively breaking specific bonds of resin to achieve controlled degradation[22].Excellent mechanical properties of fibers are retained,and the recovered monomers or oligomers can be used to make recycled fiber-reinforced composites.In addition, there is a trend to develop high-performance recyclable polymers due to increasing concerns about sustainability and environmental protection.The introduction of dynamic covalent bonds in cross-linked polymer to prepare new recyclable thermosetting materials is considered as a more promising solution[23,24].The purpose of this paper is to review the research progress of chemical recovery of CFRCs, and to comprehensively understand the degradation mechanism of thermosetting resins, and to provide reference for the future development direction of this field.
2 Recycling methods
2.1 Solvolysis by super- and subcritical fluids
Solvolysis using super- and subcritical fluids is a typical method for recovering waste composite materials.Supercritical fluids have unique intermediate properties between gas and liquid[25].The high diffusivity and solubility of the fluids in the supercritical state enable the polymer to undergo decomposition and partial oxidation[19,26].In particularly, water and alcohols have been used to decompose cross-linked polymers.
2.1.1 Supercritical water and its mixed systems
Water has the advantages of being harmless, inexpensive, and can be easily recycled and reused.The critical temperature of water is 374 °C and the critical pressure is 22.1 MPa.The supercritical water has the characteristics of low viscosity, high efficiency of heat and mass transfer and high diffusion rate, which helps the fluid to quickly penetrate the microcavity of the CFRCs, resulting in more efficient degradation of epoxy resin[26].Moreover, the dielectric constant of supercritical water is similar to that of a non-polar solvent, so that supercritical water can be completely mixed with organic compounds to form a single phase, which make it an effective medium for recovering resins and fibers undamaged.The specific process conditions of CFRCs degradation with supercritical water are shown in Table 2.
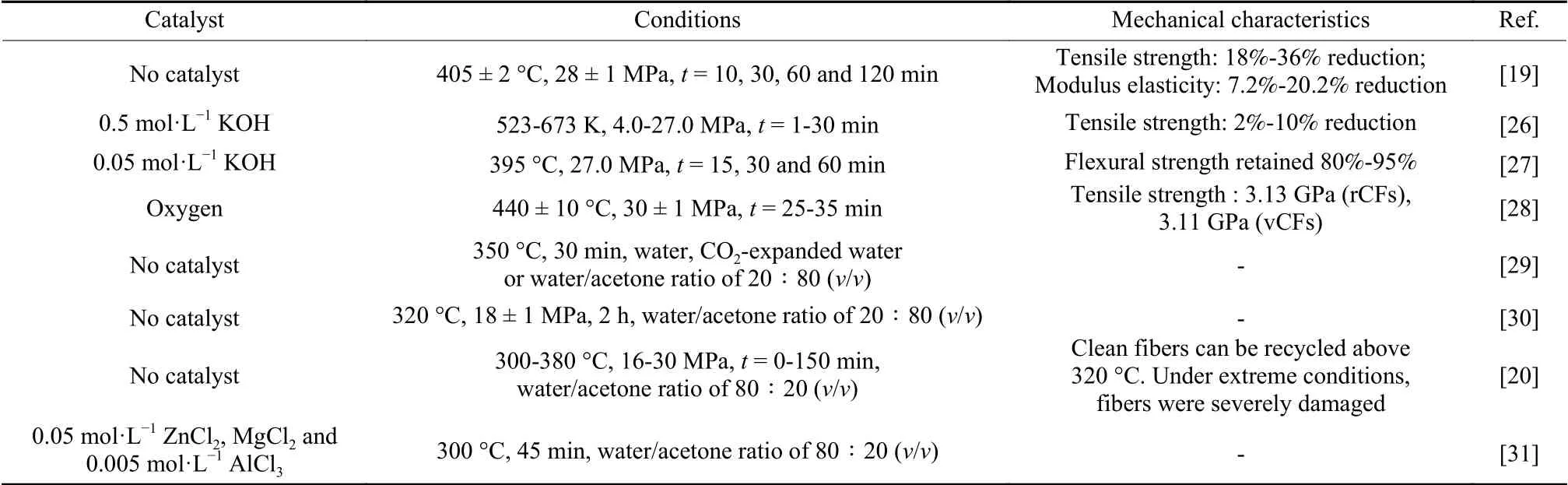
Table 2 Summary of degradation conditions of CFRCs with supercritical water
Y N Kim et al.effectively degraded CFRCs through supercritical water without catalysts[19].When the reaction time was 120 min, the degradation efficiency of epoxy resin was more than 99%.The tensile strength and the elastic modulus of the rCFs were reduced by 18%-36% and 7.2%-20.2%, respectively,compared with those of vCFs, due to the removal of resin and sizing agent from surface of fibers.Adding catalyst can obviously improve the resin removal efficiency.R Piñero-Hernanz et al.recovered carbon fibers in supercritical water without stirring[26].The percentage degradation of resin was 95.4% by adding 0.5 mol·L−1KOH.The tensile strength of the rCFs was 2%-10% lower than that of the vCFs.C C Knight et al.achieved a resin removal efficiency of 97.4%when the composite reacted in 0.05 mol·L−1KOH solution for 15 min[27].The flexural strength of the composites prepared from the rCFs retained 80%-95%of that of the original composites.However, strong alkaline environment is harmful to the mechanical properties of rCFs.Y Bai et al.added oxygen in supercritical water to increase the degradation efficiency[28].When the degradation percentage was 96.5%, the tensile strength of the rCFs was 3.13 GPa, which retained the tensile strength (3.11 GPa) of vCFs.The resin without degradation can repair the surface defects of the rCFs and avoid stress concentration that reduces the strength of the rCFs.When the degradation percentage was higher than 96.5%, the tensile strength of the rCFs declined quickly, which may be due to the excessive oxidation of the rCFs.
After a high degradation ratio is achieved, there are more and more studies on degradation reaction in mixed systems.G Oliveux et al.compared the degradation ratios of resin in three systems, including pure water, CO2-expanded water and a mixture of 80%acetone and water[29].Compared with pure water, the CO2-expanded water and the mixture of acetone and water were conducive to resin degradation.By adding CO2or acetone to water, the critical point of water canbe reduced to generate a fluid with an enhanced fluidity or a supercritical fluid, thus facilitating diffusion and mass transfer.Moreover, the addition of acetone increased the solubility of the degraded products.The degradation percentage of resin was 93% in the solution with a water to acetone ratio of 80% at 350 °C for 30 min.The effects of rCFs defects, such as discontinuous fiber with residues, fluffy fibers and imperfectly arranged fiber tows, on the mechanical properties of the composites with rCFs were evaluated[30].The results showed that the rCFs had excellent reinforcing properties, which can be comparable with the vCFs.M J Keith et al.recycled carbon fiber reinforced epoxy resin using a mixture of acetone and water and studied the effects of alkaline catalysts and weak Lewis acids on degradation of resin[20,31].The results showed that the alkaline addition could not effectively catalyze the degradation reaction, however,weak Lewis acids (ZnCl2, MgCl2and AlCl3) promoted the degradation reaction efficiently.It may be that in the presence of alkaline media, aldol condensation of acetone increased the critical point of the system, which was not conducive to form supercritical fluids.Compared with the reaction without catalyst,the temperature was significantly reduced by 40 °C.
2.1.2 Subcritical water and near-critical water
Subcritical water and near-critical water are considered as excellent solvents for degradation of polymers and are cheap, non-toxic, environmentally friendly[32].In addition, near-critical water has the characteristics of high diffusion coefficient, low density, low dielectric constant (a decreased polarity of water).The specific process conditions of CFRCs degradation with subcritical water and near-critical water are shown in Table 3.
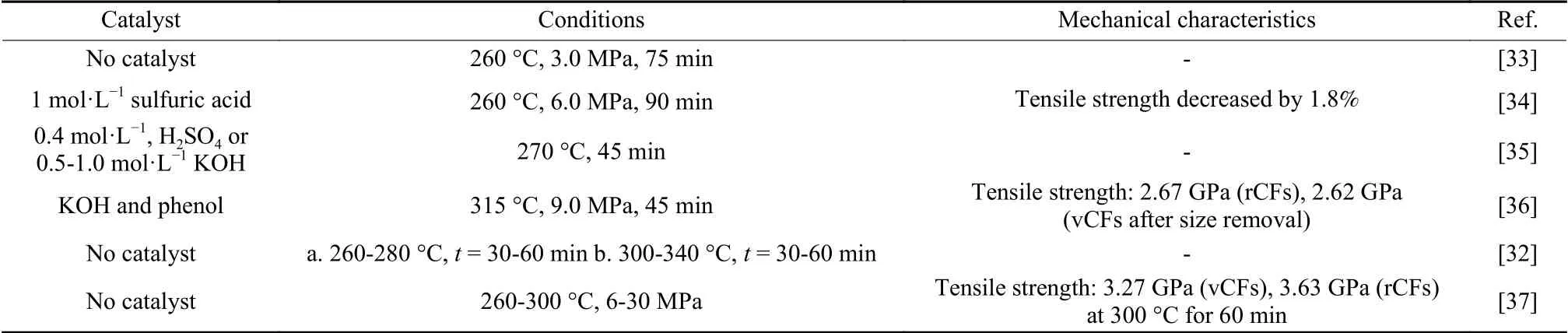
Table 3 Conditions of CFRCs degradation with subcritical/near-critical water
Y Liu et al.achieved complete degradation of the resin, and found the degradation rate of the resin was affected by temperature, reaction time and pressure,while the feedstock ratio had little effect on the degradation rate[33].The tensile strength of the rCFs decreased by 1.8% compared to vCFs.The addition of acidic or basic catalyst was beneficial to the degradation of resin matrix, and the degradation percentage can reach 97.7%-100%.The decomposition reactions could be by different paths[34,35].KOH inhibited the hydrolysis of ester bonds and had a stronger catalytic effect on the hydrolysis of ether bonds than ester bonds.H2SO4catalyst had the same promoting effect on breaking ether bonds and ester bonds.The synergistic catalysis of phenol and KOH can also improve the decomposition efficiency of resin[36].The resin can be completely degraded.Free-radical mechanism is dominant in this case.In the presence of KOH, phenol formed a phenoxy anion, which was easy to lose an electron to form phenoxy radical in subcritical water.Phenoxy radical may break C―N, C―C and C―O bonds, forming free radicals to degrade epoxy resin.There was no significant difference between the tensile strength of all the rCFs and that of vCFs after sizing agent was removed.
X Gong et al.achieved complete degradation of diglycidyl ether of bisphenol A (DGEBA) cured by different curing agents (methylhexahydrophthalic anhydride (MeHHPA), 4,4’-diaminodiphenyl sulfone(4,4’-DDS)) in near-critical water[32].It was found that the degradation mechanisms of the two resins were different, and the curing agent containing benzene ring could improve the heat resistance of the resins,which led to a relatively high degradation temperature.The degradation reactions of the two systems were first-order reactions.H U Sokoli et al.recycled mixed fiber reinforced composites using near-critical water, and achieved a resin removal efficiency of 99.5%[37].The rCFs retained the mechanical properties of vCFs, but the strength of glass fiber recovered decreased by 50%-65%.This was due to the presence of alkali oxides on the surface of the glass fibers,which can react with water to liberate alkali ions (such as Na+).Alkali ions reacted with water to generate Na-OH and other strong bases, resulting in micro-cracks on the surface of glass fibers that decreased the strength.
2.1.3 Super- and subercritical alcohols and mixed system
A large number of studies have been conducted on the degradation of CFRCs using supercritical alcohols due to the advantages of low cost, low toxicity,recyclability and the ability to dissolve organic and inorganic compounds[38–40].In addition, the critical condition of alcohols is more easily achieved than that of water and supercritical alcohols can generate a large amount of hydrogen in situ, which is conducive to the degradation reactions[41].The solvents used for recovery and reaction conditions are shown in Table 4.
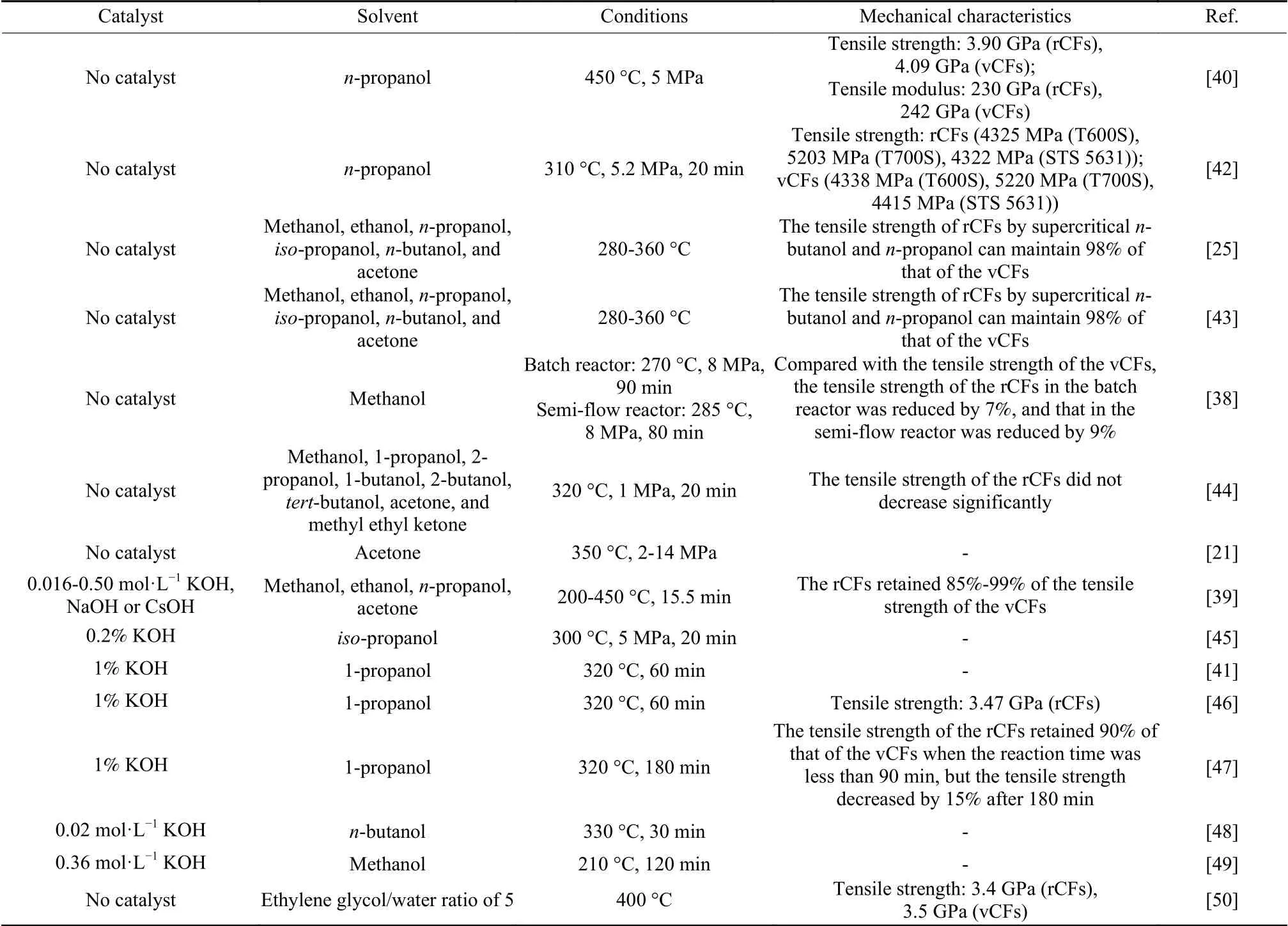
Table 4 A comparison of degradation conditions of CFRCs with super- and subcritical alcohols
J R Hyde et al.used supercritical propanol to degrade amine-cured epoxy resin matrix composites in the absence of catalyst.The tensile strength of rCFs was 4.6% lower than that of the vCFs[40].The epoxy resin can be completely degraded with supercriticalnpropanol at 310 °C and 5.2 MPa[42].The tensile strength of the rCFs was 0.3%-2% lower than that of the vCFs.The oxygen content on the surface of the rCFs was significantly reduced.H Cheng et al.also analyzed the degradation kinetics of six supercritical fluids (methanol, ethanol,n-propanol,iso-propanol,nbutanol, and acetone) and established the kinetic mod-el of CFRCs degradation by supercritical fluids[25,43].The degradation efficiency of supercriticaln-butanol on epoxy resin in CFRCs was the highest, followed by supercritical acetone.In the temperature range of 280 to 340 °C, the degradation efficiency of supercritical ethanol was similar to that of supercriticaln-propanol.However, the degradation efficiency of supercriticaln-propanol was higher than that of supercritical ethanol in the range of 340 to 360 °C.The tensile strength of rCFs by supercriticaln-butanol andn-propanol can maintain 98% of that of the vCFs.Supercritical methanol andiso-propanol were not conducive to the degradation of CFRCs.However, I Okajima et al.successfully degraded thermosetting epoxy resin in supercritical methanol[38].The rCFs recovered by the semiflow reactor retained the form of plain fabric without resin or cracks on the fiber surface.The tensile strength of the rCFs retained 91% of the vCFs.The interfacial strength of rCFs was decreased by about 20%, compared with the vCFs.Afterwards, I Okajima et al.used eight different supercritical solvents (methanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol,tert-butanol, acetone, and methyl ethyl ketone) to selectively degrade the CFRCs[21,44].Acetone was the best solvent for degradation among the selected solvents.The increase of reaction pressure and acetone density increased the degradation efficiency.The maximum degradation efficiency (95.6%) was obtained at 350 °C with the acetone density of 4.35 mol·L−1(14 MPa) for 60 min.
Alkaline additives can reduce the activation energy of the reactions and accelerate the degradation rate of resin matrix by supercritical alcohols[39,41,45–47].R Piñero-Hernanz et al.investigated the ability of methanol, ethanol, 1-propanol, and acetone solvents to degrade CFRCs at 300-450 °C[39].It was found that acetone had the best degradation efficiency at lower temperatures.When the temperature increased up to 450 °C, the degradation percentage of resin in ethanol,1-propanol, and acetone was up to 78.8%, but only 60.2% in methanol.The poor degradation efficiency of methanol is due to the fact that methanol has the highest dielectric constant among these solvents and is in the subcritical state under the conditions studied,while the other solvents are in the supercritical states.H Yan et al.degraded amine-cured epoxy resin by supercritical 1-propanol[41,46].The reaction was a free radical mechanism.KOH stimulated Guerbet reaction of 1-propanol to produce excess hydrogen, which was conducive to degradation reactions.However, the tensile strength of rCFs without adding KOH was greater than that of rCFs with adding KOH[47].When the concentration of KOH was increased, the degradation rate did not increase significantly and the mechanical properties of rCFs got worse.The rCFs without resin can be recovered at 320 °C for 180 min, but the tensile strength was reduced by 15%.H Huang et al.studied the degradation mechanism in supercriticalnbutanol with KOH catalyst[48].KOH can facilitate Guerbet reaction and accelerate the generation of hydrogen radical, thus speeding up the breaking of bonds.The degradation percentage was close to 100%in supercriticaln-butanol/KOH solution at 330 °C for 30 min.
Different from the above mechanism of alkali catalysis, J Liu et al.determined that the degradation of epoxy resin by subcritical methanol using KOH catalyst was a transesterification reaction[49].Compared with other alcohol-catalyst systems, CH3OH/KOH system had the best degradation efficiency for epoxy resin, which may be due to the higher nucleophilic capacity of CH3O−, which was conducive to break bonds of epoxy resin.Alkalinity and type of alkali metal ion can affect degradation rate of epoxy resin in methanol.The resin was completely degraded at 210 °C and 0.36 mol·L−1KOH within 120 min, which was 65 °C lower than that without catalyst.Adding a small amount of water can improve resin removal efficiency[50].With an ethylene glycol/water ratio of 5, the resin removal efficiency was the highest (97.6%) at 400 °C.The mechanical properties of the rCFs with mixed solvents declined slightly.
2.2 Chemical recycling under mild conditions
2.2.1 Oxidation methods
The most common oxidation method to recover CFRCs is to use oxidants such as nitric acid[2,3,51], oxygen (O2)[1], hydrogen peroxide (H2O2)[52,53]and peracetic acid (PAA)[54].The thermosetting amine-cured epoxy resin could be completely degraded in nitric acid solution, but not in sulfuric acid and hydrochloric acid, which was caused by the C―N bonds breaking in the epoxy resin due to the oxidation of nitric acid[2,51,55,56].Reaction conditions and degradation products are shown in Table 5.W Dang et al.completely degraded bisphenol F-type epoxy resin cured by aliphatic amine (1,8-diamino-p-menthane (MDA))and aromatic amine (4,4’-diaminodiphenylmethane(DDM)) in 4 mol·L−1nitric acid solution at 80 °C and found that T-glass fibers had higher corrosion resistance to nitric acid than E-glass fibers[57,58].T Hanaoka et al.summarized the effects of different types of amines curing agents (m-xylylenediamine (MXDA),1,3-bis(aminomethyl)cyclohexane (BAC), MDA, isophoronediamine (IPDA), norbornanediamine (NBDA),m-phenylenediamine (MPDA)) on the decomposition rate of epoxy resin and proposed the decompositionmechanisms[56].It was found that the chemical structure around the C―N bonds and the ring structures of curing agents affected the degradation rate.The decomposed products were hydrogenated[2].After nitro group was converted to amino group, the substance can replace a part of curing agent to cure epoxy resin.The tensile strength of the regenerated resin can maintain more than 90% of that of the original one after 5% curing agent was replaced by hydrogenated extract.Subsequently, T Hanaoka et al.compared several oxidants and found that nitric acid was the most effective oxidant for resin removal[3].The tensile strength and tensile modulus of rCFs were close to those of the vCFs, and the mechanical properties of the recycled composite were similar to those of the original composite.The swelling process is very important to improve the degradation efficiency and reduce the reaction temperature.F Tian et al.introduced porous structure into amine-cured epoxy resin by microwave-assisted swelling in N-Methyl pyrrolidone (NMP), as shown in Fig.1a[59].It can be seen from the scanning electron microscope (SEM)figure (Fig.1b) that a large number of uniform nanoscale pores existed in the epoxy resin after swelling,which was helpful for the degradation of the resin by catalysts and reactants.The C―N bonds of swollen epoxy resin in the presence of nitric acid were broken to obtain oligomers.Micropores were also generated during the nitric acid treatment, which was beneficial to the degradation of the resin.The swollen epoxy resin was completely degraded in nitric acid solution at 60 °C for 3 h.The recovered degradation solution was supplemented with 5.0 mL of nitric acid solution, and the degradation rate remained above 93% after 5 cycles.
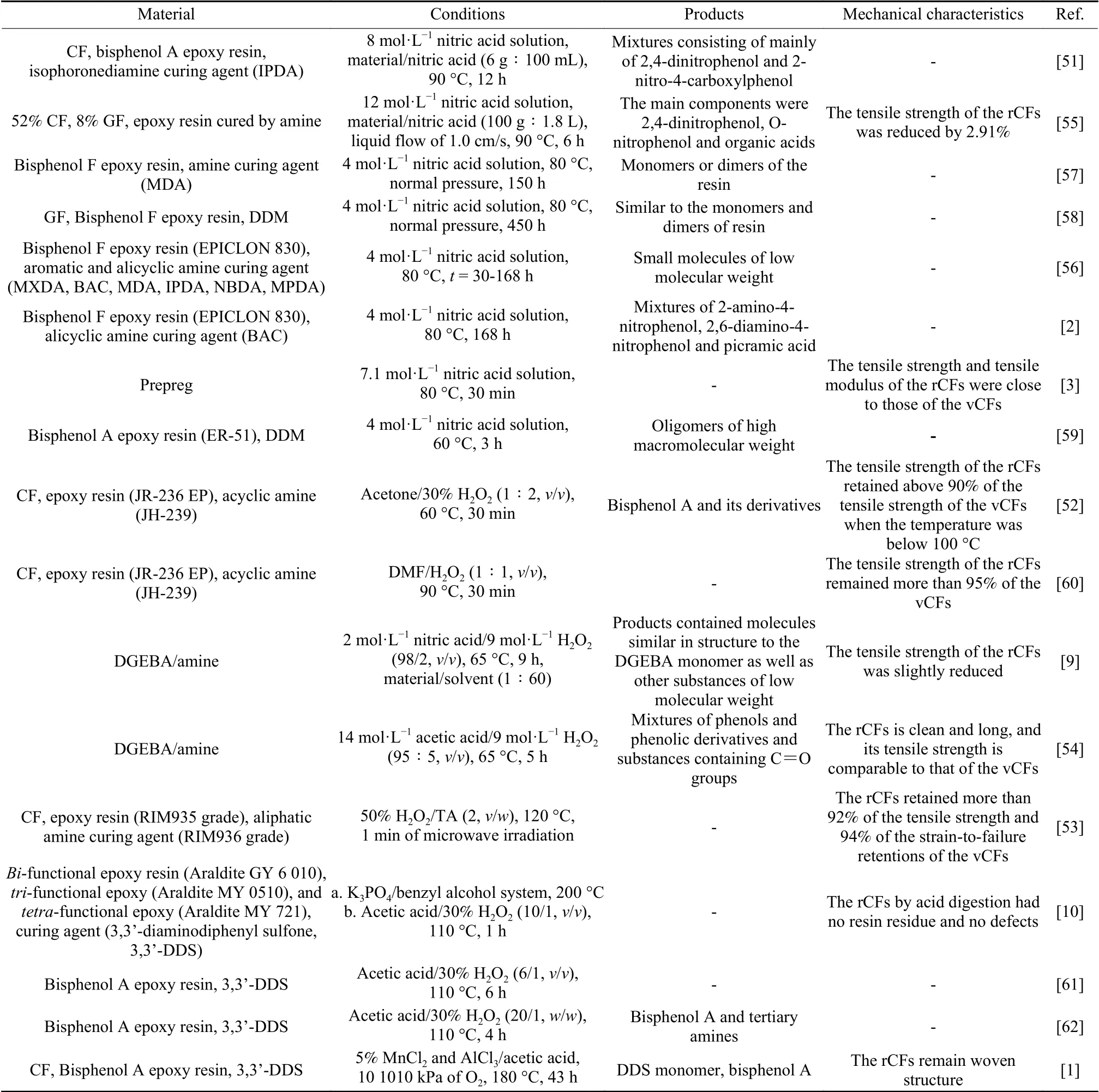
Table 5 Oxidation recovery of CFRCs and its degradation products
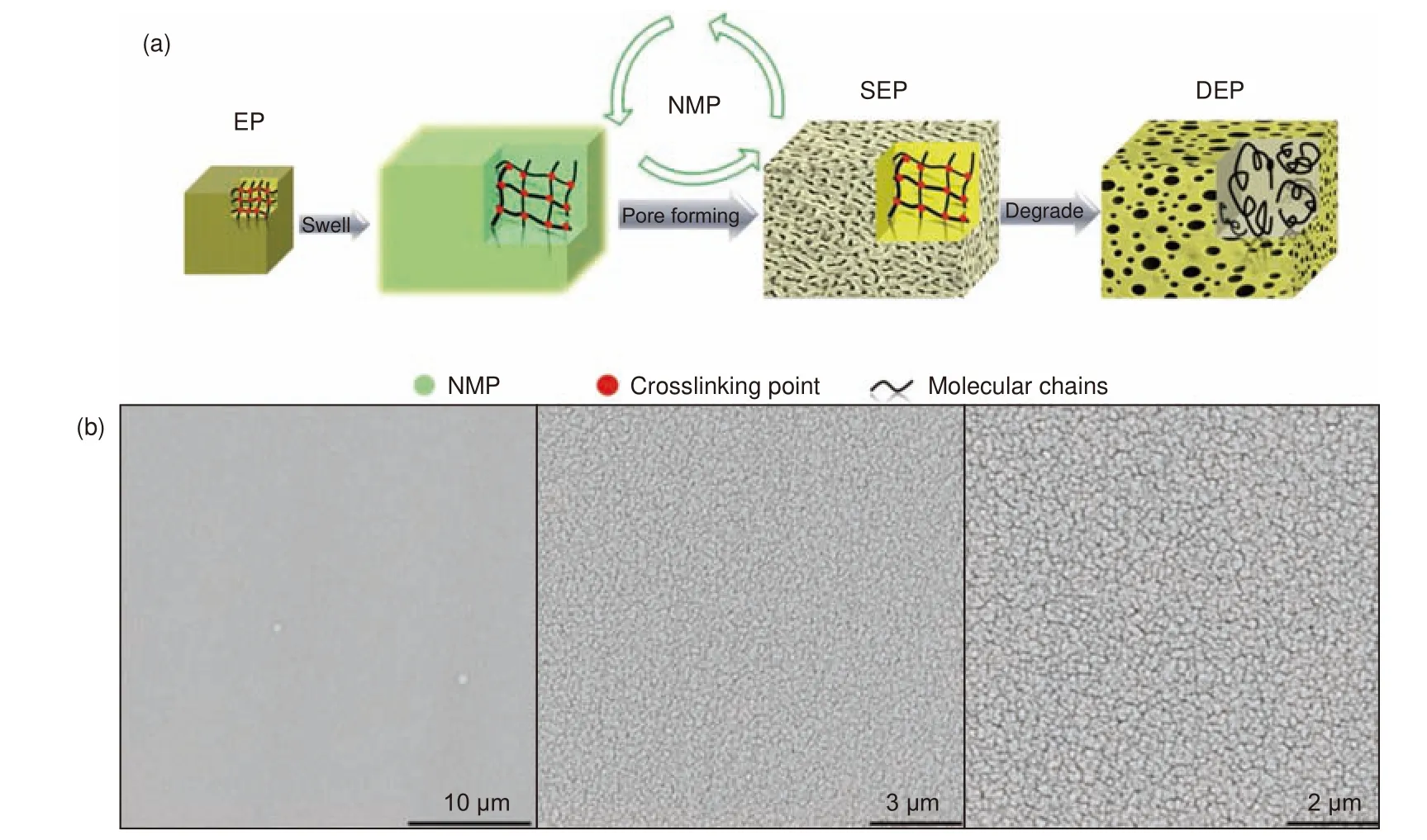
Fig.1 (a) Schematic diagram of swelling and rapid degradation of thermosetting epoxy resin.(b) SEM images of swollen epoxy resin[59].Reprinted with permission by American Chemical Society
Acid solution was also used as the pretreatment reagent to obtain the composites with expanded layer and large surface area.Then, oxidative methods using a mixture of H2O2and organic solvents, such as acetone[52], N,N-dimethylformamide (DMF)[60], nitric acid[9]and acetic acid[54], were used to degrade epoxy resin cured by amine.J Li et al.used acetic acid solution to pretreat the composite, and then recovered CFRCs in a self-accelerating oxidative degradation system[52].In the mixture of acetone and H2O2, the removal efficiency of epoxy resin was about 90%.The diameter of rCFs was similar to that of the vCFs and the tensile strength of the rCFs remained more than 90% of vCFs.The reaction was caused by the decomposition of H2O2into hydroxyl radicals and then a series of free radical transfer and propagation reactions occurred in this system.Similarly, after pretreatment with acetic acid, the composite was recovered in the mixture of DMF and H2O2.The degradation percentage of epoxy resin was more than 90% and the tensile strength of the rCFs remained more than 95%of the vCFs[60].M Das et al.pretreated CFRCs with a mixture of nitric acid and H2O2, and then recovered carbon fibers by ultrasonic treatment[9].The degradation percentage of the resin was 95% under the optimal ratio of nitric acid to H2O2(98/2,v/v).Very little resin remained on the surface of the rCFs and its tensile strength was equivalent to that of the vCFs.One-step oxidation can also be used to recover CFRCs.M Das et al.used peracetic acid (PAA) generated in situ from a mixture of acetic acid and H2O2to oxidize the CFRCs[54].The degradation percentage of the resin reached 97% at 65 °C for 5 h and the clean long fibers were recycled, whose tensile strength was comparable to that of the vCFs.Oxidation reactions were initiated by hydroxyl radicals formed by H2O2and acyloxy radicals formed by PAA.The acetic acid of more than 90% was recovered and 94.1% of the original concentration can be maintained.O Zabihi et al.recovered CFRCs with a mixture of H2O2and tartaric acid (TA) assisted by microwave, and the resin removal efficiency reached more than 90%[53].The rCFs retained more than 92% of the tensile strength and 94% of the strain-to-failure retention of the vCFs.The composites made of rCFs were recycled for three times, and the tensile strength of the rCFs for the third time was 11.7% lower than that of the vCFs.
Y Ma et al.concluded that alkali depolymerization and acid digestion were effective to recover amine-cured epoxy composites[10].In terms of alkali depolymerization, the resin dissolution rate depended mainly on the chemical reaction rate, while for acid digestion, it was controlled by the diffusion rate.The results showed that acid digestion could recover amine-cured epoxy composites more efficiently because of its fast reaction rate and low reaction temperature.The aniline groups can transfer the oxygen atoms produced by H2O2and then break the polymer by elimination reactions.Moreover, the rCFs had no resin residue and no defects.In the same year, the team investigated the acid degradation mechanism of another amine-cured epoxy resin composites[61].It was found that the reaction involved the transfer of oxygen atoms to the linking aniline group and then C―N bonds were broken by elimination and hydrolysis reactions.In the process of degrading polymer matrix,the elimination reaction was a rate-control step rather than oxygen atom transfer.In order to achieve closedloop recovery, the team prepared new epoxy resins by using the degraded matrix as an accelerator or a filler[62].TheTgof anhydride-cured materials prepared by adding 5% degraded matrix as an accelerator and filler is above 100 °C.In addition to the usual oxidant methods, the team reported the recovery of amine-cured composites by catalytic aerobic oxidation[1].Metallic salts (manganese chloride and aluminum chloride) were necessary as catalysts for the oxidation and dissolution steps to achieve the breaking of C―N bonds.The fiber length and weave structure were preserved.
2.2.2 Alcoholysis
Due to their lipophilic wettability of epoxy thermosetting resins, the degradation efficiency in organic solvent (mainly alcohols) is higher under catalysis(e.g.,N-methyl-4-piperidinol, 1,5,7-triazabicyclo[4,4,0] dec-5-ene (TBD), K3PO4, ZnCl2, etc.).The boiling point of the selected catalyst is close to that of solvent, which is convenient for distillation recovery[63].N-methyl-4-piperidinol catalyst was selected to degrade the epoxy resin cured by anhydride with ethylene glycol (EG).The resin degradation efficiency dropped from 99.7% to 90.3% after three cycles, probably due to the reduced concentration of the catalyst during distillation.The degradation reactions were mainly caused by transesterification catalyzed by organic base.The synergistic effect of alcohol and 1-methyl-2-pyrrolidinone (NMP) solvent can accelerate the depolymerization[64].X Kuang et al.selectively broke the ester bonds in anhydride cured epoxy resin and composites using a mixture of NMP and EG catalyzed by an organic catalyst, TBD.After treating CFRCs with 0.30 mol·L−1TBD-EG/NMP(10/90,v/v) solution at 170 °C for 1.5 h, the rCFs were cleaner than vCFs and almost kept the original morphology, size, and mechanical properties of vCFs.In addition, a good solvent swells resin rapidly, transferring the catalyst to enter the resin network, thus significantly increasing the dissolution rate, as shown in Fig.2a.L Ye et al.degraded an anhydride cured composite using NaOH/benzyl alcohol and K3PO4/benzyl alcohol system and proposed the degradation mechanism[65].The decomposition efficiency was over 90% at 195 °C for 40 min.The tensile strength of rCFs maintained over 90% of the vCFs.In this reaction, benzyl alcohol was used as a solvent to swell the resin, and as a nucleophile to participate in the transesterification reaction.The reaction conditions and degradation products are listed in Table 6.
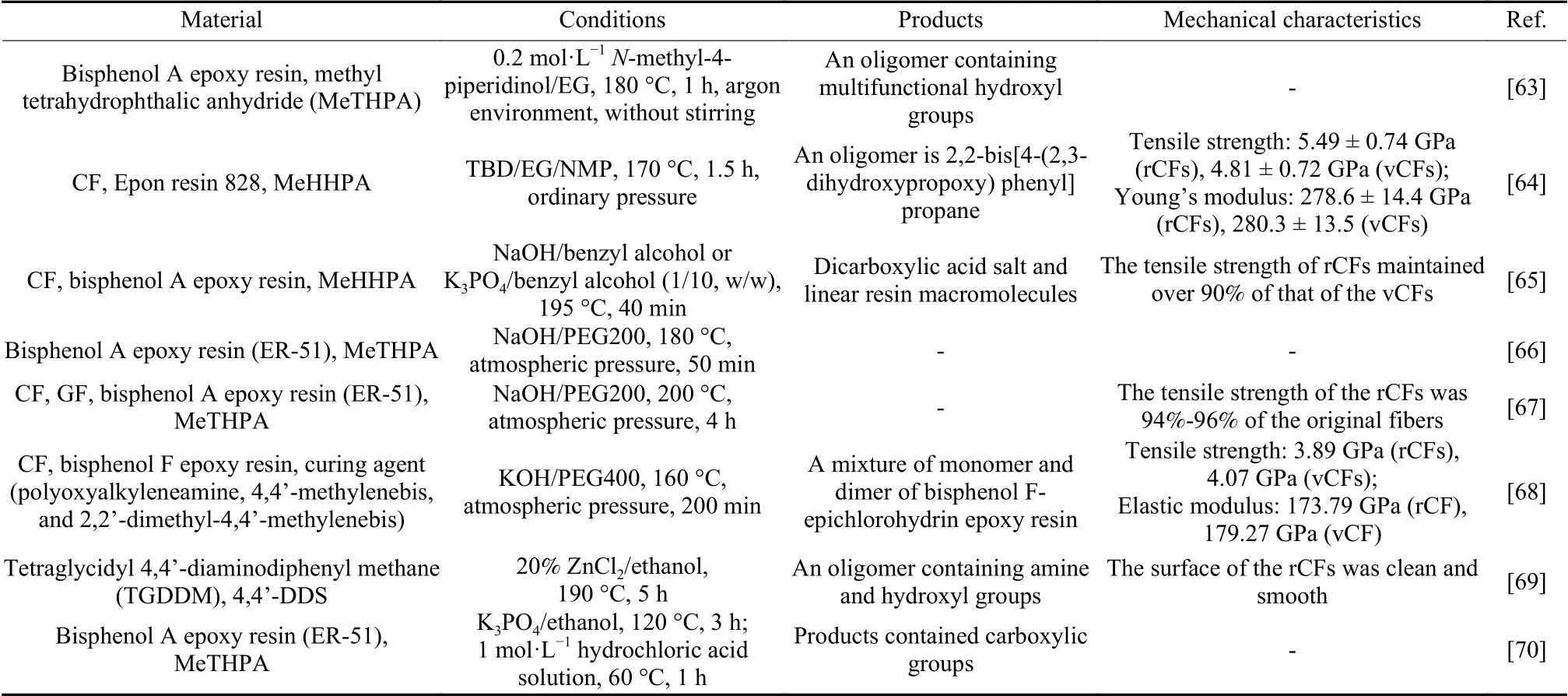
Table 6 Alcoholysis of CFRCs and their degradation products
In recent years, poly(ethylene glycol) (PEG) has also been used to degrade epoxy resins[66,67].P Yang et al.studied the dissolution of epoxy resins in PEG with different molecular weights and found that PEG200/NaOH system had the highest dissolution capacity for resin matrix.Its highest solubility was due to the formation of chelates with the coronal structure at the end of PEG chain, which can change the helical conformation with cations, improving the solvation of cation and thus increasing the activity of alkali.The main dissolution reaction was hydrolysis of ester bonds, accompanied by a small amount of transetherification reaction.The degradation efficiency was up to 84.1%-93.0% at 200 °C for 4 h.The texture and diameter of the rCFs and alkali-free glass fibers were similar to the original fibers, but there was some residual resin on the surface.The tensile strength of the three types of fibers was 94%-96% of the original fibers.The recycled medium-alkali glass fibers had obvious corrosion, cracking, and other damage on the surface.Accordingly, the tensile strength decreased to 89% of the original.J Jiang et al.soaked CFRCs in nitric acid before using PEG400/KOH system to degrade resin matrix, as shown in Fig.2b[68].The pretreatment process reduced the energy required in the degradation stage and speeded up the degradation rate.After the reaction at 160 °C for 200 min, the resin removal efficiency was 97% and the mechanical properties of the rCFs remained 95% of the vCFs.
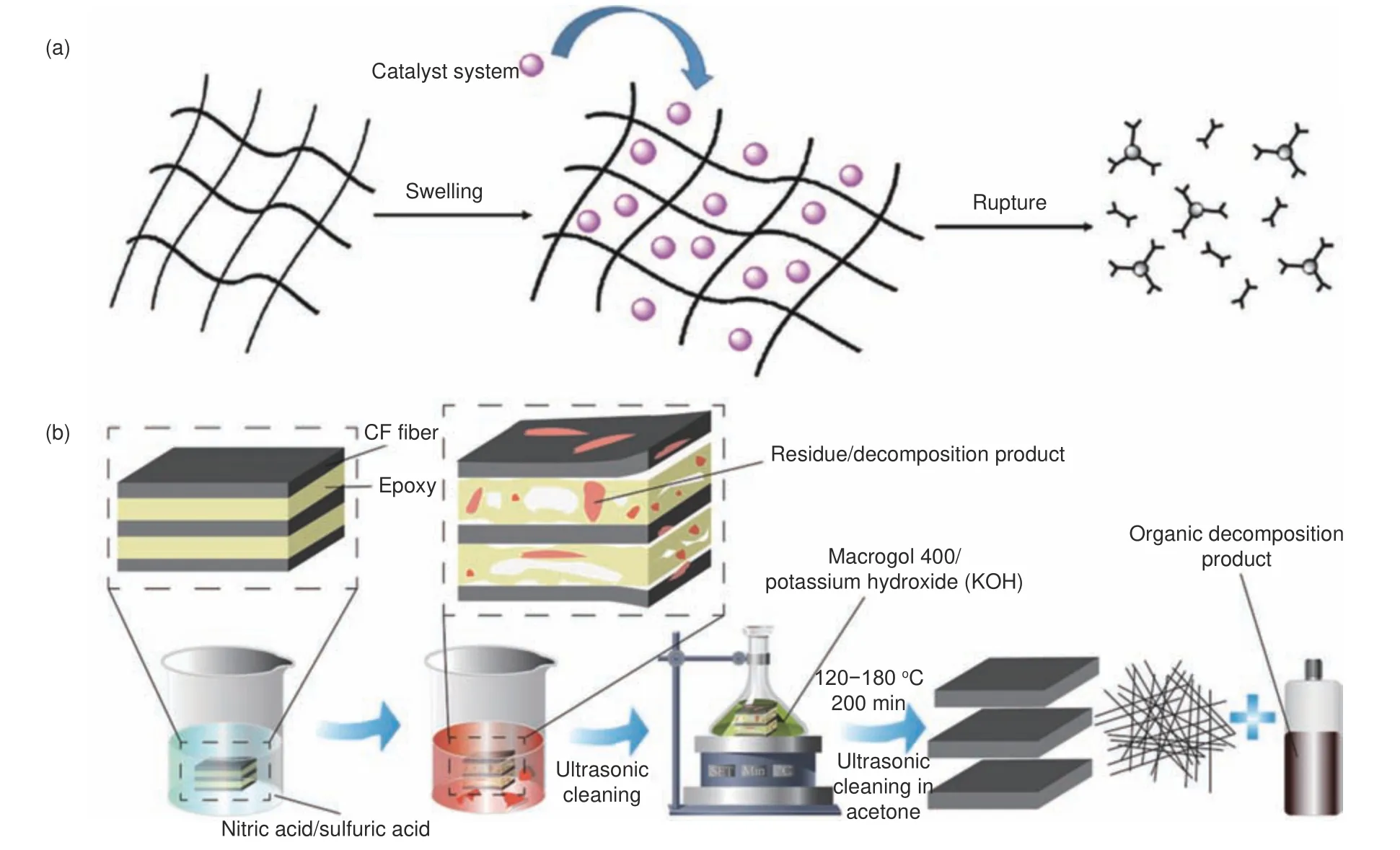
Fig.2 (a) Schematic diagram of swelling and depolymerization[69].(b) Schematic diagram of pretreatment and degradation of CFRCs[70].Reprinted with permission by Elsevier
It should be noted that the use of high boiling solvents (benzyl alcohol, NMP, EG, PEG, diethyleneglycol, etc.) can adversely affect the separation and reuse of products.Ethanol with a low boiling point has strong swelling ability for epoxy resin and is also a transesterification solvent.T Liu et al.achieved complete degradation of epoxy resin in the ZnCl2/ethanol system[69].The degradation degree of resin increased with the increase of temperature and catalyst concentration.The degradation degree of the recycled catalyst directly used in the degradation reaction was up to 90%.The surface of the rCFs was clean and smooth.X Zhao et al.reported the two-step process of alcoholysis and hydrolysis of anhydride-cured epoxy resins[70].The degradation efficiency of epoxy resin exceeded 95% using the K3PO4/ethanol catalytic system at 120 °C for 3 h.After further acidic hydrolysis,the degraded products contained a large number of carboxyl reactive groups, which can be used as curing agents to prepare a new anhydride-cured epoxy resin.
2.2.3 Electrochemical recycling
Recently years, electrochemical recycling systems for CFRCs recovery have been proposed, as shown in Table 7.The recycling device is shown in Fig.3, including a direct current power supply, a stainless-steel plate or carbon rod as the cathode,waste CFRCs as the anode, and electrolyte[71,72].J H Zhu et al.reported electrolytic oxidation at low voltages.The reaction was carried out by degrading the epoxy resin.The degradation rate of epoxy resin was proportional to the current.The tensile strength of rCFs decreased with the increase of the electrolyte concentration and the decrease of the current, which may be due to the improvement of tensile strength of rCFs by residual resin.Under the condition of 3%NaCl solution and 25 mA applied current, the tensile strength of rCFs retained 80% of the vCFs.The SEM results showed that the amount of residual resin was proportional to the applied current (Fig.4).The composites were treated in 4 mA, 3% NaCl solution to obtain clean carbon fibers without cracks or fissures.In the electrolysis process, Cl−in the solution oxidized to form Cl2at the anode and some gases reacted with water to produce chlorinated water.There were 4 pHdependent oxidizing substances, Cl2, HClO, ClO−and O2.The process of electrochemical recovery method is simple, but the processing time is long (21 days).In order to improve the recovery efficiency, adding KOH catalyst or raising the reaction temperature was considered feasible[73].The addition of KOH catalyst promoted the reaction between Cl2and water to generate more ClO−, thus improving the degradation efficiency of epoxy resin.In addition, the intercalation of OH−caused the expansion of carbon fiber cortex, whichwas conducive to the degradation of resin matrix and recovery of CFs.The electrochemical recovery process involves breaking the C―N bonds, followed by breaking the ether, benzyl, secondary and tertiary amine bonds.Under the condition of a NaCl concentration of 2%, current of 20 mA and KOH concentration of 1%, the tensile strength and interfacial shear strength (IFSS) of the rCFs were 90% and 120%, respectively, of those of vCFs.K Oshima et al.reported the removal of epoxy resin at high voltages[74,75].The neutral electrolyte with a concentration of 0.1 mol·L−1facilitated the electrical treatment.High voltage can also lead to a large weight loss of unidirectional CFRCs laminate.The weight loss at high voltage was due to the oxygen produced by the electrolysis of water at the anode, which caused the resin of the CFRCs laminates to fall off.In the recovery process, resin stripping began with voids formed by oxidation of the resin.High voltage can induce the damage of CFs.A few carbon fibers were broken and had irregular fiber ripples, and there were small voids on the surface of the rCFs.Electrochemical method is only suitable for the recovery of carbon fiber reinforced composites because of the high electrical conductivity of CFs.
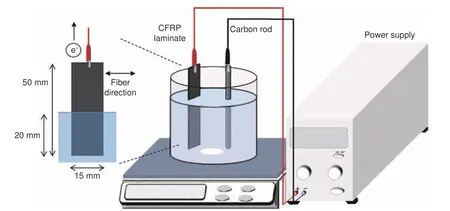
Fig.3 Schematic diagram of electrochemical recovery of CFRCs[74].Reprinted with permission by Elsevier
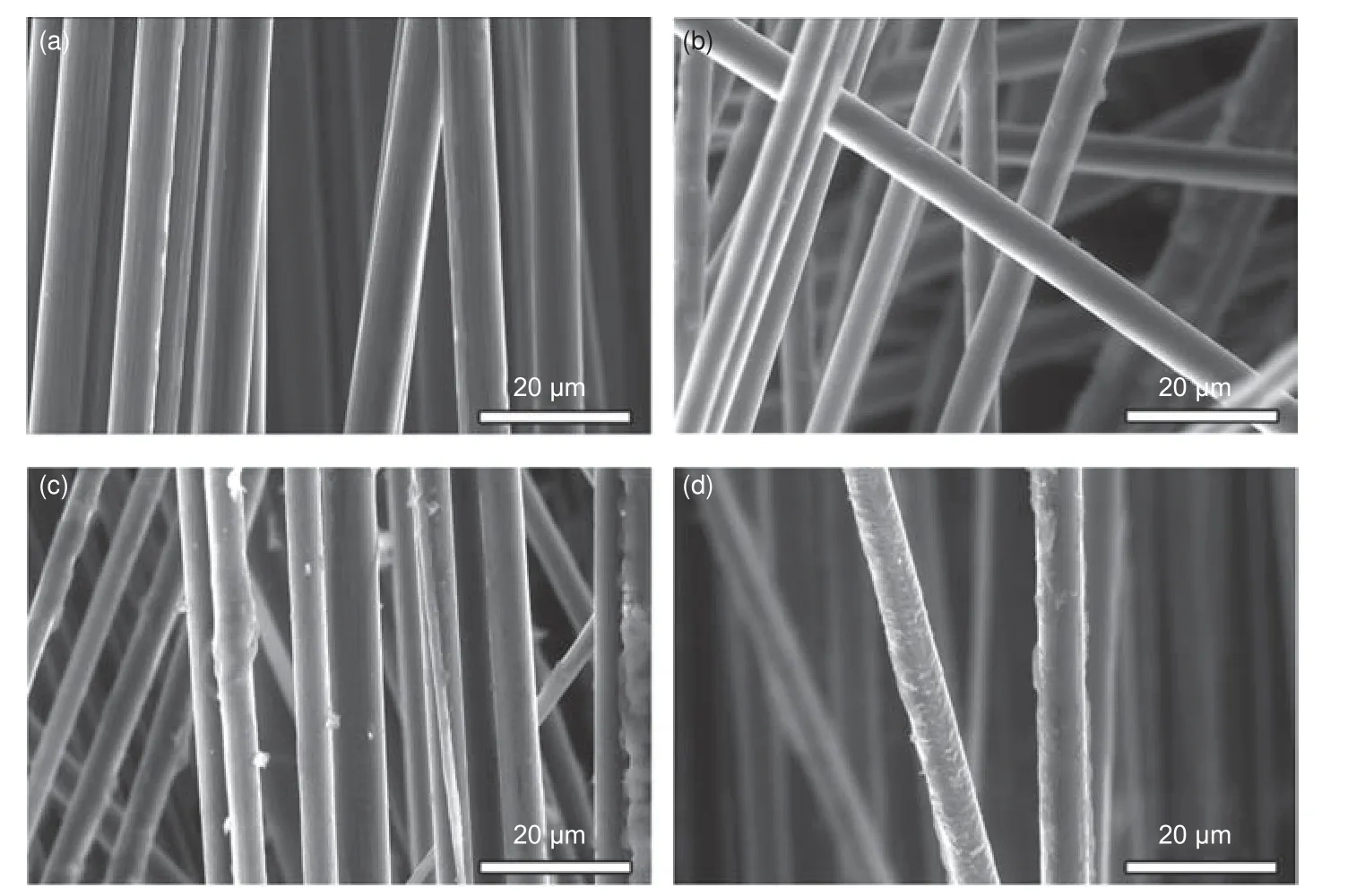
Fig.4 SEM images of (a) vCFs, (b) rCFs with a current of 4 mA, (c) rCFs with a current of 20 mA and (d) rCFs with a current of 25 mA[71].Reprinted with permission by Elsevier
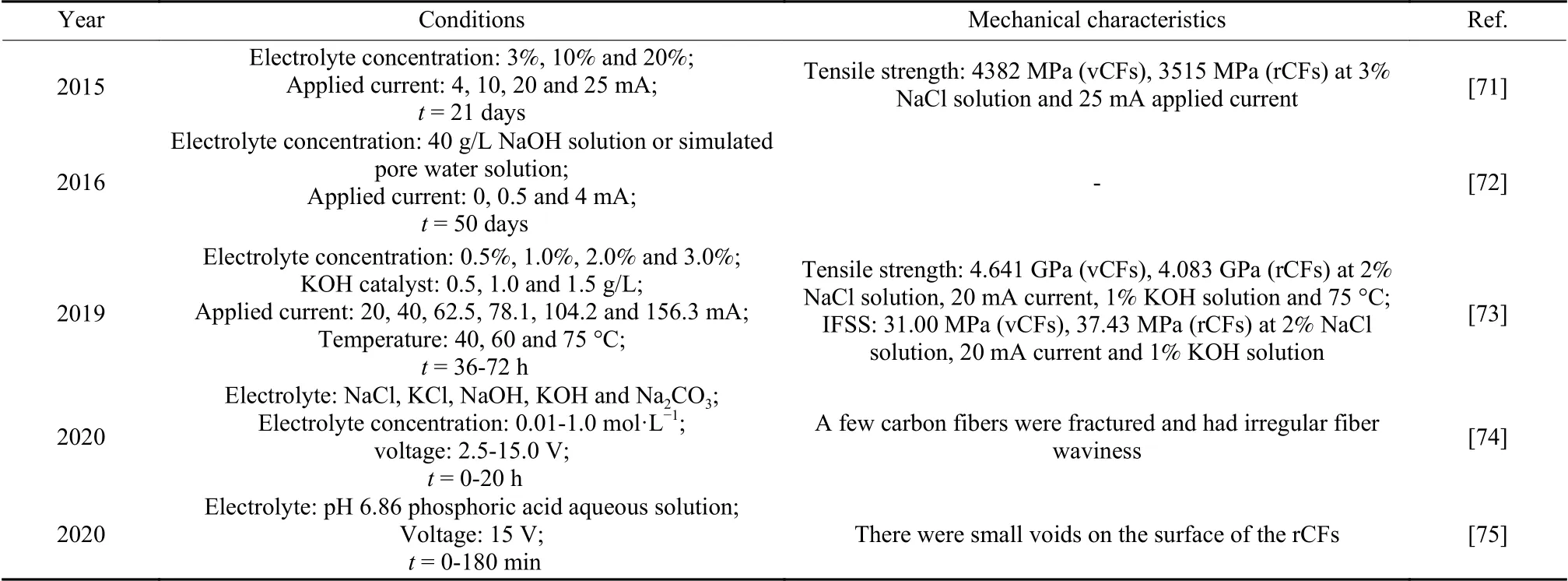
Table 7 Specific conditions of electrochemical recovery of CFRCs
2.2.4 Other methods
Hydrogenolysis with hydrogen donors (tetraline and 9,10-dihydroanthracene) had also successfully used to degrade epoxy resin cured by phthalic anhydride (PA)[76], as shown in Table 8.The yield of soluble products was greater than 99% after the reaction at 340 °C for 2 h.The secondary alcohol groups in epoxy resin were first dehydrated to form allyl bonds,which were prone to homolysis to form free radicals that could be saturated with hydrogen from a hydrogen donor.The rate-determining step was the homolysis of bonds.The tensile strength of rCFs reached 3 950 MPa, which was close to that of vCFs.Using a mixture of tetraline and ethanolamine, the crosslinked epoxy resin was able to dissolve at a lower temperature of 280 °C for 24 h, as a part of the resin was dissolved by aminolysis in the presence of the amine.Phosphotungstic acid (HPW) aqueous solution can be used to selectively break the ester bonds of epoxy resin[77].And the recovered oligomer contained muti-functional active groups (hydroxyl and carboxyl), which could replace up to 40% of the resin to prepare the new cured epoxy resin.The recovered heteropoly acid solution could be directly used in the degradation reaction and the degradation degree did not change significantly after 6 times of reuse.Microwave-assisted technology can enhance mass and heat transfer and speed up the degradation of resin.The pretreatment reduced the interaction between epoxy resin chains, resulting in a loose and porous structure that facilitated the diffusion of catalyst and solvent during degradation.X Zhao et al.first immersed anhydride-cured epoxy resin in dichloromethane (DCM) and then degraded the pretreated composite under catalysis of diethylenetriamine (DETA) with the assistance of microwave[78].The degradation de-gree of epoxy resin was 96% at 130 °C for 50 min.The degradation liquid contained oligomers and excess DETA, which could be used as curing agents for preparing new epoxy resin.
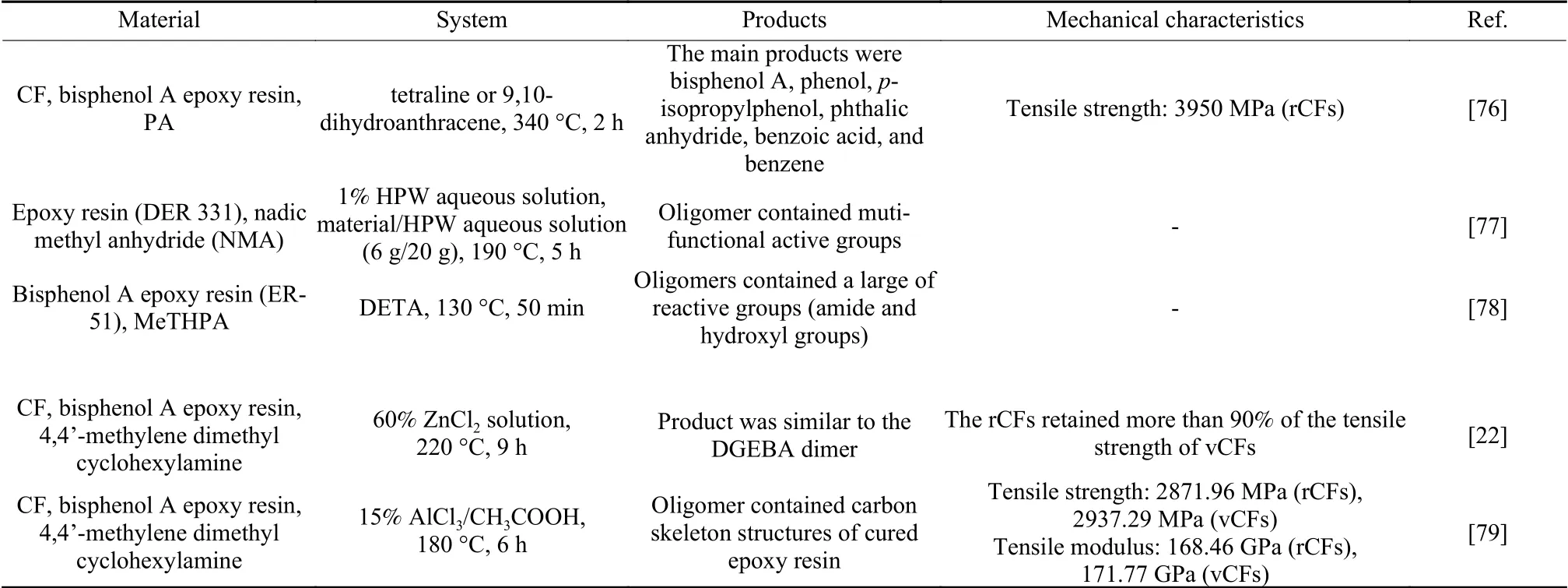
Table 8 Recycling methods and degradation products of CFRCs
Our previous work found that the concentrated ZnCl2aqueous solution can also break the secondary C―N bonds, which had an effect on the epoxy resin cured with aliphatic amine[22].Unsaturated coordinated Zn2+can degrade epoxy resin in CFRCs at a fast rate.This can be attributed to the presence of a large number of pores among the layers of the composites,which enlarged the contact area between epoxy resin and unsaturated coordinated Zn2+.The degradation percentage of epoxy resin was 97.3% under the condition of 9 h at 220 °C, 60% ZnCl2solution.The rCFs were clean, smooth, and retained more than 90% of the tensile strength of vCFs.In the untreated case, Zn-Cl2solution still showed high catalytic activity after six times of recycling.In addition, we also found that acetic acid can swell the cured epoxy resin, which facilitated the contact of AlCl3with resin, thereby selectively cleaving the C―N bonds[79].The resin was completely degraded at 180 °C.The surface of the rCFs was very clean, with only a few spots remaining for the epoxy resin, as shown in Fig.5.The tensile strength and tensile modulus of the rCFs retained 97.77% and 98.07% of the vCFs, respectively.
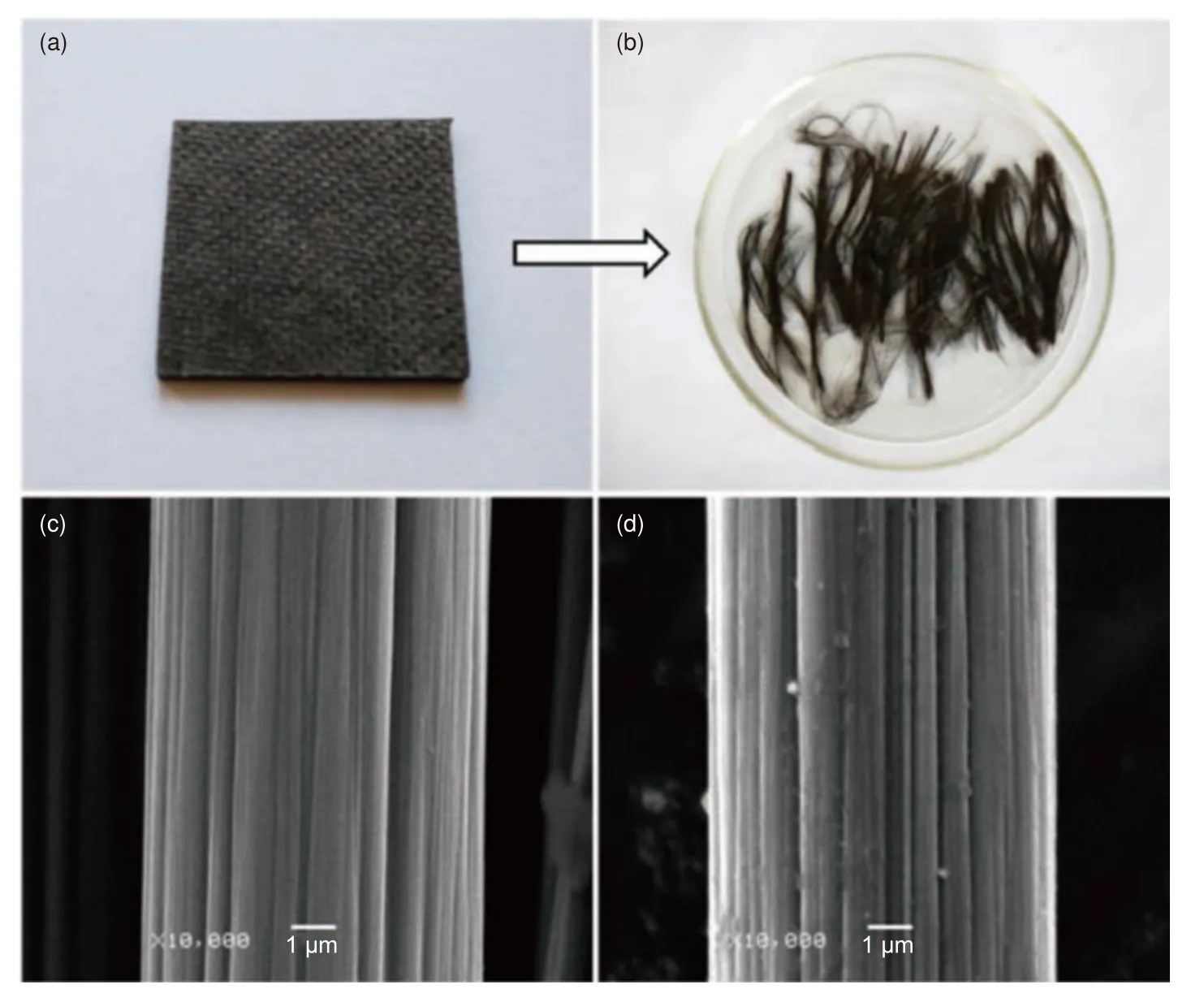
Fig.5 Photos of (a) CFRP waste and (b) rCFs.SEM images of (c) vCFs and (d) rCFs[79].Reprinted with permission by American Chemical Society
3 Utilization of recycled epoxy resins and preparation of recyclable epoxy resins
3.1 New uses for recycled epoxy resins
A new method to treat waste polymer was developed and a new idea was provided for the use of recycled epoxy resins in efficient oil-water separation materials[80].F Tian et al.transformed the waste epoxy resin into an oil/water separation material with adjustable pore sizes by a microwave assisted method.The prepared porous material has the advantages of high flux, high strength, and high separation efficiency, as well as excellent oil-water separation performance, which is suitable for the oil separation in micro-nano oil-water emulsions.Swollen epoxy resin(SEP) was obtained by swelling epoxy resin with NMP at 150 °C under microwave assistance for 30 min and then porous epoxy resin (PEP) was obtained by microwave heating.Adding dimethylacetamide (DMA) to NMP could change the polarity of the solvent and thus adjust the pore size.The criterion of good separation effect of PEP is that the oil droplet size of the emulsion is close to PEP.The separation efficiency was greater than 99.99%.In addition, this material could withstand considerable pressure in the process of oil-water separation.The separation flux and separation efficiency did not change significantly after PEP was used for 7 cycles.In 2020, the team prepared an oil absorption material by simple dipcoating the degradation products of epoxy resin(DEPs) on melamine foam (MF), as shown in Fig.6[81].The material (DEPs@MF) has excellent adsorption performance for various oils or organic solvents.Its fastest adsorption rate is 2 s and the oil absorption capacity is 116 g/g.Furthermore, the material can be recovered by simple mechanical extrusion, and it still has excellent adsorption capacity after repeated use for 10 times.
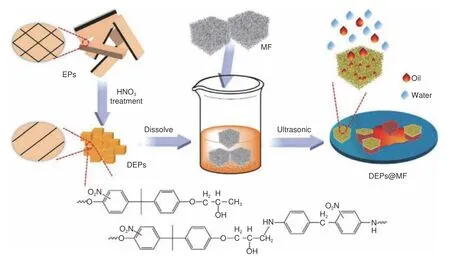
Fig.6 Schematic diagram of preparation of oil absorbing material[81].Reprinted with permission by Elsevier
3.2 Degradable and recyclable epoxy resin
In 2011, Leibler’s group proposed the concept of“vitrimer” based on dynamic bonds exchange reactions[23].Like traditional thermosetting polymers, vitrimer has excellent mechanical properties, chemical resistance, and dimensional stability.At a certain temperature, the reversible chemical bonds of the polymer exchange dynamically, allowing the polymer to be reprocessed and repaired.Preparation of recyclable polymers can solve the problem of recycling polymer materials and provide a closed-loop method for the application of thermosetting materials.A large number of degradable epoxy resins have been reported based on reversible covalent bonds, such as ester bonds, disulfide bonds, Schiff base bonds and acetal bonds.The degradation mechanisms of recyclable thermosetting materials and the recycling process of composites are introduced.
3.2.1 Ester bonds
Transesterification reactions between hydroxyl group and ester group is the most representative, as shown in Fig.7[24].Transesterification reactions can occur at high temperatures, allowing the vitrimer to be recycled and reshaped.K Yu et al.realized almost complete recovery of CFRCs using EG as a solvent[82].The hydroxyl group of EG and the ester group of the fatty acid linker underwent transesterification reactions, which successfully broke the long chain of polymer into short chain, and clean carbon fiber fabric was recovered.Under the conditions of 180 °C and 5% Zn(Ac)2catalyst, the epoxy resin was completely dissolved after the composite was immersed in 3 g EG for 160 min.Evaporating the EG solvent caused the repolymerization of dissolved polymer, and a new composite with basically the same thermo-mechanical properties and malleability as those of the original epoxy resin was obtained.According to the dynamic properties of the reactions, the CFRCs with surface damage can be completely repaired by heating, as shown in Fig.8.And the size and mechanical properties of the composite can be repaired.Recycled carbon fibers of the same size as the original fibers was not damaged, and the tensile modulus and tensile strength of the rCFs maintained 97% and 95% of the vCFs, respectively.Each generation of the recovered composites had the same elastic modulus (within the first 2% stretch) and ultimate strength.It was noteworthy that theTgof epoxy matrix was low (≈ 30 °C).In 2019, the team prepared vitrimer with adjustable mechanical properties using epoxy resin oligomers with different rigidities and curing agent (sebacic acid,SA)[83].Increasing temperature and adding the catalyst can significantly accelerate the dissolution of the vitrimer.After stirred at 170 °C for 6 h, the epoxy vitrimer can be dissolved in 0.21 mol·L−1Zn(Ac)2/2-EH solution by transesterification reaction to extract biolubricant.J H Chen et al.prepared epoxy vitrimer with adjustable properties by changing the ratio of two curing agents SA to 1,4-cyclohexanedicarboxulic acid(CHDA) in the presence of TBD catalyst[84].With the increase of the CHDA content, the stiffness andTg(36.2-50.3 °C) of vitrimer increase and the stress relaxation decreases.Using TBD as a transesterification catalyst, the team prepared a flame-retardant epoxy vitrimer with recyclability, malleability and shape memory using adipic acid and [(6-Oxido-6Hdibenz[c,e][1,2]oxaphosphorin-6-yl)methyl]butanedioic acid (DDP)[85].When the content of DDP increased to 30%, theTgand limiting oxygen index(LOI) increased to 110.7 °C and 34.5%, respectively.The high malleability of vitrimer allows it to be reprocessed in less than 15 min at 200 °C with no reduction in other properties.
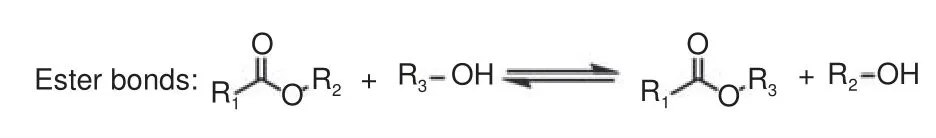
Fig.7 Transesterification reaction in vitrimer[24]
A vitrimer containing palm oil was obtained by curing palm oil-based epoxy resin with citric acid without using any catalyst[86].Materials can be reshaped at 150 °C or higher.For example, a heartshaped sample can be remolded into a spiral shape by heating it to 150 °C for 2 h.J Han et al.synthesized a catalyst-free epoxy vitrimer (Tg, 70-96 °C) by the reaction of hyperbranched epoxy (HBE) prepolymers with succinic anhydride[24].The curing process and dynamic transesterification reaction could be carried out without the addition of catalyst, which was attributed to the fact that HBE had a large number of free hydroxyl group as reaction sites to activate the reaction.The prepared materials exhibited stress relaxation above 120 °C, allowing the material to have characteristics such as self-healing and welding.The scratches on the surface of the film almost disappeared after 90 min treatment at 150 °C.In order to obtain sufficient molecular mobility at high temperatures, the existing vitrimer mostly adopts soft epoxy monomer or curing agent, which ultimately leads to the lowTgof material[87].The relatively lowTgof epoxy vitrimer may limit the application of the material.T Liu et al.prepared a vitrimer with highTg(187 °C) using triepoxy and anhydride curing agent[87].The properties of resin can be adjusted by changing the structure of the curing agent and resin matrix.Under the action of Zn(acac)2catalyst, dynamic transesterification reaction occurred by increasing the temperature.The samples with surface crack were hot-pressed at 220 °C, and the width of the crack decreased by more than 90% within 5 min.
3.2.2 Disulfide bonds
Disulfide bonds are dynamic and unstable, and it can undergo thiol-disulfide exchange and disulfide exchange reactions under certain conditions (Fig.9)[88].Epoxy thermosetting resins containing disulfide bonds are stable in harsh environments and offered mechanical durability.L M Johnson et al.prepared degradable epoxy resin containing disulfide bonds synthesized from DGEBF and 4-aminophenyl disulfide (4APDS)[89].The conventional epoxy resin prepared from DGEBF and 4,4’-methylenebiscyclohexanamine(PACM) was used as a reference.The study showed that the mechanical properties of epoxy resins with disulfide bonds were comparable to those without disulfide bonds.The mechanical properties of the sample exposed to mineral oil or retarded cement slurry at pH 12 for 2 weeks at 100 °C and 69 MPa were basically retained and these extreme conditions did not affect its ability to degrade in the presence of thiols.In general, when it was immersed in 2-mercaptoethanol (2-ME) for the same time, its weight gain and swelling were proportional to the content of 4APDS in the resin, which supported the thiol-based degradation mechanism that the fracture of the disulfide bonds created diffusion pathway for absorbing solvent.At 80 °C, the epoxy resin containing DGEBF and 100% 4APDS was degraded after immersed in 2-ME for 18 h and eventually split into fragments.A R de Luzuriaga et al.prepared CFRCs with reprocessability, reparability and recyclability[90].DGEBA was cured with a curing agent containing disulfide bonds(4APDS) and a conventional curing agent (diethyltoluenediamine, DETDA).It was found that the thermal and mechanical properties of the thermosetting epoxy resin and CFRCs with disulfide bonds were comparable with those of materials cured by DETDA.The prepared CFRCs could be easily modified and repaired by applying heat and pressure.The resin dissolved easily in the presence of 2-ME after magnetic agitation for 24 h at room temperature due to the thiol-disulfide exchange reaction.The recycled glass fibers or carbon fibers were not damaged.Q Zhou et al.synthesized an aromatic amine curing agent containing dynamic disulfide bonds and C―N bonds for curing epoxy resin, resulting in a degradable thermosetting resin with good solvent resistance,highTg(148.9 °C) and remoldability[91].The sample can be remolded by pressing it at 200 °C and 8 MPa for 2 h.After 3 remolding, the thermal stability of resin was almost unchanged.The degradation process of samples was divided into two steps (disulfide bonds reduction and C―N bonds fracture).The material was first degraded into fragments in a 2-ME/DMF (2∶8)solution, and then the fragments were degraded to small particles in a HCl/THF (2∶8) solution.The surface of rCFs was clean.A.Takahashi et al.reported that diamine containing disulfide bonds had little effect on the degradation efficiency, while the introduction of disulfide bonds into epoxy resin was beneficial to achieve effective degradation[92].Degradable epoxy resin with dynamic disulfide bonds was synthesized using bis(4-glycidyloxyphenyl)disulfide(BGPDS) and diamine (p-toluidine).The mechanical properties of the crosslinked epoxy resin prepared by BGPDS were comparable to those of traditional crosslinked epoxy resin without disulfide bonds.In the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU),the prepared sample was degraded to the soluble part.
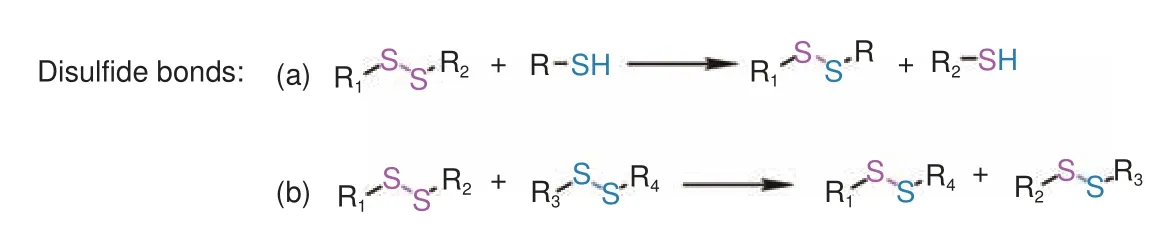
Fig.9 Dynamic disulfide bonds in vitrimer and their cleavage mechanisms[88,90]
3.2.3 Schiff base structure
The reversible Schiff base structure has attracted extensive attention.Schiff base realizes reversible covalent interaction through two different processes, including transimination and imine metathesis[93].P Taynton et al.prepared a recyclable CFRCs containing Schiff base and achieved closed loop recovery[94].The synthesis route of polyimines is shown in Fig.10.The type of diamine had an effect on the crosslinking density of polymer, which affected theTg, tensile strength and tensile modulus of thermosetting resin.Through a simple hot-pressing process, multiple layers of composites with perfectly bonded interfaces could be obtained.Completely cured composites could be reshaped into 3D curved shapes.The CFRCs material can be recovered by immersion in pure DETA, which was based on the transimination reaction between diamine and imine-linked network.The recycled fibers retained their original length and fabric structure.The layered damage could be well repaired by simple hot-pressing and the mechanical properties could be more than 100% restored.S Wang et al.prepared a high-performance and recyclable epoxy resin with a Schiff base structure in situ through the reaction of a monoepoxide (3-methoxy-4-(oxiran-2-ylmethoxy) benzaldehyde, MB) with a diamine curing agent (PACM)[95], as shown in Fig.11.MB-PACM had a highTgof 172 °C, a 5% weight loss(Td5%) of 323 °C, an elongation at break of 15%, and its tensile strength and tensile modulus were 81 MPa and 2 112 MPa, respectively.Compared with traditional thermoset resin, theTgand modulus of MBPACM were higher due to its highly π-conjugated Schiff base structure and its associated hydrogen bond.The tensile strength, tensile modulus and elongation at break of CF/MB-PACM were 763 MPa,35.3 GPa and 3.02%, respectively, which were close to CF/DGEBA/PACM.MB-PACM showed excellent malleability and reprocessing ability through imine exchange or imine metathesis of Schiff base.The resin in the composite was completely removed in 0.1 mol·L−1HCl solution (methanol/water = 8∶2,v/v)at room temperature for 15 h.The rCFs were clean and retained the fabric structure and mechanical properties of vCFs.However, the Schiff base structure with high polarity is sensitive to water.In order to achieve the high performance of epoxy resin and solve the stability problem of the Schiff base, X Xu et al.synthesized an epoxy thermosetting resin with excellent degradability and antibacterial properties by using aromatic diamine as curing agents (Fig.12)[96].Compared with the traditional DGEBA/DDM, the two thermosetting resins containing a Schiff base structure conjugated by two benzene rings had higherTg(about 206 °C), tensile strength (about 122 MPa) and tensile modulus (about 2 646 MPa), which is explained by their large cross-link density and rigidity.The epoxy resins containing the Schiff base structure had excellent resistance to acidic solution at room temperature and the degradation could be controlled by simply raising the temperature.In addition, the two epoxy resins were stable under neutral and alkaline conditions and possessed good hydrothermal stability.The two epoxy resins were stable below 330 °C and possessed excellent coating properties, making them high-performance antibacterial coatings.
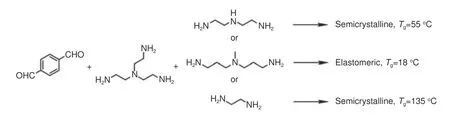
Fig.10 Synthetic route of polyimines[94]
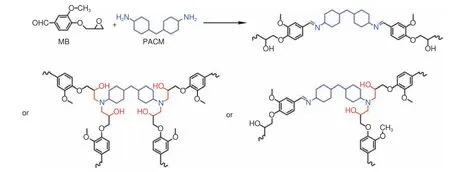
Fig.11 Synthetic route and 3 repeating units of MB-PACM[95]
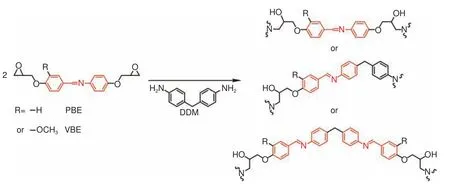
Fig.12 Synthetic route and 3 repeating units of PBE-DDM or VBE-DDM[96]
3.2.4 Acetal bonds
Acetals bonds are stable in neutral and basic conditions, but readily hydrolyzed in acidic conditions(Fig.13).T Hashimoto et al.prepared four types of epoxy resins containing acetal bonds and applied them to fabricate CFRCs[97,98].Bisphenol A (BA) or cresol novolak (CN) were used as resin skeletons, in which the phenolic hydroxy reacted with vinyl ethers containing the glycidyl group (4-vinlyoxybutyl glycidyl ether (VBGE) and cyclohexane dimethanol vinyl glycidyl ether (CHDMVG)) to form the epoxy resin containing an acetal structure and then cured with amine to obtain BA-VBGE, BA-CHDMVG, CN-VBGE and CN-CHDMVG.CN-CHDMVG epoxy resin had the highestTg(91 °C), because the rigidities of CN and cyclohexane dimethylene were greater than those of the bisphenol A and butylene, respectively.The thermal decomposition temperatures of these resins at 5% weight loss were all lower than those of traditional bisphenol A epoxy resins.In 2021, the team prepared composites containing acetal bonds through the addition reaction of 2,2-bis(4-hydroxycyclohexyl)propane (HBA) or 2,2-bis[4-(2-hydroxyethoxy)phenyl]propane (HOBA) with CHDMVG (Fig.14)[99].The thermal decomposition temperatures of HBA-CHDMVG and HOBA-CHDMVG were 283 and 319 °C, respectively.Based on the difference in thermal stability, it is concluded that the type of acetal linkages may affect the thermal stability, and thermal decomposition temperatures increase as follows: hemiacetal ester < phenol derivative < alcohol derivative.The composites containing acetal bonds were degraded completely with 0.1 mol·L−1HCl in a mixture of THF and water with a volume ratio of 9∶1 for 24 h.
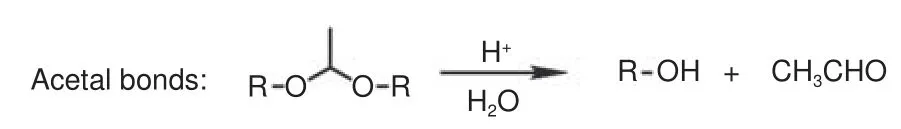
Fig.13 Hydrolysis of acetal bonds[95]
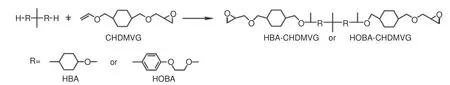
Fig.14 Synthetic route of HBA-CHDMVG or HOBA-CHDMVG[99]
4 Conclusions and outlook
Thermosetting polymers are used as matrices for composites due to their excellent thermal and chemical stabilities.Recycling these composites is very difficult because they form permanent cross-linked networks through irreversible chemical reactions.This review summarizes the various recycling methods for carbon fiber-reinforced composites and briefly point out the advantages and disadvantages of each recycling methods.The details of the recovery methods and their rationale are described, especially the reaction conditions and the mechanical properties of rCFs.In addition, the synthesis and depolymerization mechanism of thermosetting resins with reversible dynamic chemical bonds are also described.
Chemical recycling, which can transform waste composites into high value-added substances, is considered as a more promising method to achieve waste recycling and utilization.The chemical recovery process under super- and subcritical condition has high energy consumption and the degradation products of epoxy resin are complex.Catalytic recovery under mild conditions is seen as a future solution to recover waste CFRCs.Adding suitable catalyst or using mixed solvent in the system is beneficial to the reaction.The reaction can be carried out at low temperatures and pressures, and the chemical bond can be selectively broken to obtain high value-added chemical products.Clean carbon fibers can also be recycled.In addition,pretreatment and the microwave-assisted method can improve the degradation efficiency of resins.At present, the recycling and utilization of composite materials has not been industrialized, and it still faces challenges.It must be realized that solvent recovery and utilization in the reaction is more troublesome.Most of the rCFs are discontinuous short fibers, which limits the scope of subsequent use.A small amount of resin remains on the surface of the rCFs, and the mechanical properties are reduced, although efforts have been made to completely maintain the mechanical properties of the vCFs.Currently, it is difficult to use composites made from the rCFs for structure applications.In the future, if the rCFs can retain the length and mechanical properties of the vCFs, it may greatly improve the value of the rCFs.If the recycled degradation products can completely replace the raw material for the preparation of composites, and the mechanical properties of the composites can be comparable to the composites prepared from the raw material, it will be practical to realize the waste recovery.Further development of methods for recycling composites is needed to produce reusable products and carbon fibers comparable to the vCFs in a milder and more environmentally friendly way.
In recent years, the insertion of reversible chemical bonds into resins has opened opportunities for thermoset recycling, allowing composites to be reprocessed, reshaped, and recycled.The excellent performance of composites should be combined with their recyclability.Dynamic exchange reactions require the presence of some active groups, so the vitrimer is not fully cured, which affects the crosslinking density of cured polymers.Therefore, theTgof the material is low, which limits the application of the composite and only has obvious advantages in recycling.The preparation of recyclable epoxy composites requires further research to produce materials whose mechanical properties can be close to or even better than those of conventional composites.In addition,due to the shortage of petroleum resources and the enhancement of environmental protection awareness, the development of recyclable epoxy resin and curing agents using biomass resources may attract more and more attention.
Acknowledgements
This work was supported financially by the Youth Innovation Promotion Association CAS(2021173) and the National Natural Science Foundation of China (51703237) .
杂志排行
新型炭材料的其它文章
- Coal-based graphene as a promoter of TiO2 catalytic activity for the photocatalytic degradation of organic dyes
- 咖啡渣成型制备生物质炭及其CH4/N2分离性能
- A flexible hard carbon microsphere/MXene film as a high-performance anode for sodium-ion storage
- Preparation and lithium storage of anthracite-based graphite anode materials
- Oxygen-incorporated carbon nitride porous nanosheets for highly efficient photoelectrocatalytic CO2 reduction to formate
- 炭纸衬底上化学气相沉积直立型二维过渡金属硫化物及其电催化产氢性能
