单相、双相及多相离子掺杂的羟基磷灰石研究进展
2022-11-08刘莹杨瑞刘井来刘佳欣张靖羚
刘莹,杨瑞,刘井来,刘佳欣,张靖羚
单相、双相及多相离子掺杂的羟基磷灰石研究进展
刘莹1a,杨瑞1b,刘井来2,刘佳欣1b,张靖羚1b
(1.辽宁工程技术大学 a.理学院 b.材料科学与工程学院,辽宁 阜新 123000;2.甘李药业股份有限公司,北京 101102)
羟基磷灰石是天然骨组织成分中的重要组成部分,一直是生物医学领域关注和研究的重点。天然骨成分中的磷灰石,是一种结合着多种离子的羟基磷灰石,维持着生命系统的正常生长与发育。本研究阐述了羟基磷灰石的晶体结构;概述了研究者针对羟基磷灰石性能探究,其中包括生物相容性、生物活性、适宜的机械强度、优异的成骨性能和耐腐蚀性能。同时归纳了离子掺杂羟基磷灰石取代位点。在此基础上,重点阐述了近五年来单相、双相以及多相掺杂羟基磷灰石材料的研究,其中包括单相、双相以及多相离子掺杂对羟基磷灰石在结构性能、机械强度、抗菌性、降解性和成骨性等性能的影响,同时简单总结了离子掺杂对机械强度的影响规律。近年来虽然羟基磷灰石生物陶瓷材料在临床上作为植入物涂层、缓释药物的载体、骨移植物代替材料等被应用,但在临床方面广泛运用还面临着许多问题与挑战,所以本文同时展望了离子掺杂羟基磷灰石未来的的发展方向,有望在临床应用及发展方面有一定的指导意义。
羟基磷灰石;离子掺杂;抗菌性;机械强度;植入物涂层;治疗癌症
羟基磷灰石(HA,Ca10(PO4)6(OH)2)是一种与哺乳动物硬组织组成非常相似的合成生物陶瓷材料,因此被广泛应用于骨移植、骨修复、硬组织工程支架和金属基材的涂层等方面[1-2]。HA中钙磷比为1.67,晶型常态属于六方晶系,稳定相为单斜晶相(一般高温热处理后),L6PC对称型和63/空间群。晶胞参数为==0.943~0.938 nm,=0.688~0.686 nm,==90°,=120°,每个晶胞中包含10个Ca2+、6个PO43‒和2个OH‒,图1为HA晶体结构示意图。
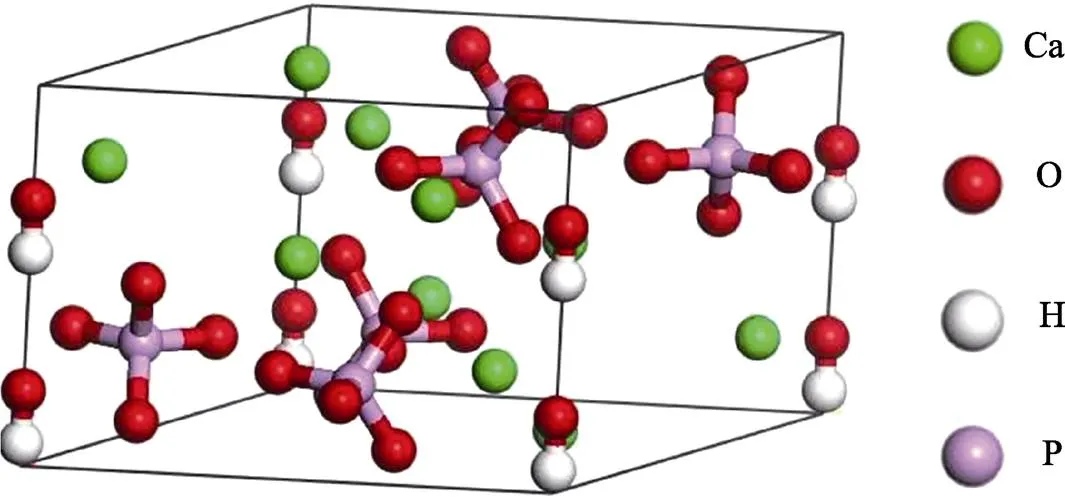
图1 HA晶体结构示意图
早在1951年,RD Ray等合成了适用于骨缺损治疗领域的HA;直至1980年,de K Groot等相继合成了HA,并应用于整形外科[3]。人工合成的HA具有良好的生物活性、生物相容性、适宜的机械强度、成骨性和耐腐蚀性等[4]。王艳玲等[5]通过微弧氧化法在金属钛无牙区种植体表面制备了HA涂层,发现HA可以促进成骨细胞的黏附和外周骨质的生成,从而诱导骨的形成。T. M等[6]通过射频磁控溅射法在AZ91镁合金表面制备了HA涂层,研究发现涂层可以控制降解速率,提高AZ91合金的生物相容性和生物矿化能力。杨蕾等[7]利用微弧氧化技术(MAO)在AZ31镁合金表面制备出含有纳米级别的n-HA涂层,该涂层能对多孔形态进行填充,体现优异的耐腐蚀效果,在体外细胞培养实验中,细胞粘附和细胞诱导效果明显强于无涂层的样品,能够促进成骨细胞的增殖分化。Jiang等[8]通过水热法在钛合金表面覆盖了一层HA涂层,研究发现涂层样品耐蚀性优于未涂层样品,因此植入材料具有良好的生物相容性和骨诱导性。但是HA涂层的脆性和不易降解性限制了其应用[9-10]。
研究发现,生物体内的磷灰石是一种晶体结构不完善的HA,其中结合一些很多微量元素,例如Na+、Mg2+、Zn2+、Cl‒、CO32‒、F‒等离子[11-12],因此研究者们在人工合成HA时会有选择性的掺入一些离子,通过离子半径的变化改变晶格参数,从而在模拟生物矿化的基础上改进HA的各项性能。
离子掺杂可以分为阴离子掺杂与阳离子掺杂。阳离子掺杂是指HA中的Ca2+可被某些二价的阳离子(如Mg2+[13-15]、Zn2+[16-19]、Sr2+[20-23]、Fe2+[24-27]、Cu2+[28-32]、Ni2+[33-34]等)部分或全部取代,也可被单价和三价的阳离子(如Na+[35-37]、K+[35,38]、Fe3+ [24-25,39]、Eu3+[40-44]等)部分取代。阴离子掺杂可分为A和B两种类型的取代,A是在羟基位点的取代,一般为OH‒被Cl‒、F‒[45-46]、Br‒等离子的取代;而B则是PO43‒被CO32‒[24,44-45]、VO43‒[46-47]和SiO44‒[48]等部分或全部取代,此取代方式为磷酸根位点的取代,当然A、B同时取代也是存在的。研究者们通过单相掺杂综合分析,对双相及多相离子掺杂的HA进行了多方向多角度的设想、制备与研究。
本文选材多为离子掺杂HA作为涂层材料改善金属植入物性能的研究,以更好地满足骨移植和骨修复领域的临床需求。合金材料表面附着的离子掺杂HA涂层材料,为合金材料提供机械强度和可降解性能,而离子掺杂在通过改变HA涂层结构和调控涂层与合金之间的关系来增强植入物的生物活性、生物相容性和抗菌性等生物性能。
1 单相离子掺杂羟基磷灰石的制备与发现
目前,关于单相离子掺杂已经形成系统的研究,下面简单对几种常见且代表性较强的单相离子掺杂HA的案例进行叙述。
M等[45]通过低温共沉淀法和水热辅助溶胶凝胶法相结合分别制备了HApMn、HApFe、HApCo、HApNi、HApCu、HApZn和纯HAp,发现HAp-Mn、HAp-Fe和HAp-Ni与CaHAp相的PDF文件(00- 009-0432)一致,当Mn4+、Fe2+加入与CaHAp相对应的主峰强度降低,而当Co+、Cu2+和Zn2+加入时主峰相发生偏移至2=26.04°,说明离子的加入在一定程度会降低羟基磷灰石的结晶度。研究表明离子的加入一般会降低HA的结晶度,但均无其他杂相存在(见图2)。Mg2+掺杂HA可以促进骨和血管形成[46-47],对铜绿假单胞菌、金黄色葡萄球菌和白色念珠菌有较好的的抗菌功效,但Mg-HA在形成涂层的过程中存在着稳定性较差的弱点[48]。Fe2+掺杂会使HA纳米粒子具有超顺磁性,通过外加磁场力的作用可以促进降解和推动矿化过程,从而促进细胞的增殖与 分化[49-52]。

图2 CaHA、HAMn、HAFe、HACo、HANi、HACu和HAZn的X射线衍射图谱[45]
Balak等[53]通过水热法制备纯HA和Fe-HA(Fe2+为变量),发现Fe2+的加入改善了HA的晶粒尺寸,最小的尺寸可达19.7 nm,显现为球形结构(见图3)。磁性测试发现Fe-HA饱和磁化强度最强为0.029 emu/g;但是微量Fe的加入可能会产生轻微的溶血现象,以及研究中只体现了硬度的改善,不足以体现Fe2+的加入提高了HA的力学性能。
Yazici等[54]采用MAO技术在可降解的Mg-Sr-Ca合金表面制备了Ag-HA涂层。该涂层具有良好的生物活性和耐腐蚀性能,Ag+的增加明显增强了HA涂层的抑菌性,但抗菌实验中抗菌率达到100%时,Ag+的浓度已经超过其在生物体中的极限浓度,会产生严重的细胞毒性。而且在体液浸泡实验中,随着Ag+浓度的增加,表面沉积的Sr-HA与Ag-HA之间可能存在应力作用,使涂层表面出现了明显的裂纹。
Taolei等[55]采用水热法在ZK60镁合金表面制备了Sr-HA纳米棒/纳米线涂层。ZK60镁合金为植入物提供了良好的可生物降解性,Sr-HA涂层具有较好的耐腐蚀性能,Sr2+的存在提高了HA涂层的成骨性和生物相容性。但是水热法合成的HA与镁合金之间是否存在应力缺陷还值得考究。
Sergi等[56]采用前驱体等离子喷涂法制备了锌掺杂羟基磷灰石涂层(Zn-HA),发现Zn-HA涂层对人成骨细胞Saos-2细胞无细胞毒性,对金黄色葡萄球菌()和大肠杆菌()均表现出较强的抗菌作用(见图4)。
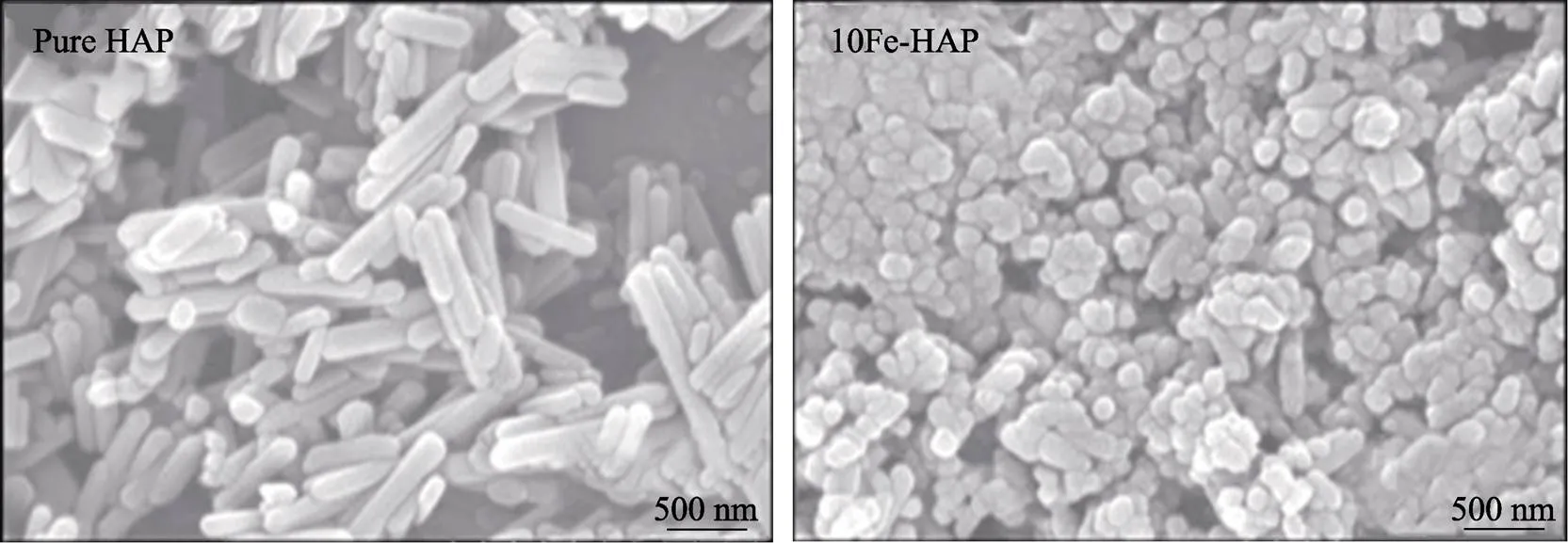
图3 水热合成的HA与10FeHA的形貌[53]
Zhou等[57]采用水热法在ZK60镁合金表面制备了Zn-HA涂层。研究发现锌的加入改变了镀层的形貌,使其表面出现了纳米晶须结构,并发现此植入物具有良好的成骨分化能力、抑菌能力和耐腐蚀特性。但是Zn2+的掺入量明显影响HA的稳定性,过量会有α-磷酸三钙相存在,这个结果可能与温度有关[58-59]。
Benjamín等[60]采用水热法制备了Si-HA。研究发现Si-HA的结晶度较高,呈现为35~80 nm的棒状结构,当SiO44‒达到一定浓度时,高温烧结后的HA颗粒之间会出现SiO2的小玻璃区域。但Si的实际掺入量仅为目标掺入量的60%左右,并且没有表明小玻璃区域出现对涂层性能的影响。
Maryam等[61]采用热物理混合烧结法制备了SiO2-HA,发现有SiO44‒掺入到HA中,得到的结论是适当的Si掺杂量会提高HA的生物可降解能力和矿化能力,原因是制备过程中有溶解度高于HA的磷酸三钙(TCP)和硅酸钙(Ca2SiO4)相的产生,使得到的类羟基磷灰石材料致密性增强。但物理混合法可能会导致材料部分位置存在应力缺陷,且在烧结过程中温度变化对SiO2-HA产生的Si-HA的分解程度不明确。还有研究发现Si-HA涂层具有较好的生物活 性[62],它可以增强成骨细胞活性,同时降低破骨细胞活性,说明Si-HA能减缓破骨细胞分化和骨重塑速率,这对骨质疏松症有着十分重要的意义[63]。
Nasker等[64]采用水热法制备了不同浓度氟掺杂n-HA(F-HA)和纯n-HA,合成的纳米颗粒形貌大多呈均匀规则的纳米棒状,虽然有氟化钙杂相出现,但是F‒的存在显著的改善了n-HA的结晶度与结构稳定性,还发现F-HA有一定的杀菌效果并且粉末无细胞毒性(见图5)。而且F‒掺杂n-HA在一定程度上可增加成骨细胞活性,促进骨细胞生长、增殖与分化,从而起到诱导骨形成的作用[65-66]。
碳酸根掺杂HA(CHA),CO32‒可以取代OH‒或PO43‒分别进行A或B取代,也可以同时进行AB取代,研究发现人工合成的CHA一般以B取代为主[67-68]。CO32‒取代可以降低HA的结晶度和热稳定性,提高HA的溶解性[69],CO32‒掺杂羟基磷灰石块体和涂层样品在模拟生理溶液中均可形成类骨碳酸羟基磷灰石矿化层[70]。
上述单相离子掺杂HA可以在一定程度上修饰HA的性能,但还不能满足临床应用的需求,笔者认为尚有下述问题需要解决:(1)在产物为纯相的前提下,结晶度和晶粒尺寸是否均可被改善;(2)抗菌性作用是否可以同时高效针对多种细菌;(3)生物可降解性是否可以调控;(4)离子掺杂是否可以有效改善HA的脆性。
这些问题可以在单相离子掺杂HA的基础上,通过调整制备工艺、反应物浓度和反应条件等来探究,与此同时为得到优良的综合性能,尝试复合离子掺杂HA也已经成为了研究的热点。
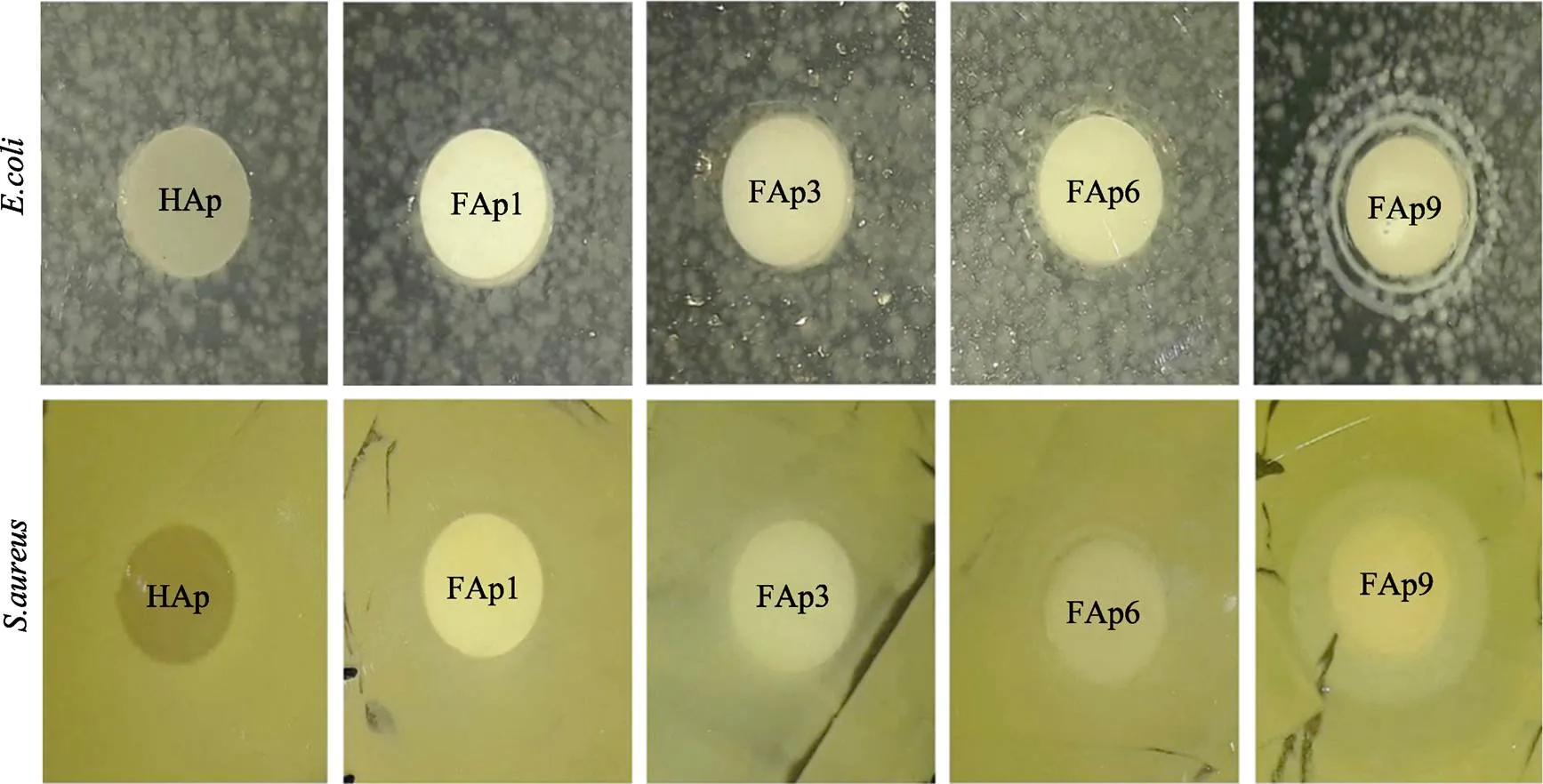
图5 48 h内各个样品在E.coli和S.aureus两种菌株存在环境中的培养情况[64]
Fig.5 The culture situation of each sample in the presence of E.coli and S.aureus within 48 h[64]
2 双相离子掺杂羟基磷灰石的发展与应用
基于现代生物材料的理念,对于毒性、安全性、机械强度、降解性、成骨性、可塑性等性能的把控,是满足机体不同部位骨修复与骨移植的必要条件。双相离子掺杂HA通过制备技术优化和应用效果改善,来适应天然骨性能和临床的要求。
Cao等[71]通过脉冲激光沉积技术在金属钛植入物表面制备Mg-F-HA涂层。模拟体液浸泡7 d后涂层内部结构为类似于松质骨的有序线性结构,浸泡14 d后,涂层完全被新形成的磷灰石层覆盖,表面光滑,干燥过程中应力释放出现少量裂痕,Mg-F-HA涂层可以长期保持良好的生物相容性与生物活性,可以在一定程度上调节生物降解速率和促进细胞增殖,但涂层裂痕会使涂层与基体之间存在较大程度的应力隐患(见图6)。
Veerla等[72]采用共沉淀法制备了Ag-Fe-HA纳米颗粒,研究发现控制浓度变化会使样品形貌显现为球形、棒状和片状,Ag-Fe-HA样品具有超顺磁性,将纳米颗粒分别在普通细胞(HEK-293)培养皿和癌细胞(HeLa)培养皿上进行培养(见图7),发现Ag-Fe-HA的存在几乎对细胞没有影响,而癌细胞长度明显变长,在50Ag50Fe-HA的存在下HeLa细胞的细胞膜似乎被破坏,细胞发生了剧烈变化如图8b4,表明Ag-Fe-HA纳米颗粒能够有效靶向杀伤癌细胞,且对正常细胞几乎无毒副作用。但是此研究中银离子与亚铁离子的实验组尚未确定Ag+和Fe2+的最佳组合浓度,可以在后续研究中对其进行深入。
Seyed等[73]采用溶胶凝胶法制备了一种原位硅包覆硅酸根与碳酸根掺杂的HA纳米粉体(Si-S- C-HA)。通过表征发现非晶态SiO2包覆层的存在促进HA在生理盐水中离子的释放,使S-C-HA纳米颗粒悬浮在溶液中且带负电荷,说明非晶态SiO2层可以加速S-C-HA的生物矿化与改善细胞附着,但是正是由于表面带负电荷会吸引样品中的阳离子,这样会不会影响样品中HA结构和性能的变化有待研究。
Abbas等[74]采用机械化学合成方法制备了HA- Cl-F,随着阴离子的双重取代OH‒,样品的晶粒尺寸、结晶度和单位细胞体积呈非线性增大,晶格应变逐渐减小,由于粉末的比表面积大和范德华力相互作用,研磨后的样品具有较高的团聚倾向,纳米粒子呈现为多边形和球形组成的簇状结构,而在高温煅烧后颗粒逐渐呈等轴状,在退火过程中,由于残余弹性应变的大幅度减小,导致晶粒长大;惊奇的是TEM观察得到的晶粒尺寸范围类似于天然骨的20~40 nm范围[75](见图8)。而且发现样品在碱性、酸性和中性条件下zeta电位均为负,负zeta电位的存在在一定程度上有利于骨整合、磷灰石成核和骨再生。

图6 SBF溶液浸泡7、14 d后Mg-F-HA涂层微观结构[71]

图7 HEK-293、HeLa与纳米颗粒培养24 h的高倍(100倍)相位差显微镜图像(a1、b1表示细胞本身,a2、b2表示HA,a3、b3表示25Ag25Fe-HA,a4、b4表示50Ag50Fe-HA)[72]
Yong等[76]通过电沉积与阳极氧化在利用二氧化钛纳米管(TN)改性的金属Ti表面上沉积了一种新型的掺银羟基磷灰石/硅酸钙(AgHA/CS)层,此涂层实际上存在的离子交换形式是Ag+和SiO32‒与HA中离子进行替换,即是另一种意义上的Ag-Si-HA涂层,AgHA/CS涂层表面形貌呈现多孔网状结构,具有这样的网络结构膜适合于关节和牙齿的修复[77](见图9),与纯HA涂层相比,该涂层具有优异的耐腐蚀性,在对S.aureus的抑制实验中体现出较强的抗菌作用,除此之外,Ag-Si-HA涂层还有利于成骨细胞的附着、增殖与分化。但这样的结构是否存在较大程度的应力缺陷未能体现。电沉积的HA层是均匀的,形态显现为花簇或针状晶体,表面形貌的变化可能是由于Ag+的取代CS(壳聚糖)在HA中的结合。尽管复合涂层中的多孔结构可以降低其机械性能,但它通常有利于成骨细胞向陶瓷复合物中的生长,从而增加骨整合性,这说明在多孔网状结构存在的情况下材料依然可以保持良好的机械性能。这是否说明复合涂层与基体之间存在着某种界面效应影响了涂层与基体的性能?
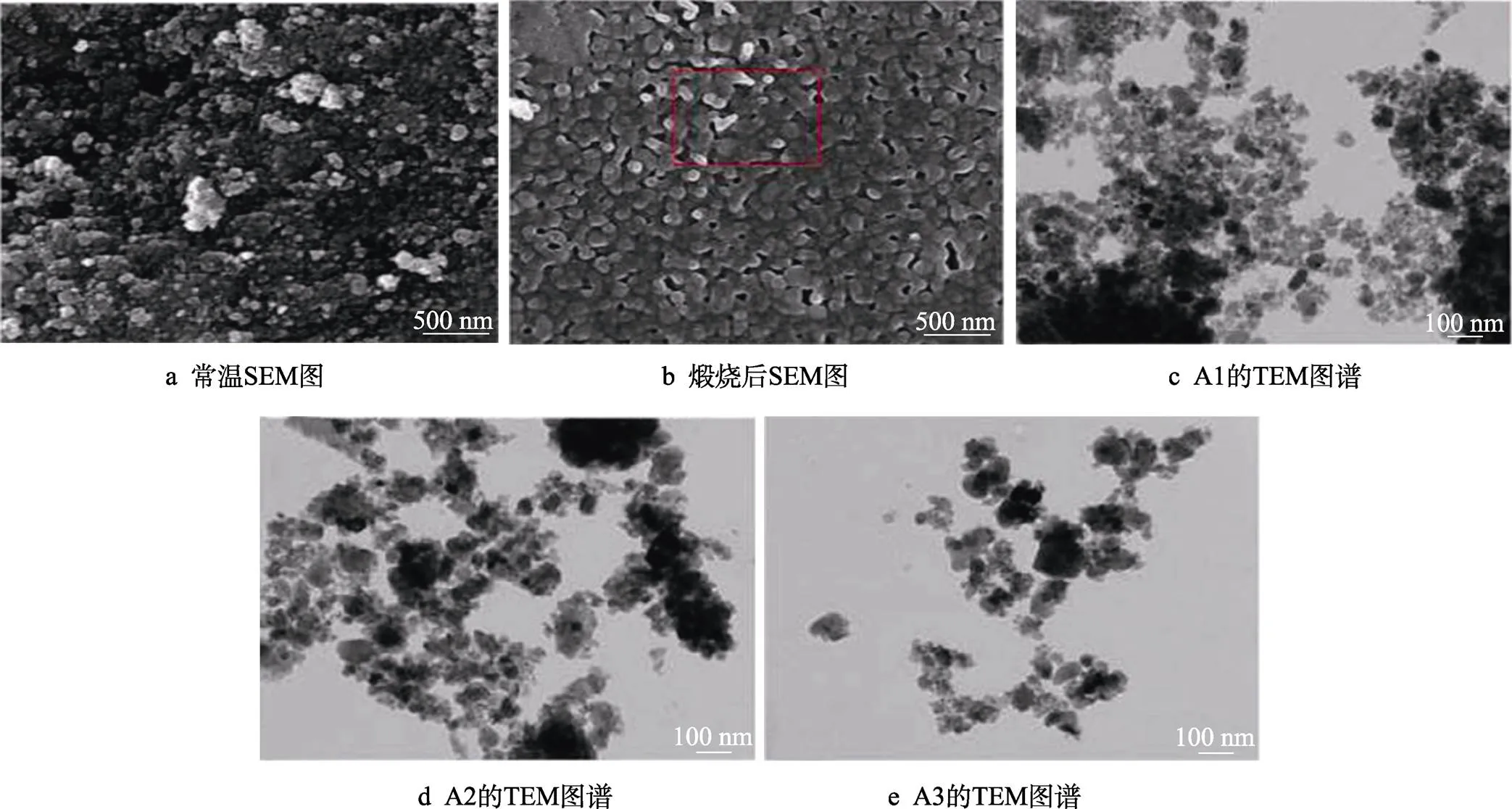
图8 机械研磨60 min的样品常温和煅烧后的形貌SEM图与样品A1、A2和A3的TEM图谱[74]
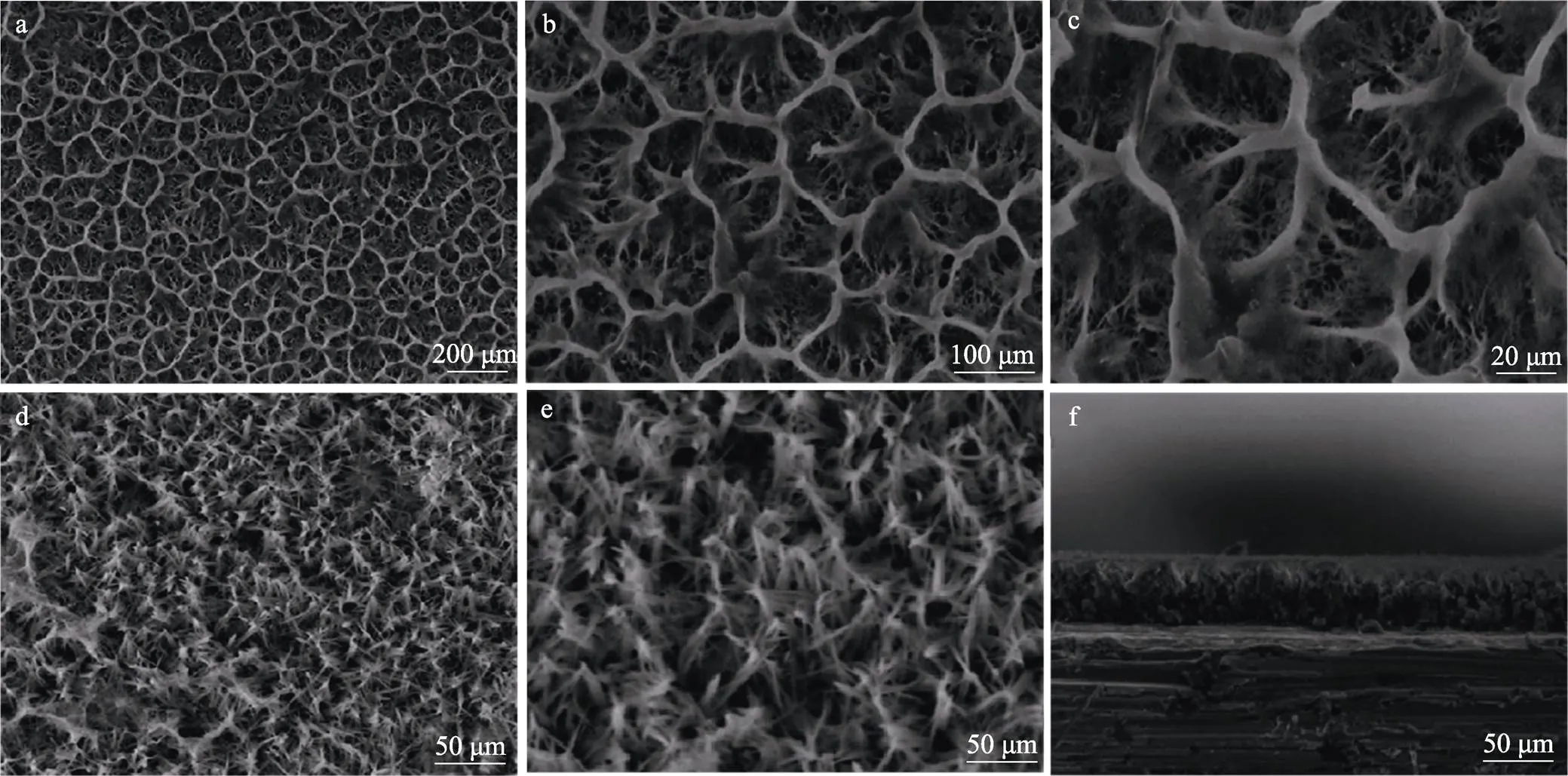
图9 AgHA/CS涂层表面形貌(a—c),HA涂层表面(d,e),AgHA/CS涂层厚度的横截面图像(f)[76]
M. K等[78]通过脉冲激光沉积法在氧化铝表面制备了金和碳酸根掺杂的羟基磷灰石(Au-C-HA)涂层。研究发现,HA粒子呈现为250~450 nm的球状结构。随着Au+的增加,剪切模量有明显变化的趋势(先变大后减小),弹性模量逐渐变小但变动很小,而显微硬度会变大,变化范围为31~34 Gpa,说明Au+的加入可以在一定程度上改善HA涂层的力学性能(见表1)。在对HFB4细胞系培养的实验中发现,Au+的加入增强了支架表面的细胞粘附性,使细胞不仅在涂层的表面扩散与生长,同时在涂层的孔隙结构中也不断地进行着增殖和分化。
Wei等[79]研究了化学沉淀法制备的Se-Sr-HA结构性能和生物性能,发现SeO32‒和Sr2+的共同加入得到了纯羟基磷灰石相、降低了Se-Sr-HA的结晶度、减小了晶粒尺寸,Sr2+的加入影响了样品的热稳定性;细胞培养实验显示,Se-Sr-HA对小鼠骨髓间充质干细胞(mBMSCs)无毒副作用,而其对人成骨肉瘤细胞(MG63)有明显的抑制作用,并发现Sr2+对MG63无影响,真正抑制它的是SeO32‒,而Sr2+却可以促进SeO32‒的释放,间接的对MG63起到了双重抑制作用,所以Se-Sr-HA有望成为骨癌患者的治疗及修复的双功能材料。Sr2+是否对其他离子的释放均有促进作用,这将是后续研究中较为重要的方向。
表1 各个涂层的剪切模量()、体积模量()、杨氏模量()和显微硬度()的变化[78]
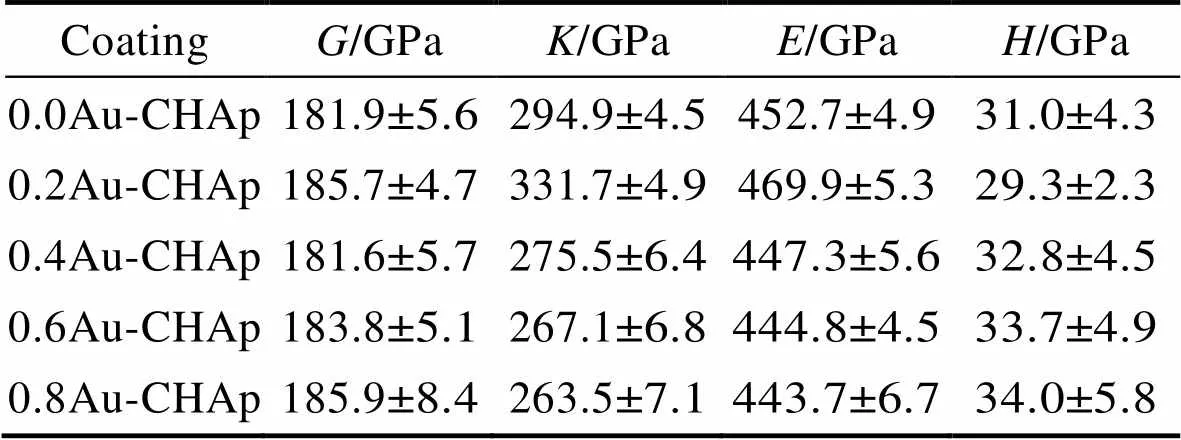
Tab.1 Changes in shear modulus (G), bulk modulus (K), Young's modulus (E) and microhardness (H) of each coating[78]
综上所述,双相离子掺杂的羟基磷灰石(X-Y- HA,X和Y表示未知离子)可以在多个角度影响HA性能,X-Y-HA根据临床的实际需要可以应用到植入物表面涂层、药物缓释、骨修复、牙齿美容和癌症治疗等方面,虽然与天然骨材料仍然有一定程度的差距,但双相离子掺杂在医学和科研领域必定会有深远的应用前景和发展前景。
3 多相离子掺杂羟基磷灰石的研究与展望
单相和双相离子在结构性能、力学性能、抗菌性能和成骨性能等多个方面体现了离子掺杂对HA影响,然而多相离子掺杂可以站在仿生的角度改性HA,多个离子同时存在的HA拥有和天然骨磷灰石更加接近的结构与性能,将可能是未来骨修复领域研究与应用的重点。在双相离子掺杂HA中Sr-F-HA在一定条件下可以促进成骨细胞的生长、增殖和分化,那么在其基础上多一相或者(≥2)相是否依然存在这样的性能甚至更多的影响呢?
Gao等[80]通过水热法合成了Sr2+、F‒和SiO44‒共掺杂的羟基磷灰石(Sr-F-Si-HA)。研究发现制备产物为纯HA且结晶度优于HA,在对MG63细胞进行培养过程中,发现其促进MG63的粘附和增殖的能力强于HA,说明Sr2+、F‒和SiO44‒的加入确实增加了HA的骨传导性。然而前面说过SiO44‒在单独掺杂羟基磷灰石时还会增加HA的抗断裂韧性与弯曲强度,今后可以对Sr-F-Si-HA的机械性能进行进一步的研究。
Monika等[81]使用脉冲电流在钛合金材料(Ti6Al4V)表面沉积制备了Ag-Zn-Mg-Sr-HA(m-HA)涂层。涂层显现为和天然骨的结构相似的多孔海绵状结构(见图10),经计算产物中Ca/P为1.55,说明HA晶体结构被破坏或出现CaP其他杂质相,通过将成骨MG-63细胞接种到样品表面的实验发现,细胞活力值明显高于裸植入物(Ti6Al4V)和HA涂层的钛合金材料,且活细胞在涂层表面致密性与黏附度良好,形态正常且均匀,说明m-HA涂层可以促进成骨细胞的生长发育。
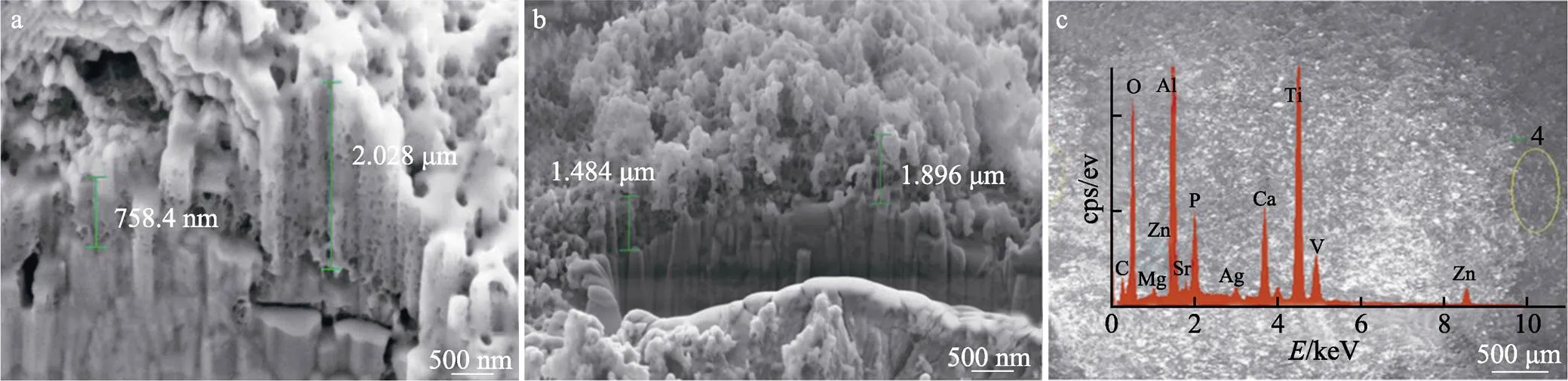
图10 m-HA涂层的不同位置的横断面形貌图(a,b)和EDX能谱图像(c)[81]
Mirna等[82]采用简单沉淀法分别制备了Mg- Mn-Sr-HA。发现Mg2+和Mn5+在掺杂过程中很难进入HA晶格中,即使有进入,量也是相对较少的,而Sr2+很容易加入HA晶格中,并且三者同时掺杂HA时MnO43−和Mg2+的掺入量浓度很高,而Sr2+在Mg-Mn-Sr-HA中的掺入量却明显低于Sr-HA,这恰恰表明HA的多相离子共掺杂是一个高度复杂的过程,所以在多相离子掺杂HA的研究过程中对其掺杂结构变化与机理的分析是十分必要的,有利于更多相离子掺杂HA在类骨仿生骨修复领域的研究。然而,多相离子的共同掺杂是否存在着离子之间的相互作用致使性能的改变,Sr2+的促进作用是否时刻存在,并且是否存在先后顺序,其他金属阳离子是否也存在这种作用,或者同时掺入单相甚至多相阴离子结果又如何呢?
Vu等[83]采用等离子体喷涂方法在金属钛合金材料表面制备HA涂层和ZnSiAg-HA涂层。研究发现涂层与基材之间有很强的结合且在基材上保持完整,在磷酸盐缓冲液(PBS)和pH为5的醋酸缓冲液(ABS)中Ag+总释放量分别为0.000 171%、0.001 870%,这是由于酸性条件下增加了HA的降解速率。掺杂涂层在中性环境下同HA涂层均可长期存在,在酸性条件下14 d后HA涂层完全消失,而ZnSiAg-HA涂层仍有部分存在,说明ZnSiAg-HA涂层具有更好的结晶度;通过大鼠股骨远端模型分析基材本身、HA涂层和ZnSiAg-HA涂层假体的早期骨整合。到第5周,掺杂涂层显示68%的总骨形成,而HA涂层只有55%,ZnSiAg-HA涂层能产生32%的骨矿化,而HA涂层仅能产生11%的骨矿化,说明ZnSiAg-HA涂层的骨结合能力相对较强。该系统可应用于矫形、牙齿置换以及翻修手术,以促进早期种植体骨整合,缩短愈合时间。
多相离子掺杂HA的研究并不丰富,原因可能是多离子取代HA的过程与结构变化太为复杂,但是由于天然骨磷灰石中多种微量离子的存在,那么人工合成的多相离子掺杂HA的研究就必须深入。
4 总结
本研究总结了单相、双相及多相离子掺杂羟基磷灰石对HA的性能的影响,但是和天然骨磷灰石相比仍然存在差距,多相离子掺杂HA的研究还不够深入,对于天然骨磷灰石的多种离子成分的存在,我认为后续的研究可以根据天然骨磷灰石中各离子所占比例进行掺杂制备类骨磷灰石材料,还可以通过控制大多数离子的占比,进行单相离子或多相离子比例的动态浮动改变HA的晶粒尺寸、拓扑结构、化学成分、结晶度和表面电荷,从而达到深层次改性的效果,而且在此基础上改变制备方法和反应条件或许可以更加接近仿生的目的。总之,在未来骨的再生和修复临床中,纳米羟基磷灰石材料将会起到关键性的作用,所以离子掺杂改性HA达到符合临床要求的研究更加需要进一步开展。
[1] 范依然, 闫文馨, 韩颖超. 羟基磷灰石纳米粒子表面修饰的研究进展[J]. 表面技术, 2020, 49(7): 17-27.
FAN Yi-ran, YAN Wen-xin, HAN Ying-chao. Research Progress on Surface Modification of Hydroxyapatite Nanoparticles[J]. Surface Technology, 2020, 49(7): 17-27.
[2] 谭思民, 王帅星, 赵晴, 等. 水热时间对钛合金微弧氧化膜合成羟基磷灰石的影响[J]. 表面技术, 2014, 43(3): 20-24.
TAN Si-min, WANG Shuai-xing, ZHAO Qing, et al. Effects of Hydrothermal Time on Hydroxyapatite Synthesis of Microarc Oxidized Titanium[J]. Surface Technology, 2014, 43(3): 20-24.
[3] 李亚莹, 白艳洁, 曹婷. 羟基磷灰石在硬组织修复中的应用进展[J]. 中国美容整形外科杂志, 2020, 31(3): 190-191, 199.
LI Ya-ying, BAI Yan-jie, CAO Ting. Application Progress of Hydroxyapatite in Hard Tissue Repair[J]. Chinese Journal of Aesthetic and Plastic Surgery, 2020, 31(3): 190-191, 199.
[4] CHEN Liang, MCCRATE J M, LEE J C M, et al. The Role of Surface Charge on the Uptake and Biocompatibility of Hydroxyapatite Nanoparticles with Osteoblast Cells[J]. Nanotechnology, 2011, 22(10): 105708.
[5] 王艳玲, 丁虹, 何巍, 等. 微弧氧化羟基磷灰石涂层增强金属钛种植体骨整合的体内研究[J]. 口腔医学研究, 2019, 35(11): 1070-1073.
WANG Yan-ling, DING Hong, HE Wei, et al. Experimental Study on Osseointegration of Micro-Arc Oxidized Hydroxyapatite Coated Titanium Implants[J]. Journal of Oral Science Research, 2019, 35(11): 1070-1073.
[6] MUKHAMETKALIYEV T M, SURMENEVA M A, VLADESCU A, et al. A Biodegradable AZ91 Magnesium Alloy Coated with a Thin Nanostructured Hydroxyapatite for Improving the Corrosion Resistance[J]. Materials Science and Engineering: C, 2017, 75: 95-103.
[7] 杨蕾, 梁军, 许益蒙, 等. AZ31镁合金表面含纳米羟基磷灰石微弧氧化涂层的制备及性能研究[J]. 表面技术, 2018, 47(4): 153-159.
YANG Lei, LIANG Jun, XU Yi-meng, et al. Preparation and Properties of Micro-Arc Oxidation Coating Containing Nano-Hydroxyapatite on AZ31 Magnesium Alloy[J]. Surface Technology, 2018, 47(4): 153-159.
[8] IANG Jia-wei, HAN Gui, ZHENG Xue-song, et al. Characterization and Biocompatibility Study of Hydroxyapatite Coating on the Surface of Titanium Alloy[J]. Surface and Coatings Technology, 2019, 375: 645-651.
[9] 华帅, 陶庆爽, 郭亚鑫, 等. 镁合金表面电化学沉积制备羟基磷灰石涂层及耐腐蚀性能的研究[J]. 功能材料, 2017, 48(11): 11216-11220.
HUA Shuai, TAO Qing-shuang, GUO Ya-xin, et al. Preparation and Corrosion Resistance of Hydroxyapatite Coatings on Magnesium Alloy by Electrochemical Deposition[J]. Journal of Functional Materials, 2017, 48(11): 11216-11220.
[10] 贾理男, 梁成浩, 黄乃宝, 等. 镁基羟基磷灰石/微弧氧化层制备的研究进展[J]. 表面技术, 2013, 42(1): 109-112.
JIA Li-nan, LIANG Cheng-hao, HUANG Nai-bao, et al. Progresses in Preparation of Hydroxyapatite/MAO Coating Supported on Magnesium Substrate[J]. Surface Technology, 2013, 42(1): 109-112.
[11] DOROZHKIN S V. Nanodimensional and Nanocrystalline Calcium Orthophosphates[J]. American Journal of Biomedical Engineering, 2012, 2(3): 48-97.
[12] LIM P N, SHI Zhi-long, NEOH K G, et al. The Effects of Silver, Silicon-Containing Apatite towards Bacteria and Cell Responses[J]. Biomedical Materials (Bristol, England), 2014, 9(1): 015010.
[13] PREDOI D, ICONARU S L, PREDOI M V, et al. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions[J]. Nanomaterials (Basel, Switzerland), 2019, 9(9): 1295.
[14] YUAN Xiao-ya, ZHU Bang-shang, TONG Gang-sheng, et al. Wet-Chemical Synthesis of Mg-Doped Hydroxyapatite Nanoparticles by Step Reaction and Ion Exchange Processes[J]. Journal of Materials Chemistry B, 2013, 1(47): 6551-6559.
[15] 马晓雨, 刘永佳, 朱邦尚. 镁掺杂纳米羟基磷灰石的制备及其在载药方面的应用[J]. 无机化学学报, 2018, 34(5): 917-924.
MA Xiao-yu, LIU Yong-jia, ZHU Bang-shang. Preparation and Drug Delivery Properties of Mg-Doped Hydroxyapatite Nanoparticles[J]. Chinese Journal of Inorganic Chemistry, 2018, 34(5): 917-924.
[16] UYSAL I, SEVERCAN F, TEZCANER A, et al. Co- Doping of Hydroxyapatite with Zinc and Fluoride Improves Mechanical and Biological Properties of Hydroxyapatite[J]. Progress in Natural Science: Materials International, 2014, 24(4): 340-349.
[17] ZHANG Wan-dong, CAO Na-na, CHAI Ya-min, et al. Synthesis of Nanosize Single-Crystal Strontium Hydroxyapatite via a Simple Sol-Gel Method[J]. Ceramics International, 2014, 40(10): 16061-16064.
[18] 袁秋华, 石鑫, 万磊, 等. HA-PLA和Zn掺杂HA-PLA的界面结构及性能模拟[J]. 深圳大学学报(理工版), 2018, 35(6): 603-610.
YUAN Qiu-hua, SHI Xin, WAN Lei, et al. Simulation of Interfacial Structures and Mechanical Properties of Hydroxyapatite-Polylactic Acid and Zn-Doped Hydroxyapatite- Polylactic Acid[J]. Journal of Shenzhen University (Science and Engineering), 2018, 35(6): 603-610.
[19] NEGRILA C, PREDOI M, ICONARU S, et al. Development of Zinc-Doped Hydroxyapatite by Sol-Gel Method for Medical Applications[J]. Molecules, 2018, 23(11): 2986.
[20] CAPUCCINI C, TORRICELLI P, BOANINI E, et al. Interaction of Sr-Doped Hydroxyapatite Nanocrystals with Osteoclast and Osteoblast-Like Cells[J]. Journal of Biomedical Materials Research Part A, 2009, 89A(3): 594-600.
[21] CURRAN D J, FLEMING T J, TOWLER M R, et al. Mechanical Parameters of Strontium Doped Hydroxyapatite Sintered Using Microwave and Conventional Methods[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2011, 4(8): 2063-2073.
[22] AVCI M, YILMAZ B, TEZCANER A, et al. Strontium Doped Hydroxyapatite Biomimetic Coatings on Ti6Al4V Plates[J]. Ceramics International, 2017, 43(12): 9431- 9436.
[23] CHENG Guo-feng, ZHANG Yong, YIN Han-di, et al. Effects of Strontium Substitution on the Structural Distortion of Hydroxyapatite by Rietveld Refinement and Raman Spectroscopy[J]. Ceramics International, 2019, 45(8): 11073-11078.
[24] JIANG Ming, TERRA J, ROSSI A M, et al. Fe2+/Fe3+Substitution in hydroxyapatite: Theory and Experiment[J]. Physical Review B, 2002, 66(22): 224107.
[25] MERCADO D F, MAGNACCA G, MALANDRINO M, et al. Paramagnetic Iron-Doped Hydroxyapatite Nanoparticles with Improved Metal Sorption Properties. a Bioorganic Substrates-Mediated Synthesis[J]. ACS Applied Materials & Interfaces, 2014, 6(6): 3937-3946.
[26] ZUO Kai-hui, ZENG Yu-ping, JIANG Dong-liang. Synthesis and Magnetic Property of Iron Ions-Doped Hydroxyapatite[J]. Journal of Nanoscience and Nanotechnology, 2012, 12(9): 7096-7100.
[27] DROUET C. Applied Predictive Thermodynamics (). Part 2. Apatites Containing Ni2+, Co2+, Mn2+, or Fe2+Ions[J]. The Journal of Chemical Thermodynamics, 2019, 136: 182-189.
[28] LIU Guo-jing, TALLEY J W, NA Chong-zheng, et al. Copper Doping Improves Hydroxyapatite Sorption for Arsenate in Simulated Groundwaters[J]. Environmental Science & Technology, 2010, 44(4): 1366-1372.
[29] HUANG Yong, ZHANG Xue-jiao, ZHAO Ran-lin, et al. Antibacterial Efficacy, Corrosion Resistance, and Cytotoxicity Studies of Copper-Substituted Carbonated Hydroxyapatite Coating on Titanium Substrate[J]. Journal of Materials Science, 2015, 50(4): 1688-1700.
[30] HADIDI M, BIGHAM A, SAEBNOORI E, et al. Electrophoretic-Deposited Hydroxyapatite-Copper Nanocomposite as an Antibacterial Coating for Biomedical Applications[J]. Surface and Coatings Technology, 2017, 321: 171-179.
[31] UNABIA R B, BONEBEAU S, CANDIDATO R T, et al. Investigation on the Structural and Microstructural Properties of Copper-Doped Hydroxyapatite Coatings Deposited Using Solution Precursor Plasma Spraying[J]. Journal of the European Ceramic Society, 2019, 39(14): 4255- 4263.
[32] GHOSH R, SWART O, WESTGATE S, et al. Antibacterial Copper-Hydroxyapatite Composite Coatings via Electrochemical Synthesis[J]. Langmuir, 2019, 35(17): 5957-5966.
[33] SALAVATI-NIASARI M, BANAIEAN-MONFARED G, EMADI H, et al. Synthesis and Characterization of Nickel Sulfide Nanoparticles via Cyclic Microwave Radiation[J]. Comptes Rendus Chimie, 2013, 16(10): 929-936.
[34] ANU PRIYA B, SENTHILGURU K, AGARWAL T, et al. Nickel Doped Nanohydroxyapatite: Vascular Endothelial Growth Factor Inducing Biomaterial for Bone Tissue Engineering[J]. RSC Advances, 2015, 5(89): 72515-72528.
[35] CHAVAN P N, BAHIR M M, MENE R U, et al. Study of Nanobiomaterial Hydroxyapatite in Simulated Body Fluid: Formation and Growth of Apatite[J]. Materials Science and Engineering: B, 2010, 168(1-3): 224-230.
[36] 喻志武, 李东北, 许帅, 等. 钠离子掺杂羟基磷灰石的合成及其微结构的固体NMR研究[C]//第十九届全国波谱学学术会议论文摘要集. 兰州, 2016: 86-87.
[37] ZHANG Lei-lei, PEI Li-na, LI He-jun, et al. Preparation and Characterization of Na and F Co-Doped Hydroxyapatite Coating Reinforced by Carbon Nanotubes and SiC Nanoparticles[J]. Materials Letters, 2018, 218: 161-164.
[38] SINGH B, KUMAR S, BASU B, et al. Conductivity Studies of Silver-, Potassium-, and Magnesium-Doped Hydroxyapatite[J]. International Journal of Applied Ceramic Technology, 2015, 12(2): 319-328.
[39] KANDORI K, TOSHIMA S, WAKAMURA M, et al. Effects of Modification of Calcium Hydroxyapatites by Trivalent Metal Ions on the Protein Adsorption Behavior[J]. The Journal of Physical Chemistry B, 2010, 114(7): 2399-2404.
[40] YANG Chun, YANG Piao-ping, WANG Wen-xin, et al. Solvothermal Synthesis and Characterization of Ln (Eu3+, Tb3+) Doped Hydroxyapatite[J]. Journal of Colloid and Interface Science, 2008, 328(1): 203-210.
[41] HAN Ying-chao, WANG Xin-yu, DAI Hong-lian, et al. Synthesis and Luminescence of Eu3+Doped Hydroxyapatite Nanocrystallines: Effects of Calcinations and Eu3+Content[J]. Journal of Luminescence, 2013, 135: 281-287.
[42] SILVA F R, DE LIMA N B, BRESSIANI A H A, et al. Synthesis, Characterization and Luminescence Properties of Eu3+ -Doped Hydroxyapatite Nanocrystal and the Thermal Treatment Effects[J]. Optical Materials, 2015, 47: 135-142.
[43] ZENG Guang-jian, LIU Mei-ying, JIANG Ru-ming, et al. Surface Grafting of Eu3+Doped Luminescent Hydroxyapatite Nanomaterials through Metal Free Light Initiated Atom Transfer Radical Polymerization for Theranostic Applications[J]. Materials Science and Engineering: C, 2017, 77: 420-426.
[44] SZYSZKA K, TARGONSKA S, GAZINSKA M, et al. The Comprehensive Approach to Preparation and Investigation of the Eu3+Doped Hydroxyapatite/Poly(L-Lactide) Nanocomposites: Promising Materials for Theranostics Application[J]. Nanomaterials (Basel, Switzerland), 2019, 9(8): 1146.
[45] ROBLES-ÁGUILA M J, REYES-AVENDAÑO J A, MENDOZA M E. Structural Analysis of Metal-Doped (Mn, Fe, Co, Ni, Cu, Zn) Calcium Hydroxyapatite Synthetized by a Sol-Gel Microwave-Assisted Method[J]. Ceramics International, 2017, 43(15): 12705-12709.
[46] BLUM C, BRÜCKNER T, EWALD A, et al. Mg: Ca Ratio as Regulating Factor for Osteoclastic in Vitro Resorption of Struvite Biocements[J]. Materials Science and Engineering: C, 2017, 73: 111-119.
[47] DENG Li-qing, LI Dong-hai, YANG Zhou-yuan, et al. Repair of the Calvarial Defect in Goat Model Using Magnesium-Doped Porous Hydroxyapatite Combined with Recombinant Human Bone Morphogenetic Protein-2[J]. Bio-Medical Materials and Engineering, 2017, 28(4): 361-377.
[48] PREDOI D, ICONARU S L, PREDOI M V, et al. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions[J]. Nanomaterials (Basel, Switzerland), 2019, 9(9): 1295.
[49] MENG Jie, ZHANG Yu, QI Xiao-jin, et al. Paramagnetic Nanofibrous Composite Films Enhance the Osteogenic Responses of Pre-Osteoblast Cells[J]. Nanoscale, 2010, 2(12): 2565-2569.
[50] MENG Jie, XIAO Bo, ZHANG Yu, et al. Super-Paramagnetic Responsive Nanofibrous Scaffolds under Static Magnetic Field Enhance Osteogenesis for Bone Repair in Vivo[J]. Scientific Reports, 2013, 3: 2655.
[51] HEIDARI F, BAHROLOLOOM M E, VASHAEE D, et al. In Situ Preparation of Iron Oxide Nanoparticles in Natural Hydroxyapatite/Chitosan Matrix for Bone Tissue Engineering Application[J]. Ceramics International, 2015, 41(2): 3094-3100.
[52] 禹怡君. 静磁场作用下铁掺杂的羟基磷灰石复合电纺膜诱导MSCs骨向分化的研究[D]. 南京: 南京大学, 2016.
YU Yi-jun. Electrospun Fibrous Scaffolds with Iron-Doped Hydroxyapatite Induce Osteogenic Differentiation of Mesenchymal Stem Cells with Static Magnetic Field Exposure[D]. Nanjing: Nanjing University, 2016.
[53] BALAKRISHNAN S, PADMANABHAN V P, KULANDAIVELU R, et al. Influence of Iron Doping towards the Physicochemical and Biological Characteristics of Hydroxyapatite[J]. Ceramics International, 2021, 47(4): 5061-5070.
[54] YAZICI M, GULEC A E, GURBUZ M, et al. Biodegradability and Antibacterial Properties of MAO Coatings Formed on Mg-Sr-Ca Alloys in an Electrolyte Containing Ag Doped Hydroxyapatite[J]. Thin Solid Films, 2017, 644: 92-98.
[55] WANG Tao-lei, YANG Guang-zheng, ZHOU Wu-chao, et al. One-Pot Hydrothermal Synthesis, in Vitro Biodegradation and Biocompatibility of Sr-Doped Nanorod/ Nanowire Hydroxyapatite Coatings on ZK60 Magnesium Alloy[J]. Journal of Alloys and Compounds, 2019, 799: 71-82.
[56] SERGI R, BELLUCCI D, CANDIDATO R T Jr, et al. Bioactive Zn-Doped Hydroxyapatite Coatings and Their Antibacterial Efficacy Againstand[J]. Surface and Coatings Technology, 2018, 352: 84-91.
[57] ZHOU Wu-chao, HU Zhen-rong, WANG Tao-lei, et al. Enhanced Corrosion Resistance and Bioactivity of Mg Alloy Modified by Zn-Doped Nanowhisker Hydroxyapatite Coatings[J]. Colloids and Surfaces B: Biointerfaces, 2020, 186: 110710.
[58] TÕNSUAADU K, BOGDANOVICIENE I, TRAKSMAA R. Purity Test of Precipitated Apatites by TG/DTA/EGA- MS[J]. Journal of Thermal Analysis and Calorimetry, 2016, 125(2): 919-925.
[59] PAWŁOWSKI L, BLANCHART P. Industrial Chemistry of Oxides for Emerging Applications[M]. Chichester, UK: John Wiley & Sons Ltd, 2018.
[60] MORENO-PEREZ B, MATAMOROS-VELOZA Z, RENDON-ANGELES J C, et al. Synthesis of Silicon- Substituted Hydroxyapatite Using Hydrothermal Process[J]. Boletín De La Sociedad Española De Cerámica y Vidrio, 2020, 59(2): 50-64.
[61] TAVAFOGHI M, KINSELLA J M, GAMYS C G, et al. Silicon-Doped Hydroxyapatite Prepared by a Thermal Technique for Hard Tissue Engineering Applications[J]. Ceramics International, 2018, 44(15): 17612-17622.
[62] LI Ke-zhi, GUO Qian, ZHANG Lei-lei, et al. Synthesis and Characterization of Si-Substituted Hydroxyapatite Bioactive Coating for SiC-Coated Carbon/Carbon Composites[J]. Ceramics International, 2017, 43(1): 1410-1414.
[63] KE Dong-xu, BANERJEE D, BOSE S.Characterizations of Si4+and Zn2+Doped Plasma Sprayed Hydroxyapatite Coatings Using Osteoblast and Osteoclast Coculture[J]. ACS Biomaterials Science & Engineering, 2019, 5(3): 1302-1310.
[64] NASKER P, MUKHERJEE M, KANT S, et al. Fluorine Substituted Nano Hydroxyapatite: Synthesis, Bio-Activity and Antibacterial Response Study[J]. Ceramics International, 2018, 44(17): 22008-22013.
[65] HIDALGO-ROBATTO B M, AGUILERA-CORREA J J, LÓPEZ-ÁLVAREZ M, et al. Fluor‑Carbonated Hydroxyapatite Coatings by Pulsed Laser Deposition to Promote Cell Viability and Antibacterial Properties[J]. Surface and Coatings Technology, 2018, 349: 736-744.
[66] O’NEILL E, AWALE G, DANESHMANDI L, et al. The Roles of Ions on Bone Regeneration[J]. Drug Discovery Today, 2018, 23(4): 879-890.
[67] CHU Cheng-lin, HU T, YIN L H, et al. Microstructural Characteristics and Biocompatibility of a Type-B Carbonated Hydroxyapatite Coating Deposited on NiTi Shape Memory Alloy[J]. Bio-Medical Materials and Engineering, 2009, 19(6): 401-408.
[68] 仇满德, 王晓燕, 李旭, 等. 水热法合成羟基磷灰石的微分析研究[J]. 人工晶体学报, 2013, 42(9): 1965-1971.
QIU Man-de, WANG Xiao-yan, LI Xu, et al. Microanalysis of Hydroxyapatite Synthesized by Hydrothermal Method[J]. Journal of Synthetic Crystals, 2013, 42(9): 1965-1971.
[69] SUCHANEK W L, SHUK P, BYRAPPA K, et al. Mechanochemical-Hydrothermal Synthesis of Carbonated Apatite Powders at Room Temperature[J]. Biomaterials, 2002, 23(3): 699-710.
[70] 朱庆霞, 宋健平, 练爱民, 等. 碳酸根替代对磷灰石块体和涂层生物矿化的影响研究[J]. 硅酸盐通报, 2019, 38(12): 3959-3963, 3971.
ZHU Qing-xia, SONG Jian-ping, LIAN Ai-min, et al. Effect of Carbonate Substitution on the Biomineralization of Apatite Bulk and Coating[J]. Bulletin of the Chinese Ceramic Society, 2019, 38(12): 3959-3963, 3971.
[71] CAO Jin-xing, LIAN Rui-zhe, JIANG Xiao-hong. Magnesium and Fluoride Doped Hydroxyapatite Coatings Grown by Pulsed Laser Deposition for Promoting Titanium Implant Cytocompatibility[J]. Applied Surface Science, 2020, 515: 146069.
[72] VEERLA S C, KIM D R, KIM J, et al. Controlled Nanoparticle Synthesis of Ag/Fe Co-Doped Hydroxyapatite System for Cancer Cell Treatment[J]. Materials Science and Engineering: C, 2019, 98: 311-323.
[73] LATIFI S M, FATHI M, SHARIFNABI A, et al.Characterisation of a Sol-Gel DerivedSilica- Coated Silicate and Carbonate Co-Doped Hydroxyapatite Nanopowder for Bone Grafting[J]. Materials Science and Engineering: C, 2017, 75: 272-278.
[74] FAHAMI A, BEALL G W, BETANCOURT T. Synthesis, Bioactivity and Zeta Potential Investigations of Chlorine and Fluorine Substituted Hydroxyapatite[J]. Materials Science and Engineering: C, 2016, 59: 78-85.
[75] RUKSUDJARIT A, PENGPAT K, RUJIJANAGUL G, et al. Synthesis and Characterization of Nanocrystalline Hydroxyapatite from Natural Bovine Bone[J]. Current Applied Physics, 2008, 8(3-4): 270-272.
[76] HUANG Yong, XU Zhi-wei, ZHANG Xue-jiao, et al. Nanotube-Formed Ti Substrates Coated with Silicate/ Silver Co-Doped Hydroxyapatite as Prospective Materials for Bone Implants[J]. Journal of Alloys and Compounds, 2017, 697: 182-199.
[77] CATAURO M, BOLLINO F, PAPALE F, et al. Investigation on Bioactivity, Biocompatibility, Thermal Behavior and Antibacterial Properties of Calcium Silicate Glass Coatings Containing Ag[J]. Journal of Non-Crystalline Solids, 2015, 422: 16-22.
[78] AHMED M K, RAMADAN R, AFIFI M, et al. Au-Doped Carbonated Hydroxyapatite Sputtered on Alumina Scaffolds via Pulsed Laser Deposition for Biomedical Applications[J]. Journal of Materials Research and Technology, 2020, 9(4): 8854-8866.
[79] WEI Lu-xi, YANG Hui-fang, HONG Jia-dan, et al. Synthesis and Structure Properties of Se and Sr Co-Doped Hydroxyapatite and Their Biocompatibility[J]. Journal of Materials Science, 2019, 54(3): 2514-2525.
[80] GAO Jian-yong, WANG Ming, SHI Chao, et al. A Facile Green Synthesis of Trace Si, Sr and F Multi-Doped Hydroxyapatite with Enhanced Biocompatibility and Osteoconduction[J]. Materials Letters, 2017, 196: 406-409.
[81] FURKO M, HAVASI V, KÓNYA Z, et al. Development and Characterization of Multi-Element Doped Hydroxyapatite Bioceramic Coatings on Metallic Implants for Orthopedic Applications[J]. Boletín De La Sociedad Española De Cerámica y Vidrio, 2018, 57(2): 55-65.
[82] MOREIRA M P, DE ALMEIDA SOARES G D, DENTZER J, et al. Synthesis of Magnesium- and Manganese- Doped Hydroxyapatite Structures Assisted by the Simultaneous Incorporation of Strontium[J]. Materials Science and Engineering: C, 2016, 61: 736-743.
[83] VU A A, ROBERTSON S F, KE Dong-xu, et al. Mechanical and Biological Properties of ZnO, SiO2, and Ag2O Doped Plasma Sprayed Hydroxyapatite Coating for Orthopaedic and Dental Applications[J]. Acta Biomaterialia, 2019, 92: 325-335.
Hydroxyapatite Nanomaterials Doped with Single-phase,Dual-phase and Multiphase Ions
1a1b,2,1b,1b
(1. a. College of Science, b. College of Materials Science and Engineering, Liaoning Technical University, Liaoning Fuxin 123000, China; 2. Ganli Pharmaceutical Co., Ltd., Beijing 101102, China)
Hydroxyapatite is an important part of natural bone tissue composition and has always been the focus of attention and research in the field of biomedicine. The apatite in the natural bone composition is a kind of hydroxyapatite combined with a variety of ions, which maintains the normal growth and development of life systems. With the systematic research on single-phase ion doping, the preparation and performance of dual-phase and multi-phase doped hydroxyapatite are being continuously expanded. The preparation and performance of phase and heterogeneous ion-doped hydroxyapatite are systematically elaborated and summarized.
This study expounds the crystal structure of hydroxyapatite, describes the advantages and performance advantages of synthetic hydroxyapatite itself, hydroxyapatite with good biological properties as a coating has been widely used in experiments to replace bone, but Some performance deficiencies limit its application. The study outlines the researchers' research on the effects of different ion doping or different forms of doping on the properties of hydroxyapatite, including biocompatibility, biological activity, suitable mechanical strength, excellent bone formation performance and corrosion resistance performance. At the same time, the different substitution sites of anion and cation doped hydroxyapatite are summarized.
On this basis, starting from the preparation and performance research of single-phase ion-doped hydroxyapatite, the performance changes caused by different ion doping and the corresponding mechanism are explained. Prospects and shortcomings. Based on the single-phase ion-doped hydroxyapatite in the past five years, it is necessary to explore the effects of two-phase and multi-phase ion-doped hydroxyapatite on the structural properties, mechanical strength, antibacterial properties, degradability, and osteogenic properties. A certain pavement. At the same time, two-phase and multi-phase ion doping of hydroxyapatite found that the doping ions will promote each other. By doping hydroxyapatite in different phases, it is found that the performance of multi-phase doping is based on the synergistic effect of single-phase or multi-phase doping. In addition, the doping of hydroxyapatite with trace ions can promote the activity of bone cells, promote the growth, proliferation and differentiation of bone cells, and thus play a role in inducing bone formation. However, doping with different ions will produce different properties. The doping of hydroxyapatite with magnesium, iron, zinc, strontium, and fluorine will produce different degrees of antibacterial properties against Escherichia coli or Staphylococcus aureus. Part of the ions doped with hydroxyapatite will increase the mechanical properties of the material and increase the degradation performance.
Through more systematic preparation and performance research of single-phase and dual-phase doped hydroxyapatite, a more effective preparation method has been explored for realizing multiphase doped hydroxyapatite. In recent years, although hydroxyapatite bioceramic materials have been used clinically as implant coatings, sustained-release drug carriers, and bone graft replacement materials, they still face many problems and challenges in their widespread use in clinical practice. Therefore, this article also looks forward to the future development direction of ion-doped hydroxyapatite, paving the way for exploring the preparation method and performance of heterogeneous ion-doped hydroxyapatite, making the preparation closer to natural crystal structure and performance The bionic bone material of apatite is expected to have certain guiding significance in clinical application and development.
TB34
A
1001-3660(2022)10-0143-12
10.16490/j.cnki.issn.1001-3660.2022.10.014
2021–08–03;
2021–11–08
2021-08-03;
2021-11-08
辽宁省教育厅项目(LJ2020JCL033)
Liaoning Provincial Department of Education Project (LJ2020JCL033)
刘莹(1970—),男,硕士,教授,主要研究方向为生物化学及天然高分子材料。
LIU Ying (1970-), Male, Master, Professor, Research focus: biochemistry and natural polymer materials.
刘莹, 杨瑞, 刘井来, 等. 单相、双相及多相离子掺杂的羟基磷灰石研究进展[J]. 表面技术, 2022, 51(10): 143-154.
LIU Ying, YANG Rui, LIU Jing-lai, et al. Hydroxyapatite Nanomaterials Doped with Single-phase, Dual-phase and Multiphase Ions[J]. Surface Technology, 2022, 51(10): 143-154.
