Screening of Brown Planthopper Resistant miRNAs in Rice and Their Roles in Regulation of Brown Planthopper Fecundity
2022-10-25JunLiuJinhuiChenLinSunJiaweiSuQinLiShihuiYangJianhuaZhangWenqing
Lü Jun, Liu Jinhui, Chen Lin, Sun Jiawei, Su Qin, Li Shihui, Yang Jianhua, Zhang Wenqing
Research Paper
Screening of Brown Planthopper Resistant miRNAs in Rice and Their Roles in Regulation of Brown Planthopper Fecundity
Lü Jun#, Liu Jinhui#, Chen Lin, Sun Jiawei, Su Qin, Li Shihui, Yang Jianhua, Zhang Wenqing
(State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China; These authors contributed equally to this work)
MicroRNAs (miRNAs) can participate in plant-insect interactions, which regulate plant defense networks. In this study, we analyzed the miRNA expression profiles of six rice varieties before and after brown planthopper (BPH)-feeding. We identified 45 differentially expressed miRNAs between BPH- susceptible and BPH-resistant rice varieties and 144 miRNAs that responded to BPH-feeding. Thus, miRNAs may be involved in multiple pathways regulating rice defense response against BPH. In addition, we found that the genetic history of rice varieties determined the regulation mode of the miRNA and affected the amounts, types, changing trends and response periods of miRNAs in response to BPH- feeding. To conclude, we scanned seven potential cross-kingdom miRNAs, of which miR5795 may target thegene in BPH, causing a 16.07% reduction in BPH oviposition. The results provide new miRNA information of rice-BPH interactions and BPH-resistant rice variety breeding.
; insect-resistant rice; miR5795; fecundity
Brown planthopper (BPH) is one of the most devastating pests, severely reducing rice yield (Jena and Kim, 2010). Currently, chemical pesticides are often used to control BPH (Wuet al, 2018). However, insecticides have caused several ecological and environmental problems in recent years (Baoet al, 2012). Therefore, the cultivation of resistant rice varieties has ecological benefits and is the most economical and environ- mentally friendly measure for BPH control (Yang and Zhang, 2016).
After long-term adaptation, rice has gradually developed mechanisms to defend BPH attacks (Chenet al, 2012). It is generally believed that rice resistance to BPH is controlled by resistance genes (Chenget al, 2013; Fujitaet al, 2013). Currently, around 40 major BPH-resistance genes have been identified in rice (Akankshaet al, 2019). Nine genes,,/,,,,and,have been characterized by map-based cloning approaches. Different resistance genes confer different levels of insect resistance in rice, and their mechanisms of action and resistant spectrum are also varied (Chenget al, 2013).encodes a nucleotide- binding and leucine-rich repeat protein that activates the salicylic acid signaling pathway and induces callose deposition in phloem cells and trypsin inhibitor production after planthopper infestation, thus reducing BPH fitness (Duet al, 2009).encodes three plasma membrane-localized lectin receptor kinases (OsLecRK1‒OsLecRK3), which combine to confer broad-spectrum and permanent insect resistance in rice (Liuet al, 2015).encodes a protein in the exocyst, interacts with, and participates in cell wall maintenance and reinforcement (Guoet al, 2018). Since less than 18% of the genome encodes protein- coding genes, most genomic landscapes are comprise of non-coding elements. Studies of only coding genes are not sufficient to reveal the molecular mechanism underlying rice resistance to BPH infestation.
MicroRNAs (miRNAs) are a class of 21–24-nucleotide- long endogenous non-protein-coding small RNAs present in eukaryotes (Bartel, 2004). As essential post- transcriptional regulators, miRNAs play significant roles in plant-insect interactions. For example, miR396 and miR156 negatively regulate rice resistance against BPH through regulation of flavonoid and jasmonic acid biosynthesis (Geet al, 2018; Daiet al, 2019). In recent years, evidence of cross-kingdom miRNAs has been reported in several studies (Zhanget al, 2012; Zhouet al, 2015; Chinet al, 2016; Zhuet al, 2017). In particularly, plant miR162a can target the TOR (target of rapamycin) gene of honeybee () (Zhuet al, 2017), which motivates investigations on the interactions between rice and BPH.
This study focused on analyzing the miRNA expression profiles of six rice varieties (including two BPH-susceptible and four BPH-resistant varieties) before and after BPH-feeding. We predicted and analyzed the functions of miRNAs to clarify the modulatory defense roles of miRNAs in the interactions between rice and BPH. In addition, we selected a group of potential cross-kingdom miRNAs from differentially expressed miRNAs, and predicted their targets in BPH to screen out rice miRNAs that can regulate BPH genes. Subsequently, rice miR5795 was observed to possibly target thegene in BPH.
RESULTS
Differentially expressed miRNAs between BPH-susceptible and BPH-resistant rice varieties
We performed deep sequencing and characterization of miRNAs in two BPH-susceptible rice varieties (TN1 and Nipponbare) and four BPH-resistant rice varieties [R476, Mudgo, IR36 and Rathu Heenati (RH)] before and after BPH-feeding. Total reads of the 47 libraries were filtered to remove low-quality reads, incorrect adaptors, poly-A and those shorter than 18 nt, and then the clean reads were obtained (Table S1). The length distribution of the small RNAs was mostly concentrated in the range of 19–24 nt, as previously reported (Fig. S1-A) (Bartel, 2004). Subsequently, the total small RNAs were aligned with the miRNA database in miRBase (release 22) to determine the known miRNAs. In total, 575 known miRNAs were identified, which were dominatly 21 nt- long with 5′-U as the first base (Fig. S1-B), consistent with the characteristics of Argonaute (Ago) 1 protein in plants (Miet al, 2008).
To determine whether there was a difference in the expression of miRNAs between BPH-susceptible and BPH-resistant rice varieties, principal component analysis (PCA) was performed on the miRNA expression of samples before BPH-feeding. The result of PCA showed that the four BPH-resistant rice varieties were concentrated in the area above PC2. In comparison, two BPH-susceptible rice varieties were located in the area below PC2, indicating differences in the miRNAs expression between BPH-susceptible and BPH-resistant rice varieties (Fig. 1-A).
In addition, a total of 45 differential miRNAs were identified through differential expression analysis (Fig. 1-B). Among them, 25 miRNAs were substantially expressed in BPH-susceptible rice varieties, while 20 miRNAs were highly expressed in BPH-resistant rice varieties (Fig. 1-B and -C). Furthermore, target prediction and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichments of the predicted targets were carried out to understand the possible defense modulatory roles of the differentially expressed miRNAs. The KEGG pathway enrichments revealed that some of the targets were involved in common rice defense responses such as plant hormone signal transduction, MAPK signaling pathways and secondary metabolite biosynthesis (Fig. 1-D).
Identification of putative miRNAs that respond to BPH-feeding
During the interactions between rice and BPH, BPH- feeding may induce the specific expression of miRNAsin rice, which will help to regulate the defense responseof rice against BPH. To investigate the effects of BPH- feeding on miRNA expression in rice, the miRNA expression profiles of the six rice varieties before and after BPH-feeding at 8 and 32 h were analyzed, respectively. A total of 144 miRNAs (TN1, 13; Nipponbare, 5; IR36, 28; Mudgo, 32; RH, 55; and R476, 53) that responded to BPH-feeding were identified with some of them being identified repeatedly (Table 1). In terms of quantity, the miRNAs of BPH-resistant rice varieties in response to BPH-feeding were more than those of BPH-susceptible rice varieties, indicating that BPH-resistant rice varieties were more sensitive to BPH-feeding and had a stronger response. Regarding the changing trend of miRNAs, the number of up- regulated miRNAs after BPH-feeding was higher than that of down-regulated miRNAs in BPH-susceptible rice varieties, while the miRNAs of RH and Mudgo were predominantly down-regulated. Comparing the two time points, R476 and Mudgo showed no or slight response at the early stage (8 h) of BPH-feeding but a strong response at the late stage (32 h). In contrast, RH and IR36 had an intense response at the early stage and a mild response at the late stage.
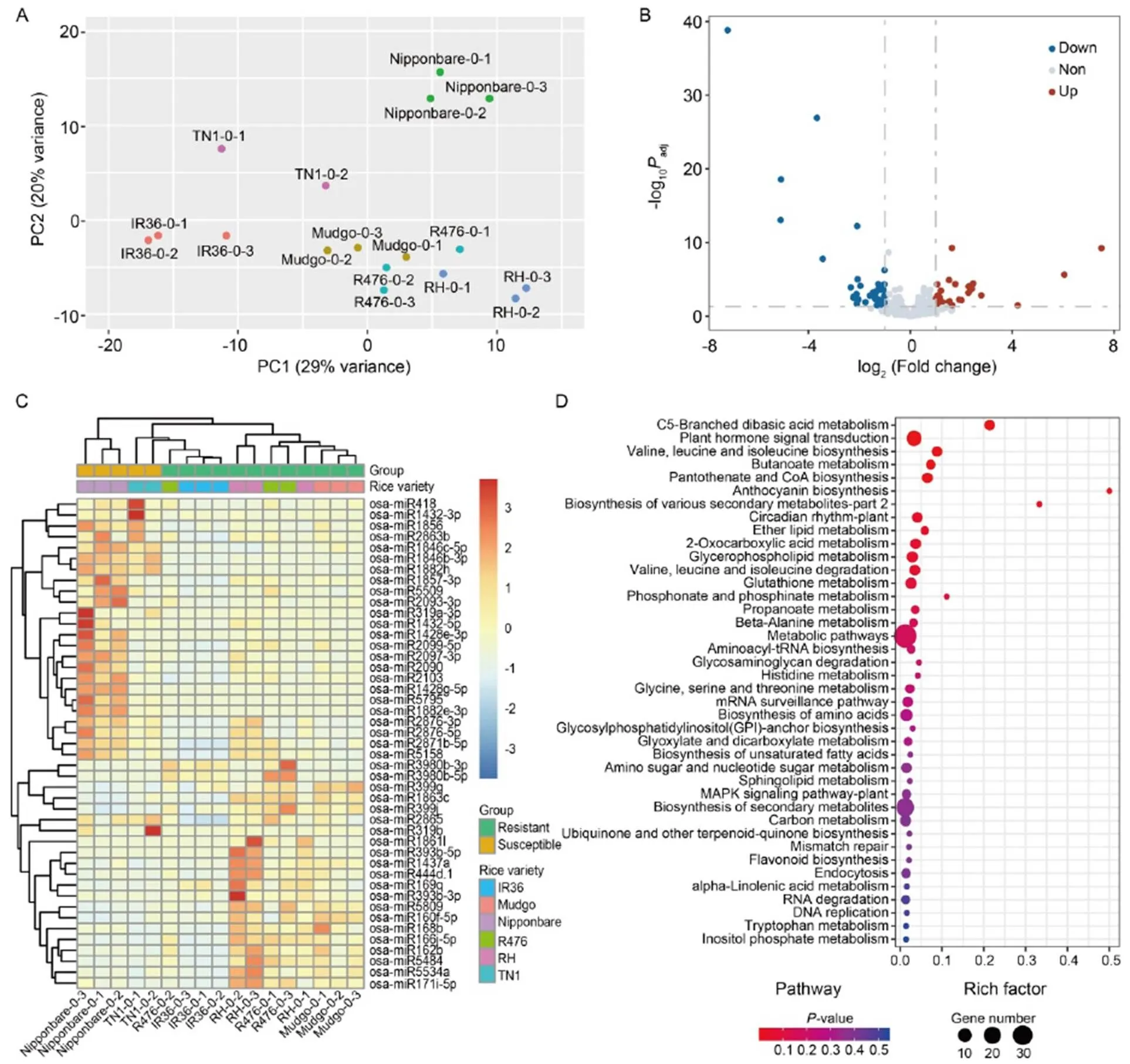
Fig. 1. miRNAs differentially expressed in brown planthopper (BPH)-susceptible and BPH-resistant rice varieties before BPH-feeding.
A, Principal component analysis (PCA) of miRNAs in rice samples before BPH-feeding.
B, Volcano plot of differential miRNA expression in BPH-resistant and BPH-susceptible rice varieties. ‘down’ and ‘up’ mean miRNA expression was down-regulated and up-regulated in BPH-resistant rice varieties compared with BPH-susceptible rice varieties, respectively, and ‘non’ means the expression of miRNA was not significantly different between the BPH-resistant and BPH-susceptible rice varieties.
C, Heatmap of differential miRNA expression profile.
D, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment of target genes of differential miRNAs.
RH, Rathu Heenati.
Furthermore, we found only a small number of miRNAs (32, 22.22%) involved in the response of two or more rice varieties to BPH-feeding, while most miRNAs (112, 77.78%) were involved in response of only one rice variety (Fig. 2-A). The prediction results of target genes indicated that a single miRNA can regulate the expression of many genes, and the different miRNAs regulated different target genes (Fig. 2-B).However, the target genes of miRNAs that responded to BPH-feeding in four BPH-resistant rice varieties shared 12 identical KEGG pathways (Fig. 2-C). The results indicated that different miRNAs were involved in the rice-BPH interactions, and the genetic back- ground of rice determined its miRNA regulation mode after BPH-feeding. There were differences in the amounts, types, changing trends and response periods of miRNAs in response to BPH-feeding in rice varieties with different BPH-resistance genes. However, these miRNAs responded to BPH-feeding by regulating common signaling pathways, such as the synthesis and metabolism of secondary metabolites, flavonoids and amino acids, as well as the regulation of plant hormones.
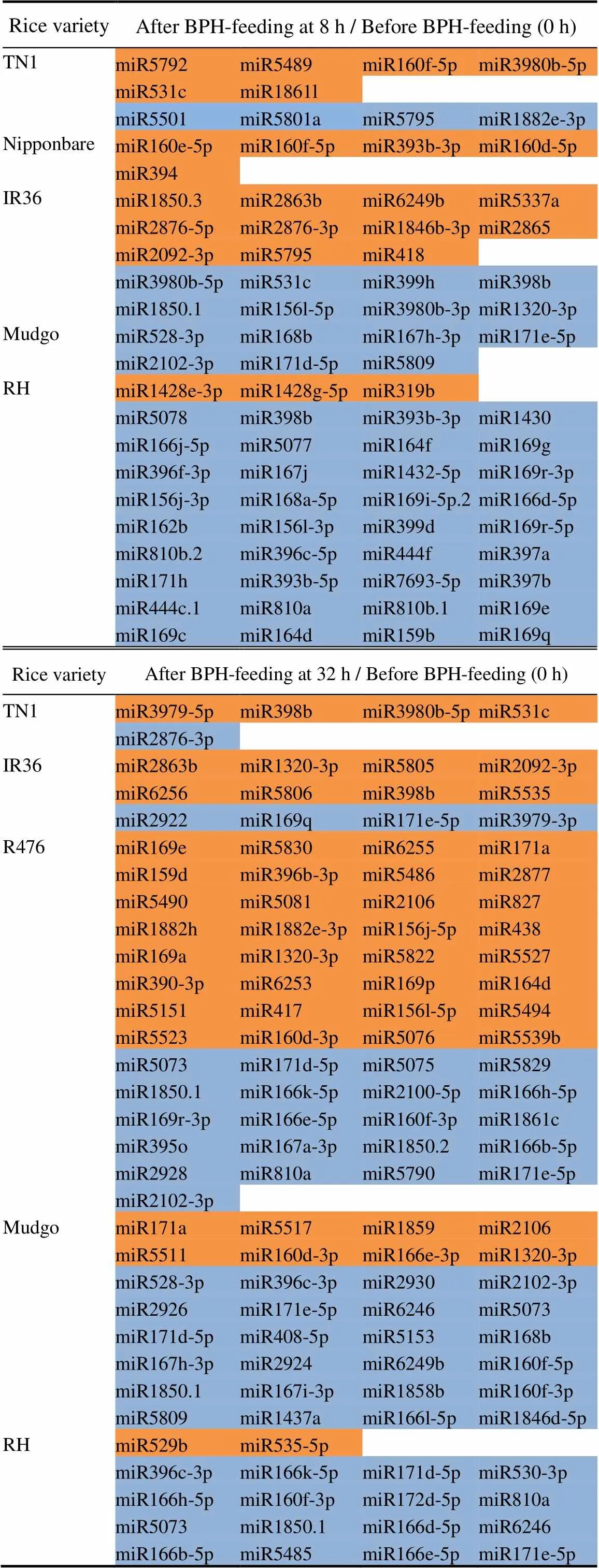
Table 1. Summary of rice miRNAs in response to brown planthopper (BPH)-feeding.
The backgrounds with orange and blue colors indicate miRNA expression was up-regulated and down-regulated, respectively.
RH, Rathu Heenati.
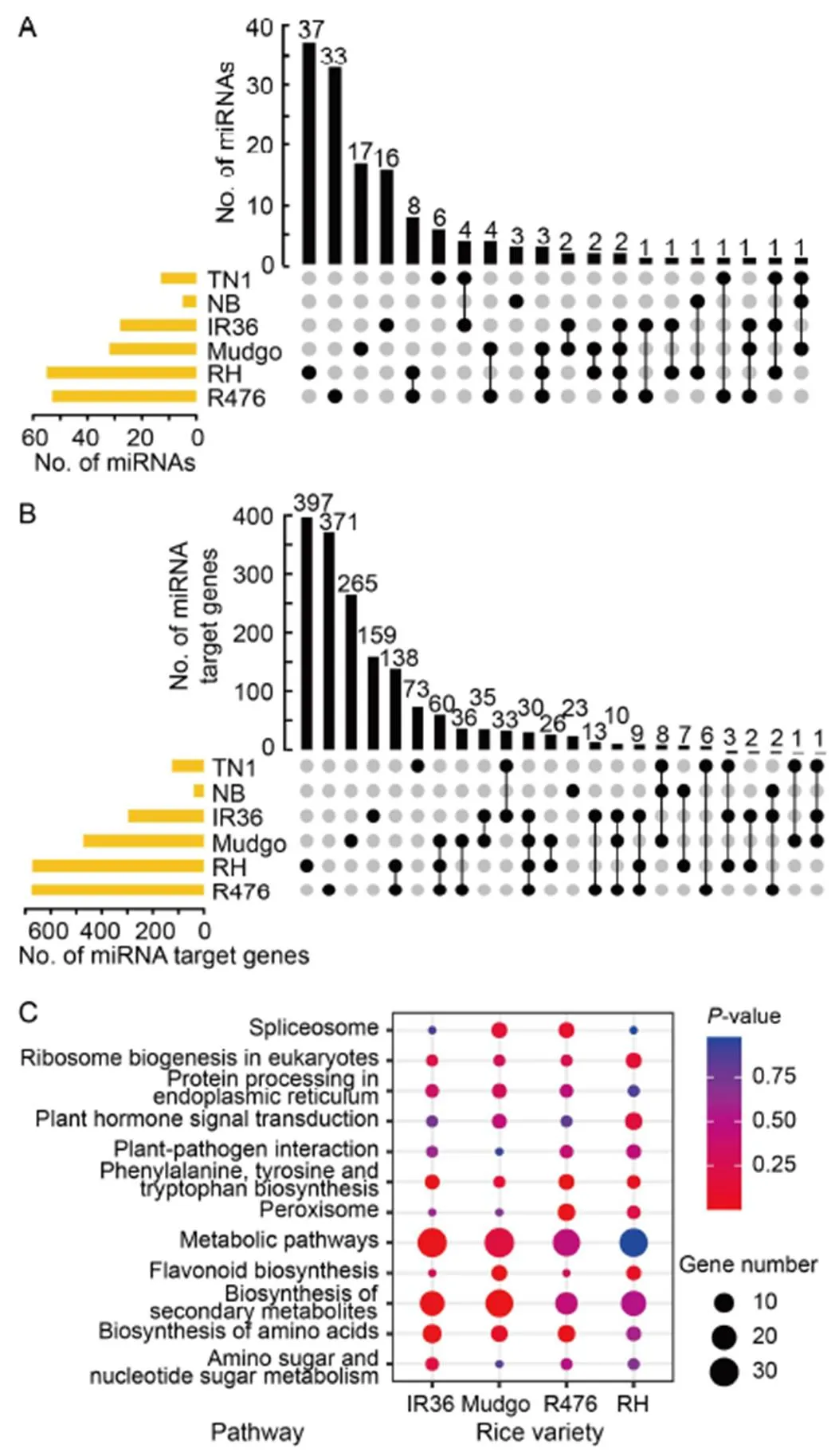
Fig. 2. Different miRNAs participate in rice-brown planthopper (BPH) interactions after BPH-feeding.
A, UpSet diagram of miRNAs in rice response to BPH-feeding. ‘●’ indicates that the miRNAs appeared in only one rice variety, and ‘●‒●’ indicates that the miRNAs appeared in two or more rice varieties.
B, UpSet diagram of miRNA target genes in rice response to BPH- feeding.‘●’ indicates that the miRNA target genes appeared in only one rice variety, and ‘●‒●’ indicates that the miRNA target genes appeared in two or more rice varieties.
C, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment pathway is shared by four BPH-resistant rice varieties.
NB, Nipponbare; RH, Rathu Heenati.
Screening of potential cross-kingdom miRNAs in rice
Fisher’s exact test results showed that the miRNAs differentially expressed in BPH-susceptible and BPH- resistant rice varieties and the miRNAs that responded to BPH-feeding were significantly correlated, meaning that 25 miRNAs shared in 2 groups were more likely to be directly involved in the rice-BPH interactions (Fig. 3-A). Among them, seven miRNAs (miR168b, miR1428g-5p, miR1432-5p, miR5795, miR3980b-5p, miR1882h and miR2876-5p) terminated in a 5′-A residue. These types of miRNAs tended to bind to the Ago2 protein rather than to the Ago1 protein that plays an important role in plants (Miet al, 2008). In addition, Ago2 protein can promote the secretion of miRNAs outside the cell (Lvet al, 2014).
We speculated that miRNAs terminating with 5′-A residue can be secreted extracellularly and ingested by BPH. Therefore, we predicted the targets of these seven miRNAs in BPH. A total of 3 861 miRNA- target pairs were predicted using three bioinformatic algorithms (miRanda, TargetScan and RNA22) (Fig. 3-B). It was noted that these targets were involved in multiple pathways related to the fecundity of BPH, including the pathways related to the vitellogenin biosynthesis such as PI3K-Akt, AMPK and insulin signaling pathways, and the pathways related to oocytedevelopment such as oocyte meiosis and progesterone-mediated oocyte maturation. In contrast, other pathways such as pancreatic secretion, bile secretion, protein digestion and absorption may be related to feeding, digestion and detoxification (Fig. 3-C).
Rice miR5795 mediates fecundity of BPH
To verify whether the miRNAs in rice have the potential to mediate the fecundity of BPH, we scanned 15 representative genes related to the fecundity of BPH for potential binding sites in 7 miRNAs (Table S2). The results showed that miR5795 and miR1428g-5p had potential binding sites in the CDS (coding sequence) and 3′-UTR (untranslated region) of multiple genes, respectively (Table 2). Subsequently, the fecundity of BPH was tested in three seasons (winter, spring and summer) after injection of miRNA mimics. In order to exclude the effects of seasonal differences, a two-way analysis of variance (ANOVA) was used to analyze the fecundity data. The results showed that miR5795 reduced the number of eggs laid by 16.07% (< 0.01) and the hatching rate by 16.45% (< 0.01) of BPH (Fig. 4-A to -C), while miR1428g-5p did not affect the fecundity of BPH (Fig. S2-A to -C). Additionally, ovarian development was inhibited significantly at 72 h after injection of miR5795 mimics (Fig. S3).
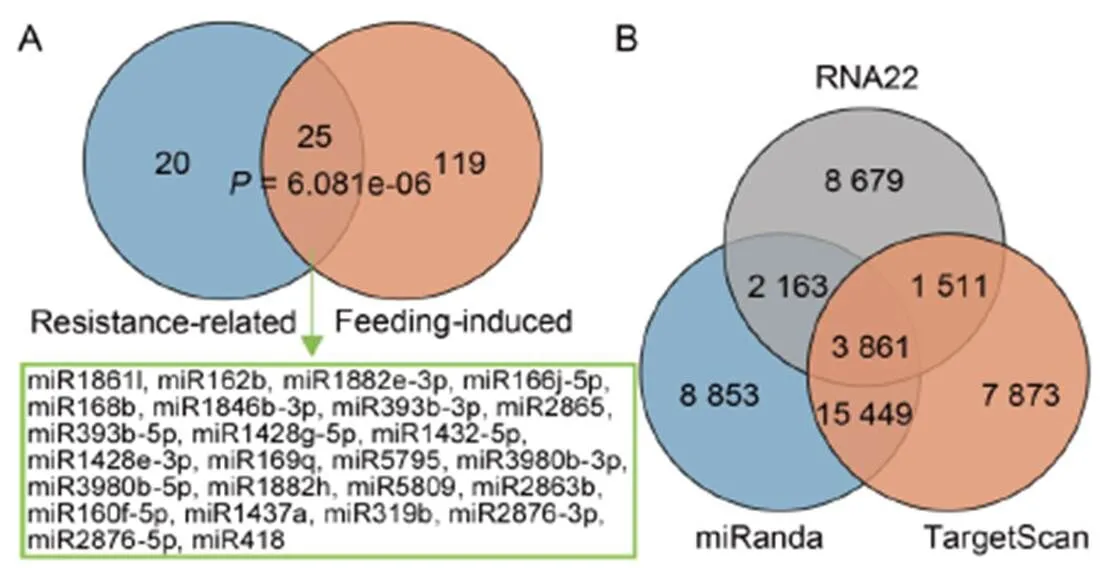
Fig. 3. Screening of potential cross-kingdom miRNAs in rice.
A, Venn diagram of two sets of miRNAs.
B, Venn diagram of target prediction of rice miRNAs using three bioinformatic algorithms (miRanda, TargetScan and RNA22) based on the total transcripts of
C, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment of target genes inof miRNAs.
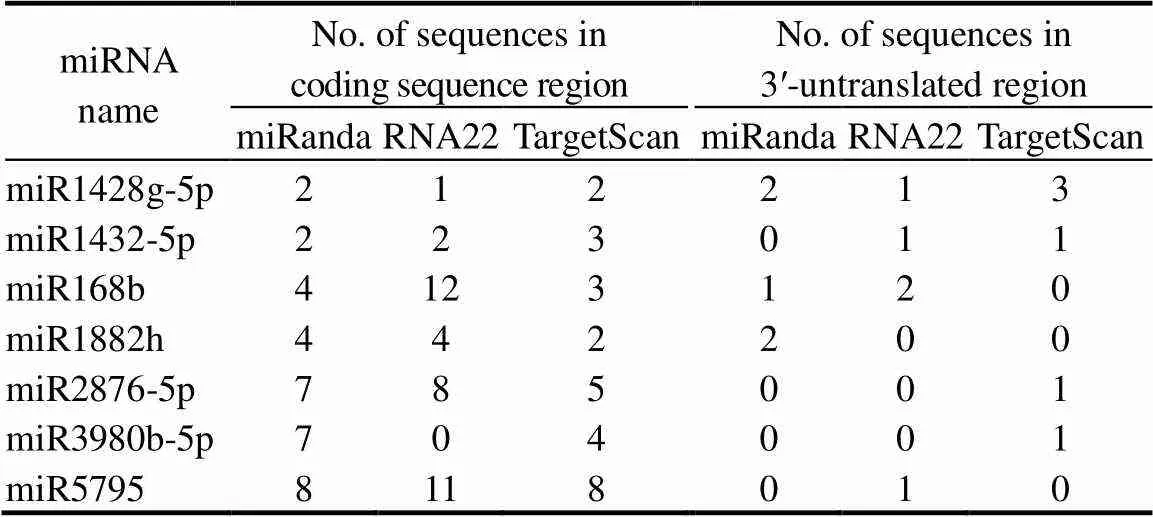
Table 2. Number of targets of rice miRNAs on fecundity of Nilaparvatalugens.
The target prediction of miR5795 showed that it had a potential binding site on the()gene in BPH, which is usually used as a molecular marker for insect reproduction (Fig. 4-D). Therefore, we fused the potential binding site oninto a reporter plasmid. Thereafter, the fused plasmid was co-transfected into a cell line in combination with miR5795 mimics. Results indicated that miR5795 resulted in a 24.30% decrease in luciferase activity (Fig. 4-E). However, when the predicted ‘seed site’ was mutated, the fused luciferase reporters were unaffected by miR5795 (Fig. 4-E). Thus, miR5795 can specifically recognize the potential binding site of NlVg in BPH. Furthermore, to determine the potential effects of miR5795 onexpression, we detected the mRNA and protein levels ofin BPH after injection of miR5795 mimics. Although the mRNA levels ofat 48 and 72 h after miR5795 mimics injection were significantly higher than those in the control (Fig. 4-F), the protein translation ofwas inhibited (Fig. 4-G and -H). Given the incomplete base pairing of miR5795 with(Fig. 4-D), miR5795 mediated the fecundity of BPH possibly by inhibiting NlVg protein translation.
DISCUSSION
Plants and insects interact in complex ways (Lucas- Barbosa, 2016). To resist the invasion of insects, plants have evolved constitutive and inducible defense mechanisms to reduce tissue damage (Liet al, 2018). miRNAs are involved in rice-BPH interactions (Wuet al, 2017; Nandaet al, 2020; Tanet al, 2020). In contrast with previous studies, we analyzed the miRNA expression of two BPH-susceptible rice varieties, TN1 and Nipponbare, as well as four rice varieties carrying different BPH-resistance genes, Mudgo (), IR36 (), RH () and R476 (). To the best of our knowledge, this is the first report to analyze the miRNA expression of multiple BPH-susceptible and BPH-resistant rice varieties.
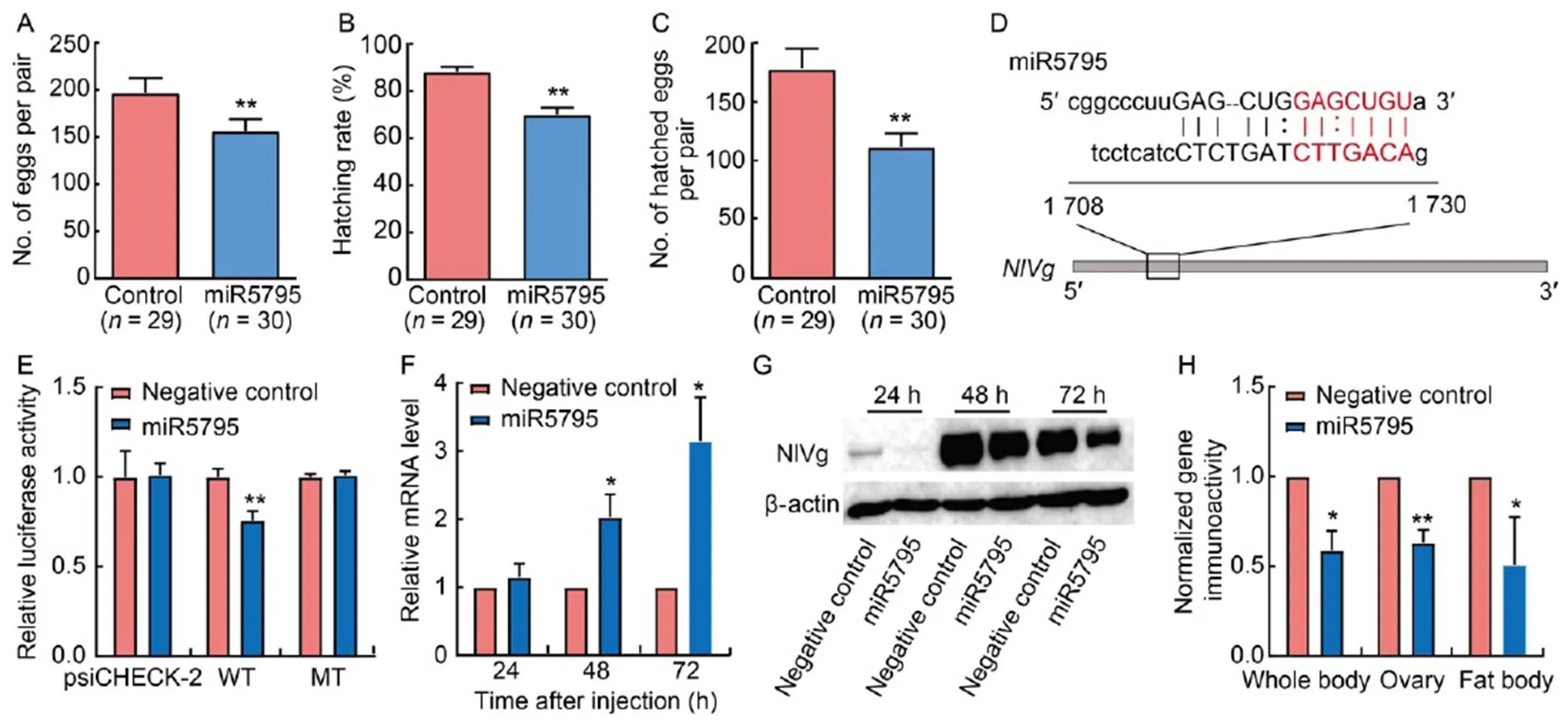
Fig. 4. miR5795 in rice may targetgene in brown planthopper (BPH) and subsequently decrease its fecundity.
A‒C, The number of eggs per pair (A), hatching rate (B) and the number of hatched eggs per pair (C) ofafter injection of miR5795 mimics.
D, Predicted binding sites between miR5795 andwith energy of -87.65 kJ/mol.
E, Dual-luciferase assay between miR5795 and NlVg. psiCHECK-2, Vector without insertion sites; WT, Wild type vector; MT, Mutation type vector.
F, mRNA expression level ofin whole bodies of BPH after injection of miR5795 mimics at 24, 48 and 72 h.
G, Western blot for NlVg in whole bodies of BPH after injection of miR5795 mimics at 24, 48 and 72 h. β-actin was used as an internal reference.
H, Protein expression levels of NlVg in whole bodies, ovaries and fat bodies of BPH after injection of miR5795 at 72 h.
Data are Mean ± SE.= 3 in A‒C, F and H, and= 9 in E. *,< 0.05; **,< 0.01.
In this study, we found that the miRNA expression profiles of BPH-susceptible rice varieties differed from those of BPH-resistant rice varieties, and these differences may be the reason for their different resistances (Fig. 1-A to -C). KEGG enrichment results indicated that the differential expression of miRNA might lead to differences in the synthesis of secondary metabolites, plant hormones and MAPK signaling pathways (Fig. 1-D). Dai et al (2019) reportedthat miR396 can regulate the biosynthesis of flavonoids, thereby negatively regulating BPH resistance in rice. Our analysis showed that flavonoid biosynthesis might participate in the defense response of rice to BPH. Moreover, we found that the expression levels of miR396 in RH and Mudgo were down-regulated, implying that BPH resistance in rice increased, which was consistent with Dai et al (2019).
It was noted that different rice varieties had significant differences in the amounts, types, changing trends and response periods of miRNAs in response to BPH-feeding (Table 1), which was consistent with previous studies. For example, P15 rice variety (carrying with) responds to BPH-feeding with far more miRNAs than susceptible rice variety PC, and only a few miRNAs between the two varieties are identical (Wuet al, 2017). In terms of changing trends and response periods, most of the differentially expressed miRNAs in IR36 are up-regulated, and the early stage response of BPH-feeding is stronger than that at late stage, which is similar to P15 (Wuet al, 2017). In addition, Nanda et al (2020) found that the genetic history of BPH affects the rice-BPH interactions. In general, the rice-BPH interactions mediated by miRNAs involve complex regulatory networks, which are affected by the genetic history of rice and BPH. However, we also found that the four BPH-resistant rice varieties shared multiple defense pathways, revealing the similarity in defense strategies mediated by BPH-resistance genes (Fig. 2-C).
Plant miRNAs are passed into animals feeding on respective plants, which can regulate the gene expression of the animal (Zhouet al, 2017). Therefore, rice miRNAs could have been transferred to BPH during feeding, which may regulate gene expression of BPH. In this study, we investigated the potential of cross- kingdom miRNAs in rice to expand the understanding of miRNA transfer between species. In cross-kingdom miRNA screening, miRNAs terminating with 5′-U residue were not selected because they tend to bind to the Ago1 protein that is important in plants (Miet al, 2008). In addition, BPHs are phloem-feeders,so they can ingest miRNAs that are secreted extracellularly (Liet al, 2018). However, the miRNAs bind to the Ago1 protein are unlikely to be secreted extracellularly (Dunoyeret al, 2010). In contrast, the miRNAs terminating with 5′-A residue bind to the Ago2 protein, which play an important role in plant biological and abiotic stress responses. The plant Ago2 protein has functions similar to animal Ago2 protein, which promotes miRNAs to load into vesicles, secretes miRNAs out of the cell, and functions outside the cell (Lvet al, 2014). As a result, miRNAs terminating with 5′-A residue are more likely to be secreted into the ligament and ingested by BPH, allowing for cross-border regulation of the BPH genes. The KEGG pathway enrichment results showed that the targets of the seven miRNAs were mainly involved in fecundity, feeding, digestion and detoxification (Fig. 3-C).
The target sites of miR1428g-5p and miR5795 were mainly located in the CDS region of the gene, which was consistent with most plant miRNA target sites (Llaveet al, 2002; Rhoadeset al, 2002). Our results showed that miR5795 might target thegene in BPH and subsequently decreased BPH fecundity (Fig. 4). In a related study, plant miR162a was reported to regulate the honeybeegene and inhibit the ovarian and overall development of juveniles in a transboundary manner (Zhuet al, 2017). However, in this study,mRNA expression level was increased after miR5795 injection (Fig. 4-F), while miR162a resulted in the reduction of honeybeemRNA expression level. Therefore, the mechanism of gene regulation by miR5795 and miR162a may be distinct and required further investigation. In addition, the degrees of base complementarity of miRNA and mRNA determine regulation (Zhanget al, 2007). miR162a is more complementary to its target gene, which may regulateexpression through mRNA degradation, and miR5795 was complementary only in the seed region and may be regulated in translation inhibition (Fig. 4).
Studies of cross-kingdom miRNAs have progressed in recent years. However, it is still unknown whether BPH can ingest miR5795 under natural conditions, and further research is needed. Overall, our study provides a better understanding of the regulatory mechanisms of rice-BPH interactions and gives data references and identifies new genetic materials for breeding of rice varieties containing insect-resistance genes.
METHODS
Plant, insect and cell lines
The seeds of six rice varieties, i.e., TN1, Nipponbare, IR36, R476, Mudgo and RH, were sown in pots (12 cm in diameter and 12 cm in height), and rice seedlings were grown in a greenhouse under standard growth conditions. The BPH population was obtained from rice fields in Guangdong Province, China. All BPHs were reared in the same walk-in chamber at 26 ºC ± 1 ºC under a photoperiod of 16 h light and 8 h dark with a relative humidity of 80% ± 10% for a susceptible rice variety Huanghuazhan. The 293T cells were cultured in 1× Dulbecco’s Modification of Eagle’s Medium (Corning Inc., NY, USA) supplemented with 10% fetal bovine serum premium (PAN Biotech GmbH, Aidenbach, Germany) at 37 ºC under 5% CO2.
miRNA sequencing analysis
Three-week-old individual rice plants were infested with twenty 3rd, 4th and 5th instar BPH nymphs that had been starved for 2 h. The stems were collected at 0, 8 and 32 h in triplicate after BPH-feeding. RNA was extracted and purified, and adaptors were added to the 5′- and 3′-ends using T4 ligase. RNA was then amplified for library construction and sequence using BGISEQ-500 (Beijing Genomics Institute, Shenzhen, China). Raw reads were filtered to remove low quality reads, incorrect adaptors, poly-A and those shorter than 18 nt. The clean reads were used to search against the miRBase database (release 22, http://www.mirbase.org/) for known rice miRNA identification. The frequency of miRNA counts was normalized as transcripts per million (TPM). PCA analysis was conducted using TPM of all miRNAs by the R package (http://www.r- project.org/).
Differential expression analysis of miRNAs
The-value of differential expression was calculated using the R DESeq2 package (Loveet al, 2014). We used the absolute value of log2(Fold change) ≥ 1 and< 0.05 as the threshold to determine significant differences between miRNA expression of BPH-susceptible and BPH-resistant rice varieties.
Prediction and functional annotation of miRNA target genes
We used the psRNATarget (v2) software (Daiet al, 2018) to predict miRNA target genes on rice, while used miRanda (v3.3a) (Johnet al, 2004), TargetScan (v7.0) (Agarwalet al, 2015) and RNA22 (v2) (Loher and Rigoutsos, 2012) softwares to predict miRNA target genes on BPH. The known fecundity- related genes in BPH are listed in Table S2. KEGG (https:// www.kegg.jp/) was used to identify the functions of target genes.
Fecundity bioassay
Each one-day-old female BPH injected with a drop of 20 μmol/L miRNA mimics (50 nL) was paired with two untreated male BPHs and then transferred to fresh rice plants. The pairs of adults were removed after 7 d, and then the number of hatched nymphs was counted daily. When the nymphs no longer hatched for two consecutive days, the number of unhatched eggs was counted. The experiment was replicated three times during three seasons (winter, spring and summer). Ten ovaries of female BPHs were dissected in PBS solution (0.02 mol/L, pH 7.4) at 72 h after injection. The ovaries were viewed and photographed with an Olympus photomicroscope (Olympus Corporation Company, Kyoto, Japan). The ovaries of brachypterous BPH were divided into five stages according to ovariole development (Dong et al, 2011).
Luciferase reporter assay
The binding site was fused into the downstream position of the firefly luciferase gene in the psiCHECK-2 reporter plasmid. Cultured cells were prepared for transfection by seeding 1 × 106cells/mL in a 96-well plate. After culturing the cells for 12–18 h, transfection was performed using FuGENE HD Transfection Reagent (Promega, Madison, WI, USA). The transfection mixture per well contained 0.3 μL FuGENE reagent, 100 ng fused plasmid and 0.5 μL miR5795 mimics. The cells were collected at 48 h after transfection and lysed using 50 μL passive lysis buffer in a linear shaker at 1 500 r/min for 15 min. The luciferaseactivity detection was performed using a multifunctional microplate reader (TriStar LB941; Berthold Technologies, Bad Wildbad, Germany) and a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) following the manufacturer’s instructions.
qRT-PCR analysis
Total RNA was extracted from samples using MagZolTM Reagent (Angen Biotech, Guangzhou, China) following the manufacturer’s instructions. One microgram of RNA was used for the first-strand complementary DNA (cDNA) synthesis using Color Reverse Transcription Kit (EZB Bioscience, Roseville, MN, USA). qRT-PCR was performed using a Light Cycler 480 (Roche Diagnostics, Indianapolis, IN, USA) with 2× Color SYBR Green qPCR Master Mix (EZB Bioscience, Roseville, MN, USA) following the manufacturer’s instructions. Each reaction mixture included 1 μL of cDNA template equivalent to 1 ng of total RNA, 0.3 μL of each primer (10 μmol/L) and 5 μL of SYBR Mix in a total volume of 10 μL. Reactions were performed in triplicate for each sample and three reactions for each biological replicate (= 3) were performed. The gene expression levels were normalized to the expression level ofgene (Chenet al, 2013). The specific primers used for qRT-PCR are listed in Table S3. The amplification conditions were as follows: 95 ºC for 30 s, followed by 40 cycles of 95 ºC for 5 s, 60 ºC for 30 s and 72 ºC for 5 s.
Western blot analysis
Total protein from whole bodies ofwas extracted from three females at 24, 48 and 72 h after miRNA mimics injection. Fat bodies and ovaries were extracted from 20 females in each of the three replicates performed. Whole or fat bodies or ovaries were lysed in RIPA Lysis Buffer (Yeasen Biotech, Shanghai, China). The homogenate was centrifuged at 12 000 ×at 4 ºCfor 15 min, and protein content in the supernatant was measured using the Bradford method. The western blot technique was modified according to Mitsumasuet al (2008). Total protein (10 μg) were separated using 10% SDS-PAGE and transferred to poly (vinylidene difluoride) membranes (0.45 μm, Millipore-Sigma, Burlington, MA, USA), and the membranes were immunoblotted with anti-NlVg (vitellogenin, 1:500; Abmart, Berkeley Heights, NJ, USA) and anti-β-actin (1:4 000; Abcam, Cambridge, UK). The secondary antibody was immunoglobulin G (IgG) goat anti-rabbit antibody conjugated to horseradish peroxidase (HRP) (1:20 000; TransGen Biotech, Beijing, China). The membranes were visualized using electrochemi-luminescence (MilliporeSigma, Burlington, MA, USA) and Image Lab (Bio-Rad Laboratories, Hercules, CA, USA). The protein bands were quantified by importing the images into the ImageJ analysis software (v1.52a).
Statistical analysis
For the statistical analysis of the fecundity bioassay of BPH, the number of eggs laid was transformed by square root, the hatching rate values were transformed by arcsine square root, and the differences between the two groups were analyzed using two-way ANOVA to exclude the effects of seasonal differences. All results are expressed as Mean ± SE, and statistical differences were considered significant at< 0.05 and< 0.01.
ACKNOWLEDGEMENTS
This study was supported by the Key Realm Research and Development Program of Guangdong Province, China (Grant No. 2020B0202090001) and the Foundation of Guangzhou Science and Technology Key Project, China (Grant No. 201904020041).
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1. Length and 5′-terminal nucleotide distribution of miRNAs.
Fig. S2. miR1428g-5p in rice did not affect fecundity of brown planthopper.
Fig. S3. Developmental stages of ovaries at 72 h after injection of miR5795 mimics.
Table S1. Sequencing data for rice samples (quoted BGI-tech).
Table S2. Known fecundity-related genes in.
Table S3. Primer sequences used in this study.
Agarwal V, Bell G W, Nam J W, Bartel D P. 2015. Predicting effective microRNA target sites in mammalian mRNAs., 4: e05005.
Akanksha S, Lakshmi V J, Singh A K, Deepthi Y, Chirutkar P M, Ramdeen, Balakrishnan D, Sarla N, Mangrauthia S K, Ram T. 2019. Genetics of novel brown planthopper(Stål) resistance genes in derived introgression lines from the interspecific crossvar. Swarna ×., 98: 113.
Bao Y Y, Wang Y, Wu W J, Zhao D, Xue J, Zhang B Q, Shen Z C, Zhang C X. 2012.intestine-specific transcriptome of the brown planthopperrevealed potential functions in digestion, detoxification and immune response., 99(4): 256–264.
Bartel D P. 2004. MicroRNAs: Genomics, biogenesis, mechanism, and function., 116(2): 281–297.
Chen H, Stout M J, Qian Q, Chen F. 2012. Genetic, molecular and genomic basis of rice defense against insects., 31(1): 74–91.
Chen J, Liang Z K, Liang Y K, Pang R, Zhang W Q. 2013. Conserved microRNAs miR-8-5p and miR-2a-3p modulate chitin biosynthesis in response to 20-hydroxyecdysone signaling in the brown planthopper,., 43(9): 839–848.
Cheng X Y, Zhu L L, He G C. 2013. Towards understanding of molecular interactions between rice and the brown planthopper., 6(3): 621–634.
Chin A R, Fong M Y, Somlo G, Wu J, Swiderski P, Wu X W, Wang S E. 2016. Cross-kingdom inhibition of breast cancer growth by plant miR159., 26(2): 217–228.
Dai X B, Zhuang Z H, Zhao P X. 2018. psRNATarget: A plant small RNA target analysis server (2017 release)., 46(W1): W49–W54.
Dai Z Y, Tan J, Zhou C, Yang X F, Yang F, Zhang S J, Sun S C, Miao X X, Shi Z Y. 2019. The OsmiR396-OsGRF8-OsF3H- flavonoid pathway mediates resistance to the brown planthopper in rice ()., 17(8): 1657–1669.
Dong S Z, Ma Y, Hou Y, Yu X P, Ye G Y. 2011. Development of an ELISA for evaluating the reproductive status of female brown planthopper,, by measuring vitellogenin and vitellin levels., 139(2): 103–110.
Du B, Zhang W L, Liu B F, Hu J, Wei Z, Shi Z Y, He R F, Zhu L L,Chen R Z, Han B, He G C. 2009. Identification and characterization of, a gene conferring resistance to brown planthopper in rice., 106(52): 22163–22168.
Dunoyer P, Schott G, Himber C, Meyer D, Takeda A, Carrington J C, Voinnet O. 2010. Small RNA duplexes function as mobile silencing signals between plant cells., 328: 912–916.
Fujita D, Kohli A, Horgan F G. 2013. Rice resistance to planthoppers and leafhoppers., 32(3): 162–191.
Ge Y F, Han J Y, Zhou G X, Xu Y M, Ding Y, Shi M, Guo C K, Wu G. 2018. Silencing of miR156 confers enhanced resistance to brown planthopper in rice., 248(4): 813–826.
Guo J P, Xu C X, Wu D, Zhao Y, Qiu Y F, Wang X X, Ouyang Y D, Cai B D, Liu X, Jing S L, Shangguan X X, Wang H Y, Ma Y H, Hu L, Wu Y, Shi S J, Wang W L, Zhu L L, Xu X, Chen R Z, Feng Y Q, Du B, He G C. 2018.encodes an exocyst- localized protein and confers broad resistance to planthoppers in rice., 50(2): 297–306.
Jena K K, Kim S M. 2010. Current status of brown planthopper (BPH) resistance and genetics., 3(2/3): 161–171.
John B, Enright A J, Aravin A, Tuschl T, Sander C, Marks D S. 2004. Human microRNA targets., 2(11): e363.
Li C D, Wong A Y P, Wang S, Jia Q, Chuang W P, Bendena W G, Tobe S S, Yang S H, Chung G, Chan T F, Lam H M, Bede J C, Hui J H L. 2018. miRNA-mediated interactions in and between plants and insects., 19(10): 3239.
Liu Y Q, Wu H, Chen H, Liu Y L, He J, Kang H Y, Sun Z G, Pan G, Wang Q, Hu J L, Zhou F, Zhou K N, Zheng X M, Ren Y L, Chen L M, Wang Y H, Zhao Z G, Lin Q B, Wu F Q, Zhang X, Guo X P, Cheng X N, Jiang L, Wu C Y, Wang H Y, Wan J M. 2015. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice., 33(3): 301–305.
Llave C, Xie Z X, Kasschau K D, Carrington J C. 2002. Cleavage ofmRNA targets directed by a class ofmiRNA., 297: 2053–2056.
Loher P, Rigoutsos I. 2012. Interactive exploration of RNA22 microRNA target predictions., 28(24): 3322–3323.
Love M I, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2., 15(12): 550.
Lucas-Barbosa D. 2016. Integrating studies on plant-pollinator and plant-herbivore interactions., 21(2): 125–133.
Lv Z Y, Wei Y, Wang D, Zhang C Y, Zen K, Li L M. 2014. Argonaute 2 in cell-secreted microvesicles guides the function of secreted miRNAs in recipient cells., 9(7): e103599.
Mi S J, Cai T, Hu Y G, Chen Y M, Hodges E, Ni F R, Wu L, Li S, Zhou H Y, Long C Z, Chen S, Hannon G J, Qi Y J. 2008. Sorting of small RNAs intoArgonaute complexes is directed by the 5′ terminal nucleotide., 133(1): 116–127.
Mitsumasu K, Azuma M, Niimi T, Yamashita O, Yaginuma T. 2008. Changes in the expression of soluble and integral-membrane trehalases in the midgut during metamorphosis in., 25(7): 693–698.
Nanda S, Yuan S Y, Lai F X, Wang W X, Fu Q, Wan P J. 2020. Identification and analysis of miRNAs in IR56 rice in response toBPH infestations of different virulence levels., 10(1): 19093.
Rhoades M W, Reinhart B J, Lim L P, Burge C B, Bartel B, Bartel D P. 2002. Prediction of plant microRNA targets., 110(4): 513–520.
Tan J Y, Wu Y, Guo J P, Li H M, Zhu L L, Chen R Z, He G C, Du B. 2020. A combined microRNA and transcriptome analyses illuminates the resistance response of rice against brown planthopper., 21(1): 144.
Wu S F, Zeng B, Zheng C, Mu X C, Zhang Y, Hu J, Zhang S, Gao C F, Shen J L. 2018. The evolution of insecticide resistance in the brown planthopper (Stål) of China in the period 2012‒2016., 8(1): 4586.
Wu Y, Lv W T, Hu L, Rao W W, Zeng Y, Zhu L L, He Y Q, He G C. 2017. Identification and analysis of brown planthopper- responsive microRNAs in resistant and susceptible rice plants., 7(1): 8712.
Yang L, Zhang W L. 2016. Genetic and biochemical mechanisms of rice resistance to planthopper., 35(8): 1559–1572.
Zhang B H, Wang Q L, Pan X P. 2007. MicroRNAs and their regulatory roles in animals and plants., 210(2): 279–289.
Zhang L, Hou D X, Chen X, Li D H, Zhu L Y, Zhang Y J, Li J, Bian Z, Liang X Y, Cai X, Yin Y, Wang C, Zhang T F, Zhu D H, Zhang D M, Xu J, Chen Q, Ba Y, Liu J, Wang Q, Chen J Q, Wang J, Wang M, Zhang Q P, Zhang J F, Zen K, Zhang C Y. 2012. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA., 22(1): 107–126.
Zhou G Y, Zhou Y, Chen X. 2017. New insight into inter-kingdom communication: Horizontal transfer of mobile small RNAs., 8: 768.
Zhou Z, Li X H, Liu J X, Dong L, Chen Q, Liu J L, Kong H H, Zhang Q Y, Qi X, Hou D X, Zhang L, Zhang G Q, Liu Y C, Zhang Y J, Li J, Wang J, Chen X, Wang H, Zhang J F, Chen H L, Zen K, Zhang C Y. 2015. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses., 25(1): 39–49.
Zhu K G, Liu M H, Fu Z, Zhou Z, Kong Y, Liang H W, Lin Z G, Luo J, Zheng H Q, Wan P, Zhang J F, Zen K, Chen J, Hu F L, Zhang C Y, Ren J, Chen X. 2017. Plant microRNAs in larval food regulate honeybee caste development., 13(8): e1006946.
24 February 2022;
11 May2022
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2022.05.003
Zhang Wenqing (lsszwq@mail.sysu.edu.cn)
(Managing Editor: Wu Yawen)
杂志排行
Rice Science的其它文章
- Transcriptome Analysis Provides Novel Insights into Salt Stress Response in Two Egyptian Rice Varieties with Different Tolerance Levels
- QTL Mapping for Plant Height Using Introgression Lines Derived from Zhonghui 8015 and Wild Rice (Oryza rufipogon)
- Genetic Dissection of Quantitative Trait Loci for Panicle Traits and Heat Tolerance by High-Density Bin Map in Rice
- Improvement of Rice Production under Drought Conditions in West Africa:Application of QTLs in Breeding for Drought Resistance
- Characterization of a Novel Weak Allele of RGA1/D1 and Its Potential Application in Rice Breeding
- CRISPR/Cas9-Targeted Knockout of Rice Susceptibility Genes OsDjA2 and OsERF104 Reveals Alternative Sources of Resistance to Pyricularia oryzae
