Transcriptome Analysis Provides Novel Insights into Salt Stress Response in Two Egyptian Rice Varieties with Different Tolerance Levels
2022-10-25ShehabMohamedIoveneMarinaCiancioAurelioColagieroMariantoniettaFinettiSialerMariella
Shehab Mohamed, Iovene Marina, Ciancio Aurelio, ColagieroMariantonietta, Finetti-Sialer Mariella
Letter
Transcriptome Analysis Provides Novel Insights into Salt Stress Response in Two Egyptian Rice Varieties with Different Tolerance Levels
Shehab Mohamed1, Iovene Marina2, Ciancio Aurelio3, ColagieroMariantonietta3, Finetti-Sialer Mariella2
(Kafr El-Sheikh 33717, Egypt; Institute of Biosciences and Bioresources, National Research Council, Bari 70126, Italy; Sustainable Plant Protection Institute, National Research Council, Bari 70126, Italy)
The response of rice to salt stress (200 mmol/L NaCl) was investigated at the transcription level in Egyptian varieties Giza177 (salt sensitive variety) and Giza178 (salt tolerant variety). We applied a genome-wide RNA-Seq transcriptome study at 21-day-old seedlings of both varieties, exposed or not to salt stress for 24 h. Most differentially expressed genes (DEGs) between the two varieties in response to salt stress were related to the expression of genes active at the cell wall (CW) level, including wall modification, hemicellulose/cellulose synthesis and transcripts of the peroxidase family activated in response to oxidative stress/oxidation reduction, which were significantly more represented in Giza 178. Consistently, Gene Ontology (GO) analysis showed differentially expressed transcripts, involved in response to oxidative stress and chemical stimulus, directly implicated in salt stress response and up-regulated in Giza 178, as well as oxidoreductase, peroxidase and antioxidant activities. When the two varieties were directly compared in exposed or not to salt stress conditions, Giza 177 showed a higher number of differentially expressed and unique loci than Giza 178, including transposable elements (TE). However, Giza 178 showed a higher number of transcription factors (TF) expressed, mostly involving myeloblastosis (MYB) family members and bZIP elements, with annotated elements including zinc finger domain, kinase, expansin, cellulose, sucrose synthase, peroxidase precursor, dehalogenase-like hydrolase, and sodium/ calcium exchanger protein.
Salinization exerts the most negative effect on rice worldwide, acting as an important limiting factor in production (Korres et al, 2019). Tolerance to salt stress in plants is a multigenic trait, whose mechanism is not yet fully decyphered. Salinity challenges plant metabolism by mostly provoking a growth reduction due to a shortage of available water, interfering with nutrient uptake, stomatal and mesophyll conductance, and ion toxicity (Munns and Tester, 2008). Several studies highlighted physiological and molecular changes underlying salt tolerance through unique adaptation mechanisms (Baldoni et al, 2016; Ghosh et al, 2016; Acosta-Motos et al, 2017). We investigated the response to salt stress of Egyptian rice varieties Giza 177 and Giza 178 by the RNA-Seq study, to identify the mechanisms underpinning their divergent performance. Computational and experimental approaches were combined to characterize the responsive genes. The raw transcript data produced are available at NCBI (Project Accession No. PRJNA782864). Different pipelines were applied to obtain high quality base sequences (Table S1).
Global analyses and DEG mappings for the two varieties subjected to salt stress showed a diverse modulation, when compared to the corresponding control (Fig. S1). Giza 177 showed 2 629 up-regulated and 2 802 down-regulated genes in stressed plants (Table S2). Giza 178 displayed 2 997 up-regulated and 2 813 down-regulated genes in stressed plants (Table S3). Both varieties shared a common set of 1 612 genes (855 up- and 757 down-regulated), and a contrasting expression for 50 genes in Giza 178 and 43 genes in Giza 177, respectively (Fig. S1-A). MapMan analysis showed that differences in salt response between the two varieties were related to transcripts involved in the CW metabolism (Fig. S1-B and -C). In Giza 178, 30 DEGs involved in CW modification were up-regulated with at least a 2-fold change (2-FC), with only 3 genes down-regulated. Moreover, there were three and eight genes of the CW hemicellulose and cellulose synthesis pathways exceeding the 2-FC treshold, respectively. Giza 177 displayed less functional categories up-regulated in CW modification, with only 16 genes exceeding the 2-FC treshold, and 5 genes down-regulated. However, the CW hemicellulose synthesis pathway showed the same trend in bothvarieties. An affinity in regulated genes was shown for CW degradation i.e., mannan-xylose-arabinose-fucose, in which six genes were up-regulated in each variety, with two genes (and) regulated at the same extent (Tables S2 and S3). CW provides the first physical barrier to any environmental adversity. It deploys a relevant function in plant development, acting as an interface with the outer environment, mediating indispensable physiological and biochemical processes (Leschevin et al, 2021).
Further, DEGs up-regulated in Giza 178 included TFs of the MYB, bZIP and histone families (Fig. 1-A and Fig. S2), as well as transcripts of the peroxidase family. In the peroxidase family, Giza 177 showed more down-regulated genes, with only five transcripts with at least 2-FC (Fig. 1-B). Further DEGs unique for Giza 178 included four zinc finger proteins, prevalently of the C3HC4 type domain (LOC_Os03g22830, LOC_Os03g24184,LOC_Os04g10680 and LOC_Os05g25180). These proteins form a finger-like structure and have the capability to bind Zn2+. They represent one of the largest transcriptional regulators in plants and are induced during growth and development, as well as under unfavorable conditions such as water deficiency and salinity (Han et al, 2020). Consistently with the different salt tolerance reported for the two varieties tested, MapMan analysis showed only 28 TFs (14 MYB, 3 bZIP, 2 WRKY and 1 BHLH) up-regulated in Giza 177 when compared to the control (Table S4). In Giza 178, a higher number (46) of TFs was found, including 15 MYB, 7 bZIP, 1 WRKY and 3 BHLH (Table S5). The activation of several TFs was reported in a pool of 306 rice accessions tested under salt stress (Patishtan et al, 2018). TE transcripts were also differentially expressed by the two varieties when stressed, with a double number of TE-related genes uniquely expressed by Giza 177 (8) vs Giza 178 (4) (with 2 in common). Transposons are involved in genetic re-structuring, in particular in self-fertilizing plants such as rice, and may be fundamental in a stress condition (Negi et al, 2016). Although no direct indication could be derived about the target genes or processes eventually affected, our data suggested that the expression of TE could be, at least in part, responsible for the higher sensitivity (or loss of tolerance) of Giza 177 to salt stress. This hypothesis, however, needs support by additional experimental evidence. DEGs associated to stress response in Giza 178 showed different groups associated to stress signaling, such as phosphatases, which are involved in different cell functions (Xue et al, 2008). Two phosphatases, LOC_Os01g37130 and LOC_Os02g55560, were present among the 54 genes uniquely expressed in Giza 178 under salt stress (Table S6). Phosphatases are regulatory proteins that sense and transduce environmental signals, and play a key role in the abiotic stress response, acting on the expression of downstream genes. They are correlated withthe higher plasticity shown by plants in a challenging environment (Fuchs et al, 2013; Singh et al, 2016).
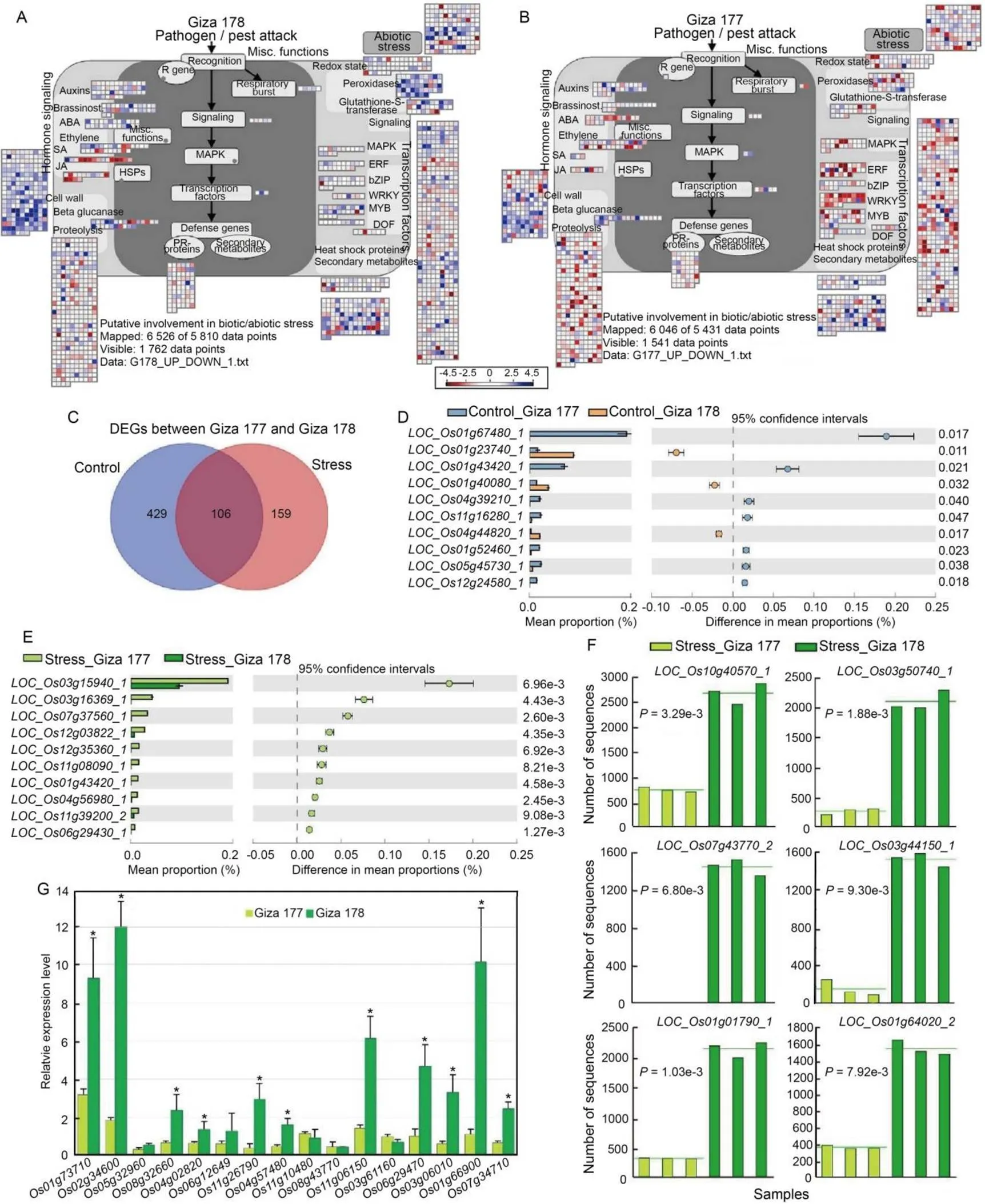
Fig. 1. Gene expression analysis between rice varieties Giza 177 and Giza 178 exposed or not to salinity stress.
A and B, Differential expression (|fold change|2 and-value0.05) of up- and down-regulated genes in Giza 178 and Giza 177 under salt stress. Colors indicate transcripts up/down regulation (see legend). ABA, Abscisic acid; SA, Salicylic acid; JA, Jasmonic acid; HSPs, Heat shock proteins; PR, Pathogenesis related; MAPK, Mitogen-activated protein kinases; ERF, Ethylene-responsive element binding factor; DOF, DNA binding with one finger. C, Venn diagram showing the repartition of differentially expressed genes (DEGs) between Giza 177 and Giza 178 in control and salt stress treatments.D and E, Top 10 (out of 535) most significant DEGs between Giza 177 and Giza 178 in control (D) and salt stressed (E) plants.F, Most significant DEGs up-regulated in Giza 178 and Giza 177 under the salt stress condition. The columns show the mean, and the horizontal line represents means of three replicates.G, qRT-PCR data from total RNA showed consistent differential expression for 11 out of 16 loci tested (asterisks).
Gene Ontology (GO) analysis of enriched terms was used to classify the DEG functions. Those involved in response to oxidative stress and chemical stimulus were directly implicated in salt stress response and were up-regulated in Giza 178, as did oxidoreductase, peroxidase and antioxidant activities (Fig. S3and Table S7). Comparisons between control and stress conditions, performed with STAMP (Statistical analysis of taxonomic and functional profiles, http://kiwi.cs.dal.ca/Software/STAMP), showed 535 (80.1%) DEGs in control and 265(67.6%) in salt stress, respectively (Fig. 1-C and Table S8), with 106 transcripts in common (Fig. 1-C). This comparative analysis showed a higher number of DEGs in Giza 177 underexposed or not to salt stress conditions (Fig. 1-D and -E). Most significant DEGs up-regulated by the salt stress in Giza 178 are shown in Fig. 1-F. Only 1 transcript annotated (LOC_Os12g36630)with the term ‘stress’ was found, expressed in both conditions, out of 79 genes with a ‘stress’ term in thegenome. The 265 DEGs betweenthe two varieties under salt stress included 14 transposons (5.2%, 8 unique for Giza 177 and 2 in common), 24 kinases (9.0%) and 15 transferases (5.6%) (Table S7). Giza 177 showed 105 of the 159 DEGs unique for the salt stress condition (Table S8). Giza 178 showed 66 (25%) up-regulated genes out of the 265 DEGs. The 54 DEGs unique for Giza 178 under salt stress showed 11 loci with annotation as expressed protein, followed by 4 loci with annotations including transposon, zinc finger domain and kinases, 2 with rust resistance and phosphatases, and 1 each with expansin, cellulose, sucrose synthase, peroxidase precursor, dehalogenase-like hydrolase and sodium/calcium exchanger protein (Table S8). In the stress treatment, their chromosome distribution showed a higher frequency for chromosomes 1, 3 and 5 (each with > 30), and a lower representation in chromosomes 7, 8 and 9 (< 12) (Fig. S4). Finally, the qRT-PCR data from total RNA showed consistent differential expression for 11 loci out of the 16 tested, shown in Fig. 1-G (annotations and corresponding FC in Table S9).
In conclusion, global data analyses indicated that the different phenotypic responses observed for the two varieties are consistent with differences in a number of key metabolic processes (Fig. S5). Further network analyses may be needed to identify the loci progressively steering the plant metabolic pathways during their response to salt stress, which can be targeted in future selection programs.
ACKNOWLEDGEMENTS
This study was supported by the Ministry of Foreign Affairs, Directorate General for Development Cooperation, Italy and National Research Council, Rome, Italy (Grant No. 1654). The authors gratefully acknowledge Nicoletta Rapanà and Domenico De Paola (Institute of Biosciences and Bioresources, National Research Council, Bari, Italy) for the support and assistance.
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
File S1. Methods.
Fig. S1. Global analysis of differently expressed genes between stressed and control plants of Giza 177 and Giza 178.
Fig. S2. Up- and down-regulated transcription factors in Giza 177 and Giza 178 under salt stress condition.
Fig. S3. Gene Onthology analysis of up-regulated molecular and cellular processes in Giza 177 and Giza 178 under salt stress condition.
Fig. S4. Chromosome distribution of 265 differentially expressed genes between Giza 177 and Giza 178 stressed plants.
Fig. S5. Crop performance of Giza 177 and Giza 178 in the field.
Table S1. Number of reads and RNA mappings.
Table S2. Differentially expressed genes in Giza 177 and their Gene Onthology analyses.
Table S3. Differentially expressed genes in Giza 178 and their Gene Onthology analyses.
Table S4. Differentially expressed genes identified by MapMan analysis in Giza 177 in different pathways.
Table S5. Differentially expressed genes identified by MapMan analysis in Giza 178 in different pathways.
Table S6. Differentially expressed loci unique for Giza 178 under salt stress condition.
Table S7. List of 265 loci differentially expressed between Giza 177 and Giza 178 under salt stress condition.
Table S8. Differentially expressed genes between Giza 177 and Giza 178, unique or in common between salt-stressed and control plants.
Table S9. Loci selected for qRT-PCR validation and related annotations.
Table S10. List of oligonucleotides used for qRT-PCR.
Acosta-Motos J R, Ortuño M F, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco M J, Hernandez J A. 2017. Plant responses to salt stress: Adaptive mechanisms., 7: 18.
Baldoni E, Bagnaresi P, Locatelli F, Mattana M, Genga A. 2016. Comparative leaf and root transcriptomic analysis of two ricecultivars reveals major differences in the root early response to osmotic stress., 9(1): 25.
Fuchs S, Grill E, Meskiene I, Schweighofer A. 2013. Type 2C protein phosphatases in plants., 280(2): 681–693.
Ghosh B, Ali Md N, Gantait S. 2016. Response of rice under salinity stress: A review update., 4: 167.
Han G L, Lu C X, Guo J R, Qiao Z Q, Sui N, Qiu N W, Wang B S. 2020. C2H2 zinc finger proteins: Master regulators of abiotic stress responses in plants., 11: 115.
Korres N E, Varanasi V, Slaton N A, Price A J, Bararpour T M. 2019. Effects of salinity on rice and rice weeds: Short- and long-term adaptation strategies and weed management.: Hasanuzzaman M, Fujita M, Nahar K, Biswas J K. Advances in Rice Research for Abiotic Stress Tolerance. Oxford, UK: Woodhead Publishing: 159–176.
Leschevin M, Ismael M, Quero A, San Clemente H, Roulard R, Bassard S, Marcelo P, Pageau K, Jamet E, Rayon C. 2021. Physiological and biochemical traits of two majoraccessions, Col-0 and Ws, under salinity., 12: 639154.
Munns R, Tester M. 2008. Mechanisms of salinity tolerance., 59: 651–681.
Negi P, Rai A N, Suprasanna P. 2016. Moving through the stressed genome: Emerging regulatory roles for transposons in plant stress response., 7: 1448.
Patishtan J, Hartley T N, de Carvalho R F, Maathuis F J M. 2018. Genome-wide association studies to identify rice salt-tolerance markers., 41(5): 970–982.
Singh A, Pandey A, Srivastava A K, Tran L S, Pandey G K. 2016. Plant protein phosphatases 2C: From genomic diversity to functional multiplicity and importance in stress management., 36(6): 1023–1035.
Xue T T, Wang D, Zhang S Z, Ehlting J, Ni F, Jakab S, Zheng C C, Zhong Y. 2008. Genome-wide and expression analysis of protein phosphatase 2C in rice and., 9: 550.
14 December 2021;
12 March 2022
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2022.09.001
Finetti-Sialer Mariella (mariella.finetti@ibbr.cnr.it)
杂志排行
Rice Science的其它文章
- QTL Mapping for Plant Height Using Introgression Lines Derived from Zhonghui 8015 and Wild Rice (Oryza rufipogon)
- Genetic Dissection of Quantitative Trait Loci for Panicle Traits and Heat Tolerance by High-Density Bin Map in Rice
- Improvement of Rice Production under Drought Conditions in West Africa:Application of QTLs in Breeding for Drought Resistance
- Characterization of a Novel Weak Allele of RGA1/D1 and Its Potential Application in Rice Breeding
- CRISPR/Cas9-Targeted Knockout of Rice Susceptibility Genes OsDjA2 and OsERF104 Reveals Alternative Sources of Resistance to Pyricularia oryzae
- Identification of Potential Zinc Deficiency Responsive Genes and Regulatory Pathways in Rice by Weighted Gene Co-expression Network Analysis
