Genetic Dissection of Quantitative Trait Loci for Panicle Traits and Heat Tolerance by High-Density Bin Map in Rice
2022-10-25LiuHongyanMaXiaosongLiEnxiZengXianjunLuoLijun
Liu Hongyan, MaXiaosong, Li Enxi, Zeng Xianjun,Luo Lijun, 4
Letter
Genetic Dissection of Quantitative Trait Loci for Panicle Traits and Heat Tolerance by High-Density Bin Map in Rice
Liu Hongyan1, 2, MaXiaosong1, 2, Li Enxi1, 3, Zeng Xianjun1, 4,Luo Lijun1, 2, 4
(Shanghai Agrobiological Gene Center, Shanghai 201106, China; Key Laboratory of Grain Crop Genetic Resources Evaluation and Utilization, Ministry of Agriculture and Rural Affairs, Shanghai 201106, China; Hainan University, Haikou 570228, China; Huazhong Agricultural University, Wuhan 430070, China)
A recombinant inbred line (RIL) with 179 lines derived from a cross between a heat tolerant line Huhan 1B and a heat sensitive line Hanhui 3 was used for QTL mapping for panicle traits and heat tolerance. A total of 52 QTLs were identified with LOD scores from 3.85 to 54.93, each explaining 0.70% to 21.48% of the phenotypic variances, including 11 for panicle length (PL), 11 for seed number per panicle (SN), 14 for spikelet number per panicle (SNPP), 6 for seed-setting rate (SSR) under normal conditions, and 5 for seed-setting rate (SSR_HT) and 5 for relative seed-setting rate (RSSR) under heat stress, respectively.located ininterval was predicted as a heat tolerant candidate gene. Haplotype analysis revealed thedifferentiation of,implying possible relation to temperature adaptability in rice. An insertion/deletion (InDel) marker HSP70-12 forand a cleavage-amplified polymorphic sequence (CAPS) marker SPKIE-299 forwere developed, and validated as co-segregating markers with heat tolerance or panicle traits in different populations. These two markers can be used to facilitate rice grain yield improvement or heat tolerance via marker-assisted selection (MAS) breeding.
Rice yield is determined by the synergistic effect of the number of panicles, SN and grain weight. Among them, SN is mainly affected by PL, SNPP and SSR. Rice yield decreases significantly under high temperature stress at the heading and grain filling stages. Particularly, high temperature can cause premature degradation of the tapetum at the early uninuclear microspore stage (Ku et al, 2003), obstruction of pollen germination and pollen tube elongation, abortion of pollen mother cells, reduction of pollen vigor, and ultimately decline of spikelet fertility (Matsui et al, 2000, 2001; Prasad et al, 2006). Several studies aiming at dissecting the genetic bases of spikelet sensitivity to high temperature have been reported in the past decade. Heat tolerance, a complex quantitative trait, was controlled by multiple genes with cumulative effects (Li et al, 2015; Lafarge et al, 2017; Shanmugavadivel et al, 2017). QTLs identified for heat tolerance based on spikelet fertility in rice were first reported by Cao et al (2003) using a doubled haploid population derived from the IR64/Azucena cross. Thereafter, a number of QTLs for SSR have been identified under controlled thermal environments using RILs (Shanmugavadivel et al, 2017), backcross inbred lines (Ye et al, 2012), F2population(Ye et al, 2012), chromosome single segment substitution lines (Liu et al, 2017) and natural population (Lafarge et al, 2017).is fine mapped to a 1.2-Mb interval, and the superior alleles ofderived from N22 can increase spikelet fertility under heat stress at the flowering stage in rice (Ye et al, 2012). The effect ofevaluated with a BC5F2population shows approximately 15% increase in spikelet fertility (Ye et al, 2015).is fine mapped to a 47.1-kb interval using a set ofintrogression lines at the booting stage (Cao et al, 2020). Among eight putative genes located in the interval of, the expression levels of two candidate genes show significant changes under heat stress.gene is isolated from CG14 () by map-based cloning and demonstrated to enhance heat tolerance in rice (Li et al, 2015).
In this study, a RIL population (HH13 RILs) derived from the cross between Huhan 1B and Hanhui 3 was grown under normal conditions in paddy fields and under heat stress in the greenhouse where the air temperatures at 10:00 am to 14:00 pm during the flowering stage reached 35 ºC to 40 ºC, with 3.5 ºC and 3.1 ºC on average higher than those in the open field in 2018 and 2019, respectively (Fig. S1 and Table S1). SSR and SSR_HT were measured under normal and heat stress conditions in 2018 and 2019, respectively (Table S2). RSSR, the ratio of SSR_HT to SSR, was used to evaluate heat tolerance (Table S2).
As shown in Table S2, Hanhui 3 had little higher SSR (around 91%) than Huhan 1B (around 85%) under normal conditions, but Huhan 1B had much higher SSR_HT (around 63%) under heat stress than Hanhui 3 (around 22%). The RSSR of Huhan 1B (72.9% and 73.0%) was higher than that of Hanhui 3 (22.9% and 20.7%) in 2018 and 2019, respectively. For the panicle traits under normal conditions in 2018, 2019, and 2020, Hanhui 3 showed a longer PL and much more SN and SNPP (Table S2). These results indicated that Hanhui 3 was a large panicle but heat sensitive variety, while Huhan 1B was a heat tolerant variety.
Both panicle traits and heat tolerant traits of HH13 RILs showed transgressive segregation (Table S2 and Fig. S2). Under the heat stress conditions, the SSR_HT of the HH13 RILs ranged from 1.6% to 90.0%, and from 1.3% to 86.2% in 2018 and 2019, respectively. The RSSR of the HH13 RILs ranged from 2.0% to 98.9%, and from 1.6% to 96.1% in 2018 and 2019, respectively. The coefficients of variation of SSR_HT and RSSR were both greater than 40%. These results indicated the drastic variations on heat tolerance among the HH13 RILs. The broad-sense heritabilities of PL, SN, SNPP and SSR were 0.31, 0.51, 0.45 and 0.96, respectively (Table S2). The highest broad-sense heritability of SSR indicated the genetical stability of SSR. Pearson’s correlation analysis of phenotypic traits showed that PL, SN, SNPP and SSR were significantly correlated (< 0.05) under normal conditions (Table S3). PL was positively correlated with SN and SNPP, but negatively correlated with SSR and RSSR in different years. RSSR was significantly positively correlated with SSR_HT, having Pearson’s correlation coefficient as high as 0.99 in both 2018 and 2019, implying that the variation in RSSR was mostly determined by the variation of SSR_HT.
A high-density genetic map of the HH13 RIL (F10) population containing 2 806 bins was constructed using 41 063 single nucleotide polymorphisms (SNPs) (Fig. 1-A and Table S4). The number of bin markers varied from 124 (chromosome 12) to 365 (chromosome 1) with an average of 233 per chromosome. The maximum and the minimum distances between bin intervals were 7 538.77 kb and 5.00 kb, respectively, and the average distance was 135.05 kb.
A total of 52 QTLs were identified for panicle traits and heat tolerance (Fig. 1-A and Table S5). Among them, 42 QTLs, including 11 QTLs for PL, 11 QTLs for SN, 14 QTLs for SNPP and 6 QTLs for SSR, were detected under normal conditions in 2018, 2019 and 2020. Under heat stress conditions, 10 QTLs for SSR_HT and RSSR were detected. Four QTLs,,,and, were repeatedly mapped in two years. The positive alleles of 35 QTLs for PL, SN, SNPP and SSR were derived from Hanhui 3. Among the 10 QTLs for heat tolerance, the positive effects of 6 QTLs were from Huhan 1B.
There were 17 QTLsco-localized in 8 intervals, and 14 QTLs in 7 overlapping or adjacent intervals (Table S5), suggesting possibly shared genetic basis or pleiotropism among phenotypically correlated traits. For example, QTLs for PL, SN and SNPP were clustered in four chromosomal regions on chromosomes 1, 2, 4 and 5 (Fig. 1-A). These results indicated that SN is determined mainly by PL and SNPP as observed by previous studies (Xing and Zhang, 2010). QTLs for SSR, SSR_HT and RSSR were clustered in chromosomes 1 and 10 (Fig. 1-A), probably reflecting common genes or mechanism controlling the tightly correlated phenotypic traits.
The chromosomal regions hosting 15 known genes were covered by 12 QTL intervals of corresponding traits (Table S6), partially endorsed the efficiency and accuracy of the QTL mapping approach used in this study. Thirty-eight QTLs have not been reported previously, for which putative candidate genes were predicted (Table S7).
was repeatedly detected in 2018 and 2019 (Fig. 1-A and Table S5) within an interval of 19.025 kb where contains only four annotated genes. OsR498G0101910700.01 in theinterval, annotated as heat shock protein 70 (), is considered as a heat tolerance candidate gene (Table S7). Hsp70 family proteins are chaperones responsible for protein folding, assembly, translocation and degradation (Lüders et al, 2000; Shin et al, 2005; Hartl et al, 2011).plays a role in plant heat stress tolerance (Wang et al,2004; Montero-Barrientos et al, 2010). A 12-bp InDel at the coding region ofwas found between Huhan 1B and Hanhui 3 (Fig. 1-B). Compared with Hanhui 3, the deleted sequence caused deletion of four amino acids in Huhan 1B (Fig. 1-B). We further analyzed the haplotype ofbased on re-sequencing data of 4 726 rice accessions at http://ricevarmap. ncpgr.cn/. Eight haplotypes (Hap) based on 40 variation sites were found (Table S8). The distinct differentiation ofbetweenandwas revealed. Haps I and IV mainly containedsubpopulation, whereas Haps II, III, VII and VIII mainly containedsubpopulation (Fig. 1-C and Table S9). Huhan 1B and Hanhui 3 belonged to Hap II and Hap I, respectively. For Asian cultivated rice,rice that mainly grown in tropical and subtropical regions, is more tolerant to high temperature thanrice that is mainly grown in temperate region.SLG1allelefrom 9311 () is more thermo-tolerant thanSLG1allelefrom KY131 () at both seedling and reproductive stages, which is consistent with the unique geographical distribution ofandrice (Xu et al, 2020). Thedifferentiation ofmight be related to temperature adaptability. An InDel marker HSP70-12 was developed across the 12-bp InDel (Table S10) and was co-segregated with RSSR in the HH13 RILs (Fig. 1-D and -F). The RSSR ofgroup (118 lines, genotype of deletion, the same as Huhan 1B) was significantly higher than that ofgroup (56 lines, genotype of insertion, the same as Hanhui 3) (Fig. 1-D). In a natural population (mainly the mini core collection of Chinese rice germplasm), the RSSR ofgroup (71 accessions) was 39.81% while that ofgroup (79 accessions) was 52.11%, showing highly significant inter-group variation (9.44E-04, the Student’s-test) (Fig. 1-E). These results confirmed that the 12-bp InDel variation inwas associated with RSSR (i.e. the heat tolerance). HSP70-12 is a functional marker and the allele offrom Huhan 1B can be used for heat tolerance improvement via MAS in rice breeding programs.
Several putative genes were screened as candidate genes based on the annotated gene functions and research reports of expression responses to heat stress or tissue-specific expression (Table S7). We noticed that 11 candidate genes encoding NB- ARC domain containing proteins were found in heat stress tolerance QTL intervals of,and. NB-ARC domain is a type of STAND (signal transduction ATPases with numerous domains) protein, which is involved in regulation of programmed cell death (PCD) (Leipe et al, 2004). Heat stress is one of the environmental factors that can trigger PCD in plant cells (Zuppini et al, 2006). Whether these NB-ARC domain genes located in the RSSR- related QTL intervals are involved in high temperature-induced PCD or other regulations in response to high temperature needs further experimental verification.located in the interval ofwas reported to be up- regulated under high temperature (Li et al, 2018).andin theinterval can be activated by H2O2signaling in rice seedlings suffering heat stress (Chou et al, 2012). A putative gene annotated as OVATE family protein 17 () located in theinterval preferentially expresses in the young panicle tissue (Yu et al, 2015). OFP proteins are plant-specific regulatory proteins (Hackbusch et al, 2005). AtOFPs as transcriptional repressors regulate multiple aspects of growth and development (Wang et al, 2011).ishighly homologous to, and overexpression ofresults in later flowering and reduced fertility in(Wang et al, 2011). We thus speculated thatmight be a candidate gene for panicle traits. Two adjacent QTLs,and, contain eight putative genes, annotated as GDSL-like lipase/acylhydrolase, express at high levels in flowers and panicles (Chepyshko et al, 2012; Shen et al, 2022). GELPs (GDSL esterase/lipase protein) have been reported to be involved in anther and pollen development inand rice (Zhu et al, 2020).
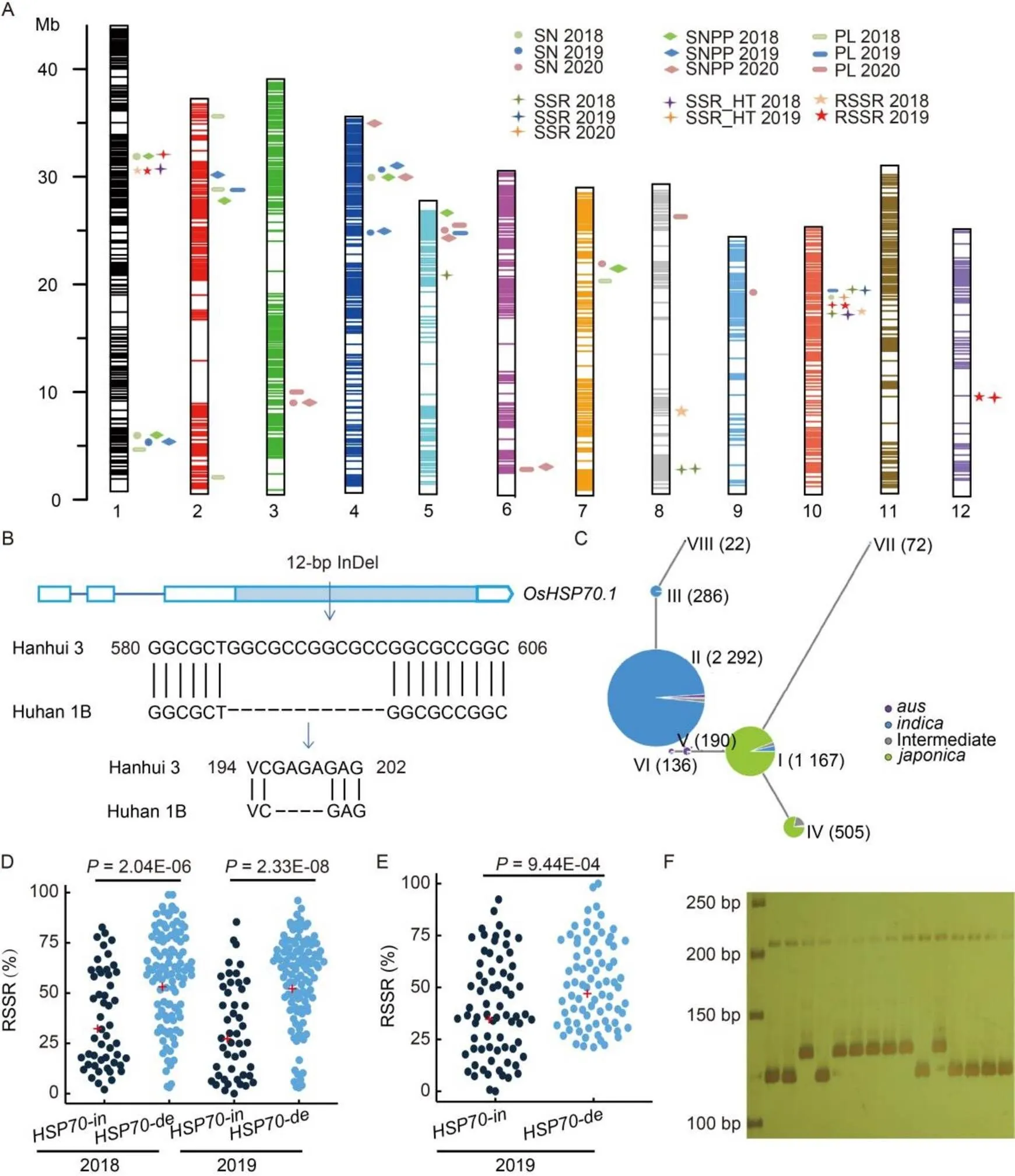
Fig. 1. QTLs detected for panicle traits and heat tolerance, and haplotype analysis and marker validation for candidate gene.
A, QTLs detected for panicle traits and heat tolerance. B, Insertion/deletion (InDel) and amino acid variations ofbetween Huhan 1B and Hanhui 3. The blue box indicates coding region, the white boxes indicate non-coding regions and the lines indicate introns. C, Haplotypes ofin rice. Numbers in parentheses indicate the number of rice accessions. D, RSSR of HH13 recombinant inbred lines in 2018 and 2019. E, RSSR of core collection population of Chinese rice germplasm in 2019. ‘+’ represents mean values in D and E. F, Schematic representation of polyacrylamide electrophoresis of InDel marker HSP70-12. The marker ladder was in the leftmost lane., Allele ofin Hanhui 3 with 12 bp insertion;, Allele ofin Huhan 1B with 12 bp deletion. SN, Seed number per panicle; SNPP, Spikelet number per panicle; PL, Panicle length; SSR, Seed-setting rate under normal conditions; SSR_HT, Seed-setting rate under heat stress conditions; RSSR, Relative seed-setting rate.
Furthermore, in the co-localized interval ofand, we found an important yield related gene, whichregulates PL, SN and SNPP (Fujita et al, 2013; Zhang et al, 2014) (Table S6). There is a single nucleotide substitution in the coding region ofbetween Hanhui 3 and Huhan 1B, forming a restriction site forI (GGTGCC/GGCGCC) in Huhan 1B. Based on this SNP, a CAPS marker SPKIE-299 was developed (Fig. S3 and Table S10). The size of the PCR product of SPKIE-299 was 299 bp. For Huhan 1B (CC-type), the PCR product can be digested withI to generate 189 bp and 110 bp bands, but not for Hanhui 3 (TT-type). SPKIE-299 was co-segregated with SN and SNPP in the HH13 RILs (Fig. S3). We also validated SPKIE-299 with HY73 RIL population (Fig. S3). Both SN and SNPP of the TT-type lines (616 lines) were significantly higher than those of CC-type lines (648 lines), withvalues of 1.35E-19 and 1.34E-26 in 2019, and 6.25E-45 and 4.44E-45 in 2020, respectively. These results clearly confirmed thatallele from Hanhui 3 exhibits higher SN and SNPP and the CAPS marker SPKIE-299 is a usable functional marker for improving SN and SNPP via MAS in rice.
QTLs and candidate genes identified in this study provide information for dissecting the genetic basis of panicle traits and heat tolerance. It has been preliminarily verified that theallele from Huhan 1B and theallele from Hanhui 3 had positive effects on heat tolerance and panicle traits, respectively. The role of these two functional markers in MAS breeding practices can be further assessed.
ACKNOWLEDGEMENTS
This study was supported by the Natural Science Foundation of Shanghai, China (Grant Nos. 18ZR1433500 and 19ZR1446700), and Runup Talent Project of Shanghai Academy of Agricultural Sciences Program, China (Grant No. ZP21231). We thank Professor Yu Xinqiao for providing seeds of Hanhui 3, Huhan 1B and Huhan 7A.
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
File S1. Methods.
Fig. S1. Temperatures under normal and heat stress conditions at heading and flowering stages.
Fig. S2. Distribution frequencies of panicle traits and heat tolerant traits in HH13 recombinant inbred line population.
Fig. S3. Genotype assay and phenotype analysis of molecular marker SPKIE-299 in HH13 and HY73 recombinant inbred lines.
Table S1. Temperatures inside and outside greenhouse at heading and flowering stages.
Table S2. Phenotypic variations of panicle traits and heat tolerant traits.
Table S3. Correlations among panicle traits and heat tolerant traits in HH13 recombinant inbred line population.
Table S4. Information of genetic linkage map.
Table S5. QTLs identified for panicle traits and heat tolerance.
Table S6. Known genes in QTL intervals.
Table S7. Candidate genes for panicle traits and heat tolerance.
Table S8. Single nucleotide polymorphism (SNP) information of.
Table S9. Haplotype ofin rice.
Table S10. Primers of gene markers.
Cao L Y, Zhao J G, Zhan X D, Li D L, He L B, Cheng S H. 2003. Mapping QTLs for heat tolerance and correlation between heat tolerance and photosynthetic rate in rice., 17: 223–227. (in Chinese with English abstract)
Cao Z B, Li Y, Tang H W, Zeng B H, Tang X Y, Long Q Z, Wu X F, Cai Y H, Yuan L F, Wan J L. 2020. Fine mapping of theQTL, which confers heat tolerance at the booting stage, using anGriff. introgression line., 133(4): 1161–1175.
Chepyshko H, Lai C P, Huang L M, Liu J H, Shaw J F. 2012. Multifunctionality and diversity of GDSL esterase/lipase gene family in rice (L.) genome: New insights from bioinformatics analysis., 13: 309.
Chou T S, Chao Y Y, Kao C H. 2012. Involvement of hydrogen peroxide in heat shock- and cadmium-induced expression of ascorbate peroxidase and glutathione reductase in leaves of rice seedlings., 169(5): 478–486.
Fujita D, Trijatmiko K R, Tagle A G, Sapasap M V, Koide Y, Sasaki K, Tsakirpaloglou N, Gannaban R B, Nishimura T, Yanagihara S, Fukuta Y, Koshiba T, Slamet-Loedin I H, Ishimaru T, Kobayashi N. 2013.allele from a rice landrace greatly increases yield in moderncultivars., 110(51): 20431–20436.
Hackbusch J, Richter K, Müller J, Salamini F, Uhrig J F. 2005. A central role ofovate family proteins in networking and subcellular localization of 3-aa loop extension homeodomain proteins., 102(13): 4908–4912.
Hartl F U, Bracher A, Hayer-Hartl M. 2011. Molecular chaperones in protein folding and proteostasis., 475: 324–332.
Ku S J, Yoon H, Suh H S, Chung Y Y. 2003. Male-sterility of thermo- sensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum., 217(4): 559–565.
Lafarge T, Bueno C, Frouin J, Jacquin L, Courtois B, Ahmadi N. 2017. Genome-wide association analysis for heat tolerance at flowering detected a large set of genes involved in adaptation to thermal and other stresses., 12(2): e0171254.
Leipe D D, Koonin E V, Aravind L. 2004. STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: Multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer., 343(1): 1–28.
Li L H, Lv M M, Li X, Ye T Z, He X, Rong S H, Dong Y L, Guan Y, Gao X L, Zhu J Q, Xu Z J. 2018. The ricefamily: OsDUF810.7 may be involved in the tolerance to salt and drought., 52(4): 489–496.
Li X M, Chao D Y, Wu Y, Huang X H, Chen K, Cui L G, Su L, Ye W W, Chen H, Chen H C, Dong N Q, Guo T, Shi M, Feng Q, Zhang P, Han B, Shan J X, Gao J P, Lin H X. 2015. Natural alleles of a proteasome α2 subunit gene contribute to thermo- tolerance and adaptation of African rice., 47(7): 827–833.
Liu Q, Yang T F, Yu T, Zhang S H, Mao X X, Zhao J L, Wang X F, Dong J F, Liu B. 2017. Integrating small RNA sequencing with QTL mapping for identification of miRNAs and their target genes associated with heat tolerance at the flowering stage in rice., 8: 43.
Lüders J, Demand J, Höhfeld J. 2000. The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome., 275(7): 4613–4617.
Matsui T, Omasa K, Horie T. 2000. High temperature at flowering inhibits swelling of pollen grains, a driving force for thecae dehiscence in rice (L.)., 3(4): 430–434.
Matsui T, Omasa K, Horie T. 2001. The difference in sterility due to high temperatures during the flowering period among- rice varieties., 4(2): 90–93.
Montero-Barrientos M, Hermosa R, Cardoza R E, Gutiérrez S, Nicolás C, Monte E. 2010. Transgenic expression of thegene increasesresistance to heat and other abiotic stresses., 167(8): 659–665.
Prasad P V V, Boote K J, Jr Allen L H, Sheehy J E, Thomas J M G. 2006. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress., 95(2/3): 398–411.
Shanmugavadivel P S, Amitha Mithra S V, Chandra P, Ramkumar M K, Ratan T, Trilochan M, Nagendra K S. 2017. High resolution mapping of QTLs for heat tolerance in rice using a 5K SNP array., 10: 28.
Shen G D, Sun W L, Chen Z C, Shi L, Hong J, Shi J X. 2022. Plant GDSL esterases/lipases: Evolutionary, physiological and molecular functions in plant development., 11(4): 468.
Shin Y, Klucken J, Patterson C, Hyman B T, McLean P J. 2005. The co-chaperone carboxyl terminus of Hsp70-interacting protein (CHIP) mediates alpha-synuclein degradation decisions between proteasomal and lysosomal pathways., 280(25): 23727–23734.
Wang S C, Chang Y, Guo J J, Zeng Q N, Ellis B E, Chen J G. 2011.ovate family proteins, a novel transcriptional repressorfamily, control multiple aspects of plant growth and development., 6(8): e23896.
Wang W X, Vinocur B, Shoseyov O, Altman A. 2004. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response., 9(5): 244–252.
Xing Y Z, Zhang Q F. 2010. Genetic and molecular bases of rice yield., 61: 421–442.
Xu Y F, Zhang L, Ou S J, Wang R C, Wang Y M, Chu C C, Yao S G. 2020. Natural variations ofconfer high-temperature tolerance inrice., 11(1): 5441.
Ye C R, Argayoso M A, Redoña E D, Sierra S N, Laza M A, Dilla C J, Mo Y, Thomson M J, Chin J, Delaviña C B, Diaz G Q, Hernandez J E. 2012. Mapping QTL for heat tolerance at flowering stage in rice using SNP markers., 131(1): 33–41.
Ye C R, Tenorio F A, Redoña E D, Morales-Cortezano P S, Cabrega G A, Jagadish K S V, Gregorio G B. 2015. Fine-mapping and validatingto increase spikelet fertility under heat stress at flowering in rice., 128(8): 1507–1517.
Yu H, Jiang W Z, Liu Q, Zhang H, Piao M X, Chen Z D, Bian M D. 2015. Expression pattern and subcellular localization of the ovate protein family in rice., 10(3): e0118966.
Zhang G H, Li S Y, Wang L, Ye W J, Zeng D L, Rao Y C, Peng Y L, Hu J, Yang Y L, Xu J, Ren D Y, Gao Z Y, Zhu L, Dong G J, Hu X M, Yan M X, Guo L B, Li C Y, Qian Q. 2014.fromcultivar, which is allelic to, increases yield ofsuper rice 93-11., 7(8): 1350–1364.
Zhu J, Lou Y, Shi Q S, Zhang S, Zhou W T, Yang J, Zhang C, Yao X Z, Xu T, Liu J L, Zhou L, Hou J Q, Wang J Q, Wang S, Huang X H, Yang Z N. 2020. Slowing development restores the fertility of thermo-sensitive male-sterile plant lines., 6(4): 360–367.
Zuppini A, Bugno V, Baldan B. 2006. Monitoring programmed cell death triggered by mild heat shock in soybean-cultured cells., 33(7): 617–627.
26 May 2022;
15 July 2022
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2022.07.008
MaXiaosong (mxs09@sagc.org.cn); Luo Lijun (lijun@sagc.org.cn)
杂志排行
Rice Science的其它文章
- Transcriptome Analysis Provides Novel Insights into Salt Stress Response in Two Egyptian Rice Varieties with Different Tolerance Levels
- QTL Mapping for Plant Height Using Introgression Lines Derived from Zhonghui 8015 and Wild Rice (Oryza rufipogon)
- Improvement of Rice Production under Drought Conditions in West Africa:Application of QTLs in Breeding for Drought Resistance
- Characterization of a Novel Weak Allele of RGA1/D1 and Its Potential Application in Rice Breeding
- CRISPR/Cas9-Targeted Knockout of Rice Susceptibility Genes OsDjA2 and OsERF104 Reveals Alternative Sources of Resistance to Pyricularia oryzae
- Identification of Potential Zinc Deficiency Responsive Genes and Regulatory Pathways in Rice by Weighted Gene Co-expression Network Analysis
